Abstract
Background
A recent genome‐wide association study in German Shepherd dogs (GSDs) with chronic enteropathy (CE) has identified polymorphisms in the Th2 cytokine genes.
Hypothesis/objective
To determine if the expression of the Th2 cytokines, interleukin‐13 (IL‐13) and interleukin‐33 (IL‐33), is altered in the duodenal mucosa of GSDs with CE compared to non‐GSDs with CE and healthy dogs.
Animals
Twenty client‐owned dogs diagnosed with CE (10 GSDs and 10 non‐GSDs) at the Bristol Veterinary School and 8 healthy Beagle dogs from the Iowa State University Service Colony.
Methods
Retrospective study using archived paraffin‐embedded duodenal biopsy samples. A novel RNA in situ hybridization technology (RNAscope) was used to hybridize IL‐13 and IL‐33 mRNA probes onto at least 10 sections from duodenal biopsy samples for each dog. RNAscope signals were visualized using a microscope and semi‐quantitative assessment was performed by a single operator.
Results
Based on duodenal villus, subvillus, epithelial, and lamina propria average expression scores, GSDs with CE had significantly lower IL‐13 and IL‐33 mRNA expression compared to non‐GSDs with CE (IL‐13, P < .04; IL‐33, P < .02) and healthy Beagle dogs (IL‐13, P < .02; IL‐33, P < .004).
Conclusions and Clinical Importance
Similar to human patients with ulcerative colitis, a subtype of human inflammatory bowel disease, these data indicate that Th2 cytokines may be involved in the pathogenesis of CE in GSDs.
Keywords: bowel, canine, cytokine, duodenum, inflammatory, Th2
Abbreviations
- CCEAI
canine chronic enteropathy activity index
- CD
Crohn's disease
- CE
chronic enteropathy
- DSS
dextran sodium sulfate
- FRE
food‐responsive enteropathy
- GI
gastrointestinal
- GSD
German Shepherd dog
- GWAS
genome‐wide association study
- IBD
inflammatory bowel disease
- IL
interleukin
- ISH
in situ hybridization
- UC
ulcerative colitis
1. INTRODUCTION
Inflammatory bowel disease (IBD) in humans is an idiopathic chronic relapsing disease,1 hypothesized to involve the interplay of 4 key components: (1) genetic susceptibility, (2) environmental risk factors, (3) intestinal dysbiosis, and (4) altered gastrointestinal (GI) mucosal immune response.2 Inflammatory bowel disease in humans is comprised of Crohn's disease (CD) and ulcerative colitis (UC), which differ in pathogenesis, localization of disease within the GI tract, and therapeutic management.2 The identification of inflammatory mediators by genome‐wide association studies (GWAS) has enabled the development of specific treatment options for either CD or UC, with efficacious biologic drugs such as infliximab (anti tumor necrosis factor‐alpha antibody) in CD3, 4, 5 and tofacitinib6, 7 (a JAK kinase inhibitor that decreases secretion of several Th2 cytokines) in UC.
Similar to IBD in humans, chronic enteropathy (CE) in dogs also is a chronic relapsing disease and the pathogenesis is considered to involve the same 4 key components.8 Chronic enteropathy is predominantly treated with corticosteroids,9, 10 but up to 50% of dogs will develop resistance, clinically important adverse effects, or both, which ultimately leads to euthanasia.11 It is therefore critical to develop more specific and efficacious treatment for long‐term management of dogs with CE. In the United Kingdom, German Shepherd dogs (GSDs) are at a significantly higher risk of developing CE compared with mixed breed dogs.12 One recent study identified several single nucleotide polymorphisms in IL‐4, IL‐5, and IL‐13 that were significantly associated with CE in GSDs.13 This novel finding is reminiscent of the strong Th2 cytokine predominance found in the intestinal mucosa in humans with UC and suggests that Th2 cytokines also might be implicated in the pathogenesis of CE in GSDs. Previous studies investigating the mucosal cytokine profile in dogs with CE have not definitively determined a Th1, Th2, or Th17 predominance.14, 15 However, many of these studies were performed on few samples and utilized different molecular methods, which makes it difficult to directly compare results among studies. Also, these studies largely included different breeds of dogs, and because of limited sample sizes, subanalysis of cytokine expression for each breed was not performed. Inflammatory profiles may differ among breeds of dogs presenting with CE, because of different etiopathogeneses.
We aimed to determine if mRNA expression of the Th2 cytokines, IL‐13 and IL‐33 was altered in the duodenal mucosa of GSDs with CE compared to non‐GSDs with CE and healthy Beagle dogs, using a novel RNA in situ hybridization (ISH) technology, RNAscope. The advantages of RNAscope include a unique patented probe (Z probe) design which increases amplification of target‐specific signals but not background noise.16 Interleukin‐13 and IL‐33 were chosen for further investigation, because recent results of GWAS in GSDs with CE implicated the IL‐13 gene, and studies in human and mouse models of IBD have highlighted the importance of IL‐33, which has not been extensively investigated to date in dogs with CE. We hoped to further unravel the pathogenesis of CE in GSDs, which might help in the development of novel therapeutic modalities, as is already the case for UC in humans.
2. MATERIALS AND METHODS
2.1. Study design
We performed a retrospective study using archived paraffin‐embedded 10% formalin‐fixed duodenal biopsy samples from dogs with CE referred to Bristol Veterinary School. Tissue specimens from 3 groups of dogs were studied: GSD CE (N = 10), non‐GSD CE (N = 10), and healthy controls (N = 8) from a population of healthy Beagle dogs at the Iowa State University.
