Abstract
Background
Data regarding invasive pulmonary aspergillosis (IPA) following respiratory viral infections (RVIs) in patients with leukemia and/or hematopoietic stem cell transplantation (LHSCT) are limited.
Methods
We conducted a retrospective case-control study of post-RVI IPA (2006–2016). Cases were patients who underwent LHSCT and had RVI due to respiratory syncytial virus (RSV), influenza virus (INF), or parainfluenza virus (PIV) followed by culture-documented IPA within 6 weeks. Controls had IPA only.
Results
We identified 54 cases and 142 controls. Among cases, 29 (54%) had PIV infection, 14 (26%) had INF infection, and 11 (20%) had RSV infection. The median time to IPA after RVI was 7 days. A greater percentage of cases (37 [69%]) than controls (52 [37%]) underwent allogeneic HSCT (P < .0001). Cases were more likely to be nonneutropenic (33 [61%] vs 56 [39%]; P = .009) and in hematologic remission (27 [50%] vs 39 [27%]; P = .003) before IPA. Cases were more likely to have monocytopenia (45 [83%] vs 99 [70%]; P = .05) and less likely to have severe neutropenia (21 [39%] vs 86 [61%]; P = .007) at IPA diagnosis. Prior use of an Aspergillus-active triazole was more common in cases (27 of 28 [96%] vs 50 of 74 [68%]; P = .0017). Median time to empirical antifungal therapy initiation was 2 days in both groups. Crude 42-day mortality rates did not differ between cases (22%) and controls (27%), but the 42-day mortality rate was higher among cases with IPA after RSV infection (45%) than among those with IPA following INF or PIV infection (13%; P = .05).
Conclusions
IPA had comparable outcomes when it followed RVI in patients who underwent LHSCT, and post-RVI IPA occurred more frequently in patients with prior allogeneic HSCT and was associated with leukemia relapse and neutropenia.
Keywords: Aspergillus, hematologic malignancy, influenza, parainfluenza, respiratory syncytial virus
Invasive pulmonary aspergillosis (IPA) uncommonly complicated respiratory viral infections (RVIs) in patients with hematologic malignancies. IPA was most frequent following allogeneic transplantation and occurred early (median, 7 days). Crude post-RVI IPA mortality was comparable to IPA mortality without prior RVI.
Respiratory viruses such as influenza virus (INF), respiratory syncytial virus (RSV), and parainfluenza virus (PIV) have emerged as major causes of morbidity and mortality among immunocompromised patients with leukemia, especially those who have undergone hematopoietic stem cell transplantation (HSCT) [1, 2]. Although bacterial pneumonias have been well described following respiratory viral infection (RVI) in this patient population [3], invasive pulmonary aspergillosis (IPA) as a superinfection has been described sporadically [4]. We conducted a case-control study of post-RVI IPA in patients with leukemia and/or HSCT (LHSCT) to determine the impact of prior RVIs on IPA outcome.
PATIENTS AND METHODS
Study Design
We conducted a retrospective case-control study in all consecutive adult patients (age, >18 years) with LHSCT who developed culture-documented IPA (proven or probable according to European Organisation for Research and Treatment of Cancer Mycoses Study Group criteria) [5] at The University of Texas MD Anderson Cancer Center between 1 January 2006 and 31 December 2016. Cases were patients with LHSCT who had a confirmed RVI caused by INF (including A[H1N1], A[H3N2], and B), RSV, or PIV types 1–3, followed by IPA up to 6 weeks after RVI. We excluded patients with documented IPA preceding RVI. Controls were those who had culture-documented IPA without prior RVI. Patients with probable IPA diagnosed on the basis of Aspergillus galactomannan detection were not included because this biomarker can cross-react with other hyalohyphomycoses, such as Fusarium species [6], further contributing to data heterogeneity. RVI patients who had IPA and a bacterial lung infection were excluded. Detailed epidemiologic, clinical, microbiological, treatment, and outcome data were collected from patient records, using a standardized case report form. The study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Definitions
RVIs were confirmed by laboratory detection of INF, RSV, or PIV infection by culture of nasal wash or swab specimens, real-time polymerase chain reaction (PCR) analysis of bronchoalveolar lavage (BAL) specimens, respiratory virus culture, or direct fluorescent antibody testing. Viral primary lower respiratory tract infection was defined as the presence of new-onset cough, fever, or hypoxia (arterial oxygen saturation, <90% in ambient air) with typical radiologic (chest radiography or computed tomography [CT]) findings consistent with an interstitial pattern of pneumonia, along with identification of RVI in upper or lower respiratory secretions. Post-RVI IPA was defined as sputum, BAL (or bronchial wash), or tissue biopsy specimen cultures positive for Aspergillus species identified within 6 weeks after RVI.
