Abstract
Introduction
Endovascular treatment for acute ischaemic stroke with large artery occlusion has become the standard of care. However, the question if a subgroup of patients, with a low cerebral blood volume Alberta Stroke Program Early CT score (CBV-ASPECTS) ≤ 7 should be excluded from endovascular treatment remains open. Therefore; we investigated the difference of outcome between patients who were treated by endovascular treatment vs patients who did not receive endovascular treatment.
Methods
We retrospectively analysed our stroke database for all patients who presented within six hours of onset with unfavourable imaging findings and who received endovascular treatment or best medical treatment alone. Unfavourable imaging was defined as a CBV-ASPECTS ≤ 7, which was an exclusion criterion for endovascular treatment at our institution before 2015.
Results
From 60 patients with an initial CBV-ASPECTS ≤ 7, 40 received best medical treatment and 20 were treated with endovascular treatment. Arterial hypertension and atrial fibrillation was more present in patients without endovascular treatment, the other baseline characteristics and percentage of patients treated with intravenous recombinant tissue plasminogen activator were not significantly different in both groups. At discharge, 40% of the interventional treated patients had a favourable outcome (eight of 20 (40%) vs six of 40 (15%; p = 0.031). The median values of the National Institute of Health Stroke Score and modified Rankin Scale at discharge were significantly lower in the treated cohort (6.5 (2.5–10.5) vs 16 (9.5–22.5); p = 0.006; 3 (0–5.5) vs 5 (4.5–5.5); p = 0.003).
Conclusion
Patients with a CBV-ASPECTS ≤ 7 are likely to benefit from therapy and therefore may not be excluded from endovascular treatment. Further randomised trials are warranted to validate the data.
Keywords: Endovascular treatment, Alberta Stroke Program Early CT Score, acute stroke, outcome
Introduction
Endovascular treatment (EVT) for large artery occlusion in stroke has been carried out for nearly two decades now.1 It has emerged as the standard of care treatment in 2015 after several trials demonstrated the benefit of EVT in stroke treatment.2–6 However, selection of the patients eligible for EVT is still an unsolved issue. Previously older patients have been excluded from EVT,5 but there has been striking evidence that especially those patients benefit from EVT.7 Even though a short time from onset to revascularization is crucial for a favourable outcome, there are patients who benefit from EVT beyond six hours of stroke onset.8,9 To improve outcome and avoid futile recanalization, several imaging strategies and criteria have been studied to identify patients who are likely to benefit from EVT. The Alberta Stroke Program Early CT Score (ASPECTS) has been used to identify patients who are likely to benefit from EVT for the treatment of a large artery occlusion of the anterior circulation,10 as well as computed tomography perfusion (CTP).4 Other studies used cerebral blood volume (CBV) maps analysed with the ASPECTS due to a higher sensitivity and specificity to select patients eligible for EVT.11 Due to the results of the three negative thrombectomy trials in 2013, rigid selection criteria for EVT were applied in our institution until 2015 to identify those patients who have a very high probability to profit from EVT.12–14 As a result of rigid image-based selection criteria patients with unfavourable pre-interventional imaging, with a CBV-ASPECTS ≤ 7, were often excluded from EVT at our institution.15 This led to a high rate of bad outcome and severe disability after stroke in the whole population, whereas the percentage of treated patients with a favourable outcome, with a modified Rankin Scale from 0–2, after treatment reached about 70% at our centre.16 There is evidence from a subgroup analysis of the Multi Center Randomised Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN), who followed a less selective inclusion protocol, that patients with an initial unfavourable ASPECTS may benefit from EVT.17 However; the question as to whether patients should be excluded from EVT based on pre-interventional imaging remains open. We therefore compared patients who presented with an unfavourable CBV-ASPECTS (≤7) treated by endovascular means vs patients who were only with treated with best medical treatment alone regarding the neurological outcome of the patients.
Materials and methods
We retrospectively analysed our prospectively kept neuro-interventional database of stroke patients who presented with a proximal anterior circulation occlusion and who underwent EVT from 2015–June 2016 with an unfavourable CBV-ASPECTS ≤ 7 within six hours of stroke onset. In addition, we retrospectively analysed all patients with a proximal anterior circulation occlusion with an unfavourable CBV-ASPECTS ≤ 7 from 2013–2014, who were not treated with EVT within six hours of stroke onset at our institution due to the former guidelines at our hospital. The study was approved by our local ethics committee (no.: 13/7/15An).
