Abstract
Purpose
Head and neck squamous-cell carcinoma (HNSCC) related to human papillomavirus (HPV) infection represents a distinct biological and prognostic subtype compared to the HPV-negative form. Prior studies suggest a correlation between the apparent diffusion coefficient (ADC) values on diffusion-weighted imaging (DWI) of primary tumor lesion and HPV status in HNSCC. In this meta-analysis, we compared the average ADC of primary lesion between HPV-positive and HPV-negative HNSCC.
Methods
A comprehensive literature search of PubMed and Embase was performed. Studies comparing the average ADC on echo-planar DWI of primary tumor lesions between HPV-positive and HPV-negative HNSCC were included. The standardized mean difference was calculated using fixed- and random-effects models. Tau-squared estimates of total heterogeneity and Higgins inconsistency index (I2 test) were determined.
Results
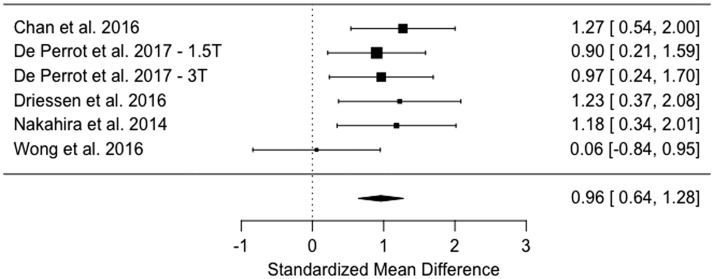
A total of five studies, pooling data of 264 patients, were included for meta-analysis. Among these five studies, three had included oral cavity, hypopharyngeal, and/or laryngeal HNSCC in addition to oropharyngeal subsite. Primary lesions were comprised of 185 HPV-negative and 79 HPV-positive HNSCC. The meta-analysis showed lower average ADC values in HPV-positive HNSCC compared to the HPV-negative form, with a standardized mean difference of 0.961 (95% confidence interval 0.644–1.279; p < 0.0001). Since there was no significant heterogeneity in analysis (p = 0.3852), both random- and fixed-effects models resulted in the same estimates of overall effect.
Conclusions
HPV-positive HNSCC primary lesions have a lower average ADC compared to the HPV-negative form, highlighting the potential application of quantitative diffusion magnetic resonance imaging as a noninvasive imaging biomarker for prediction of HPV status.
Keywords: Apparent diffusion coefficient, human papillomavirus, meta-analyses, squamous-cell carcinoma, head and neck tumors
Introduction
Infection with certain types of human papillomavirus (HPV) is a well-established risk factor for development of head and neck squamous-cell carcinoma (HNSCC), especially in the oropharynx. HPV-positive HNSCC represents a distinct biological, epidemiological, and clinical entity from the HPV-negative form.1 While the incidence of HPV-negative oropharyngeal squamous-cell carcinoma (SCC)—associated with tobacco and alcohol use—has been declining, the incidence of the HPV-positive form—related to high-risk sexual behavior—has been rising, especially among younger patients.2 Determining HPV status has significant therapeutic and prognostic implications, as HPV-positive oropharyngeal SCC tends to have a better response to radiotherapy, with longer progression-free and disease-specific survival. As a result, key changes to the staging scheme for oropharyngeal SCC have been proposed by the eighth American Joint Committee on Cancer staging manual, which distinguishes staging for HPV-positive oropharyngeal SCC from the HPV-negative form.1 In addition, recent studies have shown that even among HNSCC centered at subsites other than the oropharynx, the HPV-positive form tends to have a more favorable outcome with longer overall survival compared to the HPV-negative form, similar to oropharyngeal SCC.3
Current methods to determine the HPV status in HNSCC include tissue sampling with p16 immunohistochemistry as a surrogate marker, polymerase chain reaction (PCR) for detection of virus DNA, or in situ hybridization for HPV DNA and E6/E7 messenger RNA.4,5 The diagnostic potential of imaging biomarkers for distinction of HPV status in HNSCC remains unclear. Some studies have reported a higher ratio of nodal and primary tumor burden, well-defined tumor border, and a greater incidence of intranodal cystic degeneration in HPV-positive HNSCC.6,7 Recent studies suggest that HPV-positive HNSCC is associated with a lower apparent diffusion coefficient (ADC) compared to its HPV-negative counterpart. A noninvasive imaging biomarker such as ADC could play a substantial role in prognostication and treatment planning, with the additional benefits of being able to evaluate the whole tumor volume and having the potential for repeatability compared to tissue sampling.8–12
There are currently a limited number of studies evaluating the difference in ADC values of HPV-positive versus HPV-negative HNSCC. Therefore, we performed a meta-analysis to compare the average ADC value of primary tumor lesions between the HPV-positive versus HPV-negative forms of HNSCC.