Features of chronic or recurrent graft-versus-host disease presenting within 100 days after HSCT [7] and prior exposure to antifungal drugs within 6 months before IPA diagnosis were noted. Treatments for IPA included mold-active triazole alone or combined with an echinocandin and liposomal amphotericin B alone or combined with echinocandin or a mold-active triazole. Antimicrobial agents prescribed ≤3 months before IPA were noted.
The 42-day crude mortality rate was defined as death from any cause within 42 days of IPA diagnosis. For additional definitions, see the Supplementary Materials.
Laboratory Methods
Symptomatic patients had RVI diagnosed by analysis of nasal wash or BAL specimens; RSV and INF were detected using the direct immunofluorescence antigen method, and PIV was detected using the shell vial cultures technique. For culture and identification, the R-mix respiratory panel was used (Diagnostic Hybrids, Athens, OH). Multiplex PCR assays (BioFire Diagnostics, Salt Lake, UT) were initiated in our institution in 2014 and were used to detect the presence of ≥1 infecting respiratory virus in respiratory tract specimens. For additional laboratory methods, see the Supplementary Materials.
Statistical Analysis
Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Continuous variables were compared using the Kruskal-Wallis test (for 3-group comparisons) and the Wilcoxon rank sum test (for 2-group comparisons). A logistic regression model was used to evaluate the independent effect of RVIs on 42-day mortality. See additional information in the Supplementary Materials.
RESULTS
Patients
Detailed patient characteristics and risk factors for both groups are shown in Table 1. We identified 54 cases (1 with proven IPA and 53 with probable IPA) and 142 controls (12 with proven IPA and 130 with probable IPA). PIV was detected in 29 cases (54%); INF, in 14 (26%); and RSV, in 11 (20%). PIV type 3 was the leading PIV type detected (in 20 cases [69%]), followed by PIV type 1. The median age of cases was 59 years (range, 19–78 years), and the most common hematologic malignancies in this group were acute myeloid leukemia/myelodysplastic syndrome (in 27 [50%]) and chronic lymphocytic leukemia (in 16 [30%]). Most cases (37 [69%]) had prior HSCT (Table 1). Non-fumigatus Aspergillus species were the cause of IPA in 31 cases (57%) in this group (Table 2). The median time to IPA diagnosis was 7 days (range, 1–42 days) after RVI identification; 15 of 54 cases (28%) had IPA diagnosed on the same day as RVI. Among HSCT recipients, there was no difference between cases and controls in the median duration from engraftment to IPA diagnosis (288 days [interquartile range {IQR}, 80–472 days] and 232 days [IQR, 84–590 days], respectively; P = .7).
Table 1.