2.2. Retrospective study criteria for case selection
The histopathology archive at the Bristol Veterinary School was searched for GSD and non‐GSD cases with a histologic diagnosis of chronic inflammatory enteropathy. All medical records were then reviewed by 1 of the authors (Aarti Kathrani). Only dogs with a histologic diagnosis of chronic inflammatory enteropathy that had adequate and appropriate investigations to exclude other causes of chronic GI signs before histologic diagnosis were included.17 All dogs had intestinal biopsy samples collected during endoscopy. Complete blood count, serum biochemistry profile, serum cobalamin and folate concentrations, tests for pancreatic disease (canine pancreatic lipase immunoreactivity and trypsin‐like immunoreactivity), basal serum cortisol concentration, preprandial and postprandial serum bile acid concentrations, fecal parasitology using saturated zinc sulfate flotation, fecal culture (for Salmonella, Campylobacter, and Clostridium difficile), and abdominal ultrasound examination or computed tomography were performed in most dogs, as indicated by the history and physical examination.
The canine chronic enteropathy activity index (CCEAI),10 which is based on the presence and severity of 9 factors, including attitude or activity, appetite, vomiting, fecal consistency, frequency of defecation, weight loss, serum albumin concentration, ascites, and peripheral edema and pruritus, was retrospectively calculated for each dog based on the history collected at admission and the referring veterinarian's medical records.
Archived paraffin‐embedded duodenal biopsy samples were retrieved from the archive at Bristol Veterinary School for the selected cases, and at least 10 sections, mounted onto glass slides from each dog, were sent to Iowa State University for IL‐13 and IL‐33 RNA ISH.
2.3. Control dogs
The research colony at the Iowa State University (8 female spayed Beagle dogs, 2 years of age) was used to obtain intestinal biopsy samples from healthy control dogs. The dogs were assessed as clinically healthy, and had a CBC, serum biochemistry profile, urinalysis, and fecal parasitology performed, all of which were normal. Duodenal biopsy samples were collected from control dogs during upper GI endoscopy.
2.4. RNA in situ hybridization
The technique of RNA ISH allows detection of specific RNA sequences with cellular resolution within tissue architecture on routine formalin‐fixed paraffin‐embedded tissue specimens. We used a novel RNA ISH technology, RNAscope (ACD Biotech, Newark, California), which provides single‐molecule visualization while preserving tissue morphology.16 Two slides with ≥5 duodenal biopsy samples per dog were hybridized with specially patented probes with oligonucleotide bases. These unique patented probes were specifically designed against the canine genetic sequences of IL‐13 and IL‐33 and verified by the manufacturer (ACD Bio Techne). Slides then were examined and positive controls were included for each hybridization using ubiquitin C. Preparation and hybridization of the slides were done in accordance with the user manual's protocol provided by ACD Biotech and using RNAscope 2.5 HD Assay—RED.16
2.5. Visualization and image capture
The RNAscope signals were visualized by using a 20X Plan N lens on an Olympus BX40 microscope (Olympus Optical Co, Ltd, Tokyo, Japan) and photographed with an Olympus DP27 camera (Olympus Optical Co, Ltd). Ten images were obtained from different representative fields per slide. A representative mucosal field was defined as containing ≥3 duodenal biopsy samples containing ≥3 contiguous villi for diagnostic evaluation.18
2.6. Semi‐quantitative analysis of mRNA expression
Semi‐quantitative image analysis was performed using Fiji ImageJ on in situ hybridized slides using RNAscope assay according to ACD Bio Techne instructions. Semi‐quantitative image analysis was performed on 6 sections from each case and expressed as positive dots per cell over the total percentage area of cells by a single operator (Victor Lezcano) in a nonblinded fashion. The Semi‐quantitative assessment consisted of an estimated total count of cells expressing signal in each representative field. Mucosal fields then were evaluated for cell distribution in different regions of the duodenal mucosa. The spatial distribution of expression was defined as: (1) villus in location; (2) subvillus in location; (3) within intestinal epithelium; and (4) within the lamina propria. A histologic grading score of 0‐3 was used to define cell numbers where 0 = none; 1 = minimum expression; 2 = moderate expression; and 3 = exaggerated expression.
2.7. Ethical considerations
Archived paraffin‐embedded duodenal biopsy samples were used and the University of Bristol granted ethical approval for the study (VIN/18/014). Iowa State University granted ethical approval for the use of paraffin‐embedded duodenal biopsy samples from healthy colony Beagle dogs (9‐17‐8605‐K).
2.8. Data analysis and statistics
The Shapiro‐Wilk and Levene's tests were used to assess the assumptions of normality and homoscedasticity, respectively. A 1‐way analysis of variance (ANOVA) was performed to assess for any significant differences in average mRNA expression scores of IL‐13 and IL‐33 in the different mucosal compartments (villus, subvillus, epithelial, and lamina propria) among the 3 groups (GSD CE, non‐GSD CE, and healthy). Post hoc pairwise comparisons then were performed using Tukey's honest significant difference test. Significance was defined as P < .05. Statistical analyses were performed using IBM SPSS Statistics version 24 and R Statistical software, version 3.5.1.
