Abstract
Background
Ovarian epithelial cancer (OEC) is the second-most common gynecologic malignancy. CD109 expression is elevated in human tumor cell lines and carcinomas. A previous study showed that CD109 expression is elevated in human tumor cell lines and CD109 plays a role in cancer progression. Therefore, this study aimed to determine whether CD109 is expressed in OEC and can be useful in predicting the prognosis.
Methods
Immunohistochemical staining for CD109 and reverse transcription-quantitative polymerase chain reaction was performed. Then we compared CD109 expression and chemoresistance, overall survival, and recurrence-free survival of OEC patients. Chemoresistance was evaluated by dividing into good-response group and poor-response group by the time to recurrence after chemotherapy.
Results
CD109 expression was associated with overall survival (p = .020), but not recurrence-free survival (p = .290). CD109 expression was not an independent risk factor for overall survival due to its reliability (hazard ratio, 1.58; p = .160; 95% confidence interval, 0.82 to 3.05), although we found that CD109 positivity was related to chemoresistance. The poor-response group showed higher rates of CD109 expression than the good-response group (93.8% vs 66.7%, p = .047). Also, the CD109 mRNA expression level was 2.88 times higher in the poor-response group as compared to the good-response group (p = .001).
Conclusions
Examining the CD109 expression in patients with OEC may be helpful in predicting survival and chemotherapeutic effect.
Keywords: Ovarian epithelial carcinoma, CD109, Prognosis
Ovarian epithelial cancer (OEC) is the second most common gynecologic malignancy [1]. However, it is often found in advanced stages, which makes it difficult to treat. OEC is believed to have an insidious onset, with no early symptoms [2]. Survival rates for women with advanced disease range from 20%–30%, which are much lower than those for women with early stage disease (70%–90%) [3]. Despite tumor-debulking surgery and chemotherapy, the 50-year survival rate is as low as 40% [4]. Although factors associated with the prognosis of OEC, such as p53 and human epidermal growth factor receptor 2 expression, have been reported [5,6], only a few factors can predict poor outcome in patients with OEC.
CD109 is a glycosylphosphatidylinositol-linked cell surface glycoprotein and a member of the 2-macroglobulin-C3, C4, C5 family of thioester-containing proteins [7]. CD109 expression is limited in certain cell types in normal tissues, including myoepithelial cells of the breast, salivary gland, and basal cells of the prostate [8]. CD109 protein is a component of the transforming growth factor β1 (TGF-β1) receptor system, which is involved in cell proliferation and differentiation and has both tumor-suppressive and promoting effects during carcinogenesis [9]. However, its functions are still unknown. A previous study showed that CD109 expression is elevated in human tumor cell lines and CD109 plays a role in cancer progression [10]. CD109 expression is significantly increased in carcinomas of the lung, gallbladder, uterine cervix, and vulva and soft tissue sarcomas [10-14]. In addition, CD109 expression in lung squamous cell carcinoma is associated with the tumor stage, and its expression in myxofibrosarcomas is useful to predict recurrence [11,15]. Thus, CD109 could be expressed in OEC and may be a useful predictor of OEC prognosis.
The aim of this study was to evaluate CD109 expression by immunohistochemistry and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) using clinical specimens of human OEC; to analyze the correlation between CD109 expression and survival rate, recurrence rate, and chemotherapy response in patients with OEC; and to investigate the prognostic significance of CD109 expression.
MATERIALS AND METHODS
Patients and data
OEC patients who underwent surgical resection at Pusan National University Hospital from 1998 to 2009 were selected for this study. All patients provided written informed consents and underwent surgical procedures. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital (PNUH), a member of the Korea Biobank Network. All samples derived from the National Biobank of Korea were obtained with the approval of institutional review board. After exclusion of cases with insufficient tissue material and clinical information, a total of 120 cases were enrolled and representative formalin-fixed paraffin tissue blocks were collected. Pathological data including histological type, pathological stage, tumor histological grade, nuclear grade, and mitosis were obtained from the primary pathology report. The histological tumor type was classified according to the World Health Organization criteria [16]. Histological grades were classified as well-, moderately, and poorly differentiated according to the Silverberg grading system [17]. The tumors were staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system (Table 1) [18].
Table 1.