Demographic, Clinical, and Laboratory Characteristics of Leukemia Patients With or Without Hematopoietic Stem Cell Transplantation (HSCT) Who Had Invasive Pulmonary Aspergillosis (IPA) Alone or Following a Respiratory Viral Infection (RVI)
| Characteristic | IPA Group (n = 142) | Post-RVI IPA Group (n = 54) | P |
|---|---|---|---|
| Age, y, median (range) | 61 (18–84) | 59 (19–78) | .09 |
| Male sex | 83 (58) | 30 (56) | .71 |
| Former or current smoker | 68 (48) | 22 (41) | .30 |
| Hematologic malignancy | .42 | ||
| AML/MDS | 88 (62) | 27 (50) | |
| ALL | 12 (8) | 7 (13) | |
| CML | 6 (4) | 4 (7) | |
| CLL | 36 (25) | 16 (30) | |
| Malignancy status | .003 | ||
| Remission | 39 (27) | 27 (50) | |
| Active | 103 (73) | 27 (50) | |
| History of HSCT | 52 (37) | 37 (69) | <.0001 |
| HSCT donor | |||
| Allogeneic | 50 (35) | 33 (61) | .22 |
| Autologous | 2 (1) | 4 (7) | .05 |
| Chronic or recurrent GvHD before IPA diagnosis | 33 (23) | 23 (43) | .007 |
| History of immunosuppressant usea | |||
| Cyclophosphamide | 24 (17) | 5 (9) | .18 |
| Cytarabine | 58 (41) | 12 (22) | .015 |
| Fludarabine | 26 (18) | 8 (15) | .56 |
| Tacrolimus | 25 (18) | 27 (50) | <.0001 |
| Mycophenolate mofetil | 9 (6) | 4 (7) | .76 |
| Rituximab | 26 (18) | 6 (11) | .22 |
| Daunorubicin | 30 (21) | 4 (7) | .023 |
| Corticosteroid use (>600 mg prednisone equivalent)b | 49 (35) | 12 (22) | .12 |
| Severe neutropenia >21 d before IPA diagnosisc | 86 (61) | 21 (39) | .007 |
| Underlying medical condition | |||
| Diabetes mellitus | 45 (32) | 20 (37) | .48 |
| Chronic obstructive pulmonary disease | 15 (11) | 6 (11) | .91 |
| Antimicrobial use before IPAd | 126 (89) | 49 (91) | .68 |
| Absolute neutrophil count, neutrophils/µL | |||
| At IPA diagnosis | .05 | ||
| ≤100 | 40 (28) | 12 (22) | |
| 100–500 | 16 (11) | 1 (2) | |
| ≥500 | 86 (61) | 41 (76) | |
| Median (range) | 1.15 (0–31.58) | 2.14 (0–15.60) | .11 |
| At RVI diagnosis, median (range) | NA | 1.15 (0–14.40) | |
| Neutropenia duration at IPA diagnosis, d, median (range) | 78 (5–636) | 85 (2–344) | .96 |
| Absolute lymphocyte count, lymphocytes/µL | .26 | ||
| At IPA diagnosis | |||
| ≤100 | 37 (26) | 13 (24) | |
| 100–500 | 46 (32) | 12 (22) | |
| ≥500 | 59 (42) | 29 (54) | |
| Median (range) | 0.41 (0–49.60) | 0.57 (0–44.80) | .58 |
| At RVI diagnosis, median (range) | NA | 0.45 (0–303.0) | |
| Monocyte count at RVI diagnosis, monocytes/µL | .054 | ||
| ≤100 | 99 (70) | 45 (83) | |
| >100 | 43 (30) | 9 (17) | |
| IgG gamma globulin level at IPA diagnosis, mg/dL, median (IQR) | 651 (185–2863) | 692 (134–2040) | .85 |
| Malnutrition (serum albumin level, <3.0 g/dL) at IPA diagnosis | 90 (63) | 30 (56) | .32 |
| Galactomannan Ag OD ≥0.7 at IPA diagnosis | |||
| In serum | 63 (44) | 27 (50) | .48 |
| In BAL | 8 (6) | 5 (9) | .35 |
| Prior active triazole-based exposure before IPA diagnosise | 50/74 (68) | 27/28 (96) | .0017 |
| Duration of prior exposure to Aspergillus-active antifungal drugs, d, median (IQR) | 65 (26–141) | 142 (81–301) | <.001 |
| Coinfection with bacteremia at IPA diagnosis | 6 (4) | 15 (28) | <.0001 |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: Ag, antigen; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; BAL, bronchoalveolar lavage; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; GvHD, graft-versus-host disease; IQR, interquartile range; MDS, myelodysplastic syndrome; NA, not applicable.
aDefined as immunosuppressant use ≤12 weeks before IPA diagnosis.
bDefined as corticosteroid use (0.3 mg/kg per day prednisone equivalent) >21 days before IPA diagnosis.
cDefined as an absolute neutrophil count of <500 neutrophils/μL for at least 21 days before IPA diagnosis.
dDefined as antimicrobial prescription ≤3 months before IPA diagnosis.
eTriazole-based treatment for IPA was received by 74 controls and 28 cases.
Table 2.