3. RESULTS
3.1. Dogs
Ten GSDs with CE were included in the study: 5 female neutered and 5 male neutered dogs, with a median age of 7.2 years and a range of 1.6 to 10 years. The median CCEAI score was 9 with a range of 4 to 13. Six dogs were diagnosed with eosinophilic enteritis and 1 with each of the following: lymphoplasmacytic enteritis, lymphoplasmacytic and eosinophilic enteritis, eosinophilic and neutrophilic enteritis, and lymphoplasmacytic, eosinophilic, and neutrophilic enteritis. One dog had protein‐losing enteropathy as a consequence of its CE, another had exocrine pancreatic insufficiency, and another had an incidental gastric foreign body.
Ten non‐GSDs with CE were included in the study: 2 Border Collies, 2 Boxers, and 1 each of the following breeds: West Highland White Terrier, Beagle, Siberian Husky, Chihuahua, Border Terrier, and Cavalier King Charles Spaniel. There were 6 female neutered, 3 male neutered, and 1 male intact dog, with a median age of 7.9 years and a range of 0.8 to 11 years. The median CCEAI score was 6.5 with a range of 3 to 11. Three dogs were diagnosed with eosinophilic enteritis, 2 dogs with lymphoplasmacytic, neutrophilic, and eosinophilic enteritis, 2 dogs with lymphoplasmacytic and eosinophilic enteritis, and 1 with each of the following: lymphoplasmacytic enteritis, lymphocytic enteritis, and plasmacytic enteritis. One dog had concurrent chronic partial gastric torsion. Ten healthy Beagle control dogs were included; all were female spayed and 2 years of age.
There were no significant differences in age or CCEAI between the GSD CE and non‐GSD CE groups (P > .22).
3.2. Interleukin‐13 mRNA
One‐way ANOVA comparing IL‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores among GSDs with CE, non‐GSDs with CE, and healthy dogs showed a significant difference for all 4 distribution scores among the 3 groups (P < .03).
Post hoc analysis using the Tukey's Honest Significant Difference test showed that GSDs with CE had significantly lower average IL‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria expression scores compared to non‐GSDs with CE (mean ± SD): (1) villus score: GSD CE, 0.43 ± 0.30; non‐GSD CE, 0.81 ± 0.37; P = .03; (2) subvillus score: GSD CE, 0.06 ± 0.11; non‐GSD CE, 0.33 ± 0.26; P = .04; (3) epithelial score: GSD CE, 0.07 ± 0.19; non‐GSD CE, 0.44 ± 0.47; P = .03; and (4) lamina propria score: GSD CE, 0.43 ± 0.31; non‐GSD CE, 0.82 ± 0.24; P = .009 (Table 1 and Figure 1).
Table 1.
Post hoc analysis of interleukin (IL)‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores in German Shepherd dogs (GSDs) with chronic enteropathy (CE), non‐GSDs with CE, and healthy Beagle control dogs using RNA in situ hybridization. P‐values obtained from post hoc analysis using Tukey's Honest Significant Difference test after 1‐way analysis of variance testing of IL‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores in GSDs with CE (N = 10), non‐GSDs with CE (N = 10), and healthy Beagle control dogs (N = 8)
| Villus score | Subvillus score | Epithelial score | Lamina propria score | ||
|---|---|---|---|---|---|
| GSD CE versus | Non‐GSD CE | .03 | .04 | .03 | .009 |
| Healthy control | .07 | .01 | .99 | .02 | |
| Non‐GSD CE versus | Healthy control | .96 | .80 | .03 | .99 |
Significance was defined in bold as P < .05.
Figure 1.
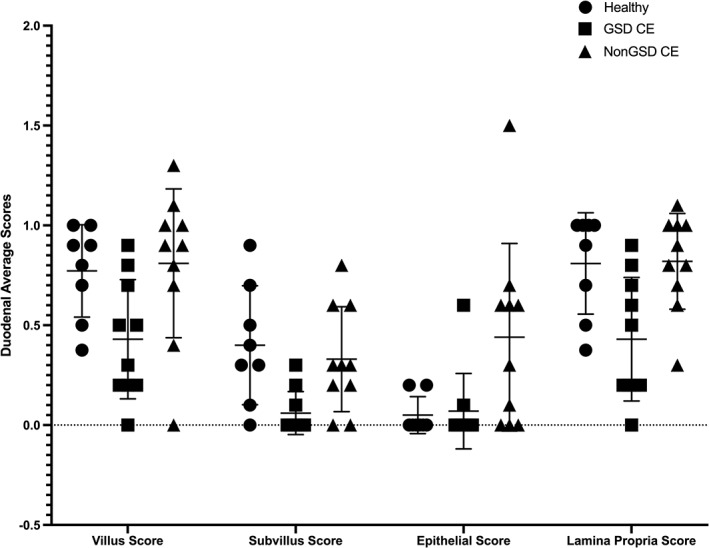
Individual scatter dot plot showing interleukin (IL)‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores for healthy Beagle control dogs (N = 8), German Shepherd dogs (GSDs) with chronic enteropathy (CE) (N = 10), and non‐GSDs with CE (N = 10). Line represents mean with SD. There were significant differences in IL‐13 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores between GSDs with CE and non‐GSDs with CE, P < .04. There also were significant differences in IL‐13 mRNA duodenal subvillus and lamina propria average expression scores between GSDs with CE and healthy Beagle control dogs, P < .02; and epithelial average expression score between non‐GSDs with CE and healthy Beagle control dogs, P = .03
In addition, GSDs with CE had significantly lower duodenal subvillus and lamina propria average expression scores compared to healthy control dogs: (1) subvillus score: GSD CE, 0.06 ± 0.11; healthy 0.40 ± 0.30; P = .01 and (2) lamina propria score: GSD CE, 0.43 ± 0.31; healthy, 0.81 ± 0.25; P = .02 (Table 1; Figures 1 and 2).
