Abstract
Background
Synbiotics decrease antibiotic‐associated gastrointestinal signs (AAGS) in cats, but data supporting synbiotic use to ameliorate AAGS in dogs are lacking.
Objectives
To determine if administration of synbiotics mitigates AAGS in dogs.
Animals
Twenty‐two healthy research dogs.
Methods
Randomized, double‐blinded, placebo‐controlled, 2‐way, 2‐period, crossover study with an 8‐week washout period. Each period included a 1‐week baseline and 3‐week treatment phase. Dogs received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by a bacterial/yeast synbiotic combination or placebo. Food intake, vomiting, and fecal score were compared using repeated‐measures crossover analyses, with P < .05 considered significant.
Results
Hyporexia, vomiting, and diarrhea occurred in 41% (95% confidence interval [CI], 21‐64), 77% (95% CI, 55‐92), and 100% (95% CI, 85‐100) of dogs, respectively, during the first treatment period. Derangements in food intake were smaller in both periods for dogs receiving synbiotics (F‐value, 5.1; P = .04) with treatment‐by‐period interactions (F‐value, 6.0; P = .02). Days of vomiting differed over time (F‐value, 4.7; P = .006). Fecal scores increased over time (F‐value, 33.5; P < .001), were lower during period 2 (F‐value, 14.5; P = .001), and had treatment‐by‐period effects (F‐value, 4.8; P = .04).
Conclusions and Clinical Importance
Enrofloxacin/metronidazole administration is associated with a high frequency of AAGS. Synbiotic administration decreases food intake derangements. The presence of milder AAGS in period 2 suggests that clinical effects of synbiotics persist >9 weeks after discontinuation, mitigating AAGS in dogs being treated with antibiotics followed by placebo.
Keywords: antibiotic‐associated diarrhea, antibiotic‐associated gastrointestinal signs, diarrhea and vomiting, probiotic, saccharomyces boulardii
Abbreviations
- AAGS
antibiotic‐associated gastrointestinal signs
- CI
confidence interval
1. INTRODUCTION
Antibiotic‐associated gastrointestinal signs (AAGS) occur commonly in people1 and cats,2, 3, 4 and they are a common cause of antibiotic noncompliance. In 1 recent report, diarrhea occurred in 56% of dogs treated with metronidazole.5 Furthermore, 26% of dog owners failed to administer at least 1 antibiotic dose in 1 study of short‐term administration, and a minority of owners followed instructions to administer antibiotics on an empty stomach.6 Noncompliance presumably is higher for longer term antibiotic administration,7 such as is often necessary for clearance of bacterial cholecystitis in dogs.8 Prevention or mitigation of AAGS in dogs might increase owner compliance during extended treatment and, thus, improve patient outcome.
Probiotic administration decreases the frequency of AAGS in people up to 3‐fold.1 Similarly, administration of a synbiotic (probiotic and prebiotic mixture) after antibiotic administration significantly decreases AAGS in cats.2 Several studies have demonstrated the efficacy of probiotic or synbiotic administration in the management of gastrointestinal diseases in dogs,9, 10, 11, 12, 13 but data are lacking regarding their impact on the development of AAGS. The purpose of our study was to determine the incidence of AAGS in healthy research dogs treated with antibiotics, followed by either placebo or a probiotic/synbiotic combination (Proviable‐Forte plus Mycequin, Nutramax Laboratories Veterinary Sciences, Inc, Lancaster, South Carolina) in a randomized, double‐blinded crossover trial. We hypothesized that administration of the probiotic/synbiotic combination 1 hour after antibiotic administration would lessen the severity or prevent the development of AAGS.
2. MATERIALS AND METHODS
2.1. Animals
The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Tennessee, Knoxville (protocol number 2544), and performed in compliance with the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals in laboratory animal facilities that are Association for Assessment and Accreditation of Laboratory Animal Care certified and exceed National Institutes of Health standards of care.
Given the lack of prior studies assessing the effects of synbiotic administration on AAGS in dogs, sample size calculation was performed using the results of a previously published study in cats.2 The sample size calculation was based on the use of an AB/BA crossover design, with a change in fecal score of 1.25 considered clinically relevant. A paired SD of 1.5 and conservative correlation of .2 were used for the calculation.2 Based on this calculation, a minimum sample size of 21 dogs would be required to achieve a power of 80% with an alpha of .05.
To accommodate the potential loss of subjects, 24 overtly healthy, purpose‐bred, research dogs were stratified by breed (Hound versus Beagle) and then randomized using a random number generator (https://www.random.org, accessed August 15, 2017) into 1 of 2 groups (A or B; Figure 1). Dogs received water ad libitum and were fed the same commercial adult dry dog food twice daily throughout the study. Dogs received imidacloprid and moxidectin (Advantage Multi for dogs; Bayer HealthCare, LLC, Shawnee Mission, Kansas), dosed according to the manufacturer's instructions, throughout the study as part of routine colony prophylaxis.
Figure 1.