Diagnosis and Outcome of Leukemia Patients With or Without Hematopoietic Stem Cell Transplantation Who had Invasive Pulmonary Aspergillosis (IPA) Infection Alone or Following a Respiratory Viral Infection (RVI)
| Diagnosis and Outcome | IPA Group (n = 142) | Post-RVI IPA Group (n = 54) | P |
|---|---|---|---|
| Aspergillus species identified | |||
| A. fumigatus | 64 (45) | 24 (44) | .74 |
| A. terreus | 20 (14) | 9 (17) | .73 |
| A. flavus | 25 (18) | 9 (17) | .78 |
| A. niger | 26 (18) | 6 (11) | .18 |
| A. versicolor | 4 (3) | 5 (9) | .12 |
| Unspecified non-fumigatus species | 11 (8) | 2 (4) | .52 |
| Diagnostic specimen used for IPA diagnosis | |||
| Culture BAL/bronchial wash | 103 (73) | 48 (89) | .01 |
| Fine needle aspirate or tissue biopsy | 12 (8) | 1 (2) | .12 |
| Pathological finding of BAL/bronchial wash cytologic analysisa | |||
| Visible hyphae | 41 (29) | 4 (7) | .001 |
| Positive result of CT imaging | |||
| Halo sign | 9 (6) | 3 (6) | > .99 |
| Cavitation | 15 (11) | 5 (9) | .79 |
| Nodule(s) | 76 (54) | 29 (54) | .98 |
| Diffuse ground glass | 49 (35) | 15 (28) | .37 |
| Ground-glass opacities | 75 (53) | 30 (56) | .73 |
| Pleural effusions | 43 (30) | 12 (22) | .26 |
| Atelectasis | 16 (11) | 5 (9) | .68 |
| Duration of hospital stay, d, median (IQR) | 18 (9–36) | 12 (7–29) | .23 |
| Lowest saturated O2 level during illness, mm Hg, median (range) | 95 (80–99) | 95 (50–99) | .84 |
| Hospital admission at or after IPA diagnosis | 11 (20) | 14 (10) | .05 |
| ICU admission for infection episode | 37 (26) | 13 (24) | .78 |
| O2 supplementation during infectious episode | 63 (44) | 29 (54) | .24 |
| SOFA score at ICU admission, median (range) | 12 (7–20) | 13 (8–18) | .87 |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; ICU, intensive care unit; IQR, interquartile range; SOFA, sequential organ failure assessment.
aBy Gomori methenamine silver staining.
Univariate analysis revealed that cases were more likely than controls to be in complete or partial hematologic remission (27 [50%] vs 39 [27%]; P = .003) and to have prior allogeneic HSCT (37 [69%] vs 52 [37%]; P < .0001). In addition, cases were more likely than controls to have chronic or recurrent graft-versus-host disease (23 [43%] vs 33 [23%]; P = .007) and to have received tacrolimus (27 [50%] vs 25 [18%]; P < .0001; Table 1). Compared with controls, cases were more likely than controls to have severe monocytopenia (45 [83%] vs 99 [70%]; P = .054) and less likely to have severe recent neutropenia at IPA diagnosis (21 [39%] vs 86 [61%]; P = .007; Table 1). There were no significant differences in the prevalence of lymphopenia (defined as an absolute neutrophil count of <500 neutrophils/μL), including severe lymphopenia (defined as an absolute neutrophil count of <100 neutrophils/μL), at IPA diagnosis between cases and controls. No differences were observed in serum immunoglobulin G hypogammaglobulinemia between the 2 groups (Table 1).
IPA Severity
The need for hospital admission at or after IPA diagnosis was more common in cases than in controls (11 [20%] vs 14 [10%]; P = .05; Table 2). However, the need for intensive care unit admission and for mechanical ventilation at or within a week of IPA, as well as the sequential organ failure assessment score at intensive care unit admission, did not differ between the 2 groups. Similarly, the 42-day crude mortality rate did not differ between cases and controls (12 [22%] and 39 [27%], respectively; P = .45; Table 2]).