Characteristic of the patients
| No. (%) (n = 120) | |
|---|---|
| Follow-up, median (range, mo) | 50 (1–115) |
| Age at diagnosis (yr) | 51 (15–82) |
| < 50 | 57 (47.5) |
| ≥ 50 | 63 (52.5) |
| Histologic type | |
| Serous | 59 (49.2) |
| Mucinous | 22 (18.3) |
| Endometrioid | 9 (7.5) |
| Clear cell | 27 (22.5) |
| Undifferentiated | 3 (2.5) |
| Histologic grade | |
| Well differentiated | 28 (23.3) |
| Moderate differentiated | 61 (50.8) |
| Poorly differentiated | 31 (25.8) |
| Nuclear grade | |
| I | 6 (5.0) |
| II | 65 (54.2) |
| III | 49 (40.8) |
| FIGO stage | |
| I | 51 (42.5) |
| II | 6 (5) |
| III | 44 (36.7) |
| IV | 19 (15.8) |
| Mitosis (per 10HPFs) | |
| 1–9 | 45 (37.5) |
| 10–19 | 41 (34.2) |
| ≥ 20 | 34 (28.3) |
| Overall survival | |
| Survival | 63 (52.5) |
| Death | 57 (47.5) |
| Recurrence | |
| Absent | 27 (22.5) |
| Present | 93 (77.5) |
FIGO, International Federation of Gynecology and Obstetrics; HPF, high-power field.
Fresh tissue samples of OEC were obtained by surgical resection and stored in the Biobank of PNUH. For this study, tissue samples from 12 patients who underwent resection were examined. We evaluated 12 patients with serous carcinoma who received chemotherapy. Six patients who did not experience relapse for 18 months after chemotherapy were included in the good-response group, and six patients who experienced recurrence within 6 months of chemotherapy initiation to 8 months of chemotherapy completion were included in the poor-response group (Table 2).
Table 2.
Fresh tissue samples of ovarian epithelial carcinoma
| Case No. | Histologic type | Stage | Chemotherapy regimen | Period before recurrence (mo) | Response |
|---|---|---|---|---|---|
| 1 | Serous | IIIc | Carbo-Taxola | 4 | Poor response |
| 2 | Serous | IV | Carbo-Taxol | 8 | Poor response |
| 3 | Serous | IIIc | Carbo-Taxol | 4 | Poor response |
| 4 | Serous | IIIc | Carbo-Taxol | 0 | Poor response |
| 5 | Serous | IV | Carbo-Taxol | 1 | Poor response |
| 6 | Serous | IV | Carbo-Taxol | 0 | Poor response |
| 7 | Serous | IIIc | Carbo-Taxol | No recur | Good response |
| 8 | Serous | IIIc | Carbo-Taxol | No recur | Good response |
| 9 | Serous | IIIc | Carbo-Taxol | No recur | Good response |
| 10 | Serous | IIa | Carbo-Taxol | 38 | Good response |
| 11 | Serous | IIIc | Carbo-Taxol | No recur | Good response |
| 12 | Serous | IV | Carbo-Taxol | 25 | Good response |
Pacilitaxel and carboplatin.
Immunohistochemistry
CD109 expression was assessed using CD109 immunohistochemical staining. The slides were then dewaxed in xylene and dehydrated in ethanol. Staining was performed using BondMax-autostainer and other reagents (Leica Microsystems, Berlin, Germany). Deparaffinization was performed automatically in the autostainer with BondWash solution (Leica Microsystems) at 72°C for 30 minutes. After washing, the slides were incubated overnight at 4°C with a rabbit polyclonal anti-CD109 antibody (dilution 1:50, cat. No., HPA009292, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (dilution 1:100, cat. No., SA00001-2, ProteinTech Group, Inc., Wuhan Sanying Biotechnology, Wuhan, China) for 90 minutes at room temperature. Antibody binding was performed by incubating the slides for 1 minute with a solution of 1 drop of 3,3'-diamino-benzidine (20 ×) per 1.0 mL diamino-benzidine substrate buffer (cat. No. ZLI-9017, Origene Technologies, Inc., Beijing, China). The slides were then counterstained with EnVision FLEX hematoxylin (Dako, Agilent Technologies, Inc., Santa Clara, CA, USA) for 1 minute and dehydrated using ethanol and xylene. For negative control, staining was performed without primary antibody.
Assessment of immunohistochemical staining
Slides were evaluated using light microscopy. CD109 expression was detected through cytoplasmic and/or membranous staining of the tumor cells. The CD109 positivity of tumor cells was determined as follows: positive staining, ≥ 10% positive tumor cells and negative staining, < 10% positive tumor cells.
