Abstract
Purpose
This study compares computed tomography angiography-based collateral scoring systems in regard to their inter-rater reliability and potential to predict functional outcome after endovascular thrombectomy, and relates them to parenchymal perfusion as measured by computed tomography perfusion.
Methods
Eighty-four patients undergoing endovascular thrombectomy in anterior circulation ischaemic stroke were enrolled. Modified Tan Score, Miteff Score, Maas Score and Opercular Index Score ratio were assessed in pre-interventional computed tomography angiographies independently by two readers. Collateral scores were tested for inter-rater reliability by weighted-kappa, for correlations with three-months modified Rankin Scale, and their potential to differentiate between patients with favourable (modified Rankin Scale ≤2) and poor outcome (modified Rankin Scale ≥3). Correlations with relative cerebral blood volume and relative cerebral blood flow were tested in patients with available computed tomography perfusion.
Results
Very good inter-rater reliability was found for Modified Tan, Miteff and Opercular Index Score ratio, and substantial reliability for Maas. There were no significant correlations between collateral scores and three-months modified Rankin Scale, but significant group differences between patients with favourable and poor outcome for Maas, Miteff and Opercular Index Score ratio. Miteff and Maas were significant predictors of favourable outcome in binary logistic regression analysis. Miteff best differentiated between both outcome groups in receiver-operating characteristics, and Maas reached highest sensitivity for favourable outcome prediction of 96%. All collateral scores significantly correlated with mean relative cerebral blood volume and relative cerebral blood flow.
Conclusions
Computed tomography angiography scores are valuable in estimating functional outcome after mechanical thrombectomy and reliable across readers. The more complex scores, Maas and Miteff, show the best performances in predicting favourable outcome.
Keywords: Collateral scores, ischaemic stroke, thrombectomy, computed tomography angiography, computed tomography perfusion
Introduction
In recent years, endovascular thrombectomy (ET) has greatly gained importance in the treatment of acute ischaemic stroke (AIS) due to arterial occlusion.1–4 In this regard, one of the outstanding goals of current research is to reliably estimate the opportunity to achieve a favourable functional outcome before initiating therapy, to reasonably consider whether ET should be performed. One of the main research topics was the influence of time until recanalization on the long-term outcome. Major studies initially indicated that patients benefited from revascularization within six1,3 or eight hours4 after symptom onset. More recently it has been shown that revascularization even after more than 12 h after symptom onset may be of benefit for patients, if certain criteria are met.5,6 However, in addition to the time since symptom onset, the major factor for estimating patient outcome in AIS is the residual blood supply of the affected brain tissue provided by collaterals.7,8 Computed tomography perfusion (CT-P) features, especially mismatch ratio and infarct core, are of major importance in this regard.9–11
However, standardised CT-P is strongly dependent on patient compliance regarding head movement and is yet not routinely performed in all institutions. In this regard, it has to be considered that CT-P is an additional time-consuming examination associated with additional radiation exposure and an increased volume of contrast agent compared with non-contrast cranial computed tomography plus computed tomography angiography (CT-A) alone. Especially in the case of a blood-brain-barrier disruption, additional volume of contrast agent may enlarge toxic effects on brain tissue. Nevertheless, if CT-P is performed, it is basically considered as reliable, safe and diagnostically important examination.
CT-A is able to capture intracranial vessel status even in moderately agitated patients and is nowadays broadly available. CT-A allows for an estimation of collateralization and may thus be a cornerstone in the process of indicating mechanical revascularization when CT-P is not performed.12–15
Different scoring systems have been introduced to assess and describe the collateralization status.10,13,15,16 However, no systematic evaluation of these scoring systems has been conducted, yet. In particular, it is unclear, how well these scores reflect parenchymal collateralization and perfusion, and if they can be used as predictors of functional outcome after recanalization in AIS.
The purpose of this study was to systematically evaluate four established and frequently used CT-A collateral scoring systems, namely the Modified Tan (mTan) Score,11 the Miteff Score,10 the Maas Score13 and the more recently introduced Opercular Index Score ratio (OISr).16
The inter-rater reliability of each score, their potential to predict functional outcome after ET in AIS and their correlation with CT-P measurements was assessed.
Material and methods
Patient selection
All patients undergoing ET in acute ischaemic stroke at the local department of Diagnostic and Interventional Radiology between June 2016–June 2017 were prospectively enrolled. The inclusion criteria for the current study were: (a) anterior circulation vessel occlusion, i.e. of the internal carotid artery, the sphenoidal or proximal insular segment of the middle cerebral artery (MCA) or tandem occlusions; (b) available and technically sufficient CT-A images (c) available three-month modified Rankin Scale (mRS). The patient sample comprised patients directly admitted to the local department as well as patients initially admitted to an external hospital for evaluation of AIS including neuro-imaging and then referred to the local department for mechanical recanalization. The procedure of ET was performed in a standardised manner.17,18 The indication for ET was determined by the neuroradiologist and neurologist on duty in accordance with current guidelines.19 The study was approved by the local institutional review board (ID: 5468R). Written informed consent for inclusion in the study was obtained from all patients or their next of kin. When patients passed away during hospitalization, written informed consent was waived.