Laboratory and Radiographic Studies
Reverse-transcription PCR was used to detect 16 of 54 RVIs (30%), including 2 of 14 INF infections (14%), 8 of 29 PIV infections (28%), and 6 of 11 RSV infections (55%). Results of viral culture were positive in 5 of 14 INF infections (36%), 12 of 29 PIV infections (41%), and 4 of 11 RSV infections (36%). Three of 14 INF-positive specimens (21%), 9 of 29 PIV-positive specimens (31%), and 5 of 11 RSV-positive specimens (45%) were simultaneously positive by direct fluorescent antibody staining and viral culture. The rate of serum or BAL galactomannan positivity did not differ between cases and controls. Specifically, among 54 cases, Aspergillus galactomannan analysis was performed for BAL specimens from 15 (with positive results for 5 [33%]) and for serum specimens from 32 (with positive results for 27 [84%]; Table 1). Similarly, among 142 controls, Aspergillus galactomannan analysis was performed for BAL specimens from 12 (with positive results for 8 [6%]) and for serum specimens from 74 (with positive results for 63 [44%]).
All patients had abnormal chest radiography findings at presentation. CT of the chest at presentation was performed for 43 cases (abnormalities were found in 39 [72%]) and for 122 controls (abnormalities were found in 110 [77%]). Interstitial pneumonia with ground glass opacities was the most common pattern detected among cases by either chest CT or chest radiography. Among cases, abnormal chest CT/radiography findings were more likely to be present bilaterally (39 [72%]), compared with controls (79 [56%]; P = .030), with a predominant ground glass opacity pattern. The frequencies of cavity formation and nodule(s) appearance were not significantly different between the 2 groups (Table 2).
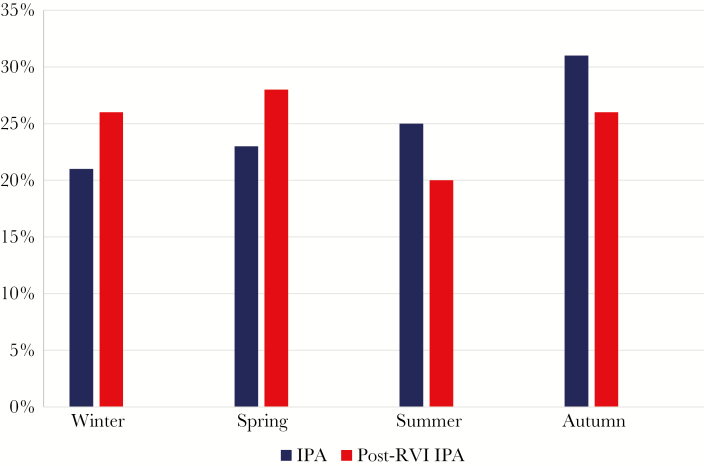
Seasonal Distribution of Post-RVI IPA
All patients were grouped into 4 seasonal groups, and distributions of IPA and post-RVI IPAs were recorded by season (Figure 1). Percentages of IPA detected in controls during winter, spring, summer, and autumn were 21%, 23%, 25%, and 31%, respectively, and percentages of post-RVI IPA detected in cases were 26%, 28%, 20%, and 26%, respectively. These rates did not significantly differ between the groups. Post-INF IPA was most prevalent between December and February (9 of 14 cases [64%]), post-PIV IPA was most prevalent between June and November (22 of 29 [76%]), and post-RSV IPA was most prevalent during January and April (8 of 11 [73%]).
Figure 1.
Distribution of invasive pulmonary aspergillosis (IPA; blue) and post–respiratory viral infection (post-RVI) IPA (red), by season, during the period studied. IPA percentages in winter, spring, summer, and autumn were 21%, 23%, 25%, and 31%, respectively, and percentages of post-RVI IPA were 26%, 28%, 20%, and 26%, respectively.
Antiviral Therapy and Antifungal Exposure Before IPA Diagnosis
All cases infected with INF and RSV were treated for their viral illness. Post-INF IPA and prior influenza vaccination was documented in only 3 of 14 IFN-infected cases (21%). The median time to initiation of antiviral medicine was 1 day (range, 1–10 days) after onset of symptoms. Cases with post-INF IPA received at least a 6-day course (range, 3–28 days) of oseltamivir. The initial oral dose of oseltamivir was 75 mg twice daily (2 cases received an initial dose of 150 mg twice daily). Cases with post-RSV IPA received at least a 10-day course (range, 4–21 days) of ribavirin (9 [82%] received the aerosolized form and 2 [18%] received the oral form). Most cases (49 of 54 [91%]) also received broad-spectrum empirical antibiotic therapy to prevent bacterial superinfection. Intravenous immunoglobulins were administered to most cases with PIV infection (17 of 29 [59%]).
