1. Considerations for HPV specific immunotherapy
Tumour immunotherapy aims to induce or enhance tumour antigen specific immune responses that are capable of killing tumour cells. Anogenital and oropharyngeal malignancies arising from cells persistently infected with “high risk” human papillomaviruses (HR-HPVs) should provide proof of concept for tumour immunotherapy, as HPV transformed tumour cells express E6 and E7 nonstructural proteins of the relevant α-HPV, and the tumour state requires continued expression of these oncoproteins [1]. E6 and E7 proteins are immunogenic in animals [2] and humans [3], as assessed by induction of specific antibodies after immunisation. Further, immunisation that induces HPV16 E7 specific cytotoxic T cells can cure mice of transplantable tumours expressing HPV16 E6 and E7 proteins [2,4]. The same immunisation, however, is less effective in achieving rejection of transplanted mouse skin transgenic for HPV16 E7 protein [5], which more faithfully resembles HPV associated human premalignancy histologically and on mRNA transcription profiling [6]. Further, immunisation of mice of many MHC haplotypes fails to induce E6 or E7 specific cytotoxic T cells [[7], [8], [9]], which has been attributed to lack of appropriate MHC Class 1 restricted epitopes within these relatively small proteins.
1.1. Natural elimination of HPV infection
Over 95% of humans infected with HR-HPVs will eliminate the HPV infection over time [10,11]. Specific immune responses are important for elimination, as immunosuppression slows eradication of HPV infection [12]. However, viral eradication is slow, even in immunocompetent individuals, and the immunological mechanisms that lead to viral clearance are not well understood. The viral antigen specific immunity demonstrated during acute infection is mostly targeted at the major capsid protein L1 with weak and infrequent antibody responses to the non-structural proteins [13,14], and there is no direct evidence that viral antigen specific immune effector mechanisms are responsible for virus elimination. Indeed, many people clear HPV infection without developing any measurable virus specific immune responses [15,16].
2. Barriers to effective immunotherapy
One challenge for effective immunotherapy against HPV associated squamous cell cancers (SCC) is that as these tumours evolve over many years in the host, they acquire many strategies to evade innate and adaptive immune responses that might target their tumour specific antigens [17,18], as evident both in animal models of cancer and in humans. Resistance to cytotoxic T cell-mediated lysis may follow down regulation of tumour cell antigen processing or MHC expression [19]. Additionally, HPV transformed cells modulate the local immune environment to suppress effector immune responses by a wide range of mechanisms including induction from fibroblasts of immunomodulatory cytokines [20], induction of local innate M2 macrophages [21], and induction of antigen specific regulatory T cells [22]. There are thus substantial barriers to successful HPV protein targeted immunotherapy for naturally arising HPV associated malignancies in humans. Many of the mechanisms of immune evasion, including cytokine and myeloid cell mediated immunoregulation and impaired antigen presentation, can be demonstrated in the hyperproliferative epithelium of HPV16 E7 transgenic mouse skin [[23], [24], [25], [26], [27]], but not in the epithelium of HPV16 E7 transgenic mouse skin with a mutation in the Rb protein preventing binding of E7 to Rb family proteins and epithelial proliferation [25], suggesting that some of the observed immunomodulatory mechanisms in HPV associated epithelial tumours can be attributed to proliferating epithelial cells rather than to expression of viral proteins.
Studies of the genetic determinants of progression of persisting HPV16 infection in humans to premalignancy demonstrate that progression associates with particular variants of the antigen presenting molecules of the MHC complex, and this linkage accounts for ~50% of the risk of progression to pre-malignancy [28,29]. These data and the MHC determined restriction of cytotoxic T cell responses to HPV E6 and E7 proteins in mice suggest that progression of HPV infection may result in part from an MHC restricted deficit in the immune repertoire to viral nonstructural proteins, a hypothesis that would if correct impact adversely on the likelihood of successful adaptive (antigen specific) immunotherapy for persisting HPV16 infection and cancer in humans.