Imaging and post-processing
Imaging protocols of the seven referring facilities differed. To ensure comparable image quality throughout the analysis, only patients with sufficiently contrasted CT-A scans and images with a slice thickness of maximally 2.0 mm in axial plane were included in the current analysis. CT-A scans were considered technically sufficient if intracranial arteries of the non-affected side showed good opacification and terminal branches of the external carotid artery were contrasted. If not provided by the referring hospital, coronal and sagittal multiplanar reformations (MPRs) as well as maximum intensity projections (MIPs) in the axial, coronal and sagittal plane were obtained for further analysis using the local Picture Archiving and Communication System (PACS; IDS7, Sectra, Linköping, Sweden).
Patients directly admitted to our local department were examined with a standardised stroke protocol including non-contrast enhanced cranial computed tomography, CT-A and CT-P.
CT-A was performed covering the brain supplying and intracranial arteries from the aortic arch to the vertex with continuous axial sections parallel to the orbitomeatal line. CT-A parameters were 120 kVp, 100 mAs, 128 × 0.6 mm collimation, 1 s/rotation, table feed of 1 mm/rotation, and active tube current modulation (SOMATOM Definition FLASH, 128-slices, Siemens, Erlangen, Germany), or 120 kVp, 175 mAs, 64 × 0.6 mm collimation, 1 s/rotation, table feed of 1 mm/rotation, and active tube current modulation (SOMATOM Definition AS with sliding gantry, 64-slices, Siemens, Erlangen, Germany).
An intravenous single bolus contrast agent injection (weight-dependent 70–80 ml iomeprol 400 mg J/ml, Imeron, Bracco, Konstanz, Germany), followed by a 30 ml saline solution bolus was injected by an automated injector via an at least 18-gauge intravenous line in an antecubital vein or a high-flow central intravenous line at 5 ml/s. Image acquisition was started by automatic bolus tracking using a region of interest (ROI) placed in the ascending aorta.
Axial CT-A images of 0.75 and 4 mm, 1 mm coronal and sagittal MPRs as well as 9 mm MIPs in the axial, coronal and sagittal plane were reconstructed.
CT-P was acquired with two adjacent slices of 1 cm thickness angled parallel to the Frankfurt horizontal line at the level of the cella media over 50 s with one image per second. CT-P parameters were 80 kVp, 180 mAs, 1 × 10 mm collimation (SOMATOM Definition FLASH) or 80 kVp, 120 mAs, 64 × 0.6 mm collimation (SOMATOM Definition AS). For CT-P, a second bolus of contrast agent of 30 ml iomeprol 400 followed by a 30 ml saline solution bolus was injected at 5 ml/s. Image acquisition started with a three-second delay after injection.
Image analysis
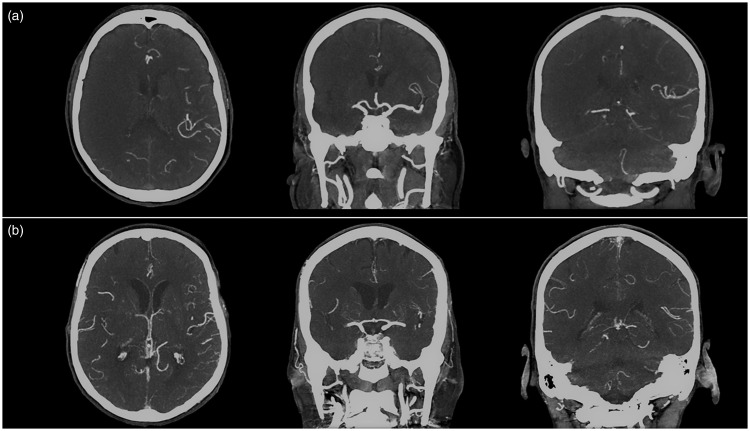
Retrospective assessment of CT-A collateral scores was performed independently by two readers with different levels of experience, i.e. one senior interventional neuroradiologist (BK) with nine years experience in (neuro-) radiology and one specifically trained final-year medical student (DW), extensively instructed and trained in the evaluation of CT-A collaterals on studies not included in the current analysis. An exemplary assessment of collateral scores in two patients is shown in Figure 1.
Figure 1.
Examples of computed tomography angiography (CT-A) scans and survey of collateral scores. Exemplary CT-A scans of two patients with right middle cerebral artery (MCA) occlusion. A representative axial and two coronal CT-A maximum intensity projections (MIPs) are shown. (a) Patient with insufficient collaterals. Modified Tan (mTan) Score was one, Miteff Score was one, Maas Score was one, Opercular Index Score ratio was >2, modified Rankin Scale (mRS) after three months was six. (b) Patient with good collaterals. mTan Score was two, Miteff Score was three, Maas Score was three, Opercular-Index-Score-ratio was one, mRS after three months was zero.