RT-qPCR
The mRNA levels of CD109 in the fresh tissue samples of OEC were measured using RT-qPCR. Total RNA was purified from cells using RNeasy mini prep kits (Qiagen, Valencia, CA, USA). cDNA was synthesized from 1 μg RNA using the ProtoScript First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA, USA). Differential RNA levels were assessed using Luna Universal qPCR Master Mix (New England Biolabs) and primers for each gene. Quantitative PCR reactions were performed using an ECO Real-Time PCR system (PCRmax, Straffordshire, UK) as follows: 95°C for 30 minutes followed by 40 cycles at 95°C for 10 seconds, 61°C for 30 seconds, and 72°C for 20 seconds. The PCR products were analyzed using ECO ware (PCRmax). All samples were normalized to the signal generated from glyceraldehyde 3-phosphate dehydrogenase using the primers presented in Table 3. The CD109 gene expression was generalized by comparing the relative expression of CD109 with the internal standard glyceraldehyde 3-phosphate dehydrogenase using the 2-ΔΔCT method [19].
Table 3.
Primer sequences
| Gene | Sequence (5'-3') |
|---|---|
| Cluster of differentiation 109 | |
| Forward | GAAGCCATCTCTCAACTTCACA |
| Reverse | CTCCTTGGAGGCCATGTG |
| GAPDH | |
| Forward | GAAGGTGGTGAAGCAGGC |
| Reverse | TTCCACTGTTAGATCCGCTCC |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
The Pearson’s chi-square test was used to assess statistical significance between CD109 expression and various clinicopathological characteristics. Overall survival and recurrence-free survival were estimated using Kaplan-Meier plots. Multivariate analyses to determine hazard ratios for overall survival and recurrence-free survival were performed using Cox regression analysis. All analyses were performed using SPSS software (IBM, Armonk, NY, USA). A p-value of < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A series of 120 OEC cases was retrieved for this study. The follow-up period ranged from 1 to 115 months (median, 50 months), and the patient age ranged from 15 to 82 years (median, 50 years). There were 59 cases of serous carcinoma, 22 cases of mucinous carcinoma, nine cases of endometrioid carcinoma, 27 cases of clear cell carcinoma, and three cases of undifferentiated carcinoma. The overall survival rate was 52.5%, and the recurrence rate was 77.5%. Other clinicopathologic parameters are presented in Table 1.
CD109 expression in OEC
In OEC samples, the malignant tumor cells exhibited brownish CD109 staining in the membrane and cytoplasm (Fig. 1). CD109 expression was detected in 63 of the 120 OEC samples (52.5%). CD109 expression differed according to the histological type of tumor (p < .001). There was a statistically significantly increase in the expression of CD109 in serous, endometrioid, and undifferentiated carcinomas (77.4%). CD109 expression was associated with a higher histological tumor grade (p = .012). Twelve of 51 FIGO stage I tumors (23.5%) and 51 of 69 FIGO stage II, III, and IV tumors (73.9%) were positive for CD109 (p < .001). Thirteen of 45 tumors with < 10 mitotic events (28.9%) and 50 of 75 tumors with more than 10 mitotic events (66.7%) were positive for CD109 (p < .001). A high nuclear grade was not associated with CD109 expression (Table 4).
Fig. 1.
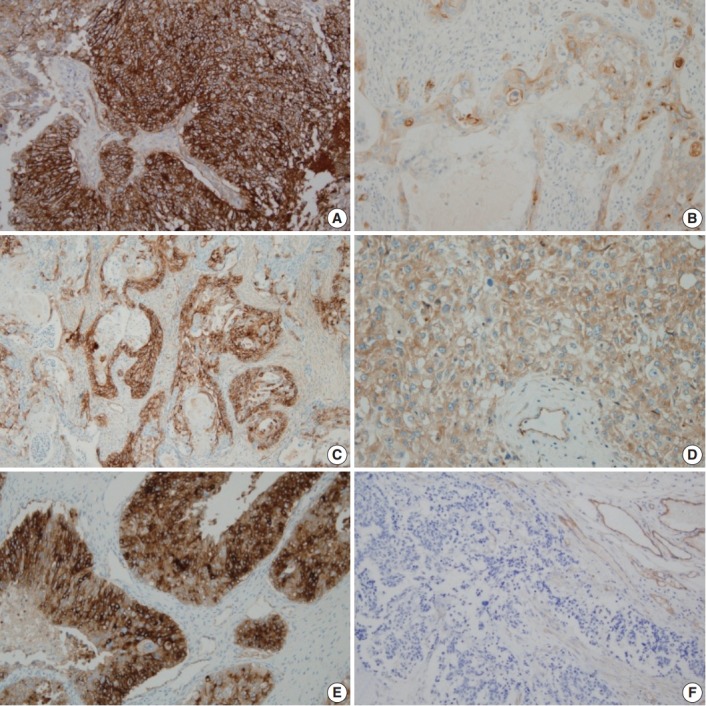
Immunohistochemical staining of CD109 in ovarian epithelial carcinoma. (A) Positive in serous carcinoma. (B) Positive in mucinous carcinoma. (C) Positive in endometrioid carcinoma. (D) Positive in clear cell carcinoma. (E) Positive in undifferentiated carcinoma. (F) Negative in serous carcinoma.