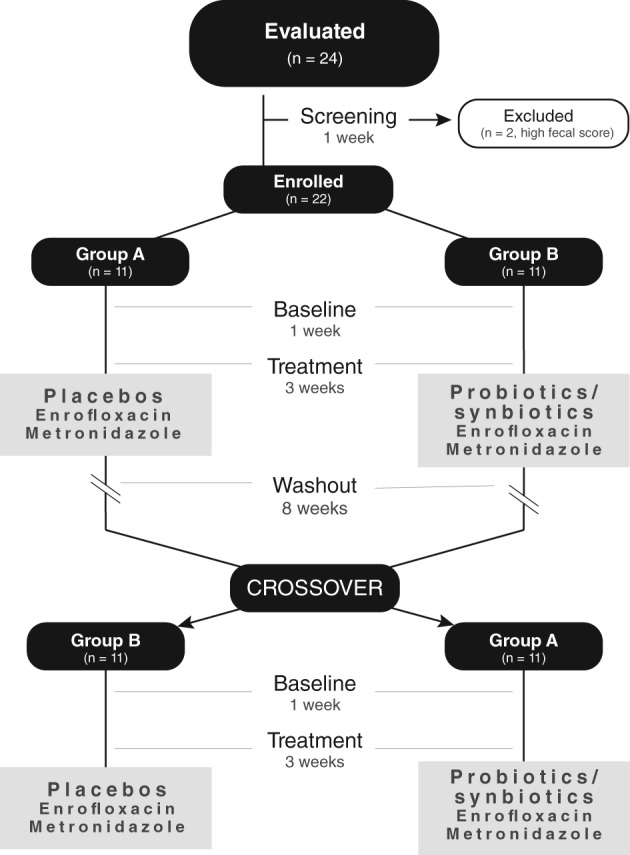
Flowchart of the study design. Healthy research dogs (n = 24) were evaluated and, after enrollment (n = 22), randomized to receive enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO, in a blinded controlled crossover trial
2.2. Initial screening
Dogs underwent physical examinations, laboratory testing, routine deworming, and twice‐daily observations (see below) for 1 week. Laboratory testing included CBCs, serum biochemistry panels, urinalyses, fecal direct smears, and sugar and zinc sulfate fecal flotations. Dogs were given ivermectin (200 μg/kg SC once) and fenbendazole (50 mg/kg, PO q24h x 5 d). Twice‐daily feeding of approximately 13 g of commercial adult canned food was initiated during screening, so that medication administration during treatment periods would not be associated with a change in diet.
2.3. Study periods
A randomized, double‐blinded, placebo‐controlled, 2‐way, 2‐period, crossover design with an 8‐week washout period was used for dogs enrolled in the study (Figure 1). Each study period was 4 weeks long and consisted of a 1‐week baseline followed by a 3‐week treatment period. After the first 7 weeks of the washout period, dogs underwent a 1‐week reacclimation to monitoring and canned food supplementation to identically match the 2 treatment periods.
2.4. Study medications
The probiotic/synbiotic combination consisted of 1 chewable multistrain bacterial probiotic tablet (Proviable‐Forte; Nutramax Laboratories Veterinary Sciences, Inc) plus 1 chewable yeast synbiotic tablet (Mycequin; Nutramax Laboratories Veterinary Sciences, Inc). Each tablet of the bacterial probiotic was formulated to contain 1 x 1010 cfu of a proprietary mixture of Bifidobacterium bifidum, Enterococcus faecium, Streptococcus thermophilus, and Lactobacillus acidophilus, bulgaricus, casei, and plantarum. Each tablet of the yeast synbiotic was formulated to contain 1 x 1010 cfu of a proprietary strain of Saccharomyces boulardii and the prebiotic beta‐glucan. Placebo tablets were provided by the manufacturer and did not differ in shape, size, smell, or flavoring from the probiotic and synbiotic tablets.
2.5. Treatments
Each dog received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h) in approximately 13 g of canned food, after which it was given its ration of commercial food. One hour after antibiotic administration, each dog was given 2 chewable tablets PO, containing either the probiotic/synbiotic combination or placebo as per group assignment. Dogs in group A received placebo in period 1 and the probiotic/synbiotic combination in period 2. Conversely, dogs in group B received the probiotic/synbiotic combination in period 1 and placebo in period 2.
2.6. Animal observations
An observer blinded to the treatment groups evaluated each dog twice daily. Food intake was quantitated daily. Vomiting was recorded as present or absent. If emesis was noted, the number of piles of vomitus was recorded, with each pile considered a separate event. Feces were scored using a published fecal scoring system.14 Feces also were photographed, with photos anonymized and randomized for fecal scoring after completion of the study by an investigator (J.C.W.) blinded to canine identity, time point, and treatment group. Body weight was measured once weekly. Dogs were removed from a treatment period if food intake was <50% of baseline food intake for >48 hours, >2 piles of vomitus were identified on 3 consecutive days, or loss of ≥6% of body weight occurred in 1 week.
