Abstract
Today, children are surviving pediatric cancer at unprecedented rates, making it one of modern medicine’s true success stories. However, we are increasingly becoming aware of several deleterious effects of cancer and the subsequent “cure” that extend beyond physical sequelae. Indeed, survivors of childhood cancer commonly report cognitive, emotional, and psychological difficulties, including attentional difficulties, anxiety, and posttraumatic stress symptoms (PTSS). Cognitive late- and long-term effects have been largely attributed to neurotoxic effects of cancer treatments (e.g., chemotherapy, cranial irradiation, surgery) on brain development. The role of childhood adversity in pediatric cancer – namely, the presence of a life-threatening disease and endurance of invasive medical procedures – has been largely ignored in the existing neuroscientific literature, despite compelling research by our group and others showing that exposure to more commonly studied adverse childhood experiences (i.e., domestic and community violence, physical, sexual, and emotional abuse) strongly imprints on neural development. While these adverse childhood experiences are different in many ways from the experience of childhood cancer (e.g., context, nature, source), they do share a common element of exposure to threat (i.e., threat to life or physical integrity). Therefore, we argue that the double hit of early threat and cancer treatments likely alters neural development, and ultimately, cognitive, behavioral, and emotional outcomes. In this paper, we (1) review the existing neuroimaging research on child, adolescent, and adult survivors of childhood cancer, (2) summarize gaps in our current understanding, (3) propose a novel neurobiological framework that characterizes childhood cancer as a type of childhood adversity, particularly a form of early threat, focusing on development of the hippocampus and the salience and emotion network (SEN), and (4) outline future directions for research.
Keywords: Childhood cancer, pediatric oncology, leukemia, brain tumor, brain
Introduction
Although cancer remains the leading disease-related cause of death among US children, recent advances in treatment for pediatric cancer have improved the outlook for many children with cancer. Today, nearly 90% of children diagnosed with cancer are surviving at least five years after diagnosis, and more than 70% will survive ten years - making it one of modern medicine’s true success stories (Howlader et al. 2016). However, survivors of childhood cancer frequently experience “late” and long-term effects associated with the disease and its intensive treatment, including chronic medical conditions and impairment in level of cognitive, behavioral, and emotional functioning (Bitsko et al. 2016; Anderson & Kunin-Batson 2009; Marcoux et al. 2016; Ehrhardt n.d.; Pogany et al. 2006; see review by Stein et al. 2008). Recent cognitive and developmental neuroscience research indicates that these late effects are due, in part, to injurious effects of therapeutic intervention (e.g., chemotherapy, cranial irradiation, surgery) during cancer treatment on the developing brain (Cheung & Krull 2015; Butler & Haser 2006; Ashford et al. 2010; Cheung et al. 2016). Here, we contend that the effects of pediatric cancer as an adverse childhood experience are also important to consider when evaluating psychological and neurodevelopmental outcomes. Childhood adversity is defined as an experience that is likely to require significant psychological, social, or neurobiological adaptation by the average child and that represents a deviation from the expectable (safe) rearing environment (McLaughlin 2016). The role of childhood adversity in pediatric cancer – namely, the presence of a life-threatening disease and endurance of invasive medical procedures – has been largely ignored in the neuroscientific literature. This is true despite compelling research by our group and others showing that other adverse childhood experiences, which involve shared elements of life threat and threat to physical integrity (e.g., violence, abuse) – but are also disparate in many important ways (e.g., context, source of threat, chronicity, onset, long-term debilitation) - strongly imprint on neural development. Moreover, these neuro developmental changes alter core cognitive and affective processes that are thought to increase risk for psychological issues and disorders (see reviews by Thomason & Marusak 2016; Teicher & Samson 2016). Lack of consideration of the additional and unique role of early threat constitutes a critical barrier to identifying pathways through which pediatric cancer impacts neural development, and ultimately, psychological outcomes.