Table 4.
Correlation between CD109 expression and clinicopathological characteristics in ovarian epithelial carcinomas (n = 120)
| Parameter | Negative | Positive | p-valuea |
|---|---|---|---|
| Histologic type | .000 | ||
| Serous | 13 (22.0) | 46 (78.0) | |
| Mucinous | 19 (86.4) | 3 (13.6) | |
| Endometrioid | 3 (33.3) | 6 (66.7) | |
| Clear cell | 22 (81.5) | 5 (18.5) | |
| Undifferentiated | 0 (0) | 3 (100) | |
| Histologic grade | .012 | ||
| Well differentiated | 19 (67.9) | 9 (32.1) | |
| Moderate differentiated | 29 (47.5) | 32 (52.5) | |
| Poorly differentiated | 9 (29.0) | 22 (71.0) | |
| Nuclear grade | .788 | ||
| I | 3 (50.0) | 3 (50.0) | |
| II | 29 (44.6) | 36 (55.4) | |
| III | 25 (51.0) | 24 (49.0) | |
| FIGO stage | .000 | ||
| I | 39 (76.5) | 12 (23.5) | |
| II, III and IV | 18 (26.1) | 51 (73.9) | |
| Mitoses (per 10HPFs) | .000 | ||
| 1–9 | 32 (71.1) | 13 (28.9) | |
| ≥ 10 | 25 (33.3) | 50 (66.7) |
Negative, less than 10% positive staining of tumor cells; Positive, more than 10% positive staining of tumor cells.
FIGO, Federation of Gynecology and Obstetrics; HPF, high power field.
Pearson’s chi-squared test.
In the above results, CD109 positivity ratio varied according to the histologic type, and the following is the CD109 expression results in serous carcinoma that accounts for the largest percentage of OEC: Six of 13 FIGO stage I tumors (46.2%) and 40 of 46 FIGO stage II, III, and IV tumors (87.0%) were positive for CD109 (p = .004). Histologic grade, high nuclear grade, and high mitotic counts were not associated with CD109 expression (Table 5).
Table 5.
Correlation between CD109 expression and clinicopathological characteristics in serous carcinoma (n = 59)
| Parameter | Negative | Positive | p-valuea |
|---|---|---|---|
| Histologic grade | .351 | ||
| Well differentiated | 2 (40.0) | 3 (60.0) | |
| Moderate differentiated | 9 (24.3) | 28 (75.7) | |
| Poorly differentiated | 2 (11.8) | 15 (88.2) | |
| Nuclear grade | .850 | ||
| I | 0 (0) | 1 (100) | |
| II | 8 (21.6) | 29 (78.4) | |
| III | 5 (23.8) | 16 (76.2) | |
| FIGO stage | .004 | ||
| I | 7 (53.8) | 6 (46.2) | |
| II, III and IV | 6 (13.0) | 40 (87.0) | |
| Mitoses (per 10HPFs) | .959 | ||
| 1–9 | 3 (25.0) | 9 (75.0) | |
| 10–19 | 5 (21.7) | 18 (78.3) | |
| ≥ 20 | 5 (20.8) | 19 (79.2) |
Values are presented as number (%).
FIGO, Federation of Gynecology and Obstetrics; HPF, high power field; Negative, less than 10% positive staining of tumor cells; Positive, more than 10% positive staining of tumor cells.
Pearson’s chi-squared test.