We chose timeframe-based selection criteria due to the different guidelines at our hospital during the years. Until 2015, patients were excluded from EVT with a CBV-ASPECTS ≤ 7. From 2015, CBV-ASPECTS ≤ 7 was no longer an exclusion criterion at our hospital and patients were treated regardless their CBV-ASPECTS within six hours. In all cases the decision for EVT was made after an interdisciplinary consensus between the neurologist and neuroradiologist.
CBV-ASPECTS was assessed by an experienced neuroradiology resident (DB) with more than one-year experience and a board-certified neuroradiology senior (MNP) with > 5 years experience in stroke diagnostics and treatment.
The collected data included neurological features such as the National Health Institute Stroke Scale (NHISS) at admission and discharge and the modified Rankin Scale (mRS) at discharge, which were assessed by a certified stroke neurologist. In addition, patient baseline data and cardiovascular risk factors (CVRFs) were collected.
Stroke imaging was performed with a 128-slice multidetector computed tomography (CT) scanner (Siemens Definition AS+; Siemens Healthcare Sector, Forchheim, Germany) including a non-enhanced cerebral CT, a CT-perfusion and a single-phase CT-angiography of the intracranial and extracranial arteries. CTP consisted of 30 near whole-brain spiral scans (96 mm coverage of the z-axis, 2 s delay after start of contrast agent injection, 45 s total acquisition time, 80 kV, 200 mAs and effective dose of ∼5 mSv). Contrast agent was injected at a rate of 6 ml/s for six seconds followed by 30 ml of saline chaser. CTP data were reconstructed with a slice thickness of 5 mm every 3 mm (H20f Kernel, 512 matrix) and analysed using a commercial analysis package (Volume Perfusion CT Neuro; Siemens) with a delay-invariant deconvolution method, automatic motion correction and a dedicated noise reduction technique for dynamic data.16 If showing no contraindications for systemic thrombolysis therapy the treatment was initiated immediately after the CT with intravenous (iv)-recombinant tissue plasminogen activator (rtPA) in a dose of 0.9 mg/kg.
Patients eligible for EVT in 2015–June 2016 were directly transferred after stroke imaging to the angiographic suite (Artis Zee (until 2015) and Artis Q (since 2015), Siemens Healthcare, Forchheim, Germany) where they underwent EVT with an aspiration catheter or a combination of an aspiration catheter and stent-retriever.18,19
Statistical analysis was performed using IBM SPSS Statistics 24 package (IBM, Armonk, New York, USA). Descriptive statistics were presented using mean and standard deviation or median and interquartile range (IQR). Group comparisons were performed using chi-squared or Fischer exact-test as appropriate. For non-normal distributed values the Mann-Whitney test was performed. The p-value to accept the hypothesis was <0.05. The normality was tested using the Kolmogorov-Smirnov test.
Results
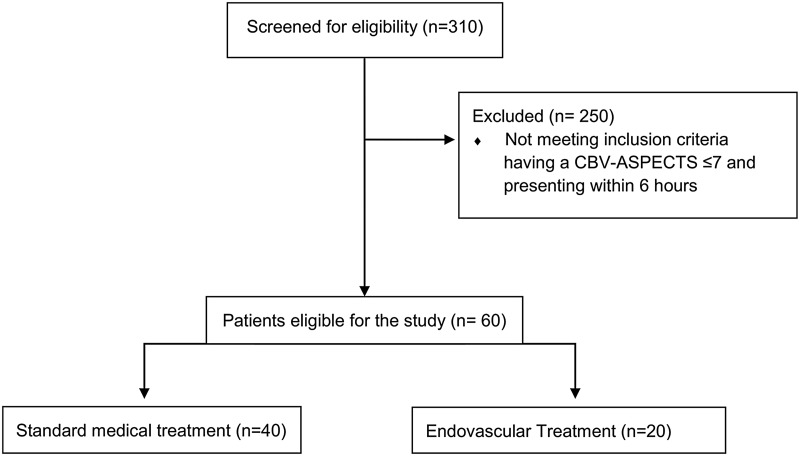
In total we screened 310 patients with an anterior circulation occlusion at our hospital from 2013 until June 2016, of these 60 patients met the inclusion criteria. Altogether 182 patients presented with an anterior circulation occlusion at our hospital from 2013–2014. Of these 182 patients, 40 patients had an initial CBV-ASPECTS ≤ 7 and arrived at the hospital within a six-hour period after symptom onset. From 2015–June 2016, 172 patients underwent EVT, of these 172 patients 20 patients had a CBV-ASPECTS ≤ 7 and presented within the six-hour time-frame (Figure 1).