2.7. Statistical analysis
Descriptive statistics were calculated for each variable. Samples were analyzed for normality using the Shapiro‐Wilk test and for the presence of outliers using box‐and‐whisker plots. Mean percent of food intake, percent days of vomiting, and mean fecal scores were determined for each week of each study period (baseline and treatment weeks 1, 2, and 3). Mean food intake for each week in each study period was calculated as a percentage of food intake during the acclimation week. Inter‐rater correlation coefficients were calculated for fecal scores recorded during daily observations and those assigned based on blinded review of photographs after study completion. Fecal scores assigned after study completion were used for all other statistical analyses. The number of dogs in each group that experienced ≥1 day of marked hyporexia (defined as food intake <50% of acclimation food intake), vomiting, or diarrhea (defined as fecal score ≥6) was determined for each period. Because diarrhea was observed in 100% of dogs (see Results section), exact binomial computations were used to determine 95% confidence intervals (CI) for frequencies of hyporexia, vomiting, and diarrhea for the whole population for the initial treatment period. Dogs that did not complete a treatment period were censored from data analyses at the point of removal from treatment.
Mean food intake, percent days vomiting per week, and mean fecal score were compared using 2‐treatment, 2‐sequence, 2‐period AB/BA crossover analyses with repeated measurements within periods that included fixed effects of treatment (placebo or probiotic/synbiotic combination), period, week, treatment‐by‐week, and period‐by‐treatment. Dog nested within sequence group was included as a random effect in all mixed model analyses of variance. A time period by dog nested within sequence was added to models in cases where significant external variability was accounted for with this effect. An unstructured Kronecker product variance/covariance structure was incorporated into each model. The Shapiro‐Wilk test of normality and QQ plots of the residuals were evaluated for each marker to confirm that the assumption of normally distributed residuals had been met. Model assumptions regarding equality of variances were verified using Levene's test for equality of variances. Based on these results, percent days of vomiting per week was rank transformed to meet model assumptions. Differences in marginal means were determined for markers with significant main effect or interaction terms. Generalized estimating equation proportional odds models were used to compare the likelihood of the occurrence of marked hyporexia, vomiting, and diarrhea between treatment groups and periods over time.
Commercial statistical software packages (MedCalc 15.8 MedCalc Software, Ostend, Belgium; SAS 9.4 release TS1M5, SAS Institute Inc., Cary, North Carolina) were used for all analyses. P < .05 was considered significant.
3. RESULTS
3.1. Animals
Two dogs, 1 from each group, were excluded from the study because of persistently high fecal scores during initial screening, leaving 22 dogs. All dogs were 1 year of age at the start of the study. There were 6 female intact Hound dogs and 5 male castrated Beagles in each group. Median weight was 9.3 kg (range, 7.3‐21.0 kg) for group A and 9.3 kg (range, 7.8‐18.5 kg) for group B.
3.2. Successful completion of treatment
One dog from group A was removed after the first week of treatment during period 1 because of loss of >6% body weight. This dog also had a waxing and waning appetite and episodic vomiting, but neither was severe enough to qualify for removal from treatment. The same dog was removed after the first week of treatment during period 2 for the same reason. All other dogs completed both treatment periods.
3.3. Other observations
Weight was not significantly associated with mean food intake, vomiting, or fecal score.
Mean food intake (Figure 2) differed significantly by treatment group (F‐value, 5.1; P = .04), with a significant period‐by‐treatment interaction (F‐value, 6.0; P = .02). Post hoc tests identified a statistically significant decrease in food intake between periods for dogs receiving the placebo (t‐value, 4.4; P < .001), with no significant differences observed between periods for dogs receiving the probiotic/synbiotic combination. Marked hyporexia occurred in 41% (95% CI, 21‐64) of the study population during the first treatment period (Table 1). No significant difference was found between treatment groups in the odds of experiencing marked hyporexia during treatment, although it was 7 times more likely to occur during treatment in period 1 (χ2 value, 4.4; 95% CI, 1.3‐38.1; P = .04).
Figure 2.
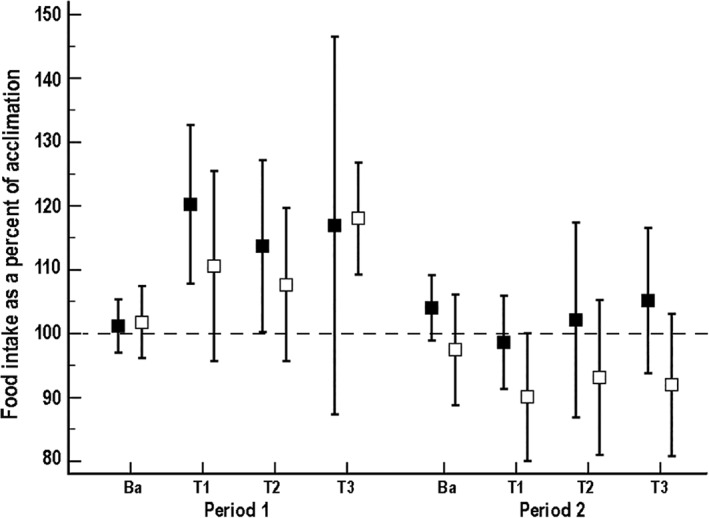
Mean (±SD) percent food intake per week compared to acclimation for 21 dogs that received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A (n = 10*, represented by solid black squares) received placebo during period 1 and the probiotic/synbiotic combination in period 2, whereas dogs in group B (n = 11, represented by open black squares) received the probiotic/synbiotic combination during period 1 and placebo during period 2. Ba, baseline; T1, treatment week 1; T2, treatment week 2; T3, treatment week 3. *Data were not available for 1 dog due to removal from treatment
Table 1.
