Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) in patients with surgically altered anatomy must be performed by a highly experienced endoscopist. The challenges are accessing the afferent limb in different types of reconstruction, cannulating a papilla with a reverse orientation, and performing therapeutic interventions with uncommon endoscopic accessories. The development of endoscopic techniques has led to higher success rates in this group of patients. Device-assisted ERCP is the endoscopic procedure of choice for high success rates in short-limb reconstruction; however, these success rate is lower in long-limb reconstruction. ERCP assisted by endoscopic ultrasonography is now popular because it can be performed independent of the limb length; however, it must be performed by a highly experienced and skilled endoscopist. Stent deployment and small stone removal can be performed immediately after ERCP assisted by endoscopic ultrasonography, but the second session is needed for other difficult procedures such as cholangioscopy-guided electrohydraulic lithotripsy. Laparoscopic-assisted ERCP has an almost 100% success rate in long-limb reconstruction because of the use of a conventional side-view duodenoscope, which is compatible with standard accessories. This requires cooperation between the surgeon and endoscopist and is suitable in urgent situations requiring concomitant cholecystectomy. This review focuses on the advantages, disadvantages, and outcomes of various procedures that are suitable in different situations and reconstruction types. Emerging new techniques and their outcomes are also discussed.
Keywords: : Endoscopic retrograde cholangiopancreatography, Surgically altered anatomy, Endoscopic retrograde cholangiopancreatography in Billroth II, Endoscopic retrograde cholangiopancreatography post-Whipple, Endoscopic ultrasonography-guided endoscopic retrograde cholangiopancreatography
Core tip: Endoscopic retrograde cholangiopancreatography (ERCP) in patients with surgically altered anatomy is really challenging and requires a well-experienced endoscopist. Understanding the type of surgery, length of the afferent limb, type of endoscope used with choice of proper approach (peroral or transgastric), and compatible ERCP accessories with various endoscopic types are the keys to success. A conventional endoscope and device-assisted enteroscope-assisted ERCP are recommend for short-limb reconstruction with/without a native papilla, while device-assisted enteroscope-assisted ERCP, ERCP assisted by endoscopic ultrasonography, and especially laparoscopic-ERCP are highly recommended for long-limb reconstruction, such as Roux-en-Y gastric bypass with concomitant cholecystectomy.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) in patients with surgically altered anatomy is challenging because of the availability of many alternative techniques with good outcome for different types of reconstruction and the relatively small number of cases. A standard technique, however, has not been established. All available procedures require a surgeon with extensive experience performing ERCP in patients with normal anatomy to increase the technical and clinical success rates in patients with altered anatomy. The three main challenges in performing these procedures are how to access the afferent limb and reach the ampulla or biliopancreatoenteric anastomosis in different types of altered anatomy, how to cannulate the bile duct or pancreatic duct in the new anatomical orientation after surgery, and how to perform diagnostic and therapeutic interventions. The optimal endoscopic technique for accessing the afferent limb and reaching the bilio-pancreatoenteric anastomosis depends on the postoperative reconstruction type; therefore, a review of the operative records is the first step. The challenges associated with this step include limited endoscopic maneuverability caused by the angulation of the anastomosis, difficult identification of the entrance of the afferent limb, determination of how to correct endoscopic looping, and management of post-operative adhesion. Successful cannulation depends on access to the papilla, availability of endoscopic accessories, adequate expertise of the endoscopist, and effective papillary and therapeutic interventions. In this review, we discuss the advantages, disadvantages, and outcomes of procedures that are suitable in different situations and for different reconstruction types. We also discuss emerging new techniques and their outcomes.
First step: Knowledge of reconstruction types for surgically altered anatomy
An understanding of the different types of postoperative reconstruction anatomy is important to determine the easiest way to access the afferent limb and reach the target (native papilla or biliopancreatoenteric anastomosis), choose the most appropriate endoscopic technique, and prevent postoperative complications, which trend to be higher than normal anatomy. Common postoperative reconstruction procedures are shown in Figures 1-5. One of the two major types of reconstruction is short afferent limb reconstruction with or without an intact papilla, in which the distance from the anastomosis to the native papilla or anastomosis is usually ≤ 50 cm. The success rate of access to the afferent limb is high even when performed with a conventional duodenoscope, gastroscope, pediatric colonoscope, or device-assisted enteroscope (DAE). Therefore, the major challenge lies in cannulation. The second major type of reconstruction is long afferent limb reconstruction, in which the afferent limb length may reach > 100, > 150, or even > 200 cm. This type of reconstruction is preserved for bariatric surgery to exclude the passage of food. The major challenge in this type of reconstruction is accessing the afferent limb and reaching the papilla, which has a low success rate when using a conventional endoscope.
Figure 1.
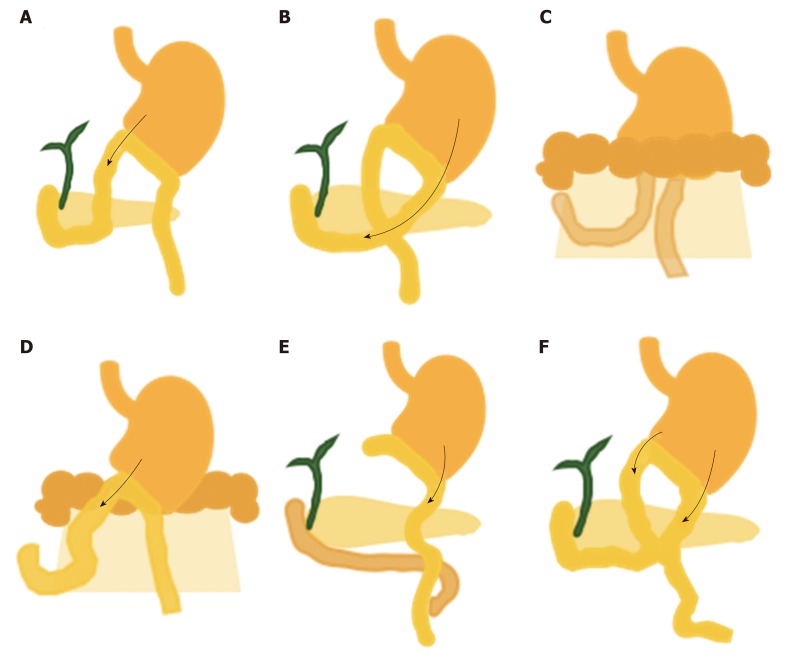
Billroth II gastrectomy and variations of reconstruction. A: Antiperistaltic type. The entry of the afferent limb is located near the lesser curvature; B: Isoperistaltic type. The entry site is located near the greater curvature; C: Retrocolic reconstruction. The afferent limb is shorter than that in antecolic reconstruction; D: Antecolic reconstruction. The afferent limb is significantly longer than that in retrocolic reconstruction; E: Roux-en-Y reconstruction involves the longest limb among all Billroth II gastrectomy techniques; F: Braun jejunojejunostomy anastomosis creates a confusing endoscopic view to reach the afferent limb.
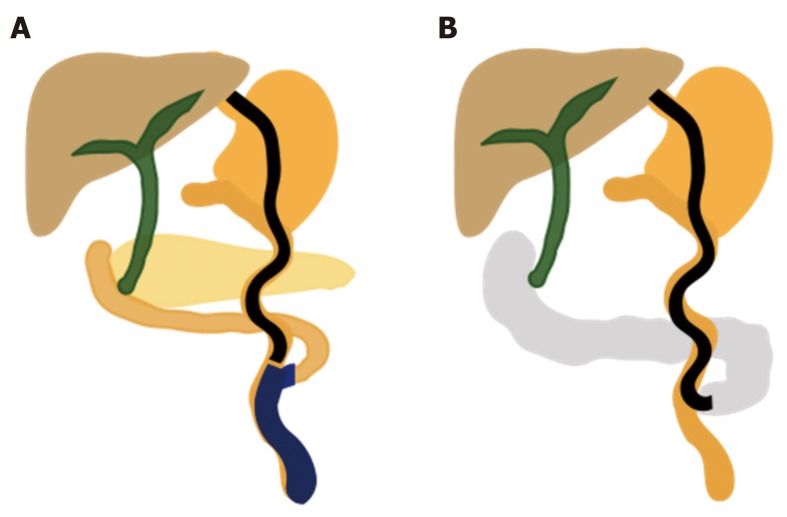
Figure 5.
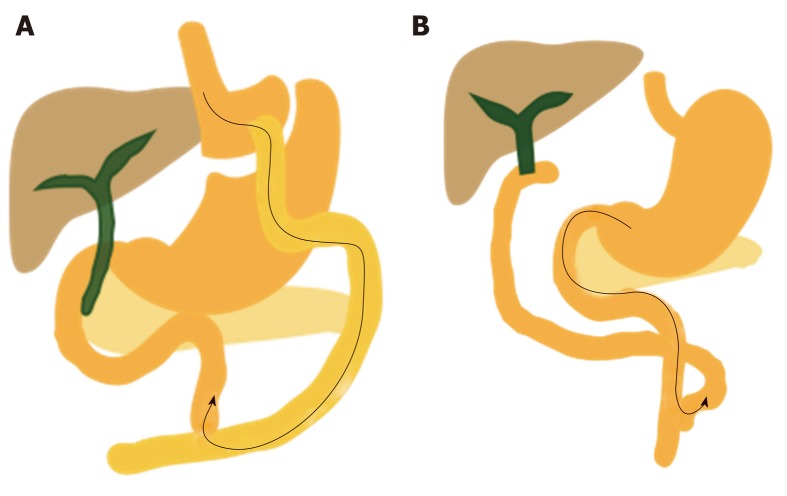
Endoscopic EUS-guided ERCP and laparoscopic-assisted ERCP in Roux-en-Y gastric bypass. A: EUS-guided transgastric fistula by luminal-apposing metallic stents; B: EUS-guided jejunogastrostomy stent with conventional ERCP; C: EUS-directed transgastric ERCP for Roux-en-Y reconstruction; D: EUS-guided sutured gastropexy for transgastric ERCP; E: Laparoscopic-assisted ERCP. ERCP: Endoscopic retrograde cholangiopancreatography; EUS: Ultrasonography.
Short afferent limb reconstruction with intact major papilla: Esophagectomy with gastric pull-up (Billroth I gastrectomy) does not involve substantial alteration of the afferent limb length; thus, ERCP can be performed with a conventional side-view duodenoscope. However, cannulation may be difficult because the anatomy is too straight and short, resulting in a very close space between the scope and papilla. Billroth II gastrectomy is a common procedure for treatment of gastric cancer and ulcer perforation. Various types of reconstruction can be performed, as shown in Figure 1. Each reconstruction technique involves a different length of and entry site for the afferent limb. In the antiperistaltic type, the afferent limb entry site is located near the lesser curvature (Figure 1A). In the isoperistaltic type, the entry site is located near the greater curvature (Figure 1B). The length of the afferent limb is approximately 30-40 cm in both techniques. In retrocolic reconstruction, however, the afferent limb is shorter than that in antecolic reconstruction, in which the afferent limb is approximately 50-80 cm (Figure 1C and D). Roux-en-Y reconstruction involves a long afferent limb of approximately 40-80 cm (Figure 1E). Braun anastomosis is a modified operation to reduce bile reflux into the stomach, but it provides a more confusing endoscopic view of the entry site to access the afferent limb (Figure 1F).