The assessment of individual scores was based on the definitions given in the respective original publications and conducted as follows:
mTan Score
The mTan Score11 classifies vessel opacification of the affected MCA territory as ≥50% (good) and less than 50% (poor) in comparison with the opposite side [15] (similar to the Miteff and Maas Score).
Miteff Score
The scoring system of Miteff et al.10 is based on the topography of opacified branches in the affected MCA territory. It is divided into three categories: (a) only distal superficial MCA branches are reconstituted; (b) some vessels in the Sylvian fissure show opacification; (c) the entire MCA distal to the occlusion is reconstituted.15
Maas Score
The scoring system of Maas et al.13 classifies vessel opacification of the affected MCA territory in five categories: (a) there is no vessel opacification in the affected MCA territory; (b) vessel opacification is lower compared with the opposite side; (c) both MCA territories are opacified similarly; (d) opacification of the affected MCA territory is higher compared with the opposite side; (e) opacification of the affected MCA territory is exuberant.15
Opercular Score
The Opercular Index Score (OIS)16 is based on the number of opacified opercular branches of the affected MCA territory compared with the opposite side in coronal images. Based on the number of opacified branches, the number of the opacified branches of the unaffected MCA territory is divided by the number of the opacified branches of the affected side to determine the OIS ratio (OISr). Two categories of the OISr are discriminated: (a) OISr ≤2 (good) and (b) OISr > 2 (poor).16
mTan, Miteff and Maas scores were evaluated in 9 mm axial MIP reconstructions of CT-A images parallel to the orbitomeatal line at basal ganglia level. OIS was assessed in 9 mm coronal MIP reconstructions of CT-A images.
For the subgroup of patients with standardised CT-P imaging available perfusion maps including time to maximum (TMax), mean transit time (MTT), relative cerebral blood volume (rCBV), and relative cerebral blood flow (rCBF) were calculated using singular value decomposition (STROKETOOL-CT, Version 2.0, H.-J. Wittsack, DIS, Frechen, Germany). The arterial input function was determined automatically or, if automatic detection failed, manually defined by choosing up to 10 reference points in the most opacified arterial vessels. Subsequent extraction of territorial rCBV and rCBF values from CT-P parameter maps was done using Angiotux CT 2D (ECCET 2006/Beck A, Aurich V, Langenfeld, Germany).20 Mean values of affected MCA territory were divided by the mean values of the unaffected MCA territory to calculate rCBV ratio and rCBF ratio.
Outcome analysis
The functional outcome of patients was evaluated using the mRS three months after ET. mRS was assessed by a standardised telephone interview performed by one investigator (DW). Patients were dichotomised by mRS into favourable outcome (mRS ≤ 2) and poor outcome (mRS ≥ 3).
Statistical analysis
Statistical analysis was performed with SPSS software environment (Statistical Package for Social Science, version 24, IBM, Armonk, New York, USA). A p value <0.05 was considered statistically significant for all analyses.
Inter-rater reliability between the two readers for each collateral score was assessed by calculating weighted-kappa statistics (k), which takes the level of disagreement between readers into account.
For further statistical analyses, the median between both investigators for each collateral score was used. Correlations between each collateral score and mRS after three months were determined using Spearman's correlation coefficients. Group comparisons between the mRS subgroups were conducted with Mann–Whitney U-tests for each collateral score.
In the subgroup of patients with standardised CT-P available, mean rCBF and mean rCBV of the affected MCA territory, as well as rCBF and rCBV ratios, were correlated with functional outcome and with each collateral score using Spearman correlations. Group comparisons for these CT-P parameters between the mRS subgroups were performed using Student's t-test.
Binary logistic regression models were calculated for each collateral score. The models included the respective collateral score, age, National Institutes of Health Stroke Scale (NIHSS) at admission and onset-to-groin-puncture time as independent variables and dichotomised three-months mRS as dependent variable. Associations between each independent variable and favourable functional outcome adjusted for the other three independent variables were analysed. For assessment of model quality Nagelkerkes R2 was used. Furthermore, odds ratios with confidence intervals as well as regression coefficients were calculated.
Collateral scores and perfusion parameters were analysed for their test quality for prediction of favourable outcome using receiver-operating characteristic (ROC) analysis with calculation of the area under the curve (AUC) and subsequent determination of Youden's J index to establish cut-off points. For unified presentation of results, ROC analysis for OISr was performed with poor outcome as state variable.
Results
Eighty-four patients were included in the current analysis. Mean age was 75 (±14) years and 53.6% of all patients were female. MCA was the most frequently occluded vessel (M1 segment, 73.8%). Median Alberta Stroke Program Early CT Score (ASPECT) Score at admission was 10 (interquartile range (IQR) 9–10) and median NIHSS at admission was 14 (IQR 10–18). Most patients achieved a level of reperfusion after mechanical recanalization of thrombolysis in cerebral infarction (TICI) 3 (36.9%). Thirty-one (37%) patients showed a favourable outcome (mRS ≤ 2) and fifty-three (63%) patients a poor outcome (mRS ≥ 3) (Table 1).