Pediatric cancer patients and their families face enormous adversity that begins at the time of diagnosis. Cancer diagnosis is surprising and life threatening, forcing children and families to re-organize their lives and relationships. At the same time, families are confronted with the tremendous burden of understanding the disease and facing the possibility of the child’s death at a young age. In addition, the procedures and treatments associated with pediatric cancer care are invasive, unfamiliar, and arduous (e.g., lumbar punctures, bone marrow aspirations, and port starts), and often cause significant physical side effects. Children frequently do not grasp why these recurrent body intrusions, pain, and hospitalizations are necessary and both parents and children often describe the stress, nausea, and fatigue associated with treatment to be more stressful than the disease itself (Hedström et al. 2003). At the same time, there may be deaths of other children known to the patient and family. Together, these take an immense emotional toll on children and families, as coping and financial resources are strained. For children who survive cancer, stress and adversity do not end after the child crosses the “finish line” and treatment concludes. The transition into survivorship brings its own set of challenges as families begin to readjust to home and family life, re-acclimate to school and social settings, and deal with physical limitations and chronic pain (Hobbie et al. 2010). Concerns about safety persist throughout the lifespan, due to emergence of various late and long-term effects and the risk of relapse or second malignancies. Childhood cancer survivors therefore require ongoing medical surveillance, which can induce hypervigilance and chronic worry about physical symptoms. Overall, the burden of receiving a life-threatening diagnosis, concerns about safety, disruptions of the family system and members, and painful treatments and medical procedures associated with cancer care constitute a significant deviation from the expectable safe rearing environment, and can be conceptualized as a form of adverse childhood experience (see Alderfer & Kazak 2006; Trentacosta et al. 2016; F. W. Harper et al. 2014b, 2015).
Pediatric cancer patients and survivors frequently experience cognitive and affective dysfunction, months, years, and even decades after cancer. Despite replacement of cranial irradiation (with chemotherapy) in many contemporary treatment protocols, estimated rates of cognitive dysfunction in survivors of childhood cancer remain as high as 67% for attentional deficits, and 3–28% for deficits in other cognitive domains (Conklin et al. 2012a, b), including executive functioning, intelligence quotient (IQ), memory, processing speed, and visual-motor integration (for a review, see Castellino et al. 2014). Emotion-related psychological problems, including anxiety, depression, and posttraumatic stress symptoms (PTSS), are frequently experienced in a subset of children (Oancea et al. 2014; Zeltzer et al. 2009; Kunin-Batson et al. 2016; Kazak et al. 2004; Price et al. 2016; Landolt et al. 2012). Of note, a recent systematic review suggests that anxiety is a relevant but understudied psychosocial outcome among pediatric cancer survivors (McDonnell et al. 2017). Estimated life-time rates of posttraumatic stress disorder (PTSD) among survivors range from 20 to 35% (see review by Bruce 2006) - nearly double what is observed in the general population and may even exceed rates reported in some military Veteran populations (National Center for PTSD 2016). These psychological problems are a major source of compromised quality of life among childhood cancer patients and survivors, and disrupt daily life, impair social functioning and academic performance, and may even increase disease morbidity, mortality, and healthcare costs by reducing children’s adherence to medical procedures.
Compelling research over the past several decades has shown that exposure to other forms of childhood adversity - including violence (e.g., domestic, community), abuse (e.g., physical or sexual abuse), and neglect - dramatically increases risk for cognitive, behavioral, and emotional problems, and for virtually all commonly occurring psychiatric disorders (e.g., anxiety, depression, PTSD; Kessler et al. 2010; Felitti et al. 1998). Although the circumstances and nature of these experiences differs dramatically from the childhood cancer experience, an early adversity framework provides a useful starting point for understanding how the developing brain adapts to adversity and early threat exposure during childhood. Indeed, similar to observations in cancer survivors, psychological issues or disorders may emerge insidiously years or even decades after exposure to other forms of childhood adversity, which is thought to reflect a latent biological vulnerability (Caspi et al. 2014; Keyes et al. 2012). Neuroscientific research suggests that this latent vulnerability is mediated by adversity-related changes in brain structure and function, which are evident even in those who do not present with psychological problems, and are remarkably similar to neural changes in clinical groups with adversity-related disorders (e.g., depression, anxiety, PTSD; see reviews by Thomason & Marusak 2016; Teicher & Samson 2016). Adversity-related changes in the brain are thought to underlie alterations in core cognitive and affective processes (e.g., elevated threat processing, decreased executive control) that increase risk for cognitive, behavioral, and emotional problems in some youth (McCrory et al. 2017).