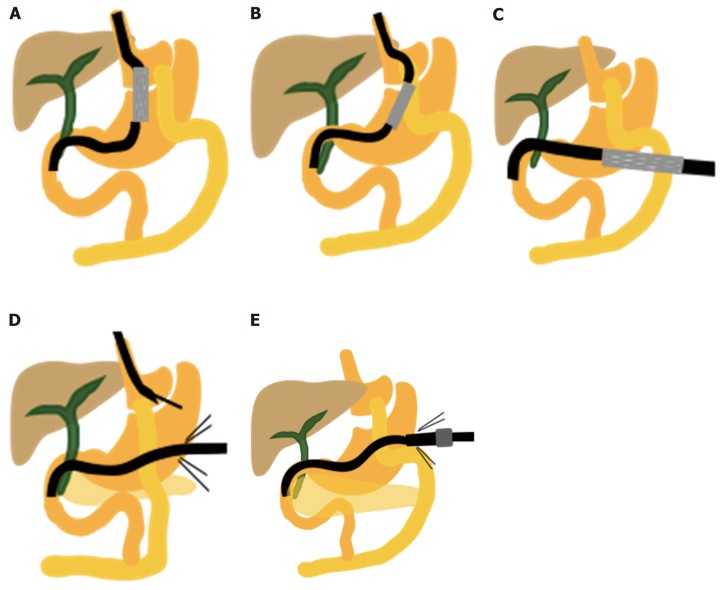
Short afferent limb reconstructions with bilio-pancreatoenteric anastomosis: Pancreaticoduodenectomy (Whipple’s operation) also has various reconstruction techniques (Figure 2). In the endoscopic view, the afferent limb entry site is commonly located at the 10 o’clock position relative to the gastrojejunostomy or duo-denojejunostomy anastomosis. For Braun anastomosis (Figure 2C), the endoscopic view can involve either two-limb entry (side-to-end) or three-limb entry (side-to-side), depending on the type of anastomosis. To identify the correct afferent limb, the surgeon should follow the bile-containing limb, the scar at the anastomosis, or the direction of peristalsis or go straight to the middle entrance in Braun anastomosis[1].
Figure 2.
Various reconstructions of pancreaticoduodenectomy (Whipple’s procedure). A: Conventional Whipple’s procedure; The afferent limb is near the lesser curve; B: Pylorus-preserving pancreaticoduodenectomy; C: Braun anastomosis may create a confusing endoscopic view.
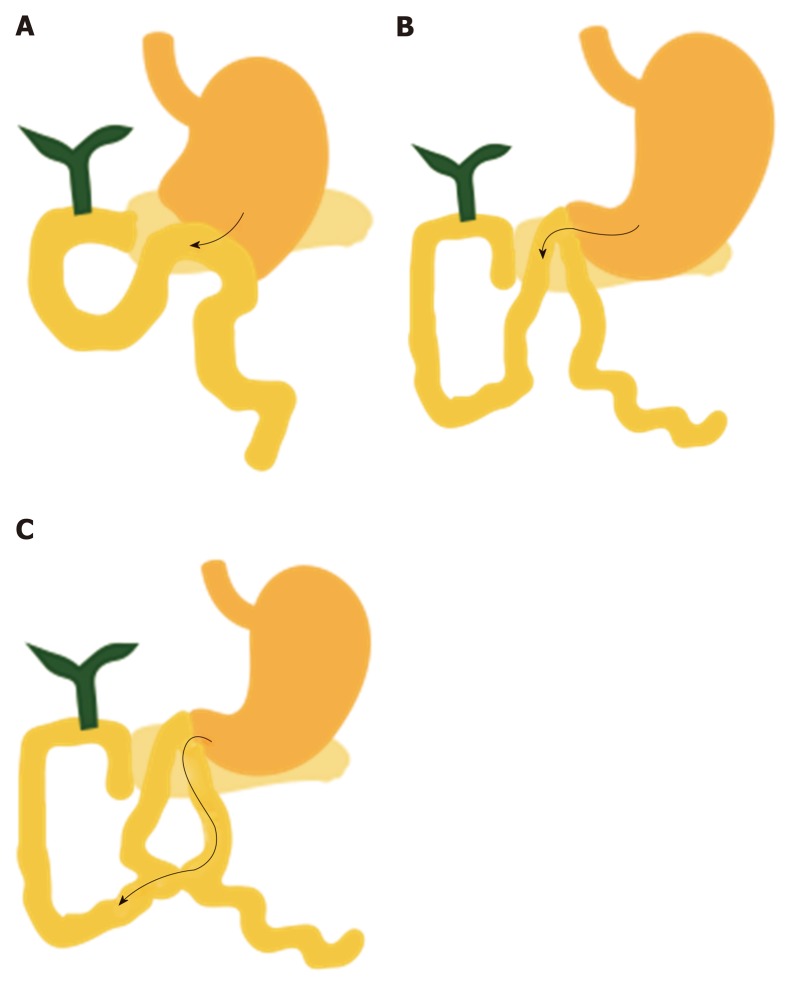
Long afferent limb reconstructions with or without major papilla: In bariatric surgery, Roux-en-Y gastric bypass (RYGB) involves a long afferent limb of > 100, > 150, or even > 200 cm (Figure 3A). Such patients are at risk of developing biliary complications from postoperative formation of gallstones due to rapid weight loss with low incidence of these complications (7%-8%)[2]. The therapeutic success rate of peroral endoscopic ERCP is very low (59%) using either a pediatric colonoscope or device-assisted ERCP because of adhesion formation, angulation of the jejunojejunal anastomosis, and figure-eight looping of the scope[3,4]. New and challenging techniques in the performance of ERCP are endoscopic ultrasonography (EUS)-guided biliary drainage (EUS-BD) and laparoscopic-assisted transgastric ERCP (LA-ERCP), which have high success rates of 80%-100%[5-7].
Figure 3.
Other types of reconstruction. A: Roux-en-Y gastric bypass; B: Hepaticojejunostomy in liver transplant, pancreaticobiliary maljunction, or bile duct cancer.
Liver transplantation in adults is usually performed in duct-to-duct or Roux-en-Y hepaticojejunostomy reconstruction with an intact stomach. No special caution is required in duct-to-duct reconstruction because no stomach or small bowel resection is performed; however, Roux-en-Y reconstruction involves a long afferent limb, as in RYGB (Figure 3B).
To insert the endoscope faster and more accurately in patients after a recon-struction, one must understand the post-surgery anatomy very well.
Second step: Selection of optimal endoscope type for different types of re-construction anatomy
Conventional duodenoscope, gastroscope, or colonoscope: The length of the afferent limb is important in selection of the endoscope. In Billroth II reconstruction with a short afferent limb, intubation is successfully achieved in most cases (62.5%-100%) with a conventional side-view duodenoscope or forward-view gastroscope with or without cap-fitting to fix the bowel wall; these should be the first-choice endoscopes (Table 1). The route of intubation to reach the entry site into the afferent limb differs depending on the reconstruction technique, as previously described. A higher perforation rate is associated with use of a duodenoscope because of limited visualization, difficulty controlling the scope, and the need to apply more pressure to overcome looping. In contrast, while the forward-view endoscope provides better visualization, cannulation is difficult due to the tangential view to the papilla. In one study, the success rate increased from 88.6%-92.5% by use of a cap fitted at the tip of the scope[8]. As shown in Table 2, Wang et al[8] and Bove et al[9] reported that the main reasons for intubation failure caused by the afferent limb are extension of the limb too far beyond the scope and the sharp angulation of the afferent limb. The success rate of gastroscopy, duodenoscopy, and colonoscopy for intubation is 84.6%, 62.5%, and 93.5%, respectively. Shah et al[10] reported a high success rate of deeper insertion by changing the patient’s position to the left lateral decubitus or supine position. In Billroth II reconstruction without Braun anastomosis, the papilla can be reached in > 80% of cases by conventional duodenoscopy or gastroscopy. In Billroth II recon-struction with Braun anastomosis, however, the success rate ranges from 29%-90%, and the failure rate is increased[1,11]. Using a conventional duodenoscope, the scope to the entry site should be at the middle entrance of the Braun anastomosis[1]. For Roux-en-Y reconstruction, entering the afferent limb of the Y anastomosis is much more difficult because of the longer afferent limb length, sharper angulation, and more severe adhesion.
Table 1.
Success rates of conventional duodenoscope and forward-view endoscope in Billroth II operation
| Ref. | Endoscope type | Operation type | Success rate of afferent loop intubation, % | Success rate of cannulation, % | Complication rate, % |
| Jang et al[44] | Conventional side-view duodenoscope | Billroth II | 100 | 100 | 0 |
| Bove et al[9] | Conventional side-view duodenoscope | Billroth II | 86.7 | 93.8 | 2.7 |
| Cicek et al[11] | Conventional side-view duodenoscope | Billroth II | 86.4 | 88.2 | 10.2 |
| Wu et al[13] | Conventional side-view duodenoscope | Billroth II | 90.5 | 88.6 | 12.5 |
| Kim and Kim[67] | Conventional side-view duodenoscope | Billroth II | 100 | 100 | 4 |
| Park et al[68] | Conventional side-view duodenoscope | Billroth II | 86.8 | 92.3 | 3.6 |
| Wang et al[8] | Conventional side-view duodenoscope | Billroth II | 62.5 | 100 | 10.3 |
| Forward-view gastroscope Standard colonoscope | Billroth II | 84.6 | 81.8 | ||
| Billroth II | 93.5 | 91.2 | |||
| Cap-fitted forward-view gastroscope/ without cap | Billroth II | 92.5/88.6 | 91.1 | 3 | |
| Park et al[38] | Cap-fitted forward-view gastroscope | Billroth II | 100 | 100 | 10 |
| Lin et al[69] | Forward-view gastroscope |
Billroth II | 76.8 | 81.4 | 0 |
DBE: Double balloon enteroscope; RYGB: Roux-en-Y gastric bypass.
Table 2.
Success rate of long and short double-balloon enteroscope-assisted endoscopic retrograde cholangiopancreatography in surgical altered anatomy
| Ref. | Endoscope type | Operation type | Successful of afferent loop intubation, % | Successful of cannulation, % | Complication, % |
| Shah et al[10] | Long DBE | Overall | 71 | 88 | NA |
| RYGB | 87 | 67 | NA | ||
| non-RYGB | 58 | 58 | NA | ||
| Katanuma et al[15] | Long DBE | Roux-en-Y reconstruction | 75 | NA | NA |
| Hepaticojejunostomy | 80 | NA | NA | ||
| Billroth II | 100 | NA | NA | ||
| Short DBE | Roux-en-Y reconstruction | 97.1 | NA | NA | |
| Hepaticojejunostomy | 87.5 | NA | NA | ||
| Billroth II | 100 | NA | NA | ||
| Whipple | 95.7 | NA | NA | ||
| Liver transplantation | 88.9 | NA | NA | ||
| Shimatani et al[70] | Short DBE | Overall | 97 | 98 | 5 |
| Billroth II | 100 | 100 | NA | ||
| Total gastrectomy | 95 | 96 | NA | ||
| Whipple | 100 | 100 | NA | ||
| Cheng et al[71] | DBE | Billroth II | 95 | 87 | 6.5 |
| Osoegawa et al[35] | Short DBE | Overall | 96 | 89 | 3.5 |
| Billroth II | 95 | 89 | NA | ||
| Roux-en-Y reconstruction | 96 | 88 | NA | ||
| Whipple | 100 | 100 | NA | ||
| Skinner et al[24] | Long DBE | RYGB | 82 | NA | NA |
| Siddiqui et al[72] | Short DBE | Overall | 81 | 90 | 8.8 |
| RYGB | 82 | 91 | NA | ||
| Billroth II | 100 | 100 | NA | ||
| Whipple | 95 | 84 | NA | ||
| Hepaticojejunostomy | 100 | 100 | NA | ||
| Shimatani et al[73] | Short DBE | Overall | 97.7 | 96.4 | 10.6 |
| Roux-en-Y reconstruction | 97 | 97 | NA | ||
| Whipple | 100 | 98 | NA | ||
| Billroth II | 96.2 | 100 | NA | ||
| Mizukawa et al[49] | Short DBE | Hepaticojejunostomy | 100 | NA | 7 |
DBE: Double balloon enteroscope; RYGB: Roux-en-Y gastric bypass.