Association between CD109 expression and OEC prognosis
CD109 expression was associated with overall survival (p = .020) (Fig. 2), but not recurrence-free survival (p = .290) (Fig. 3). The 5-year survival rate was 42.5% in patients with CD109-positive results and 64.8% in patients with CD109-negative results (p = .020) (Fig. 2). Analysis of the variables using multivariate analysis showed that CD109 expression was not an independent risk factor for overall survival due to its low reliability (hazard ratio [HR], 1.58; p = .160; 95% confidence interval [CI], 0.82 to 3.05) (Table 6). In addition, CD109 expression was not an only risk factor for recurrence-free survival (HR, 2.06; p = .110; 95% CI, 0.83 to 5.09) (Table 7). Therefore, CD109 expression is not an independent prognostic factor but is helpful for predicting the prognosis of patients with OEC.
Fig. 2.
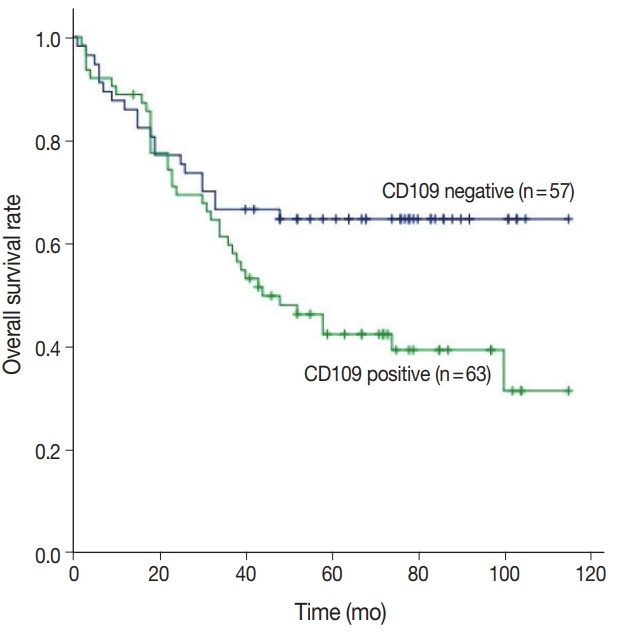
Expression of CD109 protein is associated with overall survival. Prognosis is estimated by Kaplan-Meier plots (p=.020).
Fig. 3.
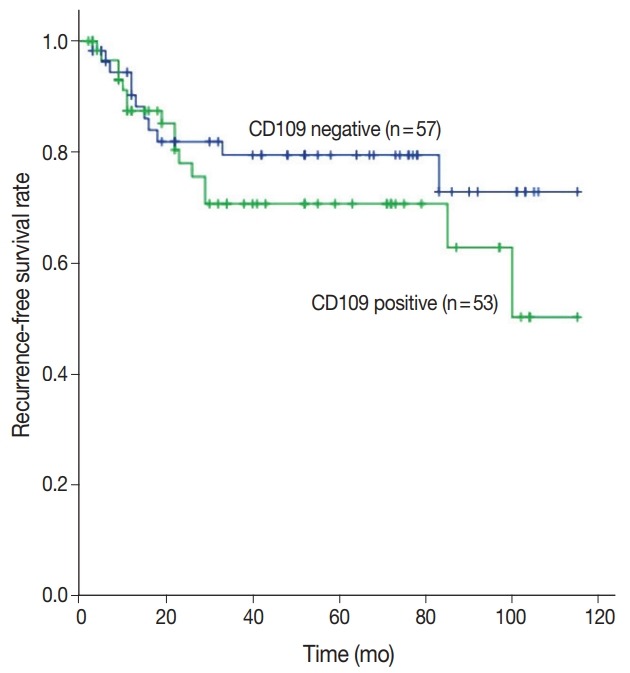
Expression of CD109 protein is not significantly associated with recurrence. Prognosis is estimated by Kaplan-Meier plots (p=.290).
Table 6.
Multivariate analysis of overall survival (n = 120)
| Variable | Hazard ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Histologic grade | |||
| Well differentiated | 1 | ||
| Moderate differentiated | 0.33 | 0.12–0.87 | .020 |
| Poorly differentiated | 0.63 | 0.34–1.14 | .130 |
| FIGO stage | |||
| I | 1 | ||
| II, III, and IV | 0.17 | 0.07–0.38 | < .001 |
| CD109 expression | |||
| Negative | 0 | ||
| Positive | 1.58 | 0.82–3.05 | .160 |
Table 7.