Number of dogs per week that had food intake <50% of acclimation for at least 1 day
| Period 1 | Period 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |||||||
| Week 1 | Week 2 | Week 3 | Weeks 1‐3 | Week 1 | Week 2 | Week 3 | Weeks 1‐3 | |||
| Group A | 1/11 | 1/11 | 3/10a | 3/10a | 5/11 | 0/11 | 1/11 | 0/10a | 0/10a | 1/11 |
| Group B | 0/11 | 2/11 | 3/11 | 0/11 | 4/11 | 1/11 | 2/11 | 1/11 | 1/11 | 2/11 |
| All dogs | 1/22 | 3/22 | 6/21 | 3/21 | 9/22 | 1/22 | 3/22 | 1/21 | 1/21 | 3/21 |
Dogs received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A received placebos during period 1 and probiotics in period 2, whereas dogs in group B received the probiotic/synbiotic combination during period 1 and placebos during period 2.
Data were not available for 1 dog due to removal from treatment.
Vomiting (Figure 3) increased in both groups during treatment, and it differed significantly over time (F‐value, 4.7; P = .006) but not by treatment group or period. Vomiting was highest during the first week of antibiotic administration, with as many as 10 piles of vomitus identified in an individual run over the course of a 24‐hour period. Vomiting generally was episodic and accompanied by hyporexia. Vomiting occurred in 77% (95% CI, 55‐92) of the study population during the first treatment period (Table 2). No significant difference in the odds of vomiting during treatment between treatment groups or periods was observed.
Figure 3.
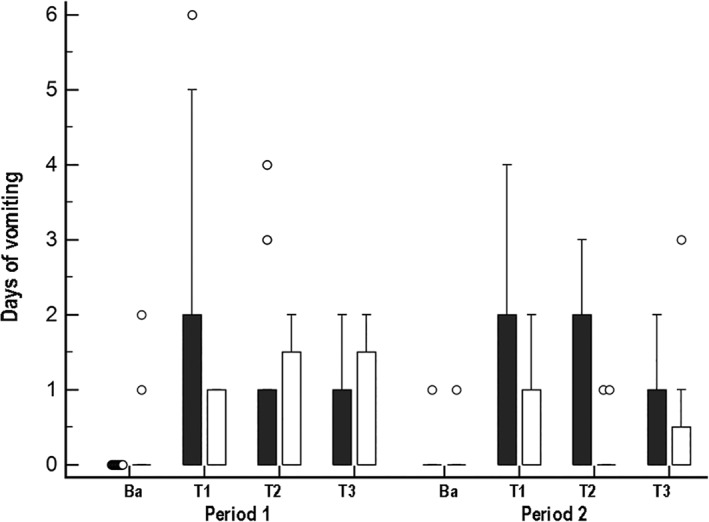
Box and whisker plots of days of vomiting per week for 21 dogs that received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A (n = 10*, represented by black boxes) received placebo during period 1 and the probiotic/synbiotic combination in period 2, whereas dogs in group B (n = 11, represented by white boxes) received the probiotic/synbiotic combination during period 1 and placebo during period 2. Ba, baseline; T1, treatment week 1; T2, treatment week 2; T3, treatment week 3. *Data were not available for 1 dog due to removal from treatment
Table 2.
Number of dogs per week that vomited at least once
| Period 1 | Period 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |||||||
| Week 1 | Week 2 | Week 3 | Weeks 1‐3 | Week 1 | Week 2 | Week 3 | Weeks 1‐3 | |||
| Group A | 0/11 | 5/11 | 6/10a | 5/10a | 10/11 | 1/11 | 4/11 | 4/10a | 3/10a | 6/11 |
| Group B | 2/11 | 5/11 | 4/11 | 5/11 | 7/11 | 1/11 | 5/11 | 2/11 | 3/11 | 7/11 |
| All dogs | 2/22 | 10/22 | 10/21 | 10/21 | 17/22 | 2/22 | 9/22 | 6/21 | 6/21 | 13/22 |
Dogs received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A received placebos during period 1 and probiotics in period 2, whereas dogs in group B received the probiotic/synbiotic combination during period 1 and placebos during period 2.