Methods
This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13 We aimed to determine if there is a significant difference in the average ADC value of primary tumor lesion between the HPV-positive versus HPV-negative forms of HNSCC.
Search strategy
A comprehensive literature search was conducted through the Embase and MEDLINE databases to identify relevant publications comparing the ADC values of HNSCC based on HPV status from 1 January 2000 to 11 January 2018. Our preliminary literature search demonstrated that some studies might have included the ADC values of HPV-positive versus HPV-negative subgroups in the results, without emphasis in the title or abstract. Thus, we broadened our literature search to identify all possible papers, using the search terms: [diffusion] AND [squamous]. During the review of articles, any relevant articles from the reference bibliography that were not identified in the original search terms were also investigated.
Study selection
Studies were included if: (1) patients had HNSCC; (2) the index test assessed ADC of the primary tumor lesion, which was determined on pretreatment magnetic resonance imaging (MRI); (3) the reference standard—either HPV status and/or p16 status (as a surrogate marker)—was determined; (4) average ADC values were reported separately for HPV-positive and HPV-negative forms; and (5) results were published as an original article. Studies were excluded if: (1) the diffusion-weighted imaging (DWI) scans were obtained after any treatment (i.e., surgery or chemoradiation); (2) there was insufficient information for comparison of average ADC values, such as missing standard deviation (SD) values or number of patients; (3) they were case reports, review articles, or conference abstracts; (4) studies included other types of tumor such as esophageal carcinoma or lymphoma; and (5) English translation was not available for the study.
Data extraction
The following data were extracted from included studies: (1) study characteristics: first author, institution, year of publication, study design, and patient enrollment; (2) patients’ characteristics: sample size, age, tumor location, and tumor stage; (3) MRI scan characteristics: magnetic field strength, DWI acquisition technique, and b-values; (4) image analysis technique: region of interest (ROI) segmentation, and slice selection; (5) quantitative metrics: the average and SD values of ADC in the primary tumor lesion, as well as possible ADC threshold cutoff used for prediction of HPV status and the corresponding sensitivity and specificity; and (6) HPV index test: the histopathological test used to determine the HPV status.
Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used for methodological evaluation of relevant study design characteristics.14 The QUADAS-2 has four key domains: patient selection, index test, reference standard, and flow and timing. All included studies were evaluated in each domain, and potential risk of bias was determined to be high, low, or unclear. The literature selection, data extraction, and quality assessment were performed independently by two reviewers. Discrepancies were discussed to reach consensus.
Data synthesis and statistical analysis
Both the random-effects model with restricted maximum likelihood and the fixed-effect model were applied for comparison of average ADC values. In order to evaluate the heterogeneity across the studies, tau-squared estimates of total heterogeneity and Higgins inconsistency index (I2 test) were determined. The presence of publication bias was assessed by a Deeks’ funnel plot asymmetry test. In a subgroup of studies that reported an average ADC threshold for prediction of HPV status, corresponding data were extracted to construct 2 × 2 contingency tables and used to calculate the pooled sensitivity and specificity with 95% confidence intervals (CI). The “metafor” and “mada” packages in R were used for statistical analyses (R Project for Statistical Computing; The R Foundation, Vienna, Austria). p-Values of <0.05 were considered statistically significant differences.