Figure 2.
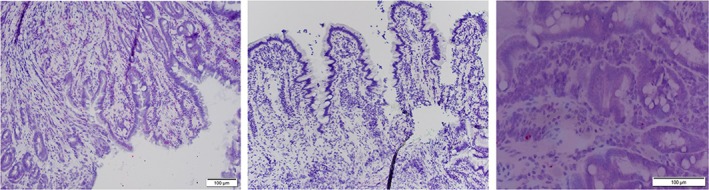
RNA in situ hybridization of interleukin (IL)‐13 in the subvillus of the duodenal mucosa of a German Shepherd dog with chronic enteropathy (left image) and a healthy Beagle control dog (right image). The central image depicts a negative control from a German Shepherd dog with chronic enteropathy
Lastly, the post hoc analysis showed that non‐GSDs with CE had significantly higher IL‐13 mRNA duodenal epithelial average expression score compared to healthy control dogs: non‐GSD CE, 0.44 ± 0.47; healthy, 0.05 ± 0.09; P = .03 (Table 1 and Figure 1).
3.3. Interleukin‐33 mRNA
One‐way ANOVA comparing average IL‐33 mRNA duodenal villus, subvillus, epithelial, and lamina propria expression scores among GSDs with CE, non‐GSDs with CE, and healthy dogs showed a significant difference for subvillus, epithelial, and lamina propria expression scores among the 3 groups (P < .01).
Post hoc analysis using Tukey's Honest Significant Difference test showed that GSDs with CE had significantly lower average IL‐33 mRNA duodenal subvillus, epithelial and lamina propria expression scores compared to non‐GSDs with CE and lower average subvillus and epithelial expression scores compared to healthy control dogs: GSD CE vs Non‐GSD CE: (1) subvillus score: GSD CE, 0.37 ± 0.49; non‐GSD CE, 1.36 ± 0.49; P < .001; (2) epithelial score: GSD CE, 0.50 ± 0.83; non‐GSD CE, 1.58 ± 0.86; P = .01; (3) lamina propria score: GSD CE, 0.73 ± 0.49; non‐GSD CE, 1.35 ± 0.39; P = .007 (Table 2). For GSD CE versus healthy: (1) subvillus score: GSD CE, 0.37 ± 0.49; healthy, 1.37 ± 0.42; P < .001; (2) epithelial score: GSD CE, 0.50 ± 0.83; healthy, 1.84 ± 0.53; P = .003 (Table 2; Figures 3 and 4). There were no significant differences in IL‐33 mRNA expression between non‐GSDs with CE and healthy control dogs (Table 2).
Table 2.
Post hoc analysis of interleukin (IL)‐33 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores in German Shepherd dogs (GSDs) with chronic enteropathy (CE), non‐GSDs with CE and healthy Beagle control dogs using RNA in situ hybridization. P‐values obtained from post hoc analysis using Tukey's Honest Significant Difference test after 1‐way analysis of variance testing of IL‐33 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores in GSDs with CE (N = 10), non‐GSDs with CE (N = 10), and healthy Beagle control dogs (N = 8)
| Villus score | Subvillus score | Epithelial score | Lamina propria score | ||
|---|---|---|---|---|---|
| GSD CE versus | Non‐GSD CE | .08 | <.001 | .01 | .007 |
| Healthy control | .16 | <.001 | .003 | .13 | |
| Non‐GSD CE versus | Healthy control | .96 | .99 | .76 | .49 |
Significance was defined in bold as P < .05.
Figure 3.
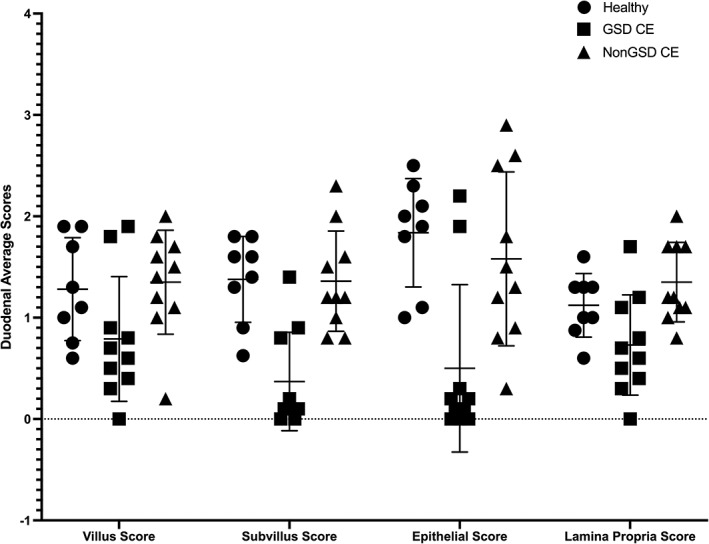
Individual scatter dot plot showing interleukin (IL)‐33 mRNA duodenal villus, subvillus, epithelial, and lamina propria average expression scores in healthy Beagle control dogs (N = 8), German Shepherd dogs (GSDs) with chronic enteropathy (CE) (N = 10), and non‐GSDs with CE (N = 10). Line represents mean with SD. There were significant differences in IL‐33 mRNA duodenal subvillus, epithelial, and lamina propria average expression scores between GSDs with CE and non‐GSDs with CE, P < .02. There were also significant differences in IL‐33 mRNA duodenal subvillus and epithelial average expression scores between GSDs with CE and healthy Beagle dogs, P < .004
Figure 4.