3. Trials of immunotherapy for HPV associated cancer
Antigen specific immunotherapy for HPV associated disease of various sorts has been under study in humans for over 20 years (reviewed in Refs. [30,31]). In these studies, HPV16 E6 or E7 proteins, and short and long peptides derived from these proteins, have been administered with a variety of intrinsic or extrinsic adjuvants, generally designed to promote cytotoxic T cell immune responses. Alternatively, plasmids or viral vectors incorporating E6 and E7 genes, generally sequence modified to avoid possible transformation of cells by integrated DNA, have been used. Most of these vaccines have induced some measurable immunity to the relevant proteins – generally reported as antibody and helper T cells, though reports of strong cytotoxic T cell responses are not common [[31], [32], [33]]. Most of the published studies are open label, with historic controls, in patients with advanced disease. Randomized trials of immunotherapy as sole treatment for premalignant disease have shown either no [3] or relatively modest efficacy [34], and no cure of metastatic HPV associated cancer by immunotherapy as sole therapy has been demonstrated to date. Where better outcomes are claimed, these are generally restricted to delay of disease progression in a small percentage of patients [[35], [36], [37]]. No potential antigen specific immunotherapy has met with government approval or been taken up for commercial development by “big pharma”. Further, where regression of cancer is seen following immunisation, the majority of the tumour specific immune response does not seem to be directed at the HPV associated antigens [38], suggesting that the vaccines are contributing as much to non-specific stimulation of immune responses as to induction of specific immunity.
4. Combination immunotherapies
Lack of efficacy of immunotherapy as a single intervention has led to trials combining specific immunotherapy with other modalities of treatment, including chemotherapy, and nonspecific immunotherapy. Chemotherapy can directly impact on the cancer, or can influence the nature of the immune response induced by immunotherapy, for example through myeloid cell or regulatory T cell depletion [39]. Non-antigen specific cancer immunotherapy through immune checkpoint blockade with monoclonal antibody (MAb) checkpoint inhibitors is held largely to strengthen or repurpose existing tumour antigen specific immune responses. Animal models support combination of nonspecific and antigen specific immunotherapy for HPV associated cancer [40,41]. Most clinical trials to date have used monoclonal antibodies to block the PD-L1/PD-1 interaction that results in T cell death. Their use over a period of one to two years has induced complete responses in up to 20% of patients with various metastatic epithelial cancers [42], though the best results have been seen with melanoma, and with non-Hodgkins lymphoma. Checkpoint blockade trials of HPV associated oropharyngeal cancers have shown some benefit [[43], [44], [45]] and as some of the 5 current commercially available checkpoint inhibiting MAbs are now licensed in some countries for HPV associated oropharyngeal cancers, later stage studies with combination of HPV specific immunotherapy and checkpoint inhibition are now underway.
5. Future developments
Each modality of immunotherapy to date has only addressed one or two of the recognized barriers to effective tumour immunotherapy. These include:
-
(1)
lack of presentation of tumour specific antigens on the tumour or to the host immune system, which can potentially be addressed with cytokine therapy,
-
(2)
failure of T cell priming, which can be addressed by antigen specific immunotherapy,
-
(3)
failure of primed T cells to access the tumour, for which we need new strategies
-
(4)
local active immune suppression by the tumour, which checkpoint inhibition and macrophage elimination can overcome, and
-
(5)
continuous generation of tumour subclones with new evasion strategies and loss of specific antigen, which will require combination therapy with non-antigen specific immunotherapy and immunotherapy targeting multiple antigens.
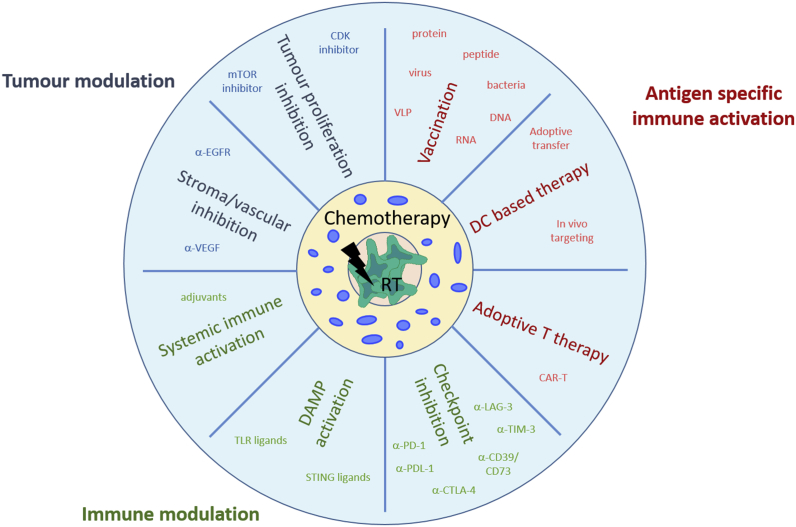
Cancer immunotherapy of the future will require a more holistic approach, combining optimized traditional radio- and chemotherapy, which in the right dose and sequence can synergistically support anti-tumour immune responses, with HPV antigen specific immunotherapy, either with immunisation or chimeric receptor targeted T cells (CAR-T). Addition of unspecific immune stimulants including cytokines and checkpoint inhibitors, and interventions to reprogram the immunoregulatory environment within the tumour and the tumour itself may also help (Fig. 1). Whatever approach to immunotherapy is adopted, HPV associated tumours will likely be used to test clinical efficacy, as these are relatively common, the tumour specific antigens are well identified, and their expression persists throughout the development of the tumour.