Results
Study search
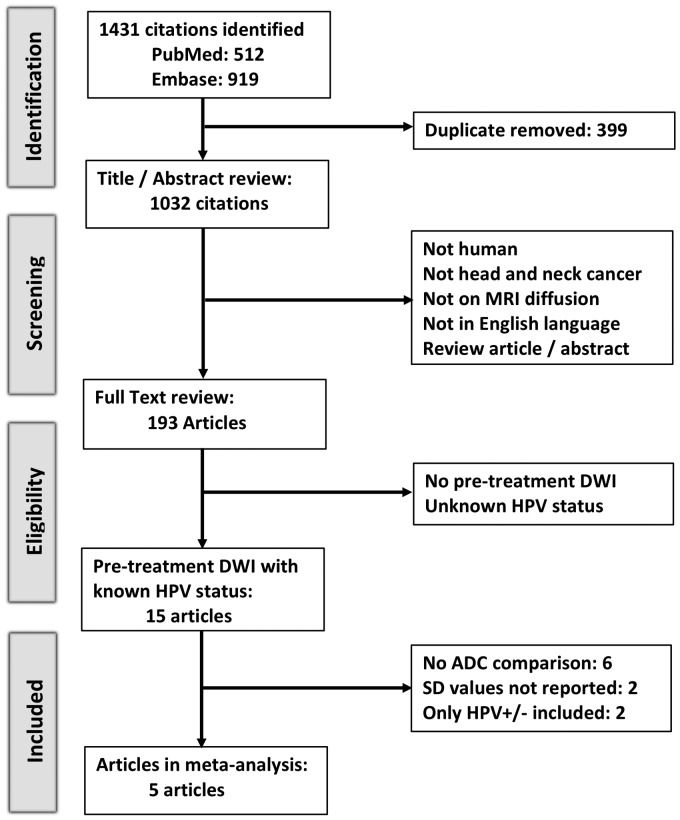
Figure 1 provides an overview of the literature search and study selection procedure. The initial search identified 1431 citations. After exclusion of duplicate records and careful review of the title/abstract and full text, we identified 15 studies with quantitative assessment of pretreatment DWI in HNSCC and reported the HPV status of their cohorts. We further excluded 10 studies, as six did not compare the ADC values between HPV-positive and HPV-negative subjects,15–20 two limited the study cohort to either HPV-positive or HPV-negative patients,21,22 and two compared the average ADC values between HPV-positive and HPV-negative HNSCC without reporting SD values.23,24 Eventually, five studies were included in the meta-analysis.8–10,25,26
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram summarizing the study search, selection, inclusion, and exclusion steps. ADC: apparent diffusion coefficient; DWI: diffusion weighted imaging; HPV: human papillomavirus; SD: standard deviation.
Study characteristics and quality assessment
Tables 1 and 2 summarize the patients’ characteristics, scanner details, imaging protocol, lesion segmentation method, and HPV status index test. All studies were retrospective. In three studies, patients with oral, hypopharyngeal, or laryngeal SCC were included in addition to oropharyngeal SCC.9,10,26 Two studies included patients scanned with both 1.5 and 3 T scanners,8,9 one of which reported the results separately for each scanner type.9
Table 1.
Patients’ characteristics from studies included in the meta-analysis.
| Study | Institution | Time span | Inclusion criteria | Exclusion criteria |
Average age
a
|
|
|---|---|---|---|---|---|---|
| HPV positive | HPV negative | |||||
| Chan et al.8 | University of Toronto, Toronto, Ontario, Canada | April 2009 to June 2015 | Newly diagnosed oropharyngeal SCC, age >18 years | Undetermined HPV status | 57.7 ± 10.6 | 62.7 ± 4.8 |
| De Perrot et al.9 | Geneva University Hospitals, Geneva, Switzerland | 3-year period (published in 2017) | Primary SCC of oropharynx and oral cavity | Lesion not seen on DWI (in situ lesions, T1 stage) | 65b | 61b |
| Driessen et al.10 | University Medical Center Utrecht, Utrecht, Netherlands | June 2009 to August 2011 | T2, T3, and T4 SCC in oral cavity, oropharynx, hypopharynx, or larynx | T1 cancer | 57 ± 10 | 62 ± 9 |
| Nakahira et al.25 | Saitama Medical University, Saitama, Japan | April 2007 to August 2012 | Biopsy-proven oropharyngeal SCC | DWI not available | 62.3b | 69.3b |
| Wong et al.26 | Royal Marsden NHS Foundation Trust, Sutton and London, UK | July 2013 to February 2015 | Oropharynx, hypopharynx, or larynx SCC, stage III–IVb planned for chemoradiotherapy | — | — | — |
Average age (years) ± standard deviation.
Standard deviation not provided.
HPV: human papillomavirus; SCC: squamous-cell carcinoma; DWI: diffusion-weighted imaging.
Table 2.
MRI and reference test characteristics of studies included in the meta-analysis.
| Study | Magnet | Scanner/vendors | DWI scan | b-Values (1000 s/mm2) | Primary lesion segmentation | HPV test |
|---|---|---|---|---|---|---|
| Chan et al.8 | 1.5 and 3 T | TwinSpeed Excite, GE; and Achieva, Philips | EPI | 0, 1000 | Largest circular ROI excluding cystic/necrotic components on b0 images with attention to T1 and STIR | p16 immunohistochemistry (>70%) |
| De Perrot et al.9 | 1.5 and 3 T | — | SS SE EPI | 0, 1000 | The two largest consecutive cross-sectional tumor areas on axial b1000 and ADC maps | p16 immunohistochemistry (>70%) and PCR |
| Driessen et al.10 | 1.5 T | Intera NT, Philips | SS SE EPI | 0, 150, 800 | Whole tumor on b0 image with additional information from other MRI series | p16 immunohistochemistry (>70%) and PCR |
| Nakahira et al.25 | 1.5 T | Achieva Nova Dual, Philips | EPI | 0, 1000 | Single slice of ADC map containing the largest available tumor area | p16 Immunohistochemistry (>25%) |
| Wong et al.26 | 1.5 T | MAGNETOM Aera, Siemens | SE EPI | 50, 400, 800 | Whole tumor on b50 images with reference to anatomic contours, excluding necrosis and cysts | — |
EPI: echo planar imaging; PCR: polymerase chain reaction; SE: spin-echo; SS: single-shot.