Multivariate analysis of recurrence-free survival (n = 120)
| Variable | Hazard ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Histologic grade | |||
| Well differentiated | 1 | ||
| Moderate differentiated | 0.34 | 0.09–1.26 | .220 |
| Poorly differentiated | 0.53 | 0.21–1.32 | .100 |
| FIGO stage | |||
| I | 1 | ||
| II, III, and IV | 1.29 | 0.41–0.41 | < .001 |
| CD109 expression | |||
| Negative | 1 | ||
| Positive | 2.06 | 0.83–5.09 | .110 |
Immunohistochemical staining was performed in 37 patients with serous carcinoma who received chemotherapy, 21 patients belonged to the good-response group and 16 patients belonged to the poor-response group. The poor-response group had higher rates of CD109 expression than the good-response group (93.8% vs 66.7%, p = .047) (Table 8). Thus, CD109 is useful for predicting the chemotherapeutic effect in patients with OEC, especially serous carcinoma.
Table 8.
Chemotherapy response in correlation with CD109 expression
| Parameter | CD109 expression |
|
|---|---|---|
| Negative | Positive | |
| Good response group | 7 (33.3) | 14 (66.7) |
| Poor response group | 1 (12.5) | 15 (93.8) |
Values are presented as number (%).
CD109 mRNA levels in OECs
All 12 fresh OEC samples were examined for CD109 expression by RT-qPCR. The samples were limited to serous carcinoma of OEC. CD109 mRNA was significantly upregulated in the poor-response group compared with the good-response group (p = .001) (Fig. 4), suggesting that high CD109 expression level is associated with chemoresistance in patients with OEC.
Fig. 4.
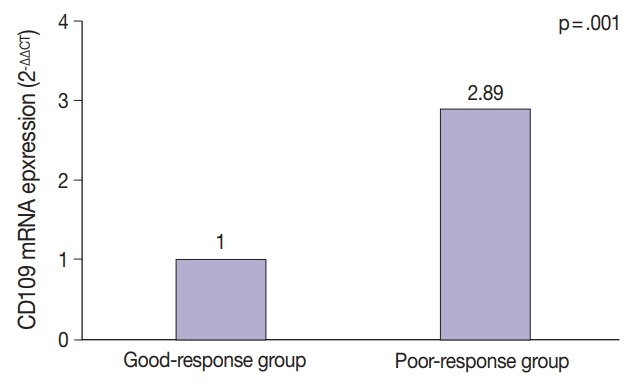
Relationship between chemoresistance and CD109 mRNA expression (n=12).
DISCUSSION
The physiological function of CD109 has not been studied, yet it is highly expressed in various types of malignancies and several normal tissues [8,20]. It has recently been reported that CD109 expression is associated with tumor development and cell proliferation using human oral tumor tissues and cancer cell lines [21]. In addition, CD109 can predict prognosis in tumors such as myxofibrosarcoma [15]. In this study, we evaluated CD109 expression in OEC samples by immunohistochemical stains and RT-qPCR and found that an increased CD109 expression was associated with the prognosis and chemoresistance of OEC.
CD109 protein is a component of the TGF-β1 receptor system [9]. Tumor suppression occurs through the TGF-β1–signaling pathway in the early tumor stage; however, in malignantly transformed cells, TGF-β1 is severely dysregulated, resulting in the loss of tumor suppression [9]. CD109 expression is followed by a complex formation with the type I TGF-β1 receptor, which is required for the regulation of TGF-β1 signaling in early tumor cells [22]. Finally, CD109 degrades the TGF-β1 receptor and blocks TGF-β1 signaling, thereby preventing tumor suppression [23], which was confirmed in a mouse model [24]. In CD109-deficient mice, the TGF-β1 signaling pathway was enhanced and it suppressed skin tumorigenesis [24].
TGF-β1 plays an important role in chemoresistance in breast cancer and squamous cell carcinoma [25,26]. During TGF-β1 expression, cancer cells proliferate slowly in early tumor stage and lead to chemoresistance because they can be protected against DNA-damaging chemotherapeutic agents [26]. In myxofibrosarcomas, tumors with high CD109 expression level showed decreased expression of TGF-β1 with a good treatment effect [27]. However, tumors with low CD109 expression and high TGF-β1 expression showed chemoresistance [27]. The current case and the other cases mentioned above indicate that there are other underlying mechanisms stronger than the CD109–TGF-β1 signaling pathway. In our study, the relationship between CD109 expression and chemoresistance was identified, but TGF-β1 expression and its role were not confirmed. If both CD109 and TGF-β1 are found to be related to the chemoresistance of OEC, it will be helpful in treating patients. Therefore, further research is needed to confirm these findings in OEC.