The most common indications for ERCP after Whipple’s operation are elimination of common bile duct (CBD) stones and resolution of anastomotic strictures[12]. Hence, the DAE is more frequently used. Moreover, the endoscopist should have a high level of experience in manipulating the scope to overcome the adhesions and angulation of the anastomosis and thus reach the afferent limb. Wu et al[13] reported a 90.5% intubation success rate using a duodenoscope with retrieval balloon-assisted enterography, which is more complicated than use of a DAE.
Device-assisted endoscopy (DAE): A double-balloon enteroscope (DBE), single-balloon enteroscope (SBE), and rotational or spiral enteroscope (SE) can increase ERCP success rate in patients with surgically altered anatomy depending on reconstruction type, limb length, en-doscopist’s familiarity, and available therapeutic accessories.
DAE-assisted ERCP provides satisfactory outcomes with an intubation success rate of 40%-100% (Tables 2-4). Table 5 compares the characteristics of each type of scope. The main objective of the balloon is to pleat the small bowel into the overtube and stabilize the scope. DBE-assisted ERCP is characterized by a long and short type comprising two separate inflatable balloons at the tip of the scope and overtube. The DBE can advance deeper into the small bowel by alternating inflation-deflation and reduction-advancement of the endoscope and overtube. The advantages are deeper insertion to the papilla (even in Roux-en-Y reconstruction and RYGB) and overcoming the sharp angulation because of the balloon at the tip, forward-view visualization, and scope stability provided by the overtube. The limitations are formation of looping because of the long length, which can soften the scope shaft; restriction of maneuverability by adhesions; limitation of orientation relative to the papilla; and lack of an elevator, making cannulation more difficult than with a conventional side-view duodenoscope. A long DBE has a 200 cm working length, which is not compatible with commercial ERCP accessories. Thus, a short DBE was developed, but the use of standard accessories for therapeutic intervention is still limited (e.g., metallic and plastic stents limited to ≤ 7 Fr) because of the 2.8-mm working channel. A short DBE with a 3.2-mm channel is now available in Japan. The overall success rate of DBE with various reconstruction types ranges from 70%-100% (Table 2). The short DBE insertion success rate in Billroth II gastrectomy is 90%-100% (Table 2). No reports have described the use of a long DBE in Billroth II reconstruction because a conventional endoscope can be successfully used in most cases (Table 1). The exceptions are Roux-en-Y and Braun anastomosis of Billroth II reconstruction, in which the longer afferent limb requires use of a DAE. The most successful afferent limb intubation by a DBE is achieved in Billroth II reconstruction (100%), then Whipple’s procedure and total gastrectomy (95%-100%), and finally hepa-ticojejunostomy (80%-100%) (Table 2). The lowest success is achieved in RYGB and after liver transplantation (80%-90%). A few reports have compared long- and short-type DBEs, Itoi et al[14] reported a significant difference in the mean time to reach the papilla between long and short DBEs (64.8 ± 24.7 and 29.0 ± 19.2 min, respectively). After a long DBE has reached the ampulla, the endoscopist must change to a conventional endoscope for standard ERCP accessories. Katanuma et al[15] reported no significant difference in the insertion success rate between a long and short DBE, but insertion tended to be easier with a short DBE because of better maneuverability, application of more pressure during insertion, and greater compatibility with therapeutic accessories.
Table 4.
Success rates of spiral enteroscope-assisted endoscopic retrograde cholangiopancreatography in surgically altered anatomy
| Ref. | Operation type | Success rate of afferent loop intubation, % | Success rate of cannulation, % | Complication rate, % |
| Lennon et al[20] | RYGB and other Roux-en-Y reconstruction | 40 | 87.5 | 3.5 |
| Ali et al[21] | RYGB and other Roux-en-Y reconstruction | 86 | 100 | 0 |
| Zouhairi et al[22] | RYGB, Billroth II, and hepaticojejunostomy | 76.2 | 81.3 | 23.8 |
| Shah[82] | RYGB, hepaticojejunostomy, Whipple, and post-gastrectomy | 88 | 79 | 12.4 |
| Wagh et al[23] | RYGB, Whipple, Billroth II, and hepaticojejunostomy | 77 | 67 | 0 |
RYGB: Roux-en-Y gastric bypass.
Table 5.
Characteristics of enteroscope types used for endoscopic retrograde cholangiopancreatography
| Scope type, release year |
Long DBE |
Long DBE |
Long SBE |
Short DBE |
Short SBE |
Short DBE |
| EN-450T5, 2004 | EN-580T, 2013 | SIF-Q260, 2007 | EI-530B, 2011 | SIF-H290S, 2017 | EN-530T, 2016 | |
| View of direction | Forward | Forward | Forward | Forward | Forward | Forward |
| Working length in mm | 2000 | 2000 | 2000 | 1520 | 1520 | 1520 |
| Total length in mm | 2300 | 2300 | 2305 | 1820 | 1840 | 1820 |
| Working channel diameter in mm | 2.8 | 3.2 | 2.8 | 2.8 | 3.2 | 3.2 |
| Outer diameter in mm | 9.4 | 9.4 | 9.2 | 9.4 | 9.2 | 9.4 |
| Angle of view | 140º | 140º | 120º | 140º | 120º | 140º |
| Water jet channel | No | No | No | No | Yes | No |
| Passive bending part | No | No | No | No | Yes | No |
DBE: Double balloon enteroscope; SBE: Single balloon enteroscope.
The SBE has a single inflatable balloon at the tip of the overtube, and the hook-shaped tip makes it easier to pass the sharply angulated anastomosis. The principle of SBE is an alternating cycle of advancement-reduction of the scope to pleat the small bowel into the overtube and achieve deeper insertion. The success and complication rates of SBE-ERCP are shown in Table 3. The overall success rate of SBE is 80%-100% (long, 80%-100%; short, 85%-100%). Use of a long SBE seems to be more successful in Roux-en-Y reconstruction and RYGB because of the insufficient length of the short SBE; however, Iwai et al[16] reported a higher success rate with a short than long SBE in Roux-en-Y reconstruction (92% and 84%, respectively) and no significant difference in reaching the blind end, the mean time to reaching the blind end, diagnostic success rate, therapeutic success rate, or complication rate between long and short SBEs.
Table 3.
Success rates of long and short single-balloon enteroscope-assisted endoscopic retrograde cholangiopancreatography in surgically altered anatomy
| Ref. | Endoscope type | Operation type | Success rate of afferent loop Intubation, % | Success rate of cannulation, % | Complication rate, % |
| Inamdar et al[74] | Long and short SBE | RYGB, hepaticojejunostomy, and Whipple | 80.9 | 61.7 | 6.5 |
| Trindade et al[75] | Long SBE | RYGB, hepaticojejunostomy, and Whipple | 87.5 | 78.57 | NA |
| Obana et al[76] | Long SBE | Total and distal gastrectomy with Roux-en-Y reconstruction | 72.7 | 85.7 | 2.4 |
| Short SBE | 87.5 | 71.4 | |||
| Shah et al[10] | Long SBE | RYGB | 73 | 59 | 12 |
| Non-RYGB | 65 | 61 | |||
| Kurzynske et al[77] | Long SBE | Overall | 100 | 88 | 0 |
| Abu Dayyeh et al[19] | Long SBE | Overall | 80.9 | 69.4 | NA |
| Lee et al[78] | Long SBE | Long-limb Roux-en-Y reconstruction | 69 | 60 | NA |
| Itokawa et al[17] | Long SBE and short SBE | Hepaticojejunostomy | 92.9 | 100 | 1.6 |
| Whipple | 82.4 | 96 | |||
| Wang et al[40] | Long SBE | Billroth II, hepaticojejunostomy, Whipple, and Roux-en-Y reconstruction | 92.3 | 90 | 12.5 |
| Kawamura et al[79] | Long SBE | Roux-en-Y gastrectomy | 91.7 | 58.3 | 2.2 |
| Iwai et al[16] | Short SBE | Billroth II | 90 | 89 | 0 |
| Roux-en-Y reconstruction | 92 | 88 | 11.5 | ||
| Yamauchi et al[80] | Short SBE | Billroth II | 88 | 86 | 14.3 |
| Roux-en-Y gastrectomy | 91 | 90 | 21.1 | ||
| Hepaticojejunostomy | 100 | 100 | 0 | ||
| Yane et al[81] | Short SBE | Overall | 92.6 | 81.8 | 12 |
| Billroth II | 100 | 95 | NA | ||
| Whipple | 97.5 | 75.9 | NA | ||
| Roux-en-Y gastrectomy | 95.6 | 88.9 | NA | ||
| Hepaticojejunostomy | 81.4 | 79.7 | NA |
SBE: Single balloon enteroscope; RYGB: Roux-en-Y gastric bypass.
A few published articles compared long and short SBEs, but no significant difference in the insertion success rate was found between the two endoscopes[17,18]. The disadvantages of the long SBE are the long length of the scope, which is incompatible with conventional ERCP accessories. The 2.8-mm working channel also limits therapeutic intervention accessories. The short SBE is more convenient because of its easier maneuverability and its 152 cm length, which is compatible with many ERCP accessories. It larger working channel (3.2 mm) allows for use of small metallic and plastic stents, conventional wire-guided devices, and the water jet function, which is very useful to maintain the operative field and manage bleeding. The newest second-generation short SBE has a passive bending section that allows for deeper and smoother advancement; thus, the short SBE may be the first choice for ERCP in patients with altered anatomy. Additionally, a few studies have shown no significant difference in the success and complication rates between the DBE and SBE. De Koning et al[18] reported an overall ERCP success rate of 73% for DBE and 75% for SBE, with no significant difference. Katanuma and Isayama[15] also reported no significant difference between DBE and SBE insertion success rates in Billroth II reconstruction; however, the DBE tended to have a lower success rate in hepaticojejunostomy but a higher success rate in Roux-en-Y reconstruction compared with the SBE (94.7% and 85.1%, respectively). This because the SBE has a slightly softer overtube system that makes insertion into the deeper part slightly more difficult; additionally, most endoscopists are more familiar with the DBE than SBE. Abu Dayyeh[19] also noted no significant difference in the mean procedure time between the two endoscopes, but the SBE was more cost-effective and less technically demanding.
The SE is characterized by a rotating overtube for gripping and pleating the small bowel onto the endoscope and advancing the scope into the lumen. Clockwise advancement of the rotating overtube is performed while the scope is pushed in for deeper insertion. The spiral overtube provides straighter and more stable manipulation, but this stiffness may cause difficult insertion and complications in cases of severe adhesion. The overall success rate of afferent limb intubation varies widely (40%-90%) because few studies have been published (Table 4). DBE- and SBE-assisted ERCP are more popular than SE-assisted ERCP because of greater familiarity in manipulating the scope; thus, the SE is the second choice for RYGB or Roux-en-Y reconstruction. Lennon et al[20] reported a low diagnostic success rate of only 40.0% and 48.3% for the SE and SBE, respectively, but a high therapeutic yield of 87.5% for the SE only in intubated cases and 100% for the SBE with no statistical difference in Roux-en-Y reconstruction (Table 4). Ali et al[21] performed a large single-center study of SE-assisted ERCP in RYGB and long-limb Roux-en-Y reconstruction. The overall success rate of reaching the papilla was 86%, and the median procedure time was 189 min, but the procedures were performed by highly experienced endoscopists in a tertiary center hospital. Zouhairi et al[22] and Wagh et al[23] also reported a high SE access rate of any type of reconstruction of 76.2% and 77.0%, respectively. Clearly, SE-assisted ERCP is feasible and safe, especially in RYGB and Roux-en-Y reconstruction, despite the fact that the success rate seems to be lower than that of the DBE and SBE.
DBE, SBE, and SE are compared in Table 6. In a large United States multicenter study, Shah et al[10] reported 74%, 69%, and 72% rates of successful access to the papilla or biliopancreatic anastomosis using a DBE, SBE, and SE, respectively, in long-limb Roux-en-Y reconstruction; no significant difference was found among the three endoscopes. The reasons for failure were sharp angulation and an inability to identify the afferent limb from the jejunojejunostomy anastomosis; these reasons did not depend on the scope type. Skinner et al[24] also compared the DBE, SBE, and SE success rates in various reconstructions and found the highest success rate in Billroth II reconstruction (96%) and the lowest in RYGB (80%) of any type endoscope used.