Table 1.
Baseline characteristics.
| Total (n = 84) | mRS ≤ 2 (n = 31) | mRS ≥ 3 (n = 53) | |
|---|---|---|---|
| Age, mean, (SD, min-max) in years | 75 (±14, 27–99) | 68 (±14, 27–93) | 79 (±12, 45–99) |
| Female sex, n, (%) | 45 (53.6) | 14 (45.2) | 31 (58.5) |
| Left hemisphere, n, (%) | 48 (57.1) | 16 (51.6) | 32 (60.4) |
| Location of occlusion, n, (%) | |||
| ICA | 10 (11.9) | 3 (9.7) | 7 (13.2) |
| T-type | 8 (9.5) | 1 (3.2) | 7 (13.2) |
| M1 | 62 (73.8) | 24 (77.4) | 38 (71.7) |
| M2 | 4 (4.8) | 3 (9.7) | 1 (1.9) |
| Local department, n, (%) | 39 (46.4) | 12 (38.7) | 27 (50.9) |
| ASPECTS at admission, median, (IQR) | 10 (9–10) | 10 (9–10) | 10 (9–10) |
| NIHSS at admission, median, (IQR) | 14 (10–18) | 11 (8–16) | 16 (13–19) |
| Door-to-groin-puncture, mean, (SD, min-max) in min | 145 (±113, 15–775) | 172 (±166, 23–775) | 130 (±65, 15–385) |
| Onset-to-groin-puncture, mean, (SD, min-max) in min | 188 (±81, 60–495) | 189 (±88, 70–495) | 188 (±78, 60–385) |
| TICI Score, n, (%) | |||
| 0 | 5 (6.0) | 0 (0) | 5 (9.4) |
| 1 | 1 (1.2) | 0 (0) | 1 (1.9) |
| 2a | 3 (3.6) | 1 (3.2) | 2 (3.8) |
| 2b | 28 (33.3) | 9 (29.0) | 19 (35.8) |
| 2c | 16 (19.0) | 7 (22.6) | 9 (17.0) |
| 3 | 31 (36.9) | 14 (45.2) | 17 (32.1) |
| mTan Score, median, (IQR)a | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | 1.5 (1.0–2.0) |
| Miteff Score, median, (IQR)a | 2.5 (1.5–3.5) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) |
| Maas Score, median, (IQR)a | 2.0 (2.0–2.5) | 2.0 (2.0–3.0) | 2.0 (2.0–2.5) |
| Operculum Index Score ratio, median, (IQR)a | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| CT-P, n, (%) | 46 (55) | 15 (48) | 31 (58) |
| rCBF ratio, mean, (SD, min-max) | 0.61 (±0.27, 0.14–1.19) | 0.70 (±0.26, 0.39–1.19) | 0.57 (±0.26, 0.14–1.13) |
| rCBV ratio, mean, (SD, min-max) | 0.79 (±0.37, 0.02–1.49) | 0.93 (±0.29, 0.48–1.49) | 0.71 (±0.39, 0.02–1.34) |
| rCBF mean, mean, (SD, min-max) | 59.81 (±46.90, 11.44–254,05) | 69.92 (±36.90, 25.39–155.63) | 54.91 (±50.87, 11.44–254.05) |
| rCBV mean, mean, (SD, min-max) | 22.94 (±13.95, 1.85–69.11) | 31.45 (±15.05, 31.45–69.11) | 18.82 (±11.50, 1.85–45.85) |
ASPECTS: Alberta Stroke Program Early CT Score; CT-P: computed tomography perfusion; ICA: internal carotid artery; IQR: interquartile range; IVT: intravenous thrombolysis; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; SD: standard deviation; TICI: thrombolysis in cerebral infarction.
Values assessed by two independent examiners and not rounded to nearest integer.
Analysis of inter-rater reliability of collateral scores indicated very good inter-rater reliability for mTan Score (k = 0.86), Miteff Score (k = 0.81) and OISr (k = 0.91), as well as a substantial reliability for Maas Score (k = 0.77).
There were no significant correlations between collateral scores and three-months mRS. However, there were significant group differences between patients with favourable and poor outcome for all collateral scores, except for mTan (Table 2).
Table 2.
Group comparisons and correlations with modified Rankin Scale (mRS) for collateral scores and computed tomography perfusion (CT-P) values.
| Group comparison (favourable vs poor outcome) | Correlation with mRS | |
|---|---|---|
| mTan Score | p = 0.08 | rs=–0.14, p = 0.12 |
| Miteff Score | p = 0.01a | rs=–0.14, p = 0.12 |
| Maas Score | p = 0.03a | rs=–0.15, p = 0.11 |
| OISr | p = 0.05a | rs=0.14, p = 0.13 |
| Mean rCBF | p = 0.16 | rs=–0.332, p = 0.01a |
| Mean rCBV | p = 0.001b | rs=–0.436, p = 0.01a |
| rCBF ratio | p = 0.06 | rs=–0.223, p = 0.07a |
| rCBV ratio | p = 0.03a | rs=–0.183, p = 0.11 |
OISr: Opercular Index Score ratio; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume.