Fig. 1.
Future immunotherapy using a holistic approach to treat HPV associated disease combining optimized traditional therapy (radio-chemotherapy) with antigen specific vaccination, immune- and tumour modulation. Abbreviations: HPV, human papillomavirus; CAR, chimeric antigen receptor; DAMP, danger associated molecular patterns; TLR, toll like receptor; DC, dendritic cell; EGFR, endothelial growth factor receptor; VEGF, vascular endothelial growth factor; VLP, virus like particle; STING, stimulator of interferon genes; mTOR, mechanistic target of rapamycin; CDK, cyclin dependent kinase; RT, radio therapy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.100176.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Graham S.V. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin. Sci. (Lond.) 2017;131(17):2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 2.van der Burg S.H. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001;19(27):3652–3660. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 3.Frazer I.H. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23(2):172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Frazer I.H., Leggatt G.R., Mattarollo S.R. Prevention and treatment of papillomavirus-related cancers through immunization. Annu. Rev. Immunol. 2011;29:111–138. doi: 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]
- 5.Bergot A.S. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells. PLoS Pathog. 2014;10(10) doi: 10.1371/journal.ppat.1004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuong Z.K. Murine HPV16 E7-expressing transgenic skin effectively emulates the cellular and molecular features of human high-grade squamous intraepithelial lesions. Papillomavirus Res. 2018;5:6–20. doi: 10.1016/j.pvr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando G.J., Tindle R.W., Frazer I.H. T-helper epitopes of the E7 transforming protein of cervical cancer associated human papillomavirus type 18 (HPV18) Virus Res. 1995;36(1):1–13. doi: 10.1016/0168-1702(94)00101-h. [DOI] [PubMed] [Google Scholar]
- 8.Azoury-Ziadeh R. T-helper epitopes identified within the E6 transforming protein of cervical cancer-associated human papillomavirus type 16. Viral Immunol. 1999;12(4):297–312. doi: 10.1089/vim.1999.12.297. [DOI] [PubMed] [Google Scholar]
- 9.Khammanivong V. Paucity of functional CTL epitopes in the E7 oncoprotein of cervical cancer associated human papillomavirus type 16. Immunol. Cell Biol. 2003;81(1):1–7. doi: 10.1046/j.1440-1711.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott M.E. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study. Int. J. Cancer. 2013;133(5):1187–1196. doi: 10.1002/ijc.28119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffman M. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol. Biomark. Prev. 2011;20(7):1398–1409. doi: 10.1158/1055-9965.EPI-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny L.A. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 13.Carter J.J. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000;181(6):1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 14.Rosales R., Lopez-Contreras M., Cortes R.R. Antibodies against human papillomavirus (HPV) type 16 and 18 E2, E6 and E7 proteins in sera: correlation with presence of papillomavirus DNA. J. Med. Virol. 2001;65(4):736–744. doi: 10.1002/jmv.2098. [DOI] [PubMed] [Google Scholar]
- 15.Carter J.J. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. JID (J. Infect. Dis.) 1996;174(5):927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 16.Goodman M.T. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68(21):8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris R.L. Immunology and immunotherapy of head and neck cancer. J. Clin. Oncol. 2015;33(29):3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbach A., Riemer A.B. Immune evasion mechanisms of human papillomavirus: an update. Int. J. Cancer. 2018;142(2):224–229. doi: 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q. Downregulation of tapasin expression in primary human oral squamous cell carcinoma: association with clinical outcome. Tumour Biol. 2010;31(5):451–459. doi: 10.1007/s13277-010-0054-4. [DOI] [PubMed] [Google Scholar]
- 20.Walch-Ruckheim B. Cervical cancer-instructed stromal fibroblasts enhance IL23 expression in dendritic cells to support expansion of Th17 cells. Cancer Res. 2019;79(7):1573–1586. doi: 10.1158/0008-5472.CAN-18-1913. [DOI] [PubMed] [Google Scholar]