Data were not available for 1 dog due to removal from treatment.
The inter‐rater correlation coefficient for fecal scores recorded during daily observations and those assigned during blinded review was .82 for period 1 and .76 for period 2. Fecal scores (Figure 4) increased significantly over time during treatment (F‐value, 33.5; P < .001). Significant period (F‐value, 14.5; P = .001) and period‐by‐treatment (F‐value, 4.8; P = .04) interactions were identified. Post hoc tests determined that dogs receiving the probiotic/synbiotic treatment during period 2 had significantly lower fecal scores than dogs receiving placebo in the same period (t‐value, 2.2; P = .04). Diarrhea occurred in 100% (95% CI, 85‐100) of the study population during the first treatment period (Table 3). No significant difference was found in the odds of diarrhea occurring during treatment between treatment groups or periods.
Figure 4.
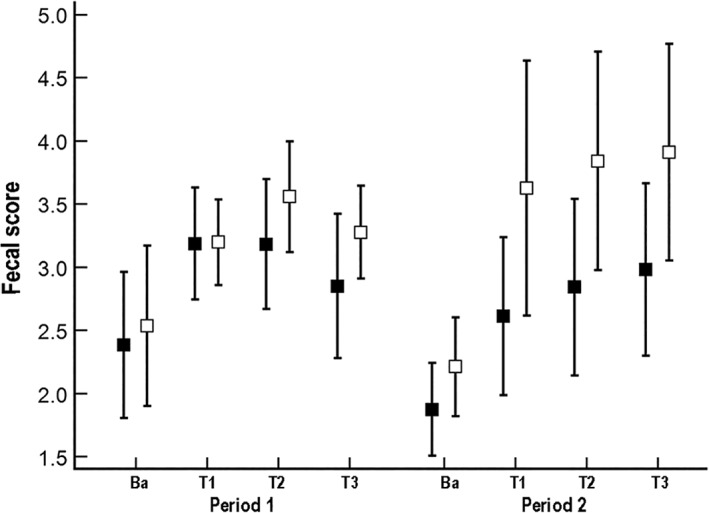
Mean (±SD) fecal scores per week for 21 dogs that received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A (n = 10*, represented by solid black squares) received placebo during period 1 and the probiotic/synbiotic combination in period 2, whereas dogs in group B (n = 11, represented by open black squares) received the probiotic/synbiotic combination during period 1 and placebo during period 2. Ba, baseline; T1, treatment week 1; T2, treatment week 2; T3, treatment week 3. *Data were not available for 1 dog due to removal from treatment
Table 3.
Number of dogs per week that had a fecal score ≥6 at least once
| Period 1 | Period 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |||||||
| Week 1 | Week 2 | Week 3 | Weeks 1‐3 | Week 1 | Week 2 | Week 3 | Weeks 1‐3 | |||
| Group A | 1/11 | 7/11 | 8/10a | 4/10a | 11/11 | 1/11 | 4/11 | 5/10a | 4/10a | 8/11 |
| Group B | 3/11 | 7/11 | 10/11 | 8/11 | 11/11 | 2/11 | 7/11 | 6/11 | 7/11 | 8/11 |
| All dogs | 4/22 | 14/22 | 18/21 | 12/21 | 22/22 | 3/22 | 11/22 | 11/21 | 11/21 | 16/22 |
Dogs received enrofloxacin (10 mg/kg PO q24h) and metronidazole (12.5 mg/kg PO q12h), followed 1 hour later by either a placebo or probiotic/synbiotic combination PO. Dogs in group A received placebos during period 1 and probiotics in period 2, whereas dogs in group B received the probiotic/synbiotic combination during period 1 and placebos during period 2.
Data were not available for 1 dog due to removal from treatment.
4. DISCUSSION
Although generally self‐limiting, the development of AAGS in dogs can result in noncompliance or premature discontinuation of antibiotic treatment.6, 7 Marked hyporexia, vomiting, and diarrhea occurred in 41%, 77%, and 100% of dogs, respectively, that were given antibiotics in period 1 of our study (Tables 1, 2, 3). Mean food intake during antibiotic administration differed significantly from acclimation intake for dogs treated with placebo, with higher intake during treatment in period 1 and lower intake during treatment in period 2. In contrast, mean food intake did not differ significantly from acclimation during treatment in either period for dogs treated with the probiotic/synbiotic combination. Days of vomiting and mean fecal score did not differ between treatment groups based on treatment and treatment‐by‐week interactions. Period effects, however, were identified despite a prolonged washout period. Dogs were 7 times more likely to experience marked hyporexia (food intake <50% of acclimation intake) during period 1. Furthermore, fecal scores were significantly lower in period 2 and a significant period‐by‐treatment interaction also was identified. Post hoc analysis confirmed that these associations were a result of significantly lower fecal scores for dogs receiving the probiotic/synbiotic combination versus the placebo in period 2 (Figure 4).