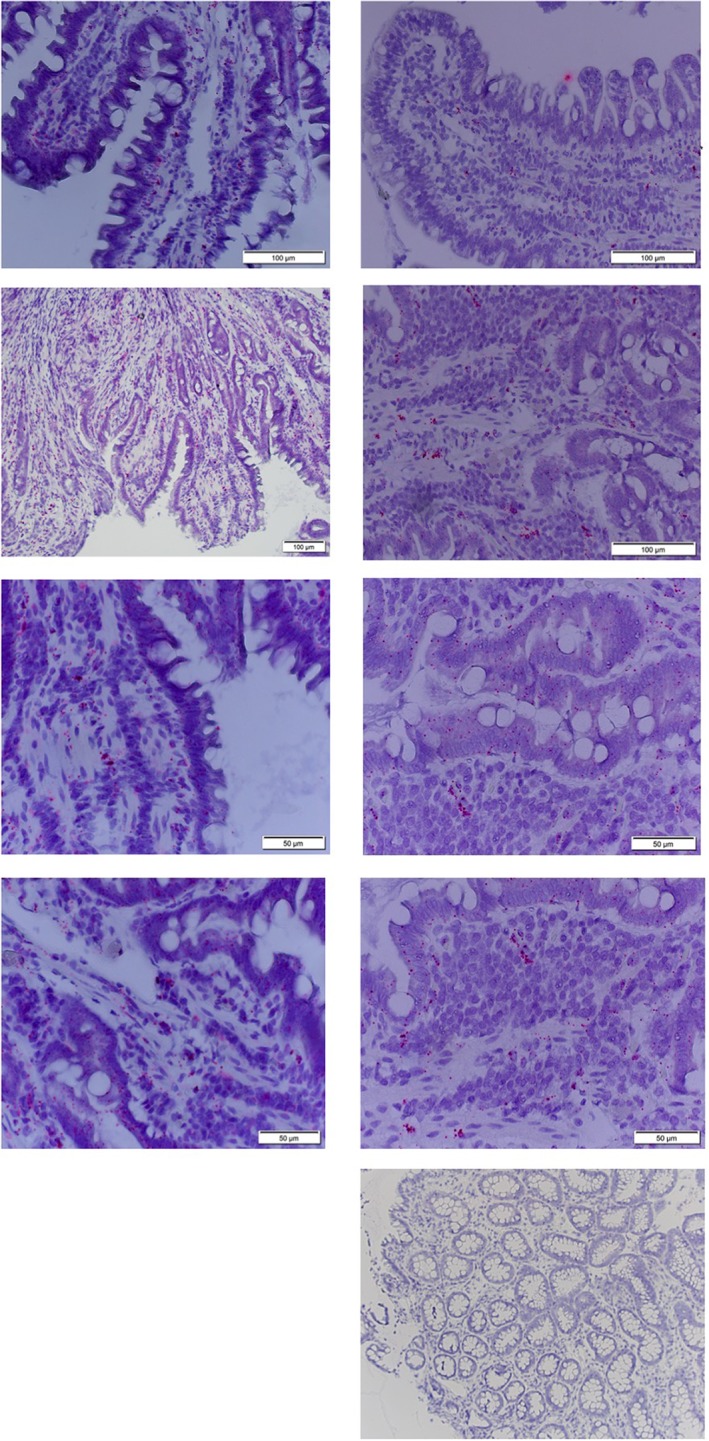
RNA in situ hybridization of interleukin (IL)‐33 in the duodenal mucosa of a German Shepherd dog with chronic enteropathy (left images; top to bottom: villus, subvillus, epithelial, and lamina propria) and a healthy Beagle control dog (right images; top to bottom: villus, subvillus, epithelial, lamina propria, and negative control)
4. DISCUSSION
Cytokines in the Th2 pathway have been implicated in the pathogenesis of UC, a subtype of IBD in humans.19 Similarly, a recent GWAS has implicated the possible role of Th2 cytokines in the pathogenesis of CE in GSDs.13 Therefore, we aimed to determine whether the mRNA expression of 2 Th2 cytokines, IL‐13 and IL‐33, was altered in the duodenal mucosa of GSDs with CE compared to non‐GSDs with CE and healthy control dogs. Our study showed that GSDs with CE had significantly decreased IL‐13 and IL‐33 mRNA expression in the duodenal mucosa compared with non‐GSDs with CE and healthy dogs.