Figure 1.
Diagram of the trial profile.
CBV-ASPECTS: Cerebral Blood Volume Alberta Stroke Program Early CT Score.
The median age of the patients was 77 years (IQR 69–87), 26 (43.3%) patients were male. The main comorbidities regarding CVRF were arterial hypertension in 78.3% (47 of 60) and 83% (50 of 60) coronary heart disease. Thirty percent (18 of 60) suffered from hyperlipidaemia and 40% (24 of 60) from diabetes mellitus. Atrial fibrillation (AF) was present in 34 of 60 patients (56.7%). Only nine of 60 patients (15%) had a history of smoking. After a median of 98 min (IQR 65–187) after symptom onset the patients were admitted to the hospital.
The median initial NIHSS at admission was 18 (IQR 14–23) which decreased to a median NHISS at discharge of 13 (IQR 5–20). The median mRS at discharge was five (IQR 3–5), 14 of 60 (23.3%) patients had a favourable mRS of 0–2 at discharge. The median CBV-ASPECTS of the patients was five (IQR 3–5), in total 28 of the sixty patients (46.7%) had a CBV-ASPECTS ≤ 4. Of the patients, 43/60 (71.7%) received intravenous thrombolysis, 7/60 patients (11.6%) received hemicraniectomy due to the development of a large cerebral infarction with a relevant oedema, and 6/60 (10%) patients died during hospitalization.
Table 1 shows the baseline characteristics of patients treated with EVT and patients without EVT. There was no significant difference in the NHISS at admission (18 (14–22) vs 18 (13.5–22.5); p = 0.900), in the CBV-ASPECTS at the initial CT scan (5 (3–7) vs 4 (2–6); p = 0.089), the initial native CT-ASPECTS (6.7 (5.2–8.2) vs 7.4 (5.9–8.9); p = 0.203) or the age (76 (66–86) vs 80.5 (72.2–88.6); p = 0.087) of the patients. There was no statistically significant difference in the rate of intravenous thrombolysis either (14 (70%) vs 29 (72.5%); p = 0.360).
Table 1.
Baseline characteristics, cardiovascular risk factors, neurological and imaging scores.
| Treated with EVT | No EVT | p-Value | |
|---|---|---|---|
| Age (IQR) | 76 (66–86) | 80.5 (72.2–88.6) | 0.087 |
| Female | 9 (45%) | 25 (62.5%) | 0.197 |
| Cardiovascular risk factors | |||
| Arterial hypertension | 12 (60%) | 35 (87.5%) | 0.015 |
| Hyperlipidaemia | 9 (45%) | 9 (22.5%) | 0.073 |
| Diabetes mellitus | 6 (30%) | 18 (45%) | 0.268 |
| Coronary heart disease | 16 (80%) | 34 (85%) | 0.624 |
| Smoking | 5 (25%) | 4 (10%) | 0.145 |
| Atrial fibrillation | 6 (30%) | 28 (70%) | 0.003 |
| Neurological and imaging scores | |||
| CBV-ASPECTS (IQR) | 5 (3–7) | 4 (2–6) | 0.089 |
| Native-ASPECTS (IQR) | 6.7 (5.2–8.2) | 7.4 (5.9–8.9) | 0.203 |
| NHISS at admission (IQR) | 18 (14–22) | 18 (13.5–22.5) | 0.900 |
| Left hemispheric stroke | 17 (43%) | 11 (55%) | 0.418 |
| Intravenous thrombolysis | 14 (70%) | 29 (72.5%) | 0.360 |
| Time from symptom onset to hospital (IQR) | 80 min (7.4–153.6) | 100 min (49.9–150.3) | 0.145 |
| Hemicraniectomy | 2 (10%) | 3 (7.5%) | 0.741 |
| Death | 3 (15%) | 5 (12.5%) | 0.778 |
| NHISS at discharge (IQR) | 6.5 (2.5–10.5) | 16 (9.5–22.5) | 0.006 |
| mRS at discharge (IQR) | 3 (0–5.5) | 5 (4.5–5.5) | 0.003 |
| Favourable mRS (0–2) at discharge | 8 (40%) | 6 (15%) | 0.031 |
ASPECTS: Alberta Stroke Program Early CT Score; CBV-ASPECTS: Cerebral Blood Volume Alberta Stroke Program Early CT Score; EVT: endovascular treatment; IQR: interquartile range; mRS: modified Rankin Scale; NHISS: National Institute of Health Stroke Score.