The QUADAS-2 scores for each study are listed in Table 3. Three studies were considered to have high risk of bias with regards to the HPV status index test.8,25,26 In addition, none of the studies documented the time gap between the HPV testing and MRI scan, which imposed an unclear risk of bias with regards to the flow and timing. There was also an unclear risk of patient selection bias in studies including HNSCC other than the oropharyngeal SCC.8,10
Table 3.
Methodological quality assessment according to the Quality Assessment of Diagnostic Accuracy Studies.
| Study | Risk of bias |
Applicability concern |
|||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Chan et al.8 | Low risk | High risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| De Perrot et al.9 | Unclear | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Driessen et al.10 | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Nakahira et al.25 | Unclear | High risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Wong et al.26 | Unclear | High risk | Low risk | Unclear | Low risk | Low risk | Low risk |
Data analysis
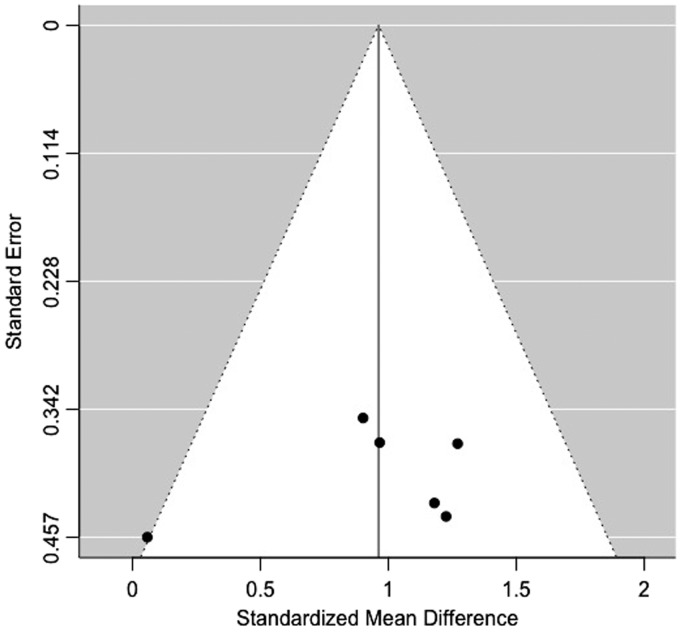
This meta-analysis included the pooled data of 264 patients, with185 HPV-negative and 79 HPV-positive tumors (Table 4). The HPV-positive primary tumors had a lower average ADC value compared to the HPV-negative type (Figure 2), with both random- and fixed-effects models resulting in same estimates of overall effect (standardized mean difference = 0.9614, 95% CI 0.6437–1.2790, p < 0.0001). There was no significant heterogeneity among studies, with tau-squared (estimated amount of total heterogeneity) of 0.00 (p = 0.3852) and I2 (total heterogeneity/total variability) of 0.00% (p = 0.3852). The Deeks’ funnel plot asymmetry test revealed no strong evidence for publication bias (Figure 3).
Table 4.
Quantitative comparison of average ADC among studies included in the meta-analysis.
| Study | Average ADC (×10–6 mm2/s) ± SD (number of patients) |
p-Values | |
|---|---|---|---|
| HPV positive | HPV negative | ||
| Chan et al.8 | 975 ± 168 (n = 28) | 1225 ± 242 (n = 12) | 0.0006 |
| De Perrot et al.9—1.5 Ta | 989 ± 188 (n = 12) | 1158 ± 183 (n = 33) | 0.004 |
| De Perrot et al.9—3 Ta | 1046 ± 167 (n = 9) | 1201 ± 157 (n = 51) | 0.02 |
| Driessen et al.10 | 1327 ± 267 (n = 6) | 1740 ± 338 (n = 67) | 0.005 |
| Nakahira et al.25 | 987 ± 156 (n = 12) | 1218 ± 214 (n = 14) | 0.002 |
| Wong et al.26 | 1150 ± 180 (n = 12) | 1160 ± 140 (n = 8) | 0.93 |
De Perrot et al. reported the average ADC values for the 1.5 and 3 T scanners separately.