CD109 expression has been associated with chemotherapeutic resistance in triple-negative breast cancers [28]. After chemotherapy was administered to patients with CD109-positive triple negative breast cancer, 50% of the patients showed disease progression and none of them showed complete response [28]. The same result was reported when examining the CD109 expression and chemotherapeutic effect in breast cancer and glioblastoma [29,30]. This may be due to the role of CD109 in the development of endothelial cells and induction of angiogenesis in contrary to the effect of chemotherapy regimen, which blocks tumor neovascularization [30]. In this study, CD109 was expressed at a higher rate in the poor-response group than in the good-response group. In addition, the expression level of CD109 mRNA was 2.88-fold higher in the poor-response group than in the good-response group. However, unlike breast cancer, most OECs are diagnosed at advanced stages, making it difficult to divide the chemotherapy response group into more detailed subgroups.
CD109 is involved in the pathogenesis and prognosis of some tumors. CD109 overexpression is known to induce cell growth in oral squamous cell carcinomas, and CD109-positive oral dysplastic lesions develop into squamous cell carcinoma within 3 years [21]. Although there is no correlation between the CD109 expression and mitosis, CD109 expression was associated with neoplastic cell growth [31]. Recently, a study suggested that CD109 expression is managed by cancer stem-like cells/cancer-initiating cells, which can initiate tumorigenesis and tumor growth in sarcomas [14]. Therefore, in the presence of cancer stem cells, CD109 expression may increase and malignancies or recurrence/metastasis may develop. In our study, CD109 expression was higher in the advanced stage, but OEC precursors such as borderline ovarian tumor is not included in the study. More research that can determine the association of CD109 with neoplastic cell growth in ovary tissue may be helpful in early detection and treatment of patients.
Based on our review, this is the first study on the relationship between CD109 expression and prognosis of OEC. The present study demonstrated that CD109 is highly expressed in OEC tissues. Immunohistochemical analysis of CD109 expression is useful for predicting overall survival but not recurrence-free survival. Regarding the chemotherapeutic effect on serous carcinoma, the poor-response group expressed increased mRNA levels of CD109 as compared to the good-response group. Therefore, identification of CD109 expression may help in predicting the survival and chemotherapeutic effect in patients with serous carcinoma of OEC. Further analysis of other factors related to CD109 is needed for careful management of patients with serous carcinoma of OEC.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1A2B4009021) and the Korea Health Technology R&D Project, Ministry of Health and Welfare (HI17C1635). The biospecimens for this study were provided by the Biobank of Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affair.
Footnotes
Author Contributions
Conceptualization: SYK, KUC.
Data curation: CH, KHK, DSS, BSK.
Formal analysis: SYK, CH, HJL.
Funding acquisition: KUC, JHK.
Investigation: SYK, KUC, DHS, JYK .
Methodology: SYK, JHL, DHS, JYK, KUC.
Project administration: SYK, KUC, JHK.
Resources: KUC, KHK, DSS, BSK.
Supervision: KUC, JHK.
Validation: DSS, JYK.
Writing—original draft: SYK, KUC.