Regarding the CVRFs, the prevalence of arterial hypertension was significantly lower in the group without EVT than in the group treated with EVT (12 (60%) vs 35 (87.5%); p = 0.015), however, the prevalence of AF was higher in the untreated group (6 (30%) vs 28 (70%); p = 0.003). The remaining CVRF did not show significant difference between the two treatment groups, as well as the mortality (3 (15%) vs 5 (12.5%); p = 0.778) or hemicraniectomy (2 (10%) vs 3 (7.5%); p = 0.741) were the same in both groups.
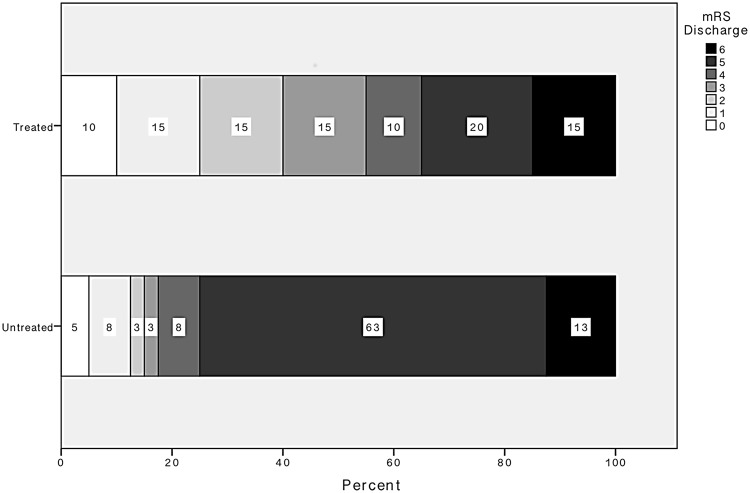
There was a significant difference in the NHISS and the mRS at discharge. Both the NIHSS and the mRS were significantly higher in the group of the patients without EVT at 6.5 (2.5–10.5) vs 16 (9.5–22.5); p = 0.006) and (3 (0–5.5) vs 5 (4.5–5.5); p = 0.003), respectively (Figure 2). Looking closer at the patients with a favourable outcome with a mRS of 0–2, there is a significant difference in favour of the group treated with EVT (8 (40%) vs 6 (15%); p = 0.031) (Figures 3 and 4).
Figure 2.
Distribution of the scores of the modified Rankin Score (mRS) in the treated and the untreated group. There was a significant difference in the treated group and the untreated group (3 (0–5.5) vs 5 (4.5–5.5); p = 0.003).
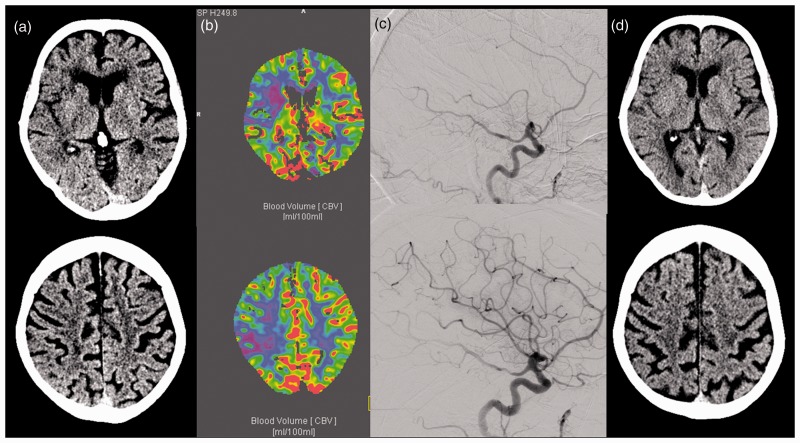
Figure 3.
Case with low Cerebral Blood Volume Alberta Stroke Program Early CT Score (CBV-ASPECTS) and endovascular treatment (EVT). A 72 year-old patient with middle cerebral artery (MCA) M1 occlusion of the right side for 64 min, initial National Institute of Health Stroke Score (NIHSS) = 15. (a) Initial non-enhanced computed tomography (CT) scan with Alberta Stroke Program Early CT Score (ASPECTS) = 8; (b) CBV-ASPECTS = 4; (c) thrombolysis in cerebral infarction (TICI) 2 b recanalization; (d) 24 h post-treatment CT-ASPECTS = 7; post-treatment NIHSS = 1.