Values of p of group comparisons (Mann–Whitney U-test for collateral scores, Student t-test for CT-P parameters) between patients with favourable and poor outcome as well as Spearmans's rho (rs) and respective p-values for correlations with mRS after three months are given for collateral scores and CT-P parameters.
ap < 0.05, bp < 0.01.
In patients with available CT-P, mean rCBF and mean rCBV showed moderate and statistically significant correlations with functional outcome. In contrast, rCBF ratio and rCBV ratio did not show significant correlations with functional outcome (Table 2). There were significant group differences between patients with favourable and poor outcome for mean rCBV and rCBV ratio, but not for mean rCBF and rCBF ratio. All collateral scores showed strong or moderate and statistically significant correlations with rCBF ratio and rCBV ratio, where highest correlations were found between mTan and rCBF ratio, and between OISr and rCBF ratio (Table 3). All collateral scores showed moderate correlations with mean rCBF and mean rCBV, whereas significance of these correlations was only reached for mTan, Miteff and Maas score, but not for OISr. Miteff Score showed the highest correlations with mean rCBF and mean rCBV.
Table 3.
Correlations between computed tomography perfusion (CT-P) parameters and collateral scores.
| mTan Score | Miteff Score | Maas Score | OISr | |
|---|---|---|---|---|
| Mean rCBF | rs=0.33, p = 0.01a | rs=0.42, p = 0.002b | rs=0.38, p = 0.005b | rs=–0.35, p = 0.009b |
| Mean rCBV | rs=0.35, p = 0.01a | rs=0.42, p = 0.002b | rs=0.40, p = 0.003b | rs=–0.37, p = 0.007b |
| rCBF ratio | rs=0.59, p < 0.001b | rs=0.45, p < 0.001b | rs=0.44, p = 0.001b | rs=–0.58, p < 0.001b |
| rCBV ratio | rs=0.48, p < 0.001b | rs=0.43, p = 0.002b | rs=0.53, p < 0.001b | rs=–0.49, p < 0.001b |
rs: Spearman's rho; OISr: Opercular Index Score ratio; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume.
ap < 0.05, bp < 0.01.
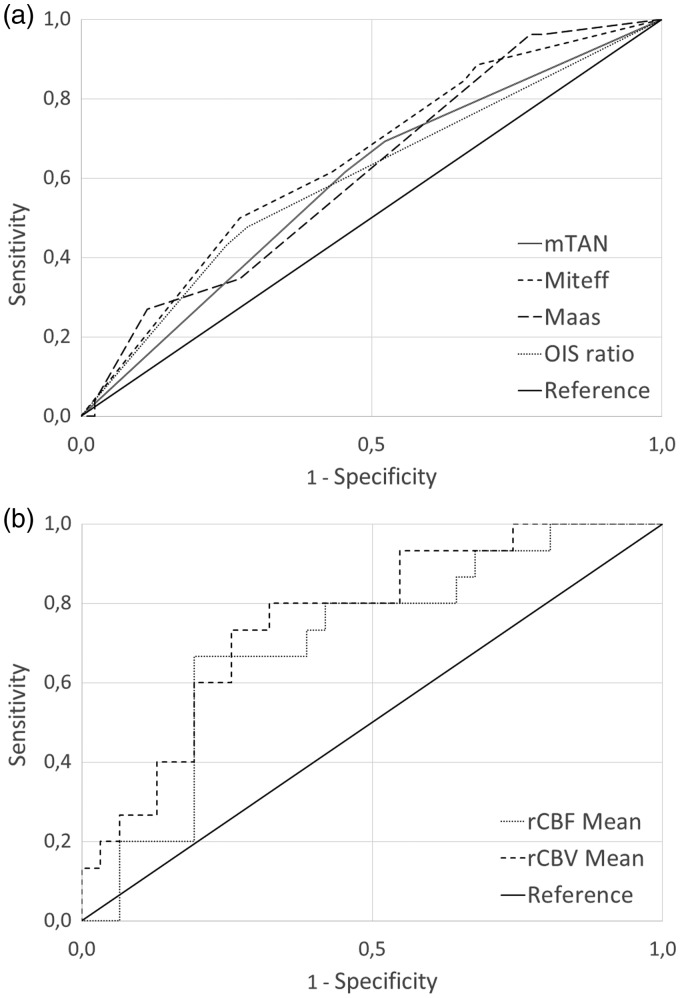
In ROC analyses (Table 4, Figure 2) highest AUC among the four collateral scores were found for Miteff Score and lowest AUC for mTan. However, mean rCBF, mean rCBV and rCBV ratio from the CT-P subsample performed even better in ROC analysis than all collateral scores, whereas the highest AUC was found for mean rCBV. Maas score reached a comparable high AUC compared with Miteff and had a very high sensitivity for good clinical outcome of 96% at the cutoff of 1.75, but low specificity. This high sensitivity was even exceeded by rCBF and rCBV ratios with 100% sensitivity.