Wide variability was noted in the occurrence and types of AAGS that individual dogs experienced. Days of decreased food intake often alternated with days of binge eating in period 1, resulting in increased mean food intake. During period 2, changes were more complex. Despite a significant decrease in the number of dogs with food intake <50% on at least 1 day, mean food intake decreased in dogs receiving the placebo, whereas it did not significantly differ from baseline for dogs receiving the probiotic/synbiotic combination. Vomiting was unexpectedly common in our study, considering its lack of occurrence in studies of healthy dogs treated with amoxicillin,15 tylosin,16 or metronidazole.5 Although antibiotics were administered at clinically relevant doses and after feeding in our study, emesis occurred in 64%‐84% and 55%‐64% of dogs treated with enrofloxacin and metronidazole followed by placebo or the probiotic/synbiotic combination, respectively. Vomiting was most frequent during the first week of antibiotic administration, but it occurred throughout both treatment periods. Although the exact frequency of vomiting could not be determined because of a lack of continuous observation, approximately half of dogs vomited at least once in the first week of antibiotic administration, several dogs vomited on >2 days in the first week, and 10 piles of vomitus were identified in each of 2 dogs’ runs over the course of 24 hours. Although differences in occurrences of vomiting between this and prior studies in dogs could be a consequence of the use of enrofloxacin, fluoroquinolones are not considered a “high‐risk” antibiotic for the development of AAGS in people.1 Administration of fluoroquinolones in combination with metronidazole, however, greatly broadens the antibiotic spectrum of activity, which is a strong risk factor for AAGS.1 Diarrhea was extremely common, occurring on at least 1 day of treatment in 100% and 73% of dogs during periods 1 and 2, respectively. Mean fecal scores also increased significantly over time regardless of period. However, fecal scores were significantly lower during period 2 in dogs given the probiotic/synbiotic mixture compared to dogs treated with placebo.
Probiotic or synbiotic administration commonly is staggered in patients receiving antibiotics to limit inactivation of antibiotic‐sensitive bacterial strains.2, 4 However, the ideal dosing schedule is unknown and can vary for patients with altered gastrointestinal motility associated with stress or hospitalization. An alternative approach to staggering treatments is to use a yeast probiotic, such as S boulardii, which is resistant to most antibiotics.17 Monotherapy with S boulardii previously has been shown to decrease the risk of AAGS in both adults and children by over 50%.18, 19 Because yeast probiotics have different potential mechanisms of action than bacterial agents, combination treatment can result in synergistic benefits.20 Thus, dogs in our study were given a synbiotic containing S boulardii in combination with a probiotic product containing the same bacterial strains and cfus as used in a study that demonstrated efficacy of synbiotics in ameliorating AAGS in cats.2
Administration of the probiotic/synbiotic combination mitigated some, but not all, AAGS induced by administration of enrofloxacin and metronidazole in our study. As noted above, the probiotic/synbiotic combination mitigated antibiotic‐associated derangements in food intake, based on significant treatment and treatment‐by‐period interactions. Period effects also were found for marked hyporexia, with a 7‐fold increase in odds of its occurrence in period 1. These results are similar to those for cats given a synbiotic 1 hour after clindamycin administration,2 supporting a role for probiotics and synbiotics in preventing antibiotic‐associated derangements in appetite. Also consistent with that study and other studies in cats,2, 3, 4 neither the number of dogs that experienced at least 1 day of diarrhea nor mean fecal score differed significantly between treatment groups in our study. Unexpectedly, fecal scores were significantly lower in period 2, as a consequence of lower scores for dogs receiving the probiotic/synbiotic combination (Figure 4). Although habituation to antibiotics cannot be ruled out, repeated exposure increases the risk of AAGS in people.18 It is more likely that our study was underpowered to detect treatment‐by‐time interactions. It previously has been shown that detection of interactions can require up to 16 times more subjects than detection of a main effect.21
Unfortunately, probiotic/synbiotic administration did not significantly alter the incidence or frequency of vomiting in our study. Faced with the repeated and sometimes marked vomiting experienced by dogs in our study, it is likely that many owners would have become noncompliant with dosing instructions or discontinued treatment entirely. This possibility is supported by a study of short‐term antibiotic administration in dogs,6 in which only 22% of owners complied with instructions to administer antibiotics on an empty stomach. Conflicting associations between probiotic and synbiotic administration and amelioration of vomiting previously have been reported for healthy cats.2, 3, 4 Neither the occurrence of vomiting nor the number of vomiting episodes differed between cats given amoxicillin‐clavulanate twice daily for 7 days when either a placebo or a probiotic (Fortiflora Probiotic Supplement; Nestle Purina PetCare, St. Louis, Missouri) was given 2 hours before the first antibiotic dose of each day.4 Similarly, although vomiting was less frequent in cats treated with both clindamycin and a synbiotic (Proviable‐DC; Nutramax Laboratories Veterinary Sciences, Inc) versus placebo in another study, the difference was not statistically significant.3 In contrast, vomiting was significantly less frequent in cats treated with clindamycin followed 1 hour later by 2 capsules of the same synbiotic versus 2 capsules of placebo.2 Because the last study also used a lower clindamycin dose and a crossover design, it is unclear whether the higher synbiotic dose or change in dosing schedule contributed to the difference in statistical outcome. Given the lack of data on potential synergism of combination treatment in dogs, dogs in our study received the same bacterial probiotic dose, regardless of weight, as was used successfully in cats to ameliorate AAGS. Because the dogs used in our study weighed substantially more than cats in the prior report,2 both the probiotic dose per administration and total daily dose were lower. Additionally, a different prebiotic, beta‐glucan, was used in our current study compared to those used in the prior report. The dose of S boulardii also was substantially lower than that used to manage inflammatory bowel disease in dogs. Although no association was found between weight and AAGS in our study, it is possible that different results would have been obtained had either a higher probiotic or synbiotic dose or both been used.