ADC: apparent diffusion coefficient; SD: standard deviation.
Figure 2.
The estimated standardized mean difference (95% confidence interval) of the average ADC in HPV-positive and HPV-negative head and neck squamous-cell carcinoma (p < 0.0001) using the random-effects model. There was no significant heterogeneity among studies, and the fixed- and random-effects models had the same results.
Figure 3.
The funnel plot showed no significant publication bias.
For subgroup analysis, the results for application of the ADC threshold for the prediction of HPV status were only available in two studies (Table 5),8,10 precluding rigorous statistical analysis. Based on these two studies, the estimated diagnostic odds ratio for prediction of HPV status applying ADC threshold cutoff was 17.04 (95% CI 4.43–65.48).
Table 5.
Application of ADC threshold for the prediction of HPV status.
| Study | ADC thresholda | Sensitivity (95% CI) | Specificity (95% CI) | Diagnostic odds ratio (95% CI) |
|---|---|---|---|---|
| Chan et al.8,b | 1072 (×10–6 mm2/s) | 80.0% (60.9–91.1%) | 80.0% (49.0–94.3%) | 16.00 (2.55–100.08) |
| Nakahira et al.25 | 1027 (×10–6 mm2/s) | 83.3% (55.2–95.3%) | 78.6% (52.4–92.4%) | 18.33 (2.52–133.26) |
Tumors with an average ADC below the threshold were considered HPV-positive.
Unpublished results provided by authors.
CI: confidence interval.
Discussion
Based on pooled data of 264 patients from five different studies, we have shown that the average ADC of the primary tumor in HPV-positive HNSCC is lower compared to those with HPV-negative status. The estimated diagnostic odds ratio of the average ADC was 17.04 for the prediction of HPV status, although the relevant data were only available in two studies (sensitivity of 80–83% and specificity of 79–80%). These findings likely reflect the histopathological differences between HPV-positive and HPV-negative HNSCC, and support the use of ADC values as a quantitative imaging biomarker for prediction of HPV status in patients with oropharyngeal SCC.
Patients with the HPV-positive form of HNSCC tend to have a more favorable outcome after (chemo)radiotherapy compared to those with the HPV-negative form.27 While the prognostic implications of HPV status in oropharyngeal HNSCC is well established and has been incorporated into the eighth American Joint Committee on Cancer staging manual,1 a recent study on 708 patients with stage 4C non-oropharyngeal HNSCC showed a longer overall survival among those with the HPV-positive form compared to those with the HPV-negative form, suggesting the prognostic importance of HPV status in non-oropharyngeal HNSCC.3 The difference in radiotherapy response may be related to the different carcinogenesis mechanism of the HPV-positive compared to the HPV-negative form of HNSCC.27 In HPV-positive HNSCC, the TP53 tumor suppressor gene is inactivated but still intact, whereas the TP53 is mutated and permanently inactive in HPV-negative tumors.27 Thus, in HPV-positive HNSCC, the TP53-mediated apoptotic response can potentially be reactivated during radiotherapy, resulting in restoration of normal cell-cycle control and favorable tumor response to treatment.
The differences in ADC values between HNSCC forms are likely the result of cellular microenvironment differences. Compared to conventional keratinizing HNSCC found in the HPV-negative subtype, the HPV-positive form tends to have poorly differentiated histologic morphology, reduced interstitial space, basaloid cells with scant cytoplasm, and few to absent keratin pearls.28 These traits likely contribute to reduced water molecular diffusivity and therefore lower ADC values among HPV-positive HNSCC. Among laryngeal and hypopharyngeal HNSCC, there is a negative correlation between cellular density, nuclear area, and nuclear–cytoplasmic ratios with ADC values, whereas the stromal area had a positive correlation with ADC values.29 A high nuclear–cytoplasmic ratio (scant cytoplasm) and low stromal component are histopathological hallmarks of basaloid HNSCC.30 Additionally, HPV-positive oropharyngeal HNSCC are often surrounded by zones of lymphoid cells, which may further contribute to lower ADC values.16,31
Two studies found no significant difference in average ADC of HPV-positive versus HPV-negative HNSCC,23,26 which could have been at least in part due to their small sample size. Nevertheless, pooled data in our meta-analysis confirm that HPV positivity in HNSCC is associated with a lower average ADC in the primary lesion. Among the two studies comparing the ADC values of HPV-positive versus HPV-negative HNSCC, which were excluded from the meta-analysis due to missing SD values (Figure 1), Schouten et al. found no significant difference in the average ADC between 22 HPV-positive versus 22 HPV-negative oropharyngeal SCC,23 whereas Ravanelli et al. found a lower average ADC in 28 HPV-positive oropharyngeal SCC compared to 31 HPV-negative subtype.24 Notably, we found no study in the literature reporting a lower average ADC in HPV-negative HNSCC compared to the HPV-positive form.