Table 4.
Receiver operating characteristics analyses for collateral scores and computed tomography perfusion (CT-P) parameters.
| AUC (95% CI) | J (Cut-off) | Sensitivity | Specificity | |
|---|---|---|---|---|
| mTan Score | 0.59 (0.45–0.78) | 0.17 (1.25) | 69% | 48% |
| Miteff Score | 0.64 (0.51–0.82) | 0.23 (2.75) | 50% | 73% |
| Maas Score | 0.61 (0.48–0.75) | 0.19 (1.75) | 96% | 23% |
| OISr | 0.60 (0.46–0.73) | 0.19 (1.25) | 48% | 71% |
| rCBF ratio | 0.61 (0.44–0.78) | 0.29 (0.38) | 100% | 29% |
| rCBV ratio | 0.67 (0.51–0.82) | 0.36 (0.48) | 100% | 35% |
| Mean rCBF | 0.70 (0.54–0.86) | 0.47 (57.15) | 67% | 81% |
| Mean rCBV | 0.76 (0.62–0.90) | 0.48 (22.05) | 80% | 68% |
AUC: area under the curve; CI: confidence interval; J: Youden's Index; mTan: Modified Tan; OISr: Opercular Index Score ratio; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume.
Figure 2.
Receiver operating characteristic analyses. Receiver operating characteristic curves for (a) Modified Tan (mTan) Score, Miteff Score, Maas Score and Opercular Index Score (OIS) ratio and for (b) relative cerebral blood flow (rCBF) mean and relative cerebral blood volume (rCBV) mean to estimate long term functional outcome by means of three-months modified Rankin Scale (mRS). For illustration purposes, receiver operating characteristic analysis for OIS ratio was referred to poor outcome prediction to facilitate visual comparability.
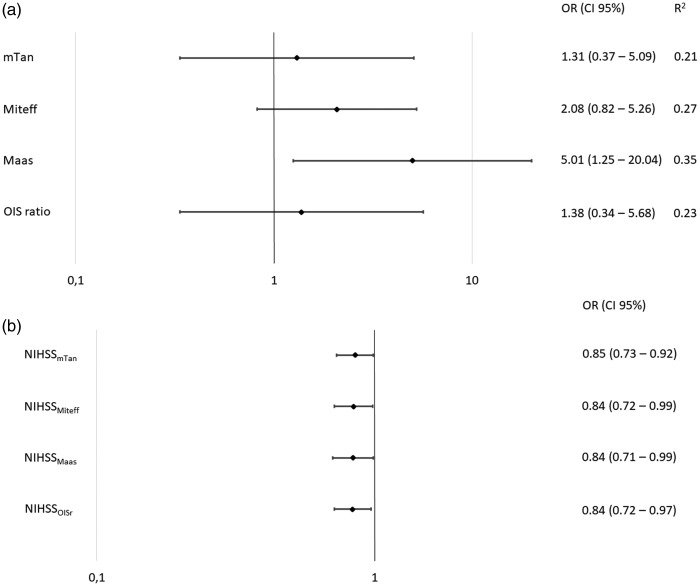
Binary logistic regression analysis yielded one model for each collateral score (Figure 3). In every model, NIHSS at admission was a significant factor. The models for mTan Score and OISr showed a moderate, and the models for Miteff Score and Maas Score a high, explanation of variance. Maas Score was the only collateral score in its corresponding model, which was a significant independent predictor for favourable outcome.
Figure 3.
Binary logistic regression analyses. Forest plots indicating odds ratios (ORs) and confidence intervals (CIs) for the binary logistic regression models for each collateral score and corresponding National Institutes of Health Stroke Scale (NIHSS) are shown. For illustration purposes, binary logistic regression for Opercular Index Score ratio (OISr) was referred to poor outcome prediction to facilitate visual comparability. mTan: modified Tan.
Discussion
The aim of this study was to evaluate the clinical applicability and usefulness of four CT-A collateral scores. Collateral scores can be of high value in management and treatment decision making in AIS, especially when CT-P is not available or is considered unfeasible. In the following sections, we will discuss the inter-rater reliability and potential of predicting a favourable outcome for each score, as well as the score value compared with CT-P features.
Reliability of CT-A collateral scores
Related to their inter-rater reliability, basically all collateral scores are useable and achieve convincing results. Other investigators have shown comparable results in inter-rater reliability for mTan Score.14,15 Minor differences may be caused by a greater experience of the readers in Yeo et al.'s study.15 Slightly lower inter-rater reliability of Miteff Score and Maas Score compared with mTan Score and OISr are probably reasoned by the more complex survey of Miteff Score and Maas Score. Best inter-rater reliability was found for OISr, which may be reasoned by the more objective assessment based on counting opacified vessels, compared with the other three scoring systems.