- 21.Petruzzi M.N. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge. Diagn. Pathol. 2017;12(1):32. doi: 10.1186/s13000-017-0623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kouketsu A. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2019 doi: 10.1016/j.ijom.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Chandra J. Epithelium expressing the E7 oncoprotein of HPV16 attracts immune-modulatory dendritic cells to the skin and suppresses their antigen-processing capacity. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo P. Recruitment of antigen presenting cells to skin draining lymph node from HPV16e7-expressing skin requires E7-Rb interaction. Front. Immunol. 2018;9:2896. doi: 10.3389/fimmu.2018.02896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo P. HPV16E7-Induced hyperplasia promotes CXCL9/10 expression and induces CXCR3(+) T-cell migration to skin. J. Investig. Dermatol. 2018;138(6):1348–1359. doi: 10.1016/j.jid.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Bashaw A.A. Modulation of antigen presenting cell functions during chronic HPV infection. Papillomavirus Res. 2017;4:58–65. doi: 10.1016/j.pvr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashaw A.A. HPV16 E7-driven epithelial hyperplasia promotes impaired antigen presentation and regulatory T cell development. J. Investig. Dermatol. 2019 doi: 10.1016/j.jid.2019.03.1162. [DOI] [PubMed] [Google Scholar]
- 28.Leo P.J. Defining the genetic susceptibility to cervical neoplasia – a genome-wide association study. PLoS Genet. 2017;13(7) doi: 10.1371/journal.pgen.1006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown M.A., Leo P.J. Genetic susceptibility to cervical neoplasia. Papillomavirus Res. 2019;7:132–134. doi: 10.1016/j.pvr.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peralta-Zaragoza O. Targeted treatments for cervical cancer: a review. OncoTargets Ther. 2012;5:315–328. doi: 10.2147/OTT.S25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.J. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016;27(5):e51. doi: 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trimble C.L., Frazer I.H. Development of therapeutic HPV vaccines. Lancet Oncol. 2009;10(10):975–980. doi: 10.1016/S1470-2045(09)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan S.R., Bharadwaj M. Gearing up T-cell immunotherapy in cervical cancer. Curr. Probl. Cancer. 2018;42(2):175–188. doi: 10.1016/j.currproblcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 34.van Poelgeest M.I. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin. Cancer Res. 2016;22(10):2342–2350. doi: 10.1158/1078-0432.CCR-15-2594. [DOI] [PubMed] [Google Scholar]
- 35.Morrow M.P., Yan J., Sardesai N.Y. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev. Vaccines. 2013;12(3):271–283. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]
- 36.Stanley M.A. Genital human papillomavirus infections: current and prospective therapies. J. Gen. Virol. 2012;93(Pt 4):681–691. doi: 10.1099/vir.0.039677-0. [DOI] [PubMed] [Google Scholar]
- 37.Nieto K., Gissmann L., Schadlich L. Human papillomavirus-specific immune therapy: failure and hope. Antivir. Ther. 2010;15(7):951–957. doi: 10.3851/IMP1665. [DOI] [PubMed] [Google Scholar]
- 38.Stevanovic S. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356(6334):200–205. doi: 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welters M.J. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016;8(334):334ra52. doi: 10.1126/scitranslmed.aad8307. [DOI] [PubMed] [Google Scholar]
- 40.Rice A.E. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015;22(9):454–462. doi: 10.1038/cgt.2015.40. [DOI] [PubMed] [Google Scholar]
- 41.Chandra J. DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J. Immunother. 2017;40(2):62–70. doi: 10.1097/CJI.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui H. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. 2018;2018:6984948. doi: 10.1155/2018/6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Li G. PD-1/PD-L1 blockade in cervical cancer: current studies and perspectives. Front. Med. 2019 doi: 10.1007/s11684-018-0674-4. [DOI] [PubMed] [Google Scholar]
- 44.Ghanizada M. The effects of checkpoint inhibition on head and neck squamous cell carcinoma: a systematic review. Oral Oncol. 2019;90:67–73. doi: 10.1016/j.oraloncology.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Massarelli E. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.