Table 6.
Success rates of double balloon enteroscope, single balloon enteroscope, and spiral enteroscope in surgically altered anatomy
| Ref. | Operation type | DBE | SBE | SE | Overall | P value |
| Shah et al[10] | RYGB, hepaticojejunostomy, post-gastrectomy and Whipple | 74% | 69% | 72% | 71% | 0.722 |
| Skinner et al[24] | RYGB, Whipple, hepaticojejunostomy and Billroth II | 89% | 82% | 72% | 74% | NA |
| Lennon et al[20] | Roux-en-Y reconstruction | NA | 100% | 87.5% | 93.8% | 1 |
| Shah et al[83] | Long-limb surgical bypass | 74% | 69% | 72% | 71% | 0.887 |
DBE: Double balloon enteroscope; SBE: Single balloon enteroscope; SE: Spiral enteroscope; RYGB: Roux-en-Y gastric bypass.
The multibending backward-oblique-viewing duodenoscope (M-D scope) and the multibending forward-viewing endoscope (M-scope) both have two bending parts to upward of distal and downward of proximal shaft to create a swan neck shape of the distal tips to facilitate an en face position of the papilla, which is beneficial for cannulation. Imazu et al[25] reported that the first M-D scope created a “look-up” view to the papilla while stabilizing the proper distance between the scope and papilla. This benefit is clear in Billroth I reconstruction, which involves a straight anatomy and close proximity to the papilla. The first-generation M-D scope is difficult to insert because of the insufficient stiffness of the scope shaft; thus, the second-generation M-D scope was developed to increase the shaft stiffness, resulting in a high overall success rate of 100%[26]. The M-D scope helps to correct the papilla position by the swan neck tip shape with an overall success rate of 100%[27]. Koo et al[26] proposed that the advantage of the M-scope for Billroth II reconstruction is that the papilla is more easily reached due to the forward view, and the success rate of papilla cannulation with a side-view endoscope with swan neck tip was 92.9%. Thus, the major advantage of the M-D scope and M-scope is obtained in cases of difficult cannulation; access to the afferent limb may be similar to other forward-view endoscopes. However, the M-D scope and M-scope are not adequate for Roux-en-Y reconstruction or pediatric patients because of their short length and poor maneuverability.
Short type SBE is very convenient and easier to control because short type does not cause much looping on insertion. It is also compatible with basic commercially-available ERCP equipment that are important for treatment procedures.
Adjunctive technique to facilitate insertion into correct direction of afferent limb: Many adjunctive techniques have been developed to enhance the success rate of intubation into the afferent limb and reach the papilla or bilioenteric or pan-creatoenteric anastomosis with various endoscope types. The afferent limb can be recognized by the bile-containing limb and antiperistalsis motility. The Roux-en-Y anastomosis can be identified by scar tissue and must be crossed to enter the correct limb, and an adjunctive technique should be added to increase the insertability[16,28,29]. Yano et al[28] reported an 80% success rate of identifying the afferent limb in Roux-en-Y reconstruction by intraluminal indigo carmine injection in the second part of the duodenum (Figure 4A). Peristalsis moves the dye to the efferent limb, and slight reflux into the afferent limb allows for identification of the afferent limb. The roux limb usually has a sharp angulation, making a side-view duodenoscope difficult to use; a forward-view enteroscope is more beneficial. The bilioenteric anastomosis is always seen before the pancreatoenteric anastomosis, which is located 10 cm ahead[3]. Iwai et al[16] reported the usefulness of CO2 insufflation at the anastomosis if radiographs confirm the scope position in the correct afferent limb and blind end expansion in the right upper quadrant. (Figure 4B) In our experience, CO2 insufflation is the easiest and quickest way to assess whether we are in the correct limb.
Figure 4.
Technique to identify afferent limb. A: Intraluminal indigo carmine injection; B: CO2 insufflation guidance.
Third step: Cannulation to native papilla or biliopancreatoenteric anastomosis
Improved endoscope insertion is a major factor of the 90%-100% success rate; if insertion to the afferent limb is successful, the cannulation is always successful (Tables 1-4). Kato et al[30] reported a similar cannulation success rate of 60%-100% among all reconstruction types which is as same as normal anatomy patients. However, questions remain regarding how the native papilla or biliopancreatoenteric anastomosis can be identified and cannulated. The position of the native papilla in surgically altered anatomy differs greatly from that in the normal anatomy. Cannulation success rates in patients with a native papilla are lower than those in patients with a biliopancreatic anastomosis because of the sphincter muscle. Knowing the position of the working channel in the endoscopic view of each endoscope type is important to rotate the papilla into the proper en face view position; if the papilla cannot be adjusted to the proper view, cannulation may be difficult. Native papilla cannulation in Billroth II reconstruction is much more difficult because the papilla is in the reverse orientation; the forward-view endoscope thus shows a tangential, oblique, and inverted papilla. The use of a catheter oriented straight out from the working channel is better. Ishii et al[31] reported a J-turn technique that advanced the scope into the inferior duodenal angle, moving it to a retroflex position to facilitate cannulation in Roux-en-Y reconstruction with a short distance from the papilla in the tangential direction; however, caution is needed because of the risk of perforation. Okabe et al[32] proposed that a softer single-lumen catheter is suitable for the native papilla, while a stiffer double-/triple-lumen catheter is suitable for anastomosis cannulation because of the larger opening.
Other catheters, such as the sphincterotome, Soehendra Billroth II sphincterotome, and rotating-tip catheters, are used when the axis of the bile duct does not allow a straight catheter to fit. For a long DBE and long SBE, a prototype catheter, standard long catheter, or endoscopic nasobiliary drainage (ENBD) tube can be used for cannulation. The biliopancreatoenteric anastomosis is usually easy to identify and cannulate except in patients with scarring stenosis. This can be located by intermittent bile flow from the opening, but it may be more difficult to identify the pinhole-like anastomosis is cases of stenosis. Thus, administration of contrast media followed by fluoroscopy can identify the anastomosis in about 67% of cases[33], and CO2 inflation can identify the anastomosis by the presence of aerobilia on radiography.
In cases of severe stricture, Tsutsumi et al[33] reported successful use of a Soehendra stent retriever for dilating the strictured anastomosis in two patients. Wang et al[8] proposed the endoscopic exchange technique when cannulation by a forward-view endoscope failed. After reaching the papilla, a guidewire was placed in the afferent limb, the forward-view endoscope was then removed, and the side-view duodenoscope was advanced over the guidewire. Itoi et al[34] also reported exchange to a side-view duodenoscope while leaving the overtube in place with a 77% clinical success rate. Although cannulation in native papillae seems to be more difficult, Osoegawa et al[35] reported no significant difference in the cannulation rate among Roux-en-Y total gastrectomy, Billroth II reconstruction, and the Whipple procedure when a DAE reached the blind end. Skinner et al[24] also reported no significant difference in the cannulation rate for native papillae and biliopancreatoenteric anastomosis (90% and 92%, respectively) or among the DBE, SBE, and SE (85%, 87% and 90%, respectively).
In another study, although a significantly higher cannulation rate was observed when using the side-view duodenoscope than the forward-view endoscope (87% and 68%, respectively), the perforation rate was lower with the forward-view endoscope[36]. In cases of difficult or failed cannulation, a rendezvous technique after percutaneous transhepatic biliary drainage (PTBD), which requires a dilated intrahepatic duct, can facilitate the cannulation. If the intrahepatic duct is not dilated, safe performance of the percutaneous transgallbladder rendezvous technique can be challenging[29,37]. Application of a cap at the tip of the forward-view endoscope (cap-fitted tip) can decrease endoscope slippage from the bowel wall during reduction of looping by mucosal suction, ensuring adequate visibility when insertion is estimated to be 2 mm from the bowel wall, stabilizing the scope, and maintaining a proper distance between the scope and papilla to facilitate successful cannulation[38]. If insertion is still difficult due to looping or a long scope length, passing a biopsy forceps or guidewire into the endoscope channel can increase the stiffness and decrease the floppiness of the scope, thereby facilitating successful insertion[39]. Wang et al[40] reported that passage of a long guidewire or long retrieval balloon into the afferent limb can facilitate scope insertion with an overall therapeutic success rate of 90%.
Cannulation with a forward-view endoscope is more difficult than side-view but if you can rotate the papilla to the en face view with the endoscope working channel it will be easier. For example, a short type SBE working channel is located at 7 o’clock, so you have to position the ampulla at 7 o’clock.
Fourth step: Papillary intervention
Papillary intervention is important and performed prior to stone extraction or other therapeutic procedures. A common technique is endoscopic sphincterotomy (EST), but in cases of surgically altered anatomy, it is difficult to keep the EST knife in the proper direction and control limit size of cutting because of the reverse position of the papilla, difficult scope maneuverability, and improper accessories. Many techniques can facilitate easier EST, such as use of an S-shaped sphincterotome, rotatable sphincterotome, push-type sphincterotome, and needle-knife sphincterotomy (either free-hand or over a biliary stent). The DBE working channel is located at the 6 to 7 o’clock position, and the papilla needs to be brought to the 6 o’clock position for safe fixation. Conversely, the SBE working channel is located at 8 to 9 o’clock, making it more difficult to fix the papilla; additionally, the cutting should be directed toward 5-o’clock. If EST is too high-risk, endoscopic papillary balloon dilatation is the first option because of its low risk of bleeding and perforation. This technique is suitable for small and multiple CBD stones because of the small balloon diameter (6-8 mm)[41]. For large and multiple CBD stones, endoscopic papillary large balloon dilatation (EPLBD) (diameter, 12-18 mm) has a satisfactory success rate even when not combined with EST, and there is no significant difference in post-ERCP pancreatitis. The main reason for performing EPLBD is to avoid additional therapeutic procedures for stone extraction. The stone clearance rate by EPLBD alone is high (Table 7); thus, EPLBD alone and EST plus EPLBD both have a higher therapeutic success rate than EST alone. Teoh et al[42] reported equal efficacy for removal of bile duct stones between EST alone and EST followed by EPLBD, which decreased the bleeding and perforation rates in the EPLBD group. In their systematic review, Kim et al[43] found that the overall success rate was 96.5% in EST with EPLBD and 97.2% in EPLBD alone with no significant difference. EPLBD alone is effective and safe for stone removal after Billroth II reconstruction with a first-session success rate of 92.5%[44]. EPLBD can be used alone for papillary intervention to decrease EST-related complications such as bleeding and perforation, but caution is still needed because of possible post-ERCP pancreatitis.
Table 7.
Success rates of stone removal in Billroth II reconstruction in different ampullary interventions
| Ref. | Number of patients | Endoscope used | Ampullary intervention | First session success rate, % | Overall success rate, % | Complication |
| Park et al[38] | 10 | Cap-fitted forward-view endoscope | EST | 30 | 100 | 0 |
| Kim et al[41] | 9 | Side-view endoscope | EST + EPLBD | 55.5 | 89 | 0 |
| Choi et al[84] | 26 | End-view and side-view endoscope | EST + EPLBD | 76.9 | 100 | 0 |
| Itoi et al[85] | 11 | End-view endoscope | EST + EPLBD | 100 | 100 | 0 |
| Lee et al[86] | 13 | Cap-fitted forward-view endoscope | EPBD | 66.6 | 100 | 23 |
| Cheng et al[71] | 77 | DBE | EPLBD | 75 | 100 | 4 |
| Jang et al[44] | 40 | Side-view endoscope | EPLBD | 92.5 | 100 | 15 |
EST: Endoscopic sphincterotomy; EPLBD: Endoscopic papillary large balloon dilatation.