Interleukin‐33 functions both as a cytokine and as a nuclear transcription factor.20 It activates both the innate and adaptive immune system and is a critical cytokine for Th2‐mediated host defense, as well as playing a key role in regulating immune responses in barrier tissues such as the intestine.20, 21 Interleukin‐33 also functions as an alarmin and is released by epithelial and endothelial cells in response to cell injury and necrosis.20 Therefore, IL‐33 acts as an important early indicator for the immune system when a breach in mucosal barrier function occurs.22 Also, because IL‐33 promotes an early Th2 immune response, it helps to regulate Th1 immune responses and prevent autoimmunity.23, 24 Interleukin‐33 has been shown to influence both T regulatory cells and Th17 cells to decrease inflammation and protect the intestinal mucosa from self‐inflicted injury.21 Studies suggest that nuclear IL‐33 can sequester nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), thereby decreasing subsequent NF‐κB‐generated proinflammatory signaling.25 Interleukin‐33 also may be involved in mucus production, because mice given exogenous IL‐33 in vivo produce more colonic mucin.26 A recent study also suggests a protective role for IL‐33 in epithelial regeneration and intestinal mucosal wound healing.27
The protective effects of IL‐33 mainly have been demonstrated in mouse models. Several studies have shown that IL‐33 knockout mice are more susceptible to dextran sodium sulfate (DSS) administration compared to wild‐type mice.28, 29, 30 Interleukin‐33 treatment ameliorated colitis in DSS, 2,4,6‐trinitrobenzenesulfonic acid, and peritoneal cavity cell transfer models.31 Interleukin‐33 treatment also increased B and T regulatory cell responses, suppressed Th17 responses, and switched Th1 to Th2 response in DSS‐induced colitis.32, 33 Interleukin‐33 has been shown to promote IgA production by B cells, which is important for maintaining intestinal homeostasis.28 Interleukin‐33 deficient mice also developed intestinal dysbiosis, which was characterized by increased numbers of mucolytic and colitogenic bacteria.28 Therefore, because IL‐33 has been shown to be important for gut homeostasis and in ameliorating intestinal inflammation, our study suggests that the significant decrease in IL‐33 expression in the duodenal mucosa of GSDs with CE may cause or contribute to the chronic intestinal inflammation seen in this disease. Interestingly, a previous study documented increased NF‐κB activity in dogs with CE.34 Because nuclear IL‐33 can sequester NF‐κB, leading to decreased proinflammatory signaling, decreased IL‐33 mRNA expression in GSDs with CE may result in increased NF‐κB activation with a subsequent increase in inflammation. However, additional studies would be needed to confirm this mechanism for increased NF‐κB activation in dogs with CE. Additionally, GSDs with CE previously have been shown to have relatively decreased IgA production, as well as intestinal dysbiosis.35, 36 However, the definitive mechanism for these findings is unknown. Our study suggests that decreased intestinal IL‐33 expression in GSDs with CE might be associated with the previously documented decreased intestinal IgA production and dysbiosis, similar to the findings seen in IL‐33 knockout mouse models.28 If IL‐33 is determined to play a role in the pathogenesis of CE in GSDs, exogenous IL‐33 could be explored as a possible specific treatment option in this breed. Therefore, further studies are warranted to investigate the consequences of decreased IL‐33 mRNA expression in GSDs with CE, such as decreased IgA production and altered intestinal microbiota.
Although the recent GWAS in GSDs with CE did not specifically implicate possible polymorphisms in the IL‐33 gene, it did implicate other Th2 genes such as IL‐13 that potentially contribute to the pathogenesis of tissue injury.13 Therefore, the expression of IL‐33 may be a consequence of altered expression of other Th2 cytokines in GSDs with CE because of polymorphisms in these genes. However, functional studies would be needed to definitively prove the role of these implicated genes and their consequent effects on other Th2 cytokines in the pathogenesis of CE in GSDs.
Our study did not show a significant difference in duodenal IL‐33 mRNA expression scores between non‐GSDs with CE and healthy dogs. This finding is in contrast to a previous study, which documented significantly decreased mRNA expression of IL‐33 in the duodenum of dogs with food‐responsive enteropathy (FRE) compared to healthy Beagle dogs.37 The lack of significance in our study might be because of the inclusion of different non‐GSD breeds of dogs with CE, whereas the previous study contained 7 dogs, each a different non‐GSD breed with FRE and only 1 of the dogs in our non‐GSD group was the same breed. In addition, our study did not subcategorize dogs with CE into treatment response, histological severity or type of inflammatory infiltrate. Therefore, the heterogeneity of breed, treatment response, and histopathologic severity and type of cellular infiltrate of CE in our non‐GSD group may have diluted any significant effects from single breeds, FRE, or histologic type resulting in nonsignificance. Therefore, future studies investigating the etiopathogenesis of CE in dogs should focus on homogenous populations using the same breeds, treatment response, and intestinal histologic type.
Interleukin‐13 is a Th2 cytokine that is produced mainly by T cells and natural killer cells.38 It also has been shown to weaken epithelial barrier function and induce epithelial cell apoptosis and downregulation of tight junction proteins.39 However, studies have produced ambiguous result with regard to its role in human and mouse models of IBD. Some studies have documented large amounts of IL‐13 in the intestinal epithelium of patients with UC.40, 41, 42, 43 Interleukin‐13 has been suggested to be a cause of inflammation, because UC patients treated with interferon‐beta‐1a had significantly downregulated IL‐13 production.44 However, other studies have documented significantly decreased IL‐13 concentrations in patients with UC compared with controls, with concentrations lower in active UC versus inactive UC.45, 46 Some earlier studies have described IL‐13 as an anti‐inflammatory agent in vitro, because it inhibited monocyte secretion of IL‐1 beta in a dose‐dependent manner.47 We determined that IL‐13 mRNA was significantly decreased in the duodenal mucosa of GSDs with CE compared with non‐GSDs with CE and healthy control dogs. A previous GWAS in GSDs with CE implicated polymorphisms in the IL‐13 gene.13 Therefore, our study suggests that polymorphisms in the IL‐13 gene in GSDs with CE may result in decreased expression of this possible anti‐inflammatory cytokine, which may cause or contribute to the increased inflammation seen in this disease. However, our study did not correlate mRNA expression of IL‐13 with the respective genotype in these dogs and therefore this possibility cannot be confirmed. Therefore, additional studies correlating IL‐13 mRNA expression with genotype profiles are warranted to confirm whether these polymorphisms have functional impact. Exogenous IL‐13 treatment then could be explored for CE in this breed if the polymorphisms in the IL‐13 gene are shown to result in a loss of function.