Given the strong and pervasive link between threat-related childhood adversity and a range of negative psychological outcomes, as well as the impact of early threat on neural development (see reviews by Thomason & Marusak 2016; Teicher & Samson 2016), we argue that research into the neurodevelopmental consequences of pediatric cancer should consider the joint impact of early threat and cancer treatments (Fig. 1). It is likely that the double hit of early threat and therapeutic intervention imprints strongly on brain development, and may thus contribute to a range of cognitive, behavioral, emotional, and also physical consequences. We assert that childhood cancer is another, largely understudied form of childhood adversity and brain injury. As we will discuss below, the majority (60%) of the existing studies on brain structure or function in childhood cancer survivors have been in patients and survivors of central nervous system (CNS) tumors (e.g., medulloblastoma, ependymoma), the second most common form of childhood cancer. This focus is likely due to their central location within the CNS and the direct effects of treatment (e.g., cranial irradiation) on brain functioning. We propose taking a broader view beyond the effects of cancer treatment itself on the brain in CNS cancer and additionally consider the cancer experience, regardless of type of cancer, as an adverse event. For example, acute lymphoblastic leukemia (ALL), the most common type of childhood cancer, is a non-CNS cancer that is now conventionally treated with an intensive chemotherapy-only approach, which may have a less direct effect on the brain than cranial irradiation. Yet, ALL patients and families undoubtedly experience enormous adversity, and several cognitive, behavioral, and emotional late effects are reported (Trentacosta et al. 2016; Harper et al. 2014b; Peterson et al. 2014; Harper et al. 2015; Cheung & Krull 2015). Characterizing the relationships among these challenges and underlying neurobiological processes should provide new insights into mechanisms of risk and novel avenues for intervention.
Fig. 1.
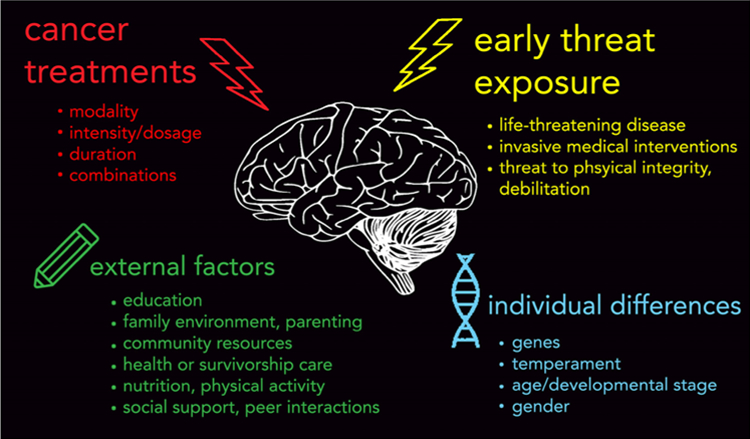
Top: Early threat exposure and cancer treatments are both developmental insults that can alter neural development and contribute to the range of cognitive, behavioral, and emotional late and long-term effects reported in childhood cancer patients and survivors. Bottom: various potential external (e.g., environment) and individual difference factors could modify these effects. While it is largely recognized that late and long-term effects are due, in part, to injurious effects of therapeutic intervention during cancer treatment (e.g., chemotherapy, cranial irradiation) on the developing brain, we assert that the role of early threat exposure in pediatric cancer – namely, the diagnosis of a life-threatening disease and endurance of invasive medical procedures – should also be considered. Early threat exposure, a form of childhood adversity, defined as an experience that is likely to require significant psychological or neurobiological adaptation by the average child and that represent a deviation from the expectable environment (McLaughlin 2016), has been shown to strongly imprint on brain development, and is one of the strongest risk factors for virtually all commonly occurring psychological disorders. Research is needed to understand neurodevelopmental consequences of the ‘double hit’ of early threat and therapeutic intervention associated with childhood cancer, and factors that may modify outcomes. Identification of such factors will be essential for guiding early intervention to mitigate these risks
The notion that childhood cancer is an adverse and potentially traumatic experience is not new (e.g., Stuber et al. 1998; Bruce 2006; Kazak et al. 2005). Although many children report at least some symptoms of anxiety, depression, or PTSS (Bitsko et al. 2016), pediatric cancer survivors generally function well despite the enormous challenge and threat that they face (Eiser et al. 2000). However, research is needed to identify pathways through which childhood cancer impacts neural development, and ultimately, psychological outcomes. Lack of consideration of the additional role of early threat exposure constitutes a critical barrier to identifying potential drivers of neurodevelopmental change. As we will demonstrate later in our review of the literature, following these early experiences, individuals experience the world with a fundamentally altered nervous system.