Laser lithotripsy, electrohydraulic lithotripsy under direct cholangioscopy, and extracorporeal shockwave lithotripsy can be used to remove difficult stones. Yamauchi et al[45] reported successful peroral direct cholangioscopy (PDCS) using a short SBE with a free-hand technique, guidewire, and large balloon anchoring and deflation in Roux-en-Y anastomosis for difficult-to-treat bile duct stones. Bile duct insertion by large balloon anchoring and deflation is very useful with a bile duct diameter of > 12 mm to prevent bile duct laceration or perforation. PDCS can improve the complete stone clearance rate from 90.1%-97.6% in the transpapillary approach and from 77.3%-100% in the transanastomotic approach without severe com-plications[46]. Matsumoto et al[46] reported an 85.7% success rate of stone removal by PDCS with replacement of the DBE by an ultraslim endoscope and leaving the balloon overtube in place during hepaticojejunostomy anastomosis without serious complications. The detection rate of residual stones by PDCS was 41.7%. Thus, PDCS can achieve complete stone clearance and reduce the stone recurrence rate. Air embolism, which might be fatal, can be avoided by using CO2 insufflation during the procedure.
One study of the treatment of stones in hepaticojejunostomy between percutaneous transhepatic cholangioscopy (PTCS) and PDCS using a short DBE showed that the 1-, 2-, and 3-year stone-free rates were 100%, 73%, and 64% for PTCS and 85%, 65%, and 59% for PDCS, respectively; however, PDCS had a lower adverse event rate (10% vs 45% in PTCS)[47]. PDCS has a lower infection rate and less hemobilia, biloma formation, and pain; additionally, the incidence of pancreatitis is very low (0.0%-8.3%). PTCS is a more difficult technique because of the anatomy of the hepatic confluence and contraindications in patients with ascites and coagulopathy.
The rate of postoperative bilioenteric anastomosis stricture can reach 12.5% at 2 years[48], and the rate of hepaticojejunostomy anastomosis stenosis can reach 3%-4% at 2.3-4.1 years after conventional pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy[31] Endoscopic balloon dilatation for this benign anastomosis stricture is an important and challenging procedure. Mizukawa et al[49] reported that the 1-, 2-, and 3-year cumulative anastomosis patency rates after endoscopic balloon dilatation (6-8 mm) by a short DBE were 73%, 55%, and 49%, respectively, which do not represent a good outcome despite a high technical success rate (100%); however, it is difficult to predict which patients will develop a recurrent stenosis. Tsutsumi et al[33] also reported successful dilation of severe bilioenteric anastomosis stricture by a 7-Fr Soehendra stent retriever over the guidewire by a short DBE. The Soehendra stent retriever can dilate severe and tight strictures over the guidewire, and a dilation catheter can subsequently pass and achieve sufficient dilation. Kamei et al[48] reported treatment of hepaticojejunostomy anastomosis stricture following living-donor liver transplantation by balloon dilatation with a DBE, and the success rate was 78%. Compared to percutaneous dilatation and stent placement, the success rate was 72%-80%, which is similar to endoscopic treatment and a highly effective short-term outcome. For pancreaticojejunostomy anastomosis following Whipple’s operation, pancreatitis usually occurs if a stenosis is present. Transient edema can occur after dilation but can be corrected by applying a 5- to 10-Fr pancreatic duct stent.
Endoscopic treatment of malignant biliary obstruction in patients with surgically altered anatomy is much more difficult. Yamauchi et al[50] reported a 100% technical success rate and 92% functional success rate with placement of an 8.5-Fr uncovered self-expandable metal stent (SEMS) for malignant biliary obstruction in altered anatomy by a short SBE, and the median time to recurrence of obstruction was 247 days. Comparison of the patency of metallic stent types showed that recurrent obstruction was longer in covered metallic stents[51]. Direct cholangioscopy has an important role in clinical investigation, and tissue biopsy of intraductal biliary carcinoma by DBE was reportedly successful after choledochojejunostomy[52]. Lenze et al[52] compared the rate of treatment failure between malignant obstruction and benign stricture in patients with altered anatomy by an SBE and found that malignant biliary obstruction had a significantly higher failure rate than benign stricture (84.2% and 14.2%, respectively). Cases of failure can be successfully treated by PTCS and surgical intervention. Thus, malignant obstruction can be successfully treated by endoscopic SEMS placement with effective short-term outcomes and a longer time to recurrence of obstruction using covered type SEMS[50,53].
EST plus EPLBD has a stone clearance rate and less complications, such as perforation or bleeding, and this intervention is not much more difficult to perform once you can cannulate the bile duct. PDCS is useful in cases with difficult-to-treat bile duct stones because it can detect retained stones in real time. However, this procedure requires advanced endoscopy skills.
Recently advanced techniques for ERCP in surgically altered anatomy: The di-fficulty of performing ERCP in altered anatomy, especially with a very long limb as in RYGB and Roux-enY reconstruction, has resulted in the adaptation and development of many endoscopic and surgical techniques. Failed cases of DAE-assisted ERCP are treated by PTBD, which has a high risk of skin infection, pain, difficult home care, decreased quality of life, and impaired enterohepatic bile circulation. Many publications have described EUS-guided ERCP, endoscopic gastropexy or gastrostomy ERCP, and LA-ERCP (Figure 5)[54-57].
Three main access techniques are used in EUS-guided biliary-pancreatic ERCP or interventions in patients with altered anatomy: the EUS-guided rendezvous technique, EUS-guided anterograde drainage, and EUS-guided transmural drainage. Good outcomes are attained by experienced surgeons in high-volume centers[54]. Because a high level of technical experience is required, EUS-guided ERCP should be reserved for patients with long (> 100 cm) or very long (> 150 cm) limbs for which conventional or DAE-assisted ERCP has failed. The anterograde or rendezvous technique may be initiated in patients with bile duct stones and failed cannulation to the native papilla or a strictured biliopancreatoenteric anastomosis. Table 8 sum-marizes the efficacy of EUS-guided ERCP and shows a high technical success rate of 75%-100% and high clinical success rate of 70%-100% with a complication rate of 10%-20%, but the complications can be managed conservatively.
Table 8.
Efficacy of endoscopic ultrasonography-guided endoscopic retrograde cholangiopancreatography in surgically altered anatomy
| Ref. | Method | Patients, n | One- or two- stage ERCP | Technical success rate, % | Clinical success rate, % | Complication rate, % |
| Bukhari et al[62] | EUS-GG-ERCP (LAMS) | 30 | One 26.7% | 100 | 100 | 10 |
| Two 73.3% | ||||||
| Hosmer et al[57] | EUS-guided HGS | 9 | One | 100 | NA | 11 |
| Iwashita et al[58] | EUS-AG for BDS | 29 | One | 79 | 72 | 17 |
| Iwashita et al[60] | EUS-guided antegrade stent | 20 | Two | 95 | 95 | 20 |
| Khashab et al[61] | EUS-guided BD | 49 | Two | 98 | 88 | 20 |
| Imai et al[56] | EUS-guided HGS | 42 | Two | 97.6 | 90.2 | NA |
Endoscopic ultrasonography-guided biliary drainage included the rendezvous technique, direct transmural ostomy formation (hepatogastrostomy, hepatoduodenostomy, hepatojejunostomy), and antegrade stenting. HGS: Hepatogastrostomy; EUS: Endoscopic ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography; EUS-BD: Endoscopic ultrasonography-guided biliary drainage; LAMS: Lumen-apposing metal stent.
Ngamruengphong et al[55] reported EUS-guided creation of a transgastric fistula from the gastric pouch or jejunum to the excluded stomach in RYGB followed by use of lumen-apposing metal stents (LAMSs). A conventional duodenoscope could then be advanced perorally via this stent. After successful ERCP, the stent was removed and the fistula was closed by over-the-scope clips or endoscopic suturing. A point of caution in this technique is the risk of perforation due to stent dislodgment into the abdominal wall and patency of the transgastric fistula with weight regain. The authors reported technical and clinical success rates of 100%, and the fistula closed in 92% of cases by endoscopic procedures without weight regain. LAMS dislodgement occurred in two patients and was managed by stent repositioning.
Hosmer et al[57] also reported a 100% technical success rate of EUS-guided hepaticogastrostomy (EUS-HGS) with antegrade clearance of bile duct stones in Roux-en-Y reconstruction, which is more suitable when urgent drainage is needed because of a single session, with no risk of stent dislodgment and no risk of patent fistula induced by weight regain. A retrospective review from four academic centers reported a 72% clinical success rate of EUS-guided antegrade bile duct stone removal in patients with altered anatomy; failure was due to insufficient bile duct dilatation. This EUS-guided antegrade procedure can be performed in the same session, while PTBD must be performed in two sessions by a radiologist and endoscopist[58]. EUS-guided antegrade removal of bile duct stones seems to be the first option for small bile duct stones, while DAE-assisted ERCP should be used for large bile duct stones if possible. Compared with the EUS-rendezvous technique, this may be easier and faster because the endoscope does not need to pass the long afferent limb for papillary intervention. Iwashita et al[58] suggested that puncture from segment 2 allows for easier manipulation of the guidewire and pushing of the balloon to treat bile duct stones because the segment 2 route to the ampulla is relatively straighter than the segment 3 route. However, the segment 2 route causes transesophageal puncture, which might introduce bile leakage into the thorax. Segment 3 puncture may therefore be safer despite the fact that the guidewire passage is slightly more difficult. EUS-HGS can resolve a benign bilioenteric anastomosis stricture with antegrade dilatation of the anastomosis with technical and clinical success rates of 100% but a dilatation success rate of only 57% because of failure to pass the guidewire through the strictured part. However, the clinical success rate can be increased to 100% by persistent hepaticogastrostomy[59]. This EUS-HGS dilatation is suitable when transpapillary access is impossible.
In malignant obstruction, EUS-guided transmural drainage is preferred because the procedure can be repeated with a conventional endoscope. Iwashita et al[60] reported a 95% clinical and technical success rate of EUS-guided antegrade biliary stenting by an uncovered metal stent for malignant obstruction in surgically altered anatomy with a 20% rate of adverse events that could be resolved by conservative management. Surgical bypass and EUS-guided drainage for malignant distal biliary obstruction show no differences in technical success, clinical success, quality of life, or survival[54]. Khashab et al[61] found that EUS-BD had a significantly higher technical success rate than DAE-assisted ERCP in patients with surgically altered upper gastrointestinal anatomy (98.0% and 65.3%, respectively). Clinical success was significantly higher in EUS-BD than ERCP (88.0% and 59.1%, respectively). EUS-BD was not dependent on the length of the surgical limb and allowed placement of larger metallic stents than DAE-assisted ERCP.
An international multicenter study compared EUS-guided gastrogastrostomy-assisted ERCP with LAMSs and enteroscopy-assisted ERCP in RYGB and found that the technical success rate was superior in EUS-guided gastrogastrostomy-assisted ERCP (100% vs 60%) with similar adverse event rates[62]. In a rare report of pancreatic duct drainage (PDD) by EUS, Chen et al[63] found that EUS-guided PDD had a significantly higher technical success rate than ERP after Whipple’s operation (92.5% and 20.0%, respectively). Although EUS-PDD had a higher adverse event rate than ERP (35.0% and 2.9%, respectively), all complications were successfully managed conservatively. Another proposed EUS technique is EUS-guided gastropexy, which has the advantages of a single procedural session and no need to wait for maturation of the gastrostomy or fistula because of performance of gastropexy. This technique may be suitable for urgent situations[64].
The EUS-HGS is a rescue procedure when you cannot access the papilla by other techniques, but it requires advance endoscopic skill. Puncturing on intrahepatic bile duct in segment 3 is safer compared to segment 2 because the working area is far from the esophagus and the thoracic cavity, but the down side is the difficulty in passing the guide wire due to the angulation.