CBV: cerebral blood volume.
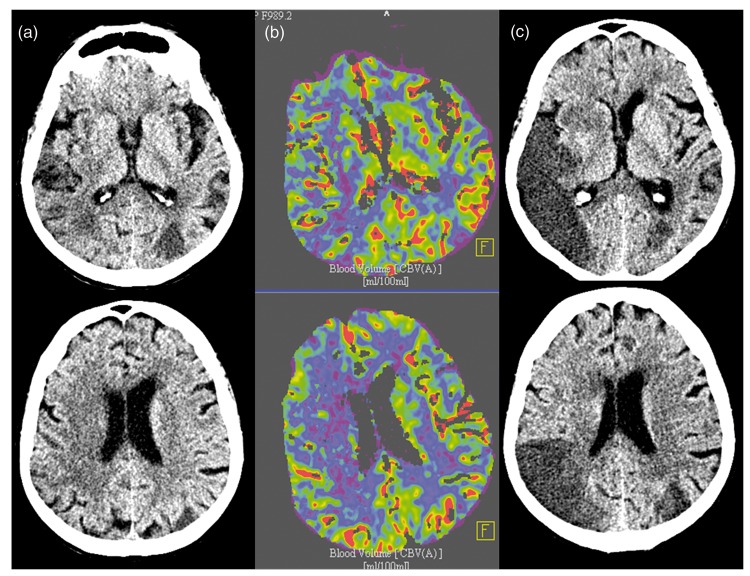
Figure 4.
Case with low Cerebral Blood Volume Alberta Stroke Program Early CT Score (CBV-ASPECTS) and no endovascular treatment (EVT) (no intravenous (iv) lysis due to cancer). An 85 year-old patient with an occlusion of the right middle cerebral artery (MCA) M1 for 98 min, initial National Institute of Health Stroke Score (NIHSS)=8. (a) Initial non enhanced computed tomography (CT) scan with Alberta Stroke Program Early CT Score (ASPECTS)=9; (b) CBV-ASPECTS = 4; (c) 24 h control CT-ASPECTS = 4, 24 h NIHSS = 8.
CBV: cerebral blood volume.
For a subgroup analysis we excluded patients with an CBV-ASPECTS of seven and two or lower. After excluding those patients there were 46 patients remaining in the study, 27 of the remaining patients underwent EVT and 19 patients underwent best medical treatment alone. There was no significant difference in initial CBV-ASPECTS (5 (4–6) vs 5 (4–6); p = 0.488) or the NIHSS at admission (15 (11–19) vs 18 (14.5–21.5); p = 0.538). The NIHSS of the patients without EVT was still significantly higher comparing to those without EVT (14 (6.5–20.5) vs 7 (2.5–11.5); p = 0.034). Whereas the mRS at discharge was no longer significantly different between the two groups (5 (3–6) vs 3 (2–5); p = 0.078). But there was a trend towards a more favourable mRS in patients with EVT. The number of patients with a favourable outcome with a mRS 0–2 was not statistically different (5 (18.5%) vs 8 (42.1%); p = 0.104), but there was a trend towards a favourable outcome in the patients treated with EVT.
Discussion
In our study we found that patients with unfavourable baseline imaging parameters, defined as CBV-ASPECTS ≤ 7, benefit from EVT within six hours of stroke onset. The treated patients had a significantly lower NHISS as well as lower mRS at discharge. Moreover, the number of patients with a favourable outcome (mRS of 0–2) was higher in the treated group. These findings are coherent with a subgroup analysis of MR CLEAN that found that patients with an ASPECTS from 5–7 do benefit of EVT.17
Interestingly mortality was not higher in the untreated group compared to patients receiving EVT, underlining that treatment of patients with an unfavourable CBV-ASPECTS does not result in only preventing death and therefore leading into a severe disability with a mRS of five. There is a shift of the mRS from five to more favourable categories as shown in Figure 2, which means an improvement of the quality of life, despite the low numbers of patients with a mRS of 0–2.
EVT in this cohort did not prevent hemicraniectomy since the rates of hemicraniectomy in both groups are the same. These results support the findings that a low CBV-ASPECTS can be used for risk stratification for the requirement of hemicraniectomy.20
Loose selection criteria for the EVT should be considered since the patients still benefit from EVT, even though the rate of a favourable outcome is lower when comparing to studies with rigid selection criteria.4,16 However, tight selection criteria might withhold the most effective therapy in stroke to patients, leading to a severe disability.