Cases had more-extended prior exposure to antifungal drugs than controls (142 days [IQR, 81–301 days] vs 65 days [IQR, 26–141 days]; P < .001; Table 1). Prior active triazole-based exposure was significantly more common in cases than in controls (27 of 28 [96%] vs 50 of 74 [68%]; P = .0017; Table 1). The median time to initiation of empirical antifungal therapy in both groups was 2 days after clinical suspicion of IPA.
Post-RVI IPA Differences by Virus
The baseline clinical characteristics and risk factors among cases with RVI due to each pathogen of interest (ie, IFN, PIV, and RSV) are shown in Supplementary Table 1. Cases with all types of RVI showed symptoms compatible with a lower respiratory tract infection at diagnosis, including shortness of breath (in 20 [37%]), cough (in 38 [70%]), and fever (defined as a temperature of >38.3°C; in 33 [61%]).
Some differences by RVI type were apparent. Specifically, IPA in cases with RSV infection was more severe and occurred at a younger median age (53 years, compared with 58 years for those with INF infection and 60 years for those with PIV infection), compared with IPA in cases with other RVIs. Those infected with RSV also had more-severe clinical symptoms than those with other RVIs, with dyspnea in 6 cases (55%), bilateral infiltrates in 5 (45%), median lowest saturated oxygen of 94% (range, 50%–99%), supplemental oxygen needed in 9 (82%), median sequential organ failure assessment score at intensive care unit admission of 14 (range, 12–16), and mechanical ventilation needed in 5 (45%; Supplementary Table 2). Although post-RVI IPA occurred typically >100 days after engraftment, those with RSV infection had IPA diagnosed sooner after engraftment than those with PIV or INF infection (median, 187 days [IQR, 51–399 days], compared with 220 days [IQR, 56–322 days) for those with PIV infection and 1336 days [IQR, 646–1727 days] for those with INF infection; P = .002; Supplementary Table 1). Serum IgG hypogammaglobulinemia was more severe in those infected with RSV (median IgG gamma globulin level, 408 mg/dL [IQR, 134–1260 mg/dL]) than those infected with PIV (943 mg/dL [IQR, 511–1570 mg/dL]) or INF (692 mg/dL [IQR, 269–2040 mg/dL]); P = .02; Supplementary Table 1). More cases with RSV (7 [64%]) had underlying diabetes at IPA diagnosis than those with INF (2 [14%]) or PIV (11 [38%]; P = .04; Supplementary Table 1).
The overall post-RVI IPA incidence density for the period studied (2006–2016) was 14.8 events per 100 000 case-days (95% confidence interval, 11.2–19.4). Crude 42-day mortality rates were 21% for post-INF IPA, 14% for post-PIV IPA, and 45% for post-RSV IPA (P = .13; Supplementary Table 2). Post-RSV IPA crude 42-day mortality rates were higher (5 [45%]), compared with that for IPA following INF or PIV (13%; P = .05).
Multivariable Analysis of 42-Day Mortality Risk Factors
Independent predictors of 42-day mortality in cases and controls were mechanical ventilation (16.45 [95%, confidence interval, 6.59–41.06]; P < .0001) and an absolute lymphocyte count of ≤100 lymphocytes/µL (5.45 [95% confidence interval, 2.24–13.27]; P < .001). RVI before IPA had no significant impact on the mortality (P = .41).
Discussion
Herein, we described the largest contemporary series of post-RVI IPA in patients with LHSCT. Post-RVI IPA followed the typical seasonal fluctuations in the activity of each virus. We found that IPA uncommonly complicated RVIs, especially in allogeneic HSCT recipients, even >100 days after engraftment, emphasizing the vulnerability of these patients to infections for an extended period. In fact, because we used a stringent, culture-based diagnosis of IPA, it is possible that we underestimated the frequency of post-RVI IPA, given that IPA is increasingly diagnosed using biomarkers [8]. Most of our cases were not neutropenic, and half were in hematologic remission. Therefore, traditional risk factors of IPA, such as severe neutropenia or relapsed leukemia, were not present in cases who underwent LHSCT and had post-RVI IPA, suggesting that virus-related immunosuppression (eg, breakdown of bronchial mucosa, disruption of mucociliary clearance, and secretion of interleukins) occurred in this population.