LA-ERCP has important roles in long-limb reconstruction (> 150 or > 200 cm) or failed DAE-assisted ERCP and EUS-guided ERCP. The LA-ERCP procedure starts by placing standard laparoscopic ports in three to four locations and connecting a hanging suture from the anterior wall of the greater curvature to the abdominal wall, then creating a gastrostomy between this suture. A 15- to 18-mm port is placed in the gastronomy site, and ERCP is performed by a conventional side-view duodenoscope via this port. After completion of the procedure, the port is removed and the defect is closed by a suture or stapler (Figure 5E). LA-ERCP is the first choice in patients with long limbs who require concomitant cholecystectomy in some institutions because standard RYGB does not include concomitant cholecystectomy in all cases due to the low incidence (only 7%-8%) of gallstone symptoms from postoperative rapid weight loss[2]. Table 9 shows that the laparoscopic and endoscopic procedure in LA-ERCP has a high success rate of 90%-100%, while the laparoscopic complication rate (e.g., port size infection and hernia) widely ranges from 1%-20%, and the endoscopic complication rate (e.g., bleeding and pancreatitis) ranges from 1%-8%. Schreiner et al[3] reported that LA-ERCP had a higher papilla identification rate, cannulation rate, and therapeutic success rate when compared with DAE-assisted ERCP (100% vs 72%, 100% vs 59%, and 100% vs 59%, respectively) because a limb length of > 150 cm is associated with a high failure rate of DAE-assisted ERCP. Thus, LA-ERCP is suitable in cases involving concomitant cholecystectomy, urgency, long-limb reconstruction, and failure of other ERCP techniques.
Table 9.
Outcome of laparoscopic-assisted endoscopic retrograde cholangiopancreatography in patients undergoing Roux-en-Y gastric bypass
| Ref. | Patients, n | Laparoscopic success rate, % | Endoscopic success rate, % | Simultaneous cholecystec-tomy, % | One- or two-stage ERCP | Median hospital stay in d | Laparoscopic complication rate, % | Endoscopic complication rate, % |
| Habenichts Yancey et al[7] | 16 | 100 | 94 | 31 | One | 3.7 | 0 | 7.6 |
| Snauwaert et al[2] | 23 | 91.3 | 100 | 56.5 | One | 2.8 | 0 | 0 |
| Paranandi et al[65] | 7 | 100 | 100 | 0 | One | 2 | 1 | 1 |
| Abbas et al[6] | 579 | 98 | 98 | 21 | One | 2 | 10 | 7 |
| Schreiner et al[3] | 24 | 100 | 100 | 0 | One | 1.67 | 8.3 | NA |
| Bowman et al[5] | 11 | 100 | 100 | 0 | One | 3.4 | 18.2 | 0 |
| Saleem et al[54] | 15 | 100 | 100 | 0 | One | 2 | 0 | 0 |
ERCP: Endoscopic retrograde cholangiopancreatography.
Limitations of LA-ERCP include the need for coordination among the surgeons, anesthesiologists, and operative room and the high risk of operative complications. As shown in Table 9, however, this is a highly successful procedure with few com-plications that can be managed conservatively. In this laparoscopic technique, the endoscope is more difficult to manipulate via the port because the shaft is outside the patient. Hence, the laparoscopic port in gastrostomy should be inserted pointing toward the pylorus[65]. Gastrostomy closure after ERCP is not complicated; sutures or surgical staples can be used without leakage. Unplanned events and complications such as bleeding and incomplete stone removal require repeating LA-ERCP without retaining the previous gastrostomy tube, making the procedure much more difficult because of adhesions from the previous operation.
Transgastric ERCP in RYGB involves the performance of ERCP by a conventional side-view duodenoscope through the gastrostomy tract. The access route to the excluded stomach may involve percutaneous, endoscopic, or surgical (laparoscopic or open) placement of the gastrostomy. Banerjee et al[66] reported a 100% gastric access rate and 98.5% duct cannulation rate with a 14.0% adverse event rate compared with a 60%-70% success rate of DAE-assisted ERCP. This can be performed in one or two stages by waiting for gastrostomy tract maturation and upsizing for 4 to 6 wk to avoid perforation, bleeding, or dislodgment of the gastrostomy tube. Therefore, this technique is suitable in patients with large stones requiring a large sphincterotomy or additional intervention through a conventional duodenoscope, while DAE-assisted ERCP requires only a single stage and can be advantageous in more urgent cases.
Table 10 summarizes the efficacy of ERCP methods, including DAE-assisted, EUS-guide biliary access, and LA-ERCP, in patients with surgically altered anatomy to help endoscopists decide method of choice.
Table 10.
Summarized efficacy of endoscopic retrograde cholangiopancreatography methods in surgically altered anatomy
| DAE-assisted ERCP | EUS-guided biliary access | Laparoscopic-assisted ERCP | |
| Cholangiography success rate | 70%-90% | 95%-100% | 95%-100% |
| Invasiveness | Minimal | Moderate | High |
| Skill requirement | Moderate | High | Moderate Cooperate with surgeon |
| Complication rate | 0%-20% | 10%-20% | 0%-10% |
| Bile duct stone removal | |||
| Small stones | Easy | Easy | Easy |
| Large stones | Easy | Fair | Easy |
| Malignant stenosis drainage | Fair | Easy | Fair |
DAE: Device-assisted enteroscope; EUS: Endoscopic ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography.
CONCLUSION
ERCP in patients with surgically altered anatomy requires high technical expertise and familiarity with the endoscope. An understanding of the type of surgery, length of the afferent limb, type of endoscope used with choice of proper approach (peroral or transgastric), and compatible ERCP accessories with various endoscopic types are the keys to success. A conventional endoscope and DAE-assisted ERCP are recommended for short-limb reconstruction with/without a native papilla, while DAE-assisted ERCP, EUS-guided ERCP, and especially LA-ERCP are highly recommended for long-limb reconstruction, such as RYGB with concomitant cholecystectomy.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: March 25, 2019
First decision: April 11, 2019
Article in press: May 18, 2019
P-Reviewer: Akbulut S, Vezakis A, Goyal H S-Editor: Ma RY L-Editor: Filipodia E-Editor: Zhang YL
Contributor Information
Chonlada Krutsri, Department of Surgery, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Mitsuhiro Kida, Department of Gastroenterology, Graduate School of Medicine, Kitasato University Hospital, Kanagawa 252-0375, Japan. m-kida@kitasato-u.ac.jp.
Hiroshi Yamauchi, Department of Gastroenterology, Graduate School of Medicine, Kitasato University Hospital, Kanagawa 252-0375, Japan.
Tomohisa Iwai, Department of Gastroenterology, Graduate School of Medicine, Kitasato University Hospital, Kanagawa 252-0375, Japan.
Hiroshi Imaizumi, Department of Gastroenterology, Graduate School of Medicine, Kitasato University Hospital, Kanagawa 252-0375, Japan.
Wasaburo Koizumi, Department of Gastroenterology, Graduate School of Medicine, Kitasato University Hospital, Kanagawa 252-0375, Japan.
References
- 1.Wu WG, Gu J, Zhang WJ, Zhao MN, Zhuang M, Tao YJ, Liu YB, Wang XF. ERCP for patients who have undergone Billroth II gastroenterostomy and Braun anastomosis. World J Gastroenterol. 2014;20:607–610. doi: 10.3748/wjg.v20.i2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snauwaert C, Laukens P, Dillemans B, Himpens J, De Looze D, Deprez PH, Badaoui A. Laparoscopy-assisted transgastric endoscopic retrograde cholangiopancreatography in bariatric Roux-en-Y gastric bypass patients. Endosc Int Open. 2015;3:E458–E463. doi: 10.1055/s-0034-1392108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748–756. doi: 10.1016/j.gie.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Shimatani M, Takaoka M, Tokuhara M, Miyoshi H, Ikeura T, Okazaki K. Review of diagnostic and therapeutic endoscopic retrograde cholangiopancreatography using several endoscopic methods in patients with surgically altered gastrointestinal anatomy. World J Gastrointest Endosc. 2015;7:617–627. doi: 10.4253/wjge.v7.i6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman E, Greenberg J, Garren M, Guda N, Rajca B, Benson M, Pfau P, Soni A, Walker A, Gopal D. Laparoscopic-assisted ERCP and EUS in patients with prior Roux-en-Y gastric bypass surgery: a dual-center case series experience. Surg Endosc. 2016;30:4647–4652. doi: 10.1007/s00464-016-4746-8. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AM, Strong AT, Diehl DL, Brauer BC, Lee IH, Burbridge R, Zivny J, Higa JT, Falcão M, El Hajj II, Tarnasky P, Enestvedt BK, Ende AR, Thaker AM, Pawa R, Jamidar P, Sampath K, de Moura EGH, Kwon RS, Suarez AL, Aburajab M, Wang AY, Shakhatreh MH, Kaul V, Kang L, Kowalski TE, Pannala R, Tokar J, Aadam AA, Tzimas D, Wagh MS, Draganov PV LA-ERCP Research Group. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 2018;87:1031–1039. doi: 10.1016/j.gie.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Habenicht Yancey K, McCormack LK, McNatt SS, Powell MS, Fernandez AZ, Westcott CJ. Laparoscopic-Assisted Transgastric ERCP: A Single-Institution Experience. J Obes. 2018;2018:4. doi: 10.1155/2018/8275965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Xu B, Li Q, Zhang X, Jiang G, Ge X, Nie J, Zhang X, Wu P, Ji J, Miao L. Endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: One single center's experience. Medicine (Baltimore) 2016;95:e5743. doi: 10.1097/MD.0000000000005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bove V, Tringali A, Familiari P, Gigante G, Boškoski I, Perri V, Mutignani M, Costamagna G. ERCP in patients with prior Billroth II gastrectomy: report of 30 years' experience. Endoscopy. 2015;47:611–616. doi: 10.1055/s-0034-1391567. [DOI] [PubMed] [Google Scholar]
- 10.Shah RJ, Smolkin M, Yen R, Ross A, Kozarek RA, Howell DA, Bakis G, Jonnalagadda SS, Al-Lehibi AA, Hardy A, Morgan DR, Sethi A, Stevens PD, Akerman PA, Thakkar SJ, Brauer BC. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video) Gastrointest Endosc. 2013;77:593–600. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Ciçek B, Parlak E, Dişibeyaz S, Koksal AS, Sahin B. Endoscopic retrograde cholangiopancreatography in patients with Billroth II gastroenterostomy. J Gastroenterol Hepatol. 2007;22:1210–1213. doi: 10.1111/j.1440-1746.2006.04765.x. [DOI] [PubMed] [Google Scholar]
- 12.House MG, Cameron JL, Schulick RD, Campbell KA, Sauter PK, Coleman J, Lillemoe KD, Yeo CJ. Incidence and outcome of biliary strictures after pancreaticoduodenectomy. Ann Surg. 2006;243:571–576; discussion 576-578. doi: 10.1097/01.sla.0000216285.07069.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WG, Mei JW, Zhao MN, Zhang WJ, Gu J, Tao YJ, Liu YB, Wang XF. Use of the Conventional Side-viewing Duodenoscope for Successful Endoscopic Retrograde Cholangiopancreatography in Postgastrectomy Patients. J Clin Gastroenterol. 2016;50:244–251. doi: 10.1097/MCG.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoi T, Ishii K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Tsuji S, Ikeuchi N, Fukuzawa K, Moriyasu F, Tsuchida A. Long- and short-type double-balloon enteroscopy-assisted therapeutic ERCP for intact papilla in patients with a Roux-en-Y anastomosis. Surg Endosc. 2011;25:713–721. doi: 10.1007/s00464-010-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katanuma A, Isayama H. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy in Japan: questionnaire survey and important discussion points at Endoscopic Forum Japan 2013. Dig Endosc. 2014;26 Suppl 2:109–115. doi: 10.1111/den.12247. [DOI] [PubMed] [Google Scholar]