Writing—review & editing: HJL, JHL, DHS, JHK, MYS.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
REFERENCES
- 1.Montes AF, Gomez JG, Viejo MN, Bermejo MA, Urrutia SA, Mata JG. Epidemiology and etiology of ovarian cancer. In: Farghaly S, editor. Ovarian cancer: basic science perspective. Vancouver: InTech; 2012. pp. 1–16. [Google Scholar]
- 2.Flam F, Einhorn N, Sjövall K. Symptomatology of ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1988;27:53–7. doi: 10.1016/s0028-2243(88)80010-8. [DOI] [PubMed] [Google Scholar]
- 3.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89:2068–75. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20:5150–6. doi: 10.1158/1078-0432.CCR-14-1312. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann LC, Podratz KC, Keeney GL, et al. Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol. 1994;12:64–9. doi: 10.1200/JCO.1994.12.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Luo H, Xu X, Ye M, Sheng B, Zhu X. The prognostic value of HER2 in ovarian cancer: a meta-analysis of observational studies. PLoS One. 2018;13:e0191972. doi: 10.1371/journal.pone.0191972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin M, Sutherland DR, Horsfall W, et al. Cell surface antigen CD109 is a novel member of the alpha(2) macroglobulin/C3, C4, C5 family of thioester-containing proteins. Blood. 2002;99:1683–91. doi: 10.1182/blood.v99.5.1683. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Hagiwara S, Sato T, et al. CD109, a new marker for myoepithelial cells of mammary, salivary, and lacrimal glands and prostate basal cells. Pathol Int. 2007;57:245–50. doi: 10.1111/j.1440-1827.2007.02097.x. [DOI] [PubMed] [Google Scholar]
- 9.Leivonen SK, Kähäri VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–24. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JM, Hashimoto M, Kawai K, et al. CD109 expression in squamous cell carcinoma of the uterine cervix. Pathol Int. 2005;55:165–9. doi: 10.1111/j.1440-1827.2005.01807.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Murakumo Y, Hagiwara S, et al. High-level expression of CD109 is frequently detected in lung squamous cell carcinomas. Pathol Int. 2007;57:719–24. doi: 10.1111/j.1440-1827.2007.02168.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Lu C, Chen X, Guo Y, Liu J. CD109 is a novel marker for squamous cell/adenosquamous carcinomas of the gallbladder. Diagn Pathol. 2015;10:137. doi: 10.1186/s13000-015-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozbay PÖ, Ekinci T, Yigˇit S, et al. Investigation of prognostic significance of CD109 expression in women with vulvar squamous cell carcinoma. Onco Targets Ther. 2013;6:621–7. doi: 10.2147/OTT.S41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emori M, Tsukahara T, Murase M, et al. High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancerinitiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS One. 2013;8:e84187. doi: 10.1371/journal.pone.0084187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emori M, Tsukahara T, Murata K, et al. Prognostic impact of CD109 expression in myxofibrosarcoma. J Surg Oncol. 2015;111:975–9. doi: 10.1002/jso.23934. [DOI] [PubMed] [Google Scholar]
- 16.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. pp. 11–40. [Google Scholar]
- 17.Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994-1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1999;65:243–9. doi: 10.1016/s0020-7292(99)00070-3. [DOI] [PubMed] [Google Scholar]
- 19.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of realtime PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto M, Ichihara M, Watanabe T, et al. Expression of CD109 in human cancer. Oncogene. 2004;23:3716–20. doi: 10.1038/sj.onc.1207418. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara S, Murakumo Y, Sato T, et al. Up-regulation of CD109 expression is associated with carcinogenesis of the squamous epithelium of the oral cavity. Cancer Sci. 2008;99:1916–23. doi: 10.1111/j.1349-7006.2008.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagiwara S, Murakumo Y, Mii S, et al. Processing of CD109 by furin and its role in the regulation of TGF-beta signaling. Oncogene. 2010;29:2181–91. doi: 10.1038/onc.2009.506. [DOI] [PubMed] [Google Scholar]
- 23.Cuppini L, Calleri A, Bruzzone MG, et al. Prognostic value of CD109+ circulating endothelial cells in recurrent glioblastomas treated with bevacizumab and irinotecan. PLoS One. 2013;8:e74345. doi: 10.1371/journal.pone.0074345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa M, Moritani S, Murakumo Y, et al. CD109 expression in basal-like breast carcinoma. Pathol Int. 2008;58:288–94. doi: 10.1111/j.1440-1827.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- 25.Tao J, Li H, Li Q, Yang Y. CD109 is a potential target for triple-negative breast cancer. Tumour Biol. 2014;35:12083–90. doi: 10.1007/s13277-014-2509-5. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso P, Calleri A, Gregato G, et al. A subpopulation of circulating endothelial cells express CD109 and is enriched in the blood of cancer patients. PLoS One. 2014;9:e114713. doi: 10.1371/journal.pone.0114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai YL, Ha DP, Zhao H, et al. Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-beta signaling. Proc Natl Acad Sci U S A. 2018;115:E4245–54. doi: 10.1073/pnas.1714866115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunagawa M, Mii S, Enomoto A, et al. Suppression of skin tumorigenesis in CD109-deficient mice. Oncotarget. 2016;7:82836–50. doi: 10.18632/oncotarget.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhola NE, Balko JM, Dugger TC, et al. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshimori N, Oristian D, Fuchs E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963–76. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vita A, Recine F, Mercatali L, et al. Myxofibrosarcoma primary cultures: molecular and pharmacological profile. Ther Adv Med Oncol. 2017;9:755–67. doi: 10.1177/1758834017737472. [DOI] [PMC free article] [PubMed] [Google Scholar]