Major advantages of crossover designs compared to parallel group trials are that they increase study power relative to sample size by decreasing between‐subject variability and facilitate simultaneous comparisons between and within groups. Both can be particularly helpful when evaluating phenomena with high interindividual variation, such as occurrences of AAGS. As such, a crossover design was selected for our study. Given the lack of prior studies evaluating the impact of synbiotics on AAGS in dogs, the washout period was extrapolated from a crossover study that used a 6‐week washout period in cats.2 At the conclusion of the washout period, cats initially treated with placebo had persistent AAGS, which then normalized during period 2. Conversely, cats initially treated with the synbiotic developed less severe AAGS when given the placebo during period 2. The washout period for our study, therefore, was extended to 8 weeks. Unfortunately, carryover still occurred, resulting in decreased occurrence of hyporexia and lower fecal scores in period 2. Both could reflect sustained protective effects of the probiotic/synbiotic combination against future AAGS, but habituation to antibiotic administration cannot be ruled out.
A few other limitations of our study should be noted. The first was use of young, healthy research dogs with minimal prior antibiotic exposure as subjects. Sensitivity to AAGS has been found to increase with repeated exposure and age.2, 22 As such, results might differ for older dogs, dogs with prior antibiotic exposure, or those with systemic disease. Furthermore, because dogs were not under continuous surveillance, each pile of vomitus was considered a separate vomiting event. This could have resulted in either underestimation or overestimation of vomiting frequency, if dogs consumed vomitus or moved about during emesis, respectively. Finally, care should be taken in extrapolation of results to other antibiotics, probiotics or synbiotics, and dosing regimens, particularly given the discordant results among studies of probiotics/synbiotics and AAGS in cats. Although recommended in people, the impact of administration of probiotics or synbiotics for several weeks after antibiotic discontinuation also was not assessed in our study.
Although enrofloxacin and metronidazole were administered at clinically relevant doses and after feeding, an extremely high incidence of AAGS was identified. In period 1, 41%, 77%, and 100% of dogs experienced at least 1 day of hyporexia, vomiting, or diarrhea, respectively. Vomiting, in particular, was frequent and severe enough that the majority of owners would be anticipated to discontinue treatment. Administration of the probiotic/synbiotic combination significantly decreased derangements in mean food intake in both periods and was associated with significantly lower fecal scores in period 2, but it did not ameliorate vomiting or changes in fecal score in period 1. Dogs were significantly less likely to experience marked hyporexia and had lower fecal scores during period 2. Further evaluation is required to determine the efficacy of probiotics and synbiotics in ameliorating AAGS in clinically ill dogs, whether potential benefits of their use outweigh the disadvantages of polypharmacy, and to clarify the role of historical probiotic or synbiotic administration on future AAGS.