Currently, p16 immunohistochemistry is most commonly used as a surrogate marker to determine the HPV status in HNSCC as the first step in diagnosis. The oncoprotein E7 of high-risk HPV binds to and destabilize the retinoblastoma protein, leading to accumulation and overexpression of p16 protein, which allows tumor cells to bypass cell-cycle arrest. However, p16 overexpression can also occur via other mechanisms, and p16-positive HNSCC may not have high-risk HPV DNA in PCR analysis.4 Notably, patients with p16-positive and HPV-negative oropharyngeal SCC tend to have worse survival compared to those with the HPV-positive form.32 The 2018 Guideline from the College of American Pathologists major recommendation is to use p16 immunohistochemistry—with a 70% nuclear and cytoplasmic staining cutoff—on samples from the primary tumor or cervical nodal metastases for evaluation of all newly diagnosed oropharyngeal HNSCC, and the results may “be reported as p16 positive with a comment specifying that the tumor is very likely HPV positive.”33 In addition, the guideline recommends using HPV-specific testing such as PCR or in situ hybridization for HPV DNA in p16-positive cervical nodes or patients with multisite tumors involving the oropharynx. The ADC can serve as a quantitative imaging biomarker of HPV status, supplementing the pathological diagnosis in case of insufficient tissue sample or equivocal p16 immunohistochemistry results where >70% immunostaining is suggestive (but not confirmatory) of HPV-positive status. Compared to tissue sampling, image-based biomarkers can evaluate the whole tumor volume and play a substantial role in prognostication and treatment decision.
In our review of literature, we found two studies applying ADC value thresholds for the prediction of HPV status, reporting a receiver operating characteristic area under the curve (AUC) of 0.858 and an accuracy of 81%.25 In addition, the combination of average ADC and smoking history in multivariable logistic regression could achieve 88% accuracy and an AUC of 0.944 in the prediction of HPV status in oropharyngeal SCC.24 In comparison, recent studies combining radiomics feature extraction from contrast-enhanced computed tomography and machine learning classifiers could achieve an AUC of 0.75–0.91 in prediction of HPV status in HNSCC.34–36 While these findings support potential applications of quantitative MRI diffusion metrics as noninvasive imaging biomarker for prediction of HPV status, the considerable overlap between the average ADC values of HPV-positive and HPV-negative forms of HNSCC (Table 4) and variation in ADC values across different studies demonstrate the challenges in universal adoption of an ADC-based biomarker. The differences in DWI acquisition protocol, magnet/scanner variabilities, and quantification methods limit the precision and accuracy of ADC measurements as a quantitative biomarker. In 2007, the Radiological Society of North America launched the Quantitative Imaging Biomarker Alliance (QIBA) to promote quantitative imaging and reduce the variability across scanners, patients, and institutes.37,38 The QIBA workgroup has recently released their recommendation to improve the precision and accuracy of quantitative DWI in multicenter clinical trials.38 In addition, Long et al. have proposed the application of Z-score transformation of ADC values to minimize inter-study variations and provided a universal cutoff for differentiation of malignant from benign lymph nodes on DWI scans.39 These efforts facilitate standardization of quantitative diffusion MRI and help devise standardized universal biomarkers in cancer imaging.
Multiple studies using a pretreatment DWI scan for the prediction of treatment response in patients with HNSCC have shown that cellular tumors with low baseline ADC values have a more favorable response to chemotherapy and radiation treatment compared to those with high pretreatment ADC values.40,41 While the HPV status was not determined in these studies, our meta-analysis suggests that reported favorable outcomes associated with lower pretreatment ADC values are at least partially attributable to positive HPV status. Thus, the prognostic imaging biomarkers designed for therapy triage and/or treatment response surveillance in patients with HNSCC should reflect the distinct diffusion phenotype of HPV-positive versus HPV-negative subtypes.
The main limitations of this meta-analysis are summarized by QUADAS-2 scores in Table 3. Reports of the time interval between the DWI scan and index test for HPV were universally absent. Application of p16 immunohistochemistry as a surrogate measure of HPV status without PCR confirmation occurred in two studies,8,25 and it was unclear how HPV status was determined in another study,26 which could be a potential source of bias (Table 2). Additionally, there were a limited number of studies available, which were all of retrospective design and only had small study cohorts. Nevertheless, there was no publication bias based on Deeks’ funnel plot (Figure 3). Finally, there is always an inherent potential publication bias for meta-analyses, since studies with positive results are more likely to be published than those with negative or unfavorable results.