Predictivity of functional outcome
We could show, that mTan Score only weakly correlates with functional outcome and could not discriminate between favourable and poor outcome on the group level. The reason for this may be found in the relatively coarse classification of collateralization into only two classes, which cannot capture gradations of collateralization at the border between full and missing collaterals and, hence, may have limited ability to predict functional outcome by design. Yeo et al.15 showed that mTan Score is able to distinguish between favourable and poor outcome in AIS patients treated with the intravenous tissue plasminogen activator (t-PA). The divergent results between this trial and our results regarding differentiation of both outcome groups may be caused by the larger population size and the different treatment. A more recent study by Nordmeyer et al.21 took use of the unmodified Tan Score, which uses more classes than the mTan Score. They found that the score is an independent predictor of death in follow-up after discharge, but, similarly to our results, could not find a significant effect in dichotomised mRS.
The Miteff Score showed the highest correlation coefficient with functional outcome among the tested collateral scores, significant differences between favourable and poor outcomes and reached the highest AUC in ROC analysis. A study examining the functional outcome of patients receiving systemic intravenous t-PA in AIS showed a better prediction of outcome using the Miteff Score compared with the Maas Score and the mTan Score.15 This is in line with our results regarding the higher correlation of Miteff with three-months mRS and its higher AUC in ROC analysis compared with both scores. However, after adjustment in binary logistic regression analysis, the Maas Score achieved better results than the Miteff Score.
The Maas Score revealed significant differences between patients with favourable and poor outcome and showed moderate, but not significant, correlation with three-months mRS, that was higher than that for the mTan and the OISr but lower compared with Miteff. Previous investigators showed that favourable collateral state measured with Maas Score is associated with favourable outcome.14,15 It obtained the best results in binary logistic regression analyses compared with the other collateral scores. Furthermore, the Maas Score reached by far the highest sensitivity for the prediction of favourable outcome in ROC analysis.
We only found a weak correlation between the OISr and functional outcome, but significant differences between patients with favourable and poor outcome. Compared with the study introducing the OISr, our findings indicate a much lower sensitivity and specificity for the prediction of functional outcome in the entire patient sample.16 A reason for these differences may be the substantially lower number of patients in the original study or differences in study design between the two studies.
The results of binary logistic regression models of Miteff and Maas Scores emphasise the relevance of collateral status for patient outcome in AIS and indicate that collateralization is even more important than the factor time, which is commonly focused on when contemplating AIS management and treatment. This appraisal is underpinned by recent large randomised trials as the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) study5 or the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) study,6 which show that the factor time is not that restrictive for indication of ET and patients could benefit from endovascular therapy even 24 h after stroke-onset depending on their collateral status in cerebral perfusion.5 Several other studies show similar results regarding the relationship between collaterals, time and functional outcome.7,22
Overall, the more complex scores, Maas and Miteff, perform best for the prediction of functional outcome. Given the high predictive value and high sensitivity of 96% at the cutoff of 1.75 for the Maas Score, this score seems to be most favourable for decision-making in endovascular treatment planning in AIS. In this regard, patients with a Maas Score of ≥2 have the potential to profit from thrombectomy, while patients with a score of one have virtually no potential to achieve good outcome and could be considered to be excluded from endovascular treatment. However, this result should be confirmed in larger study samples.
CT-P and collateral scores
A more direct assessment of brain perfusion and collateralization can be achieved with analysis of CT-P measurements.22,23 The prognostic value of CT-A and CT-P was emphasised by the results of van Seeters et al.,12 which showed that CT-A and CT-P parameters could successfully differentiate between favourable and poor outcome, which is in line with our results. It has already been shown that there are significant correlations between rCBV and collateral scores in an analysis of the Solitaire™ With the Intention For Thrombectomy as PRIMary Endovascular Treatment (SWIFT PRIME) trial data,9 which is in line with our results. Furthermore, it was shown that rCBV is able to mark cerebral collaterals and possible survival of tissue-at-risk.9,24
In this analysis, mean rCBV showed a high and statistically significant correlation with functional outcome, which was higher than for any collateral score. Furthermore, the differences between patients with favourable and poor outcome were highly significant and it showed a high AUC in ROC analysis compared with the four collateral scores. Miteff Score correlates best with rCBV mean, followed by the Maas Score, which reflects their good applicability for the prediction of functional outcome. While the means of rCBV and rCBF performed better in outcome prediction, corresponding ratios yielded better results for correlation with collateral scores due to their methodical proximity.