CONFLICT OF INTEREST DECLARATION
Dr. Whittemore has received honoraria from Nutramax Laboratories for development of educational materials and public speaking. She also has received travel support for presentation of preliminary results of this study at the annual meeting of the European College of Veterinary Internal Medicine. Nutramax had no involvement in the design or performance of the study, writing of the manuscript, or the decision to submit the manuscript for publication. Further, use of placebo controls, randomization, and blinding of investigators to treatment groups and time points were employed to prevent bias in data collection or evaluation. Unblinding to treatment groups was not performed until after completion of the statistical analyses.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the IACUC of the University of Tennessee, Knoxville (protocol number 2544) and performed in compliance with “The Guide for the Care and Use of Laboratory Animals” in laboratory animal facilities that are Association for Assessment and Accreditation of Laboratory Animal Care certified and exceed National Institutes of Health standards of care.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Whittemore JC, Moyers TD, Price JM. Randomized, controlled, crossover trial of prevention of antibiotic‐induced gastrointestinal signs using a synbiotic mixture in healthy research dogs. J Vet Intern Med. 2019;33:1619–1626. 10.1111/jvim.15553
REFERENCES
- 1. McFarland LV. Antibiotic‐associated diarrhea: epidemiology, trends, and treatment. Future Microbiol. 2008;3:563‐578. [DOI] [PubMed] [Google Scholar]
- 2. Stokes JE, Price JM, Whittemore JC. Randomized, controlled, crossover trial of prevention of clindamycin‐induced gastrointestinal signs using a synbiotic in healthy research cats. J Vet Intern Med. 2017;31:1406‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whittemore JC, Stokes JE, Laia NL, Price JM, Suchodolski JS. Short and long‐term effects of a synbiotic on clinical signs, the fecal microbiome, and metabolomic profiles in healthy research cats receiving clindamycin: a randomized, controlled trial. Peer J. 2018;6:e5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torres‐Henderson C, Summers S, Suchodolski J, Lappin MR. Effect of Enterococcus faecium strain SF68 on gastrointestinal signs and fecal microbiome in cats administered amoxicillin‐clavulanate. Top Companion Anim Med. 2017;32:104‐108. [DOI] [PubMed] [Google Scholar]
- 5. Olson E, Honneffer J, Waddle M, et al. Evaluation of the effects of a 2 week treatment with metronidazole on the fecal microbiome of healthy dogs [abstract]. J Vet Intern Med. 2015;29:1184. [Google Scholar]
- 6. Adams VJ, Campbell JR, Waldner CL, Dowling PM, Shmon CL. Evaluation of client compliance with short‐term administration of antimicrobials to dogs. J Am Vet Med Assoc. 2005;226:567‐574. [DOI] [PubMed] [Google Scholar]
- 7. Weese JS, Giguère S, Guardabassi L, et al. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med. 2015;29:487‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence YA, Ruaux CG, Nemanic S, Milovancev M. Characterization, treatment, and outcome of bacterial cholecystitis and bactibilia in dogs. J Am Vet Med Assoc. 2015;246:982‐989. [DOI] [PubMed] [Google Scholar]
- 9. Herstad HK, Nesheim BB, L'Abée‐Lund T, et al. Effects of a probiotic intervention in acute canine gastroenteritis—a controlled clinical trial. J Small Anim Pract. 2010;51:34‐38. [DOI] [PubMed] [Google Scholar]
- 10. Arslan HH, Aksu DS, Terzi G, et al. Therapeutic effects of probiotic bacteria in parvoviral enteritis in dogs. Revue Méd Vét. 2012;163:55‐59. [Google Scholar]
- 11. Rose L, Rose J, Gosling S, Holmes M. Efficacy of a probiotic‐prebiotic supplement on incidence of diarrhea in a dog shelter: a randomized, double‐blind, placebo‐controlled trial. J Vet Intern Med. 2017;31:377‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossi G, Pengo G, Caldin M, et al. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One. 2014;9:e94699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Angelo S, Fracassi F, Bresciani F, et al. Effect of Saccharomyces boulardii in dogs with chronic enteropathies: double‐blinded, placebo‐controlled study. Vet Rec. 2018;182:258. [DOI] [PubMed] [Google Scholar]
- 14. Greco DS. Diagnosis and dietary management of gastrointestinal disease. In. https://www.purinaveterinarydiets.com/media/1202/gi_quick_reference_guide.pdf 2011.
- 15. Grønvold AM, L'abée‐Lund TM, Sørum H, et al. Changes in fecal microbiota of healthy dogs administered amoxicillin. FEMS Microbiol Ecol. 2010;71:313‐326. [DOI] [PubMed] [Google Scholar]
- 16. Suchodolski JS, Dowd SE, Westermarck E, et al. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixit K, Gandhi D. Biotherapeutic properties of probiotic yeast Saccharomyces species in fermented dairy foods[Internet]. Dairy Science Food Technology; April 16, 2010. http://www.dairyscience.info/probiotics/105‐biotherapeutic‐probiotic‐yeast.html.
- 18. Szajewska H, Konarska Z, Kołodziej M. Probiotic bacterial and fungal strains: claims with evidence. Dig Dis. 2016;34:251‐259. [DOI] [PubMed] [Google Scholar]
- 19. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic‐associated diarrhea: a systematic review and meta‐analysis. JAMA. 2012;307:1959‐1969. [DOI] [PubMed] [Google Scholar]
- 20. Bisson J‐F, Hidalgo S, Rozan P, Messaoudi M. Preventive effects of different probiotic formulations on travelers' diarrhea model in Wistar rats. Dig Dis Sci. 2010;55:911‐919. [DOI] [PubMed] [Google Scholar]
- 21. Gelman A. You need 16 times the sample size to estimate an interaction than to estimate a main effect [Internet]. Statistical Modeling, Casual Inference, and Social Science; November 9, 2018. https://andrewgelman.com/2018/03/15/need‐16‐times‐sample‐size‐estimate‐interaction‐estimate‐main‐effect/.
- 22. Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B. Probiotic approach to prevent antibiotic resistance. Ann Med. 2016;48:246‐255. [DOI] [PubMed] [Google Scholar]