The subgroup analysis revealed, that when excluding the very low CBV-ASPECTS from two till zero and the high CBV-ASPECTS of seven patients, the NIHSS at discharge was significantly lower in the group treated with EVT. However, the mRS at discharge and the number of patients with a favourable outcome was not significantly different anymore. This is possibly due to the low sample size, since there was a trend towards a lower mRS at discharge and a more favourable outcome in the group treated with EVT.
Nonetheless there is still data missing in patients with a very unfavourable ASPECTS of 0–4. In the subgroup analysis of MR CLEAN, as well as in our study, the subgroups of patients with a CBV-ASPECTS ≤ 4 were too small to answer this question adequately. A slight benefit from EVT in those patients seems possible, since studies with loose inclusion criteria as MR CLEAN can show the benefit from EVT, but with a lower number of patients achieving a mRS of 0–2 comparing with studies with more rigid inclusion criteria.2,3 More data and larger cohorts are needed to definitively find an answer to this question.
Other limitations of our study are the small number of patients as well as the different sizes between the two groups. The different sizes of the two groups were due to a change in our inner clinical guidelines, as the patients with a NHISS ≥ 7 were directly transferred to the angiographic suite, bypassing the CT, to minimise door to reperfusion time since June 2016.21 Therefore, few patients of the total cohort received CTP studies. Moreover, the retrospective character of this study is a further limitation. There may be a selection bias, especially in those patients not eligible for EVT, since more criteria than just the CBV-ASPECTS may have influenced the decision whether to treat those patients with EVT or not, such as comorbidities and the patients' preferences.
Conclusion
Strict image-based inclusion criteria exclude patients from EVT who are likely to benefit from therapy and patients with a low initial CBV-ASPECTS can benefit from EVT. Further randomised trials are warranted to validate the data.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
JL received travel expenses from Stryker. MNP received honoraria and travel from grants Penumbra Inc., honoraria from Phenox GmbH, is a consultant to Stryker Neurovascular and Siemens Healthineers and has a research agreement with Siemens Healthineers. DB is a consultant for Phenox and received speaking honorary and travel grants from Stryker, Acandis and Penumbra. The other authors have no confilcts of interest.
References
- 1.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: A phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998; 29: 4–11. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2014; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC V, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2017; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016; 316: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. Epub ahead of print 11 November 2017. DOI: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 10.Hill MD, Rowley HA, Adler F, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke 2003; 34: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 11.Tsogkas I, Knauth M, Schregel K, et al. Added value of CT perfusion compared to CT angiography in predicting clinical outcomes of stroke patients treated with mechanical thrombectomy. Eur Radiol 2016; 26: 4213–4219. [DOI] [PubMed] [Google Scholar]
- 12.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psychogios M-N, Schramm P, Frölich AM, et al. Alberta Stroke Program Early CT scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke 2013; 44: 2188–2193. [DOI] [PubMed] [Google Scholar]
- 16.Psychogios M-N, Knauth M, Bshara R, et al. Computed tomography perfusion-based selection of endovascularly treated acute ischaemic stroke patients – are there lessons to be learned from the pre-evidence era? Neuroradiol J 2017; 30: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo AJ, Berkhemer OA, Fransen PSS, et al. Effect of baseline Alberta Stroke Program Early CT Score on safety and efficacy of intra-arterial treatment: A subgroup analysis of a randomised phase 3 trial (MR CLEAN). Lancet Neurol 2017; 15: 685–694. [DOI] [PubMed] [Google Scholar]
- 18.Maus V, Behme D, Kabbasch C, et al. Maximizing first-pass complete reperfusion with SAVE. Clin Neuroradiol. Epub ahead of print 13 February 2017. DOI: 10.1007/s00062-017-0566-z. [DOI] [PubMed] [Google Scholar]
- 19.Möhlenbruch MA, Kabbasch C, Kowoll A, et al. Multicenter experience with the new SOFIA Plus catheter as a primary local aspiration catheter for acute stroke thrombectomy. J Neurointerv Surg 2017; 9: 1223–1227. [DOI] [PubMed] [Google Scholar]
- 20.Maier IL, Behme D, Schnieder M, et al. Early computed tomography-based scores to predict decompressive hemicraniectomy after endovascular therapy in acute ischemic stroke. PLoS One 2017; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Psychogios M-N, Behme D, Schregel K, et al. One-stop management of acute stroke patients. Stroke 2017; 48: 3152–3155. [DOI] [PubMed] [Google Scholar]