Interestingly, our study showed that non‐GSDs with CE had significantly increased IL‐13 mRNA duodenal epithelial average expression scores compared to healthy control dogs. This observation suggests that in some non‐GSD breeds with CE, IL‐13 also may play a role in disease pathogenesis. However, IL‐13 likely plays a different role in non‐GSDs with CE because the expression was increased compared to healthy dogs, whereas in GSDs with CE the expression was significantly decreased in the duodenum. Therefore, cytokines may play different roles in the pathogenesis of CE depending on the breed, perhaps because of the different genetic backgrounds, and consequently future studies investigating different cytokines in dogs with CE should focus on individual breeds.
In addition to the lack of genotype association with mRNA expression of IL‐13, other limitations of our study included the use of partial thickness endoscopic biopsy samples for RNA ISH rather than full‐thickness biopsy samples and therefore cells expressing IL‐13 or IL‐33 mRNA in the deeper layers would have been missed. Our study assessed mRNA expression which provides no information on protein expression or function, and may have been unaltered by the decreased mRNA expression of IL‐13 or IL‐33. Also, because of financial constraints, semi‐quantitative analysis was used to determine expression of IL‐13 and IL‐33 mRNA rather than absolute quantification. The non‐GSD CE group was heterogeneous and contained only 1‐2 dogs from the same breed and therefore any significant effects from individual breeds would have been diluted. Our control group contained exclusively healthy Beagle dogs that were from a research colony and were relatively young and from a different country compared to the dogs with CE. We are unable to rule out the effect of these confounding factors on the expression of IL‐13 and IL‐33 mRNA in the duodenal mucosa of these dogs and whether they are responsible for the differences seen compared to the dogs with CE. Because our study contained small numbers in both the CE and non‐GSD CE groups, statistical correlations to disease activity, WSAVA duodenal histopathology scores, or treatment response could not be performed. Our study included only mRNA expression in the duodenum and therefore expression in other locations in the intestinal tract such as the colon or ileum may have been different. Finally, additional Th2 cytokines for which polymorphisms have been suggested in GSDs with CE, such as IL‐4 and IL‐5, were not assessed and therefore may have provided additional insights into the pathogenesis of this disease.
In conclusion, our study showed that GSDs with CE exhibited decreased expression of IL‐13 and IL‐33 mRNA in the inflamed duodenal mucosa compared to non‐GSDs with CE and healthy Beagle dogs. Further studies are needed to determine the consequences of this decreased expression, such as decreased intestinal IgA production and intestinal dysbiosis, as well as to determine if decreased expression is the cause or an effect of CE in this breed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The University of Bristol granted ethical approval for the study (VIN/18/014). The Ethics' committee at Iowa State University has approved this study (9‐17‐8605‐K).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Sharon Holt and Rachel Phillips (Iowa State University) for technical assistance.
Kathrani A, Lezcano V, Hall EJ, et al. Interleukin‐13 and interleukin‐33 mRNA are underexpressed in the duodenal mucosa of German Shepherd dogs with chronic enteropathy. J Vet Intern Med. 2019;33:1660–1668. 10.1111/jvim.15544
REFERENCES
- 1. Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14(12):739‐749. [DOI] [PubMed] [Google Scholar]
- 3. Conroy CA, Cattell R. Infliximab treatment for Crohn's disease. Postgrad Med J. 2001;77:436‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen RD. Efficacy and safety of repeated infliximab infusions for Crohn's disease: 1‐year clinical experience. Inflamm Bowel Dis. 2001;7(Suppl 1):S17‐S22. [DOI] [PubMed] [Google Scholar]
- 5. Chey WY, Hussain A, Ryan C, Potter GD, Shah A. Infliximab for refractory ulcerative colitis. Am J Gastroenterol. 2001;96:2373‐2381. [DOI] [PubMed] [Google Scholar]
- 6. Bonovas S, Lytras T, Nikolopoulos G, Peyrin‐Biroulet L, Danese S. Editorial: tofacitinib and biologics for moderate‐to‐severe ulcerative colitis‐what is best in class? Authors' reply. Aliment Pharmacol Ther. 2018;47:540‐541. [DOI] [PubMed] [Google Scholar]
- 7. Mukherjee A, Hazra A, Smith MK, et al. Exposure‐response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: results from a dose‐ranging phase 2 trial. Br J Clin Pharmacol. 2018;84:1136‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci (Elite Ed). 2012;4:1404‐1419. [DOI] [PubMed] [Google Scholar]
- 9. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178:368. [DOI] [PubMed] [Google Scholar]
- 10. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 11. Allenspach K, Rufenacht S, Sauter S, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid‐refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239‐244. [DOI] [PubMed] [Google Scholar]
- 12. Kathrani A, Werling D, Allenspach K. Canine breeds at high risk of developing inflammatory bowel disease in the south‐eastern UK. Vet Rec. 2011;169:635. [DOI] [PubMed] [Google Scholar]
- 13. Peiravan A, Bertolini F, Rothschild MF, et al. Genome‐wide association studies of inflammatory bowel disease in German shepherd dogs. PLoS One. 2018;13:e0200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jergens AE, Sonea IM, O'Connor AM, et al. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta‐analysis with critical appraisal. Comp Med. 2009;59:153‐162. [PMC free article] [PubMed] [Google Scholar]
- 15. Heilmann RM, Suchodolski JS. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet Immunol Immunopathol. 2015;168:131‐134. [DOI] [PubMed] [Google Scholar]