Conclusions
Despite some prior studies finding no significant correlation between the average ADC of HNSCC and the HPV status, our meta-analysis confirms that the average ADC of primary tumor lesions in HPV-positive HNSCC is significantly lower than for HPV-negative form. These findings suggest a potential role for quantitative DWI analysis as a noninvasive biomarker for HPV status and a prognostic predictor in patients with HNSCC. Upon standardization of imaging technique and lesion segmentation, quantitative ADC analysis on pretreatment MRI can serve as an applicable clinical tool for accurate staging, treatment decision, and therapy surveillance in day-to-day clinical practice.
Acknowledgments
Results were presented at the American Society of Head and Neck Radiology (ASHNR) 52nd Annual Meeting, September 2018, Savannah, GA.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Glastonbury CM, Mukherji SK, O’Sullivan B, et al. Setting the stage for 2018: how the changes in the American Joint Committee on Cancer/Union for International Cancer Control Cancer Staging Manual Eighth Edition impact radiologists. AJNR Am J Neuroradiol 2017; 38: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson E, Li R, Eisele D, et al. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014; 50: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burr AR, Harari PM, Ko HC, et al. HPV impacts survival of stage IVC non-oropharyngeal HNSCC cancer patients. Otorhinolaryngol Head Neck Surg 2018; 3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 2007; 121: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J, Primeaux T. Is p16 immunohistochemistry a more cost-effective method for identification of human papilloma virus-associated head and neck squamous cell carcinoma? Ann Diagn Pathol 2012; 16: 91–99. [DOI] [PubMed] [Google Scholar]
- 6.Huang YH, Yeh CH, Cheng NM, et al. Cystic nodal metastasis in patients with oropharyngeal squamous cell carcinoma receiving chemoradiotherapy: relationship with human papillomavirus status and failure patterns. PLoS One 2017; 12: e0180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantrell SC, Peck BW, Li G, et al. Differences in imaging characteristics of HPV-positive and HPV-negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol 2013; 34: 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan MW, Higgins K, Enepekides D, et al. Radiologic differences between human papillomavirus-related and human papillomavirus-unrelated oropharyngeal carcinoma on diffusion-weighted imaging. ORL J Otorhinolaryngol Relat Spec 2016; 78: 344–352. [DOI] [PubMed] [Google Scholar]
- 9.de Perrot T, Lenoir V, Domingo Ayllon M, et al. Apparent diffusion coefficient histograms of human papillomavirus-positive and human papillomavirus-negative head and neck squamous cell carcinoma: assessment of tumor heterogeneity and comparison with histopathology. AJNR Am J Neuroradiol 2017; 38: 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driessen JP, van Bemmel AJ, van Kempen PM, et al. Correlation of human papillomavirus status with apparent diffusion coefficient of diffusion-weighted MRI in head and neck squamous cell carcinomas. Head Neck 2016; 38: E613–618. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt N, Gupta N, Soni N, et al. Role of diffusion-weighted imaging in head and neck lesions: pictorial review. Neuroradiol J 2017; 30: 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agnello F, Cupido F, Sparacia G, et al. Computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: a practical approach. Neuroradiol J 2017; 30: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–94. [DOI] [PubMed] [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 15.Paudyal R, Oh JH, Riaz N, et al. Intravoxel incoherent motion diffusion-weighted MRI during chemoradiation therapy to characterize and monitor treatment response in human papillomavirus head and neck squamous cell carcinoma. J Magn Reson Imaging 2017; 45: 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartz JE, Driessen JP, van Kempen PMW, et al. Influence of tumor and microenvironment characteristics on diffusion-weighted imaging in oropharyngeal carcinoma: A pilot study. Oral Oncol 2018; 77: 9–15. [DOI] [PubMed] [Google Scholar]
- 17.Hoang JK, Choudhury KR, Chang J, et al. Diffusion-weighted imaging for head and neck squamous cell carcinoma: quantifying repeatability to understand early treatment-induced change. AJR Am J Roentgenol 2014; 203: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 18.Choi JW, Lee D, Hyun SH, et al. Intratumoural heterogeneity measured using FDG PET and MRI is associated with tumour–stroma ratio and clinical outcome in head and neck squamous cell carcinoma. Clin Radiol 2017; 72: 482–489. [DOI] [PubMed] [Google Scholar]