Although INF is the most frequently reported virus in post-RVI IPA [9–11], we found that RVIs caused by both PIV and RSV also frequently preceded IPA in cases who underwent LHSCT. In fact, PIV was the leading virus in our cohort (followed by INF), which might reflect the period of PIV circulation, which typically extends to half of the year. Despite the higher incidence of PIV, we found no significant 42-day mortality among PIV-infected cases with IPA. During the PIV circulation period, in which direct damage to the respiratory epithelium ensues after PIV replication [12], even a low inoculum of airborne conidia could lead to IPA [13]. PIV type 3 was the most commonly isolated virus in our cohort, similar to other studies that have documented a high occurrence of PIV type 3 infection in HSCT recipients [2, 14]. Although the severity of IPA for each type of virus was similar, most cases who died by day 42 had RSV infection. More cases infected with RSV than with PIV or INF had underlying diabetes and low IgG gamma globulin levels at IPA diagnosis. The median time to diagnosis of IPA after RVI was only 7 days (range, 1–42 days), and a quarter of those diagnoses occurred during the index RVI diagnosis day. Similarly, other studies highlighted the important role of diagnosing IPA coinfection early, even within 2 days, especially among critically ill patients with INF [4, 10, 11] or during the index admission with RVI infection [15].
Another interesting finding in our study was that post-RVI IPA 42-day mortality rates did not differ between cases and controls. Most cases had received mold-active triazoles before the IPA diagnosis, and together with the timely administration of the antiviral drug, this could explain the absence of excess mortality. However, the factors found to be independently associated with increased 42-day mortality rates were severe lymphopenia at the onset of IPA and the need for mechanical ventilation. Severe lymphopenia may reflect a more severe RVI and shedding, and this was previously reported as a significant risk factor for the requirement of mechanical ventilation and death in HSCT recipients infected with seasonal INF or RSV [16, 17].
Despite its case-control design, our study had several limitations. It was a single-institution, retrospective study that spanned a decade, and controls could not be perfectly matched with cases because of the heterogeneity of the 2 groups. The change in laboratory techniques used to diagnose RVI, from direct fluorescent antibody testing to molecular testing, is another limitation. In addition, the possibility of misclassification error exists as is unclear whether all the cases were classified as having lower respiratory tract viral infection had viral pneumonia. Unique epidemiologic factors of IPA in our institution, specifically the predominance of non-fumigatus Aspergillus species, the patterns of antifungal prophylaxis, and the lack of a uniform approach to diagnosis and treatment of IPA over the 10 years of the study, limit extrapolation to other institutions. Also, the strict culture-based definition of IPA used in our study could represent an underestimation of the true frequency of IPA. A bias toward diagnosis of RVI on the basis of culture for most of our cases (70%), rather than on the basis of PCR-based approaches, could also contribute to a partial underestimation of the overall RVI incidence. However, some cases with mild viral symptoms may not have sought medical attention, and, thus, their infections may have resolved on their own, which may also have contributed to selection bias. Because a significant proportion of cases had IPA diagnosed on the same day that RVI was diagnosed, the possibility of occult preexisting IPA exists in some cases, although the high rates of early coinfection are in agreement with other literature reports [4, 10, 11, 15]. Also, comparisons between the relatively small numbers of different respiratory viruses could be sensitive to small fluctuations, raising concerns about the accuracy and, thus, the usefulness of the data. We cannot extrapolate our findings to other immunosuppressed hosts such as solid organ transplantation recipients or patients in the intensive care unit who had a prior RVI [18]. Finally, we provide no attributable mortality data.