- 16.Iwai T, Kida M, Yamauchi H, Imaizumi H, Koizumi W. Short-type and conventional single-balloon enteroscopes for endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: single-center experience. Dig Endosc. 2014;26 Suppl 2:156–163. doi: 10.1111/den.12258. [DOI] [PubMed] [Google Scholar]
- 17.Itokawa F, Itoi T, Ishii K, Sofuni A, Moriyasu F. Single- and double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y plus hepaticojejunostomy anastomosis and Whipple resection. Dig Endosc. 2014;26 Suppl 2:136–143. doi: 10.1111/den.12254. [DOI] [PubMed] [Google Scholar]
- 18.De Koning M, Moreels TG. Comparison of double-balloon and single-balloon enteroscope for therapeutic endoscopic retrograde cholangiography after Roux-en-Y small bowel surgery. BMC Gastroenterol. 2016;16:98. doi: 10.1186/s12876-016-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu Dayyeh B. Single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy: getting there. Gastrointest Endosc. 2015;82:20–23. doi: 10.1016/j.gie.2015.03.1988. [DOI] [PubMed] [Google Scholar]
- 20.Lennon AM, Kapoor S, Khashab M, Corless E, Amateau S, Dunbar K, Chandrasekhara V, Singh V, Okolo PI., 3rd Spiral assisted ERCP is equivalent to single balloon assisted ERCP in patients with Roux-en-Y anatomy. Dig Dis Sci. 2012;57:1391–1398. doi: 10.1007/s10620-011-2000-8. [DOI] [PubMed] [Google Scholar]
- 21.Ali MF, Modayil R, Gurram KC, Brathwaite CEM, Friedel D, Stavropoulos SN. Spiral enteroscopy-assisted ERCP in bariatric-length Roux-en-Y anatomy: a large single-center series and review of the literature (with video) Gastrointest Endosc. 2018;87:1241–1247. doi: 10.1016/j.gie.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Zouhairi ME, Watson JB, Desai SV, Swartz DK, Castillo-Roth A, Haque M, Jowell PS, Branch MS, Burbridge RA. Rotational assisted endoscopic retrograde cholangiopancreatography in patients with reconstructive gastrointestinal surgical anatomy. World J Gastrointest Endosc. 2015;7:278–282. doi: 10.4253/wjge.v7.i3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagh MS, Draganov PV. Prospective evaluation of spiral overture-assisted ERCP in patients with surgically altered anatomy. Gastrointest endosc. 2012;76:439–443. doi: 10.1016/j.gie.2012.04.444. [DOI] [PubMed] [Google Scholar]
- 24.Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560–572. doi: 10.1055/s-0034-1365698. [DOI] [PubMed] [Google Scholar]
- 25.Imazu H, Kanazawa K, Ikeda K, Kakutani H, Sumiyama K, Ang TL, Omar S, Tajiri H. Initial evaluation of a novel multibending backward-oblique viewing duodenoscope in endoscopic retrograde cholangiopancreatography. Endoscopy. 2012;44:99–102. doi: 10.1055/s-0031-1291445. [DOI] [PubMed] [Google Scholar]
- 26.Koo HC, Moon JH, Choi HJ, Ko BM, Hong SJ, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. The utility of a multibending endoscope for selective cannulation during ERCP in patients with a Billroth II gastrectomy (with video) Gastrointest Endosc. 2009;69:931–934. doi: 10.1016/j.gie.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Toyoizumi H, Imazu H, Ikeda K, Mori N, Kanazawa K, Chiba M, Ang TL, Tajiri H. A novel second-generation multibending backward-oblique viewing duodenoscope in ERCP. Minim Invasive Ther Allied Technol. 2015;24:101–107. doi: 10.3109/13645706.2014.955030. [DOI] [PubMed] [Google Scholar]
- 28.Yano T, Hatanaka H, Yamamoto H, Nakazawa K, Nishimura N, Wada S, Tamada K, Sugano K. Intraluminal injection of indigo carmine facilitates identification of the afferent limb during double-balloon ERCP. Endoscopy. 2012;44 Suppl 2 UCTN:E340–E341. doi: 10.1055/s-0032-1309865. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi H, Kida M, Imaizumi H, Okuwaki K, Miyazawa S, Iwai T, Koizumi W. Innovations and techniques for balloon-enteroscope-assisted endoscopic retrograde cholangiopancreatography in patients with altered gastrointestinal anatomy. World J Gastroenterol. 2015;21:6460–6469. doi: 10.3748/wjg.v21.i21.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato H, Tsutsumi K, Harada R, Okada H, Yamamoto K. Short double-balloon enteroscopy is feasible and effective for endoscopic retrograde cholangiopancreatography in patients with surgically altered gastrointestinal anatomy. Dig Endosc. 2014;26 Suppl 2:130–135. doi: 10.1111/den.12251. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K, Itoi T, Tonozuka R, Itokawa F, Sofuni A, Tsuchiya T, Tsuji S, Ikeuchi N, Kamada K, Umeda J, Tanaka R, Honjo M, Mukai S, Fujita M, Moriyasu F, Baron TH, Gotoda T. Balloon enteroscopy-assisted ERCP in patients with Roux-en-Y gastrectomy and intact papillae (with videos) Gastrointest Endosc. 2016;83:377–386.e6. doi: 10.1016/j.gie.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Okabe Y, Ishida Y, Kuraoka K, Ushijima T, Tsuruta O. Endoscopic bile duct and/or pancreatic duct cannulation technique for patients with surgically altered gastrointestinal anatomy. Dig Endosc. 2014;26 Suppl 2:122–126. doi: 10.1111/den.12274. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi K, Kato H, Sakakihara I, Yamamoto N, Noma Y, Horiguchi S, Harada R, Okada H, Yamamoto K. Dilation of a severe bilioenteric or pancreatoenteric anastomotic stricture using a Soehendra Stent Retriever. World J Gastrointest Endosc. 2013;5:412–416. doi: 10.4253/wjge.v5.i8.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoi T, Ishii K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Tsuji S, Ikeuchi N, Umeda J, Moriyasu F. Single-balloon enteroscopy-assisted ERCP in patients with Billroth II gastrectomy or Roux-en-Y anastomosis (with video) Am J Gastroenterol. 2010;105:93–99. doi: 10.1038/ajg.2009.559. [DOI] [PubMed] [Google Scholar]
- 35.Osoegawa T, Motomura Y, Akahoshi K, Higuchi N, Tanaka Y, Hisano T, Itaba S, Gibo J, Yamada M, Kubokawa M, Sumida Y, Akiho H, Ihara E, Nakamura K. Improved techniques for double-balloon-enteroscopy-assisted endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:6843–6849. doi: 10.3748/wjg.v18.i46.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuno M, Iwashita T, Yasuda I, Mabuchi M, Uemura S, Nakashima M, Doi S, Adachi S, Mukai T, Moriwaki H. Percutaneous transgallbladder rendezvous for enteroscopic management of choledocholithiasis in patients with surgically altered anatomy. Scand J Gastroenterol. 2013;48:974–978. doi: 10.3109/00365521.2013.805812. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Masu K, Kanno Y, Ohira T, Noda Y. Ampullary intervention for bile duct stones in patients with surgically altered anatomy. Dig Endosc. 2014;26 Suppl 2:116–121. doi: 10.1111/den.12250. [DOI] [PubMed] [Google Scholar]
- 38.Park CH, Lee WS, Joo YE, Kim HS, Choi SK, Rew JS. Cap-assisted ERCP in patients with a Billroth II gastrectomy. Gastrointest Endosc. 2007;66:612–615. doi: 10.1016/j.gie.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Moriyasu F, Kasuya K, Tsuchida A. A newly developed variable stiffness duodenoscope for diagnostic and therapeutic endoscopic retrograde cholangiopancreatography. Diagn Ther Endosc. 2010;2010:153951. doi: 10.1155/2010/153951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang AY, Sauer BG, Behm BW, Ramanath M, Cox DG, Ellen KL, Shami VM, Kahaleh M. Single-balloon enteroscopy effectively enables diagnostic and therapeutic retrograde cholangiography in patients with surgically altered anatomy. Gastrointest Endosc. 2010;71:641–649. doi: 10.1016/j.gie.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Kim GH, Kang DH, Song GA, Heo J, Park CH, Ha TI, Kim KY, Lee HJ, Kim ID, Choi SH, Song CS. Endoscopic removal of bile-duct stones by using a rotatable papillotome and a large-balloon dilator in patients with a Billroth II gastrectomy (with video) Gastrointest Endosc. 2008;67:1134–1138. doi: 10.1016/j.gie.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Teoh AY, Cheung FK, Hu B, Pan YM, Lai LH, Chiu PW, Wong SK, Chan FK, Lau JY. Randomized trial of endoscopic sphincterotomy with balloon dilation versus endoscopic sphincterotomy alone for removal of bile duct stones. Gastroenterology. 2013;144:341–345.e1. doi: 10.1053/j.gastro.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Yang MJ, Hwang JC, Yoo BM. Endoscopic papillary large balloon dilation for the removal of bile duct stones. World J Gastroenterol. 2013;19:8580–8594. doi: 10.3748/wjg.v19.i46.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang HW, Lee KJ, Jung MJ, Jung JW, Park JY, Park SW, Song SY, Chung JB, Bang S. Endoscopic papillary large balloon dilatation alone is safe and effective for the treatment of difficult choledocholithiasis in cases of Billroth II gastrectomy: a single center experience. Dig Dis Sci. 2013;58:1737–1743. doi: 10.1007/s10620-013-2580-6. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi H, Kida M, Okuwaki K, Miyazawa S, Matsumoto T, Uehara K, Miyata E, Hasegawa R, Kaneko T, Laopeamthong I, Lei Y, Iwai T, Imaizumi H, Koizumi W. Therapeutic peroral direct cholangioscopy using a single balloon enteroscope in patients with Roux-en-Y anastomosis (with videos) Surg Endosc. 2018;32:498–506. doi: 10.1007/s00464-017-5742-3. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto K, Tsutsumi K, Kato H, Akimoto Y, Uchida D, Tomoda T, Yamamoto N, Noma Y, Horiguchi S, Okada H, Yamamoto K. Effectiveness of peroral direct cholangioscopy using an ultraslim endoscope for the treatment of hepatolithiasis in patients with hepaticojejunostomy (with video) Surg Endosc. 2016;30:1249–1254. doi: 10.1007/s00464-015-4323-6. [DOI] [PubMed] [Google Scholar]
- 47.Dimou FM, Adhikari D, Mehta HB, Olino K, Riall TS, Brown KM. Incidence of hepaticojejunostomy stricture after hepaticojejunostomy. Surgery. 2016;160:691–698. doi: 10.1016/j.surg.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamei H, Imai H, Onishi Y, Ishihara M, Nakamura M, Kawashima H, Ishigami M, Ito A, Ohmiya N, Hirooka Y, Goto H, Ogura Y. Considerable Risk of Restenosis After Endoscopic Treatment for Hepaticojejunostomy Stricture After Living-Donor Liver Transplantation. Transplant Proc. 2015;47:2493–2498. doi: 10.1016/j.transproceed.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Mizukawa S, Tsutsumi K, Kato H, Muro S, Akimoto Y, Uchida D, Matsumoto K, Tomoda T, Horiguchi S, Okada H. Endoscopic balloon dilatation for benign hepaticojejunostomy anastomotic stricture using short double-balloon enteroscopy in patients with a prior Whipple's procedure: a retrospective study. BMC Gastroenterol. 2018;18:14. doi: 10.1186/s12876-018-0742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi H, Kida M, Okuwaki K, Miyazawa S, Iwai T, Imaizumi H, Eiji M, Hasegawa R, Koizumi W. A Case Series: Outcomes of Endoscopic Biliary Self-Expandable Metal Stent for Malignant Biliary Obstruction with Surgically Altered Anatomy. Dig Dis Sci. 2016;61:2436–2441. doi: 10.1007/s10620-016-4148-8. [DOI] [PubMed] [Google Scholar]