- 19.Marzi S, Piludu F, Sanguineti G, et al. The prediction of the treatment response of cervical nodes using intravoxel incoherent motion diffusion-weighted imaging. Eur J Radiol 2017; 92: 93–102. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi N, Riaz N, Hunt M, et al. Weekly response assessment of involved lymph nodes to radiotherapy using diffusion-weighted MRI in oropharynx squamous cell carcinoma. Med Phys 2016; 43: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Hazle JD, Mohamed AS, et al. Intravoxel incoherent motion imaging kinetics during chemoradiotherapy for human papillomavirus-associated squamous cell carcinoma of the oropharynx: preliminary results from a prospective pilot study. NMR Biomed 2015; 28: 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aramburu Nunez D, Lopez Medina A, Mera Iglesias M, et al. Multimodality functional imaging using DW-MRI and (18)F-FDG-PET/CT during radiation therapy for human papillomavirus negative head and neck squamous cell carcinoma: Meixoeiro Hospital of Vigo experience. World J Radiol 2017; 9: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schouten CS, de Graaf P, Bloemena E, et al. Quantitative diffusion-weighted MRI parameters and human papillomavirus status in oropharyngeal squamous cell carcinoma. AJNR Am J Neuroradiol 2015; 36: 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravanelli M, Grammatica A, Tononcelli E, et al. Correlation between human papillomavirus status and quantitative MR imaging parameters including diffusion-weighted imaging and texture features in oropharyngeal carcinoma. AJNR Am J Neuroradiol 2018; 39: 1878–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahira M, Saito N, Yamaguchi H, et al. Use of quantitative diffusion-weighted magnetic resonance imaging to predict human papilloma virus status in patients with oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2014; 271: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 26.Wong KH, Panek R, Welsh L, et al. The predictive value of early assessment after 1 cycle of induction chemotherapy with 18F-FDG PET/CT and diffusion-weighted MRI for response to radical chemoradiotherapy in head and neck squamous cell carcinoma. J Nucl Med 2016; 57: 1843–1850. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Mann D, Sinha UK, et al. The molecular mechanisms of increased radiosensitivity of HPV-positive oropharyngeal squamous cell carcinoma (OPSCC): an extensive review. J Otolaryngol Head Neck Surg 2018; 47: 59–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendelsohn AH, Lai CK, Shintaku IP, et al. Histopathologic findings of HPV and p16 positive HNSCC. Laryngoscope 2010; 120: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 29.Driessen JP, Caldas-Magalhaes J, Janssen LM, et al. Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: association between apparent diffusion coefficient and histologic findings. Radiology 2014; 272: 456–463. [DOI] [PubMed] [Google Scholar]
- 30.Ereno C, Gaafar A, Garmendia M, et al. Basaloid squamous cell carcinoma of the head and neck: a clinicopathological and follow-up study of 40 cases and review of the literature. Head Neck Pathol 2008; 2: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol 2012; 6: S48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golusinski P, Pazdrowski J, Szewczyk M, et al. Is immunohistochemical evaluation of p16 in oropharyngeal cancer enough to predict the HPV positivity? Rep Pract Oncol Radiother 2017; 22: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med 2018; 142: 559–597. [DOI] [PubMed] [Google Scholar]
- 34.Bogowicz M, Riesterer O, Ikenberg K, et al. Computed tomography radiomics predicts HPV status and local tumor control after definitive radiochemotherapy in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2017; 99: 921–928. [DOI] [PubMed] [Google Scholar]
- 35.Leijenaar RT, Bogowicz M, Jochems A, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol 2018; 91: 20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu K, Zhang Y, Yu Y, et al. Radiomic analysis in prediction of human papilloma virus status. Clin Transl Radiat Oncol 2017; 7: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau B, Iannessi A, Hoog C, et al. How reliable are ADC measurements? A phantom and clinical study of cervical lymph nodes. Eur Radiol 2018; 28: 3362–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla-Dave A, Obuchowski NA, Chenevert TL, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging. Epub ahead of print 19 November 2018. DOI: 10.1002/jmri.26518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long M, Wang L, Mou L, et al. Z-Score transformation of ADC values: a way to universal cut off between malignant and benign lymph nodes. Eur J Radiol 2018; 106: 122–127. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res 2009; 15: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan A, Chenevert TL, Dwamena BA, et al. Utility of pretreatment mean apparent diffusion coefficient and apparent diffusion coefficient histograms in prediction of outcome to chemoradiation in head and neck squamous cell carcinoma. J Comput Assist Tomogr 2012; 36: 131–137. [DOI] [PubMed] [Google Scholar]