In conclusion, our results show that the risk for IPA following RVI in patients who underwent LHSCT should not be underestimated, especially in those who underwent allogeneic HSCT, and this diagnosis should be considered even in nonneutropenic patients who are in hematologic remission. Not only INF A/B but also PIV and RSV lung infection could precede IPA. Since IPA as a complication of RVI has not been well described in patients who underwent HSCT, a high index of suspicion is needed because IPA can develop early, even within a week after the index RVI diagnosis. Prior active triazole and prompt empirical antifungal therapy might decrease mortality rates in such patients. Nevertheless, IPA that followed RVI had comparable outcomes to IPA alone. Future multicenter registries of post-RVI IPA would be useful to better elucidate the natural history of this emerging entity and the optimal management strategies.
Supplementary Material
Acknowledgments
We thank Linda Graviss, infection control preventionist, for useful information. We thank Erica Goodoff, ELS, senior scientific editor from the Department of Scientific Publications, for clarity, organization, and editing of the manuscript. D. P. K. acknowledges the Texas 4000 Distinguished Endowed Professor for Cancer Research program.
Financial support. This work was supported by the National Cancer Institute, National Institutes of Health (award P30CA016672) and the Fulbright Foundation (scholarship support to E. E. M.).
Potential conflicts of interest. D. P. K. reports research support from Astellas Pharma and honoraria from Merck, Amplyx Pharmaceuticals, Astellas Pharma, Gilead Sciences, and Cidara Therapeutics. R. F. C. received research support from Merck, Chimerix, Shire, Oxford Immunotec, Gilead, Ansun Pharmaceuticals, and Pulmotec and received honoraria from Merck, Chimerix, Ablynx, ADMA Biologics, Pulmotec, Astellas, Shire, Oxford Immunotec, Shionogie, Janssen, Ansun Pharmaceuticals, Achaogen, and Xenex. E. E. M. certifies no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Avetisyan G, Mattsson J, Sparrelid E, Ljungman P. Respiratory syncytial virus infection in recipients of allogeneic stem-cell transplantation: a retrospective study of the incidence, clinical features, and outcome. Transplantation 2009; 88:1222–6. [DOI] [PubMed] [Google Scholar]
- 2. Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006; 85:278–87. [DOI] [PubMed] [Google Scholar]
- 3. Piñana JL, Gómez MD, Pérez A, et al. Community-acquired respiratory virus lower respiratory tract disease in allogeneic stem cell transplantation recipient: risk factors and mortality from pulmonary virus-bacterial mixed infections. Transpl Infect Dis 2018; 20:e12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crum-Cianflone NF. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis 2016; 3:ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nucci M, Carlesse F, Cappellano P, et al. Earlier diagnosis of invasive fusariosis with Aspergillus serum galactomannan testing. PLoS One 2014; 9:e87784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11:945–56. [DOI] [PubMed] [Google Scholar]
- 8. Miceli MH, Kauffman CA. Aspergillus galactomannan for diagnosing invasive aspergillosis. JAMA 2017; 318:1175–6. [DOI] [PubMed] [Google Scholar]
- 9. Martin-Loeches I, Lisboa T, Rhodes A, et al. ; ESICM H1N1 Registry Contributors Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 2011; 37:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin-Loeches I, J Schultz M, Vincent JL, et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 2017; 43:48–58. [DOI] [PubMed] [Google Scholar]
- 11. Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med 2012; 38:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nichols WG, Corey L, Gooley T, et al. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98:573–8. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Vidal C, Royo-Cebrecos C, Peghin M, et al. Environmental variables associated with an increased risk of invasive aspergillosis. Clin Microbiol Infect 2014; 20:O939–45. [DOI] [PubMed] [Google Scholar]
- 14. Marcolini JA, Malik S, Suki D, et al. Respiratory disease due to parainfluenza virus in adult leukemia patients. Eur J Clin Microbiol Infect Dis 2003; 22:79–84. [DOI] [PubMed] [Google Scholar]
- 15. Ajmal S, Mahmood M, Abu Saleh O, et al. Invasive fungal infections associated with prior respiratory viral infections in immunocompromised hosts. Infection 2018; 46:555–8. [DOI] [PubMed] [Google Scholar]
- 16. Boudreault AA, Xie H, Leisenring W, et al. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transplant 2011; 17:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vakil E, Sheshadri A, Faiz SA, et al. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis 2018; 20:e12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blot SI, Taccone FS, Van den Abeele AM, et al. ; AspICU Study Investigators A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 2012; 186:56–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.