- 51.Okabe Y, Kuwaki K, Kawano H, Kaji R, Sugiyama G, Ishida Y, Yasumoto M, Naito Y, Toyonaga A, Tsuruta O, Sata M. Direct cholangioscopy using a double-balloon enteroscope: choledochojejunostomy with intraductal biliary carcinoma. Dig Endosc. 2010;22:319–321. doi: 10.1111/j.1443-1661.2010.01013.x. [DOI] [PubMed] [Google Scholar]
- 52.Lenze F, Meister T, Matern P, Heinzow HS, Domschke W, Ullerich H. Single-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreaticography in patients with surgically altered anatomy: higher failure rate in malignant biliary obstruction - a prospective single center cohort analysis. Scand J Gastroenterol. 2014;49:766–771. doi: 10.3109/00365521.2014.904397. [DOI] [PubMed] [Google Scholar]
- 53.Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321–327.e1-3. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 54.Saleem A, Levy MJ, Petersen BT, Que FG, Baron TH. Laparoscopic assisted ERCP in Roux-en-Y gastric bypass (RYGB) surgery patients. J Gastrointest Surg. 2012;16:203–208. doi: 10.1007/s11605-011-1760-y. [DOI] [PubMed] [Google Scholar]
- 55.Ngamruengphong S, Nieto J, Kunda R, Kumbhari V, Chen YI, Bukhari M, El Zein MH, Bueno RP, Hajiyeva G, Ismail A, Chavez YH, Khashab MA. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy. 2017;49:549–552. doi: 10.1055/s-0043-105072. [DOI] [PubMed] [Google Scholar]
- 56.Imai H, Takenaka M, Omoto S, Kamata K, Miyata T, Minaga K, Yamao K, Sakurai T, Nishida N, Watanabe T, Kitano M, Kudo M. Utility of Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting for Malignant Biliary Obstruction after Failed Endoscopic Retrograde Cholangiopancreatography. Oncology. 2017;93 Suppl 1:69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 57.Hosmer A, Abdelfatah MM, Law R, Baron TH. Endoscopic ultrasound-guided hepaticogastrostomy and antegrade clearance of biliary lithiasis in patients with surgically-altered anatomy. Endosc Int Open. 2018;6:E127–E130. doi: 10.1055/s-0043-123188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwashita T, Nakai Y, Hara K, Isayama H, Itoi T, Park DH. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: a multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227–233. doi: 10.1002/jhbp.329. [DOI] [PubMed] [Google Scholar]
- 59.Miranda-García P, Gonzalez JM, Tellechea JI, Culetto A, Barthet M. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: a permanent access for repeated ambulatory dilations? Results from a pilot study. Endosc Int Open. 2016;4:E461–E465. doi: 10.1055/s-0042-103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwashita T, Yasuda I, Mukai T, Iwata K, Doi S, Uemura S, Mabuchi M, Okuno M, Shimizu M. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc. 2017;29:362–368. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 61.Khashab MA, El Zein MH, Sharzehi K, Marson FP, Haluszka O, Small AJ, Nakai Y, Park DH, Kunda R, Teoh AY, Peñas I, Perez-Miranda M, Kumbhari V, Van der Merwe S, Artifon EL, Ross AS. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open. 2016;4:E1322–E1327. doi: 10.1055/s-0042-110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bukhari M, Kowalski T, Nieto J, Kunda R, Ahuja NK, Irani S, Shah A, Loren D, Brewer O, Sanaei O, Chen YI, Ngamruengphong S, Kumbhari V, Singh V, Aridi HD, Khashab MA. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc. 2018;88:486–494. doi: 10.1016/j.gie.2018.04.2356. [DOI] [PubMed] [Google Scholar]
- 63.Chen YI, Levy MJ, Moreels TG, Hajijeva G, Will U, Artifon EL, Hara K, Kitano M, Topazian M, Abu Dayyeh B, Reichel A, Vilela T, Ngamruengphong S, Haito-Chavez Y, Bukhari M, Okolo P, 3rd, Kumbhari V, Ismail A, Khashab MA. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest Endosc. 2017;85:170–177. doi: 10.1016/j.gie.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 64.Attam R, Leslie D, Arain MA, Freeman ML, Ikramuddin S. EUS-guided sutured gastropexy for transgastric ERCP (ESTER) in patients with Roux-en-Y gastric bypass: a novel, single-session, minimally invasive approach. Endoscopy. 2015;47:646–649. doi: 10.1055/s-0034-1391124. [DOI] [PubMed] [Google Scholar]
- 65.Paranandi B, Joshi D, Mohammadi B, Jenkinson A, Adamo M, Read S, Johnson GJ, Chapman MH, Pereira SP, Webster GJ. Laparoscopy-assisted ERCP (LA-ERCP) following bariatric gastric bypass surgery: initial experience of a single UK centre. Frontline Gastroenterol. 2016;7:54–59. doi: 10.1136/flgastro-2015-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee N, Parepally M, Byrne TK, Pullatt RC, Coté GA, Elmunzer BJ. Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2017;13:1236–1242. doi: 10.1016/j.soard.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Kim KH, Kim TN. Endoscopic papillary large balloon dilation for the retrieval of bile duct stones after prior Billroth II gastrectomy. Saudi J Gastroenterol. 2014;20:128–133. doi: 10.4103/1319-3767.129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park TY, Bang CS, Choi SH, Yang YJ, Shin SP, Suk KT, Baik GH, Kim DJ, Yoon JH. Forward-viewing endoscope for ERCP in patients with Billroth II gastrectomy: a systematic review and meta-analysis. Surg Endosc. 2018;32:4598–4613. doi: 10.1007/s00464-018-6213-1. [DOI] [PubMed] [Google Scholar]
- 69.Lin LF, Siauw CP, Ho KS, Tung JC. ERCP in post-Billroth II gastrectomy patients: emphasis on technique. Am J Gastroenterol. 1999;94:144–148. doi: 10.1111/j.1572-0241.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 70.Shimatani M, Matsushita M, Takaoka M, Koyabu M, Ikeura T, Kato K, Fukui T, Uchida K, Okazaki K. Effective "short" double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy. 2009;41:849–854. doi: 10.1055/s-0029-1215108. [DOI] [PubMed] [Google Scholar]
- 71.Cheng CL, Liu NJ, Tang JH, Yu MC, Tsui YN, Hsu FY, Lee CS, Lin CH. Double-balloon enteroscopy for ERCP in patients with Billroth II anatomy: results of a large series of papillary large-balloon dilation for biliary stone removal. Endosc Int Open. 2015;3:E216–E222. doi: 10.1055/s-0034-1391480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siddiqui AA, Chaaya A, Shelton C, Marmion J, Kowalski TE, Loren DE, Heller SJ, Haluszka O, Adler DG, Tokar JL. Utility of the short double-balloon enteroscope to perform pancreaticobiliary interventions in patients with surgically altered anatomy in a US multicenter study. Dig Dis Sci. 2013;58:858–864. doi: 10.1007/s10620-012-2385-z. [DOI] [PubMed] [Google Scholar]
- 73.Shimatani M, Hatanaka H, Kogure H, Tsutsumi K, Kawashima H, Hanada K, Matsuda T, Fujita T, Takaoka M, Yano T, Yamada A, Kato H, Okazaki K, Yamamoto H, Ishikawa H, Sugano K Japanese DB-ERC Study Group. Diagnostic and Therapeutic Endoscopic Retrograde Cholangiography Using a Short-Type Double-Balloon Endoscope in Patients With Altered Gastrointestinal Anatomy: A Multicenter Prospective Study in Japan. Am J Gastroenterol. 2016;111:1750–1758. doi: 10.1038/ajg.2016.420. [DOI] [PubMed] [Google Scholar]
- 74.Inamdar S, Slattery E, Sejpal DV, Miller LS, Pleskow DK, Berzin TM, Trindade AJ. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc. 2015;82:9–19. doi: 10.1016/j.gie.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Trindade AJ, Mella JM, Slattery E, Cohen J, Dickstein J, Garud SS, Chuttani R, Pleskow DK, Sawhney MS, Berzin TM. Use of a cap in single-balloon enteroscopy-assisted endoscopic retrograde cholangiography. Endoscopy. 2015;47:453–456. doi: 10.1055/s-0034-1391077. [DOI] [PubMed] [Google Scholar]
- 76.Obana T, Fujita N, Ito K, Noda Y, Kobayashi G, Horaguchi J, Koshita S, Kanno Y, Ogawa T, Hashimoto S, Masu K. Therapeutic endoscopic retrograde cholangiography using a single-balloon enteroscope in patients with Roux-en-Y anastomosis. Dig Endosc. 2013;25:601–607. doi: 10.1111/den.12039. [DOI] [PubMed] [Google Scholar]
- 77.Kurzynske FC, Romagnuolo J, Brock AS. Success of single-balloon enteroscopy in patients with surgically altered anatomy. Gastrointest Endosc. 2015;82:319–324. doi: 10.1016/j.gie.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Lee A, Shah JN. Endoscopic approach to the bile duct in the patient with surgically altered anatomy. Gastrointest Endosc Clin N Am. 2013;23:483–504. doi: 10.1016/j.giec.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Kawamura T, Mandai K, Uno K, Yasuda K. Does single-balloon enteroscopy contribute to successful endoscopic retrograde cholangiopancreatography in patients with surgically altered gastrointestinal anatomy? ISRN Gastroenterol. 2013;2013:214958. doi: 10.1155/2013/214958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamauchi H, Kida M, Okuwaki K, Miyazawa S, Iwai T, Takezawa M, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Short-type single balloon enteroscope for endoscopic retrograde cholangiopancreatography with altered gastrointestinal anatomy. World J Gastroenterol. 2013;19:1728–1735. doi: 10.3748/wjg.v19.i11.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yane K, Katanuma A, Maguchi H, Takahashi K, Kin T, Ikarashi S, Sano I, Yamazaki H, Kitagawa K, Yokoyama K, Koga H, Nagai K, Nojima M. Short-type single-balloon enteroscope-assisted ERCP in postsurgical altered anatomy: potential factors affecting procedural failure. Endoscopy. 2017;49:69–74. doi: 10.1055/s-0042-118301. [DOI] [PubMed] [Google Scholar]
- 82.Shah RJ. Spiral enteroscopy-assisted ERCP in patients with long limb surgical biliary bypass. Endoscopy. 2009;41 Suppl 1:A25. [Google Scholar]
- 83.Shah RJ, Smolkin M, Ross AS, Kozarek RA, Howell DA, Bakis G, Jonnalagadda SS, Al-Lehibi AH, Hardy A, Morgan DR, Sethi A, Stevens PD, Akerman PA, Thakkar SJ, Yen RD, Brauer BC. 788e: A Multi-Center, U.S. Experience of Single Balloon, Double Balloon, and Rotational Overtube Enteroscopy-Assisted ERCP in Long Limb Surgical Bypass Patients. Gastrointest endosc. 2010;71:AB134–AB135. doi: 10.1016/j.gie.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 84.Choi CW, Choi JS, Kang DH, Kim BG, Kim HW, Park SB, Yoon KT, Cho M. Endoscopic papillary large balloon dilation in Billroth II gastrectomy patients with bile duct stones. J Gastroenterol Hepatol. 2012;27:256–260. doi: 10.1111/j.1440-1746.2011.06863.x. [DOI] [PubMed] [Google Scholar]
- 85.Itoi T, Ishii K, Itokawa F, Kurihara T, Sofuni A. Large balloon papillary dilation for removal of bile duct stones in patients who have undergone a billroth ii gastrectomy. Dig Endosc. 2010;22 Suppl 1:S98–S102. doi: 10.1111/j.1443-1661.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 86.Lee TH, Hwang JC, Choi HJ, Moon JH, Cho YD, Yoo BM, Park SH, Kim JH, Kim SJ. One-Step Transpapillary Balloon Dilation under Cap-Fitted Endoscopy without a Preceding Sphincterotomy for the Removal of Bile Duct Stones in Billroth II Gastrectomy. Gut Liver. 2012;6:113–117. doi: 10.5009/gnl.2012.6.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]