Organizations such as the National Institutes of Health (NIH), American Cancer Society (ACS), and Centers for Disease Control and Prevention (CDC) have emphasized the importance of research on cancer outcomes. In their seminal report in 2006, the Institute of Medicine and National Research Council identified survivorship issues as a key research priority (Institute of Medicine and National Research Council, 2006). We add to this by suggesting that the “cure” for childhood cancer should not only consider psychological wellbeing, but also neurodevelopmental consequences. Second, given that neurodevelopmental alterations as well as psychological issues and disorders frequently begin in childhood and adolescence (Kessler et al. 2005), this further emphasizes a focus on prevention. Specifically, interventions may be initiated during or after treatment to correct aberrant neurodevelopmental processes, before frank psychological problems emerge and become chronic.
Early intervention will be even more critical as this expanding population ages. Indeed, the number of pediatric cancer survivors will continue to grow, due to advances in treatments, increasing incidence, and the fact that survivors are living longer than ever before (Howlader et al. 2016). It is estimated that there will be nearly 500,000 survivors of pediatric cancer in the US by the year 2020 (Robison & Hudson 2014). Medical and research communities are challenged to meet this growing population with empirically-based services and interventions to address survivors’ psychological needs. Research into neurodevelopmental and psychological consequences of pediatric cancer should aid the development and more judicious application of targeted early interventions to improve life during and after children’s treatments for cancer.
In this paper, we (1) review the existing, yet limited, human neuroimaging research in child, adolescent, and adult survivors of childhood cancer, (2) summarize gaps in our current understanding, (3) advance a neurodevelopmental model of childhood adversity, and in particular, early threat exposures, into the area of pediatric cancer, and (4) present future directions for research. Of note, we do not discount the cognitive effects of pediatric cancer, and refer the reader to comprehensive reviews in this area (Cheung & Krull 2015; Wolfe et al. 2012; Robinson et al. 2013; Castellino et al. 2014). In addition, neurocognitive dysfunction is also observed in adults treated for cancer (Correa & Ahles 2008; Jean-Pierre & McDonald 2016; O’Farrell et al. 2013), which is not necessarily through a developmental mechanism. However, some have noted that neurocognitive dysfunction complicating pediatric cancer appears to be more frequent and severe than “chemo brain” in adults (for a review, see Castellino et al. 2014), which may be due to the sensitivity of brain systems to insults during development. Here, it is our hope that widening the lens to consider the joint impact of early threat and therapeutic intervention on cognitive, behavioral, and emotional outcomes will offer an integrative neurobiological framework for how early cancer affects the developing brain, and provide more comprehensive understanding of late and long-term effects in survivors of childhood cancer. We propose such a framework here.
Neuroimaging studies in child and adolescent cancer patients and survivors
We performed a literature review using PubMed and Google Scholar to identify neuroimaging studies that include child or adolescent patients or survivors of childhood cancer, using various combinations of the following search terms: “brain”, “MRI”, “fMRI”, “childhood cancer”, “adult survivors”, “neuroimaging”, “gray matter”, “white matter”, “cortical thickness”, “leukoencephalopathy”, “leukodystrophy”, “pediatric cancer”, “leukemia”, “chemotherapy”, “radiation”, “posterior fossa”, “medullobastoma” “neuroblastoma”, “brain tumor”. Reviewed studies are limited to structural or functional magnetic resonance imaging (fMRI) methods, but