- 16. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J Mol Diagn. 2012;14:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10‐26. [DOI] [PubMed] [Google Scholar]
- 18. Jergens AE, Evans RB, Ackermann M, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol. 2014;51:946‐950. [DOI] [PubMed] [Google Scholar]
- 19. Nemeth ZH, Bogdanovski DA, Barratt‐Stopper P, et al. Crohn's disease and ulcerative colitis show unique cytokine profiles. Cureus. 2017;9:e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller AM. Role of IL‐33 in inflammation and disease. J Inflamm (Lond). 2011;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodzic Z, Schill EM, Bolock AM, Good M. IL‐33 and the intestine: the good, the bad, and the inflammatory. Cytokine. 2017;100:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camilleri M, Madsen K, Spiller R, van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen ES, Scott IC, Majithiya JB, et al. Oxidation of the alarmin IL‐33 regulates ST2‐dependent inflammation. Nat Commun. 2015;6:8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao J, Wei J, Mialki RK, et al. F‐box protein FBXL19‐mediated ubiquitination and degradation of the receptor for IL‐33 limits pulmonary inflammation. Nat Immunol. 2012;13:651‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ali S, Mohs A, Thomas M, et al. The dual function cytokine IL‐33 interacts with the transcription factor NF‐kappaB to dampen NF‐kappaB‐stimulated gene transcription. J Immunol. 2011;187:1609‐1616. [DOI] [PubMed] [Google Scholar]
- 26. Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D. IL‐33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin‐EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762‐10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopetuso LR, De Salvo C, Pastorelli L, et al. IL‐33 promotes recovery from acute colitis by inducing miR‐320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115:E9362‐E9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik A, Sharma D, Zhu Q, et al. IL‐33 regulates the IgA‐microbiota axis to restrain IL‐1alpha‐dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469‐4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oboki K, Ohno T, Kajiwara N, et al. IL‐33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581‐18586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imaeda H, Andoh A, Aomatsu T, et al. Interleukin‐33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium‐induced colitis. Int J Mol Med. 2011;28:573‐578. [DOI] [PubMed] [Google Scholar]
- 31. Seo DH, Che X, Kwak MS, et al. Interleukin‐33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep. 2017;7:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu J, Xu Y, Zhu C, et al. IL‐33 induces both regulatory B cells and regulatory T cells in dextran sulfate sodium‐induced colitis. Int Immunopharmacol. 2017;46:38‐47. [DOI] [PubMed] [Google Scholar]
- 33. Zhu J, Wang Y, Yang F, et al. IL‐33 alleviates DSS‐induced chronic colitis in C57BL/6 mice colon lamina propria by suppressing Th17 cell response as well as Th1 cell response. Int Immunopharmacol. 2015;29:846‐853. [DOI] [PubMed] [Google Scholar]
- 34. Luckschander N, Hall JA, Gaschen F, et al. Activation of nuclear factor‐kappaB in dogs with chronic enteropathies. Vet Immunol Immunopathol. 2010;133:228‐236. [DOI] [PubMed] [Google Scholar]
- 35. German AJ, Hall EJ, Day MJ. Relative deficiency in IgA production by duodenal explants from German shepherd dogs with small intestinal disease. Vet Immunol Immunopathol. 2000;76:25‐43. [DOI] [PubMed] [Google Scholar]
- 36. Allenspach K, House A, Smith K, et al. Evaluation of mucosal bacteria and histopathology, clinical disease activity and expression of Toll‐like receptors in German shepherd dogs with chronic enteropathies. Vet Microbiol. 2010;146:326‐335. [DOI] [PubMed] [Google Scholar]
- 37. Osada H, Ogawa M, Hasegawa A, et al. Expression of epithelial cell‐derived cytokine genes in the duodenal and colonic mucosae of dogs with chronic enteropathy. J Vet Med Sci. 2017;79:393‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoshino T, Winkler‐Pickett RT, Mason AT, et al. IL‐13 production by NK cells: IL‐13‐producing NK and T cells are present in vivo in the absence of IFN‐gamma. J Immunol. 1999;162:51‐59. [PubMed] [Google Scholar]
- 39. Soufli I, Toumi R, Rafa H, Touil‐Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno‐pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d‐restricted NK T cells that produce IL‐13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T‐helper cell predominance and interleukin‐12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823‐832. [PMC free article] [PubMed] [Google Scholar]
- 42. Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058‐2069. [DOI] [PubMed] [Google Scholar]
- 43. Heller F, Florian P, Bojarski C, et al. Interleukin‐13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550‐564. [DOI] [PubMed] [Google Scholar]
- 44. Mannon PJ, Hornung RL, Yang Z, et al. Suppression of inflammation in ulcerative colitis by interferon‐beta‐1a is accompanied by inhibition of IL‐13 production. Gut. 2011;60:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kadivar K, Ruchelli ED, Markowitz JE, et al. Intestinal interleukin‐13 in pediatric inflammatory bowel disease patients. Inflamm Bowel Dis. 2004;10:593‐598. [DOI] [PubMed] [Google Scholar]
- 46. Vainer B, Nielsen OH, Hendel J, Horn T, Kirman I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine. 2000;12:1531‐1536. [DOI] [PubMed] [Google Scholar]
- 47. Kucharzik T, Lugering N, Weigelt H, et al. Immunoregulatory properties of IL‐13 in patients with inflammatory bowel disease; comparison with IL‐4 and IL‐10. Clin Exp Immunol. 1996;104:483‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]