It was shown that collaterals assessed with dynamic CT-A showed a stronger association with radiological outcome than with single-phase CT-A.25 Nevertheless, dynamic CT-A comes along with the major limitation of high radiation dose and that comprehensive availability is not given for initial diagnostics at all institutions. Regarding minor institutions or patient cases in which a higher radiation exposure should be avoided, single-phase CT-A collateral scores may be more applicable. This also concerns cases where patients are too agitated to provide a valid CT-P or when total volume of contrast agent should be limited.
Limitations
We have several limitations to admit. First, this study is limited by the total number of patients. The imaging protocols are not uniform across the sample, as some patients were referred to the local department from external hospitals. Intentionally, CT-A protocols in this study differ to reflect a realistic setting of a major institution for stroke treatment, treating internal as well as externally referred patients. Additionally, the external validity is raised by this approach and results are more generalisable. We ensured that neuroimaging of all included patients reached a minimum standard which allowed sufficient analysis. Additionally, two different CT scanner types (64 and 128 detector rows) were used for local image acquisition. Again, the use of different scanners should raise generalisability of results and represents a realistic setting in medical care. Means of rCBV and rCBF are limited in their usability due to their missing standardization across analysis software and missing global thresholds. Due to this issue, interpretation of ratios of rCBV and rCBF is preferable and decisions on absolute mean values only reliably applicable at the same institution with standardised processes. Another point that might be raised is the low level of experience of one of the two raters. We indeed chose the design with one experienced and one unexperienced rater by intention, as a valuable collateral score should be reliably reproducible for readers, independent of their level of experience.
Conclusions
CT-A collateral scores are valuable tools for estimation of collateralization, as they are highly reliable and potent to predict functional outcome after ET in AIS, although to a different degree. The more complex scores, Miteff Score and Maas Score, are preferable because of their better performance in outcome prediction. Especially the Maas Score can be particularly useful for selecting patients for endovascular treatment. However, if available, CT-P should be favoured over using single-phase CT-A collateral scores.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma R, Llinas RH, Urrutia V, et al. Collaterals predict outcome regardless of time last known normal. J Stroke Cerebrovasc Dis 2017; 27: 971–977. [DOI] [PubMed] [Google Scholar]
- 8.Christoforidis GA, Vakil P, Ansari SA, et al. Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am J Neuroradiol 2017; 38: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenillas JF, Cortijo E, Garcia-Bermejo P, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab 2017; 38: 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009; 132: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 11.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009; 30: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Seeters T, Biessels GJ, Kappelle LJ, et al. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis 2015; 40: 258–269. [DOI] [PubMed] [Google Scholar]
- 13.Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009; 40: 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan BY, Wan-Yee K, Paliwal P, et al. Good intracranial collaterals trump poor ASPECTS (Alberta Stroke Program Early CT Score) for intravenous thrombolysis in anterior circulation acute ischemic stroke. Stroke 2016; 47: 2292–2298. [DOI] [PubMed] [Google Scholar]
- 15.Yeo LL, Paliwal P, Teoh HL, et al. Assessment of intracranial collaterals on CT angiography in anterior circulation acute ischemic stroke. AJNR Am J Neuroradiol 2015; 36: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copelan A, Chehab M, Brinjikji W, et al. Opercular Index Score: A CT angiography-based predictor of capillary robustness and neurological outcomes in the endovascular management of acute ischemic stroke. J Neurointerv Surg 2017; 9: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 17.Kaschner MG, Caspers J, Rubbert C, et al. Mechanical thrombectomy in MCA-mainstem occlusion in patients with low NIHSS scores. Interv Neuroradiol 2018; 24: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gliem M, Lee JI, Barckhan A, et al. Outcome and treatment effects in stroke associated with acute cervical ICA occlusion. PLoS One 2017; 12: e0170247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015, supported by ESO, ESMINT, ESNR and EAN. Int J Stroke 2016; 11: 134–147. [DOI] [PubMed] [Google Scholar]
- 20.Turowski B, Haenggi D, Wittsack HJ, et al. [Computerized analysis of brain perfusion parameter images]. Rofo 2007; 179: 525–529. [DOI] [PubMed] [Google Scholar]
- 21.Nordmeyer H, Webering N, Chapot R, et al. The association between collateral status, recanalization and long term outcome in stroke patients treated with stent retrievers – are there indications not to perform thrombectomy based on CT angiography?. J Neuroradiol 2017; 44: 217–222. [DOI] [PubMed] [Google Scholar]
- 22.Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011; 42: 3435–3440. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer PW, Barak ER, Kamalian S, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke 2008; 39: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 24.Cortijo E, Calleja AI, Garcia-Bermejo P, et al. Relative cerebral blood volume as a marker of durable tissue-at-risk viability in hyperacute ischemic stroke. Stroke 2014; 45: 113–118. [DOI] [PubMed] [Google Scholar]
- 25.van den Wijngaard IR, Holswilder G, Wermer MJ, et al. Assessment of collateral status by dynamic CT angiography in acute MCA Stroke: Timing of acquisition and relationship with final infarct volume. AJNR Am J Neuroradiol 2016; 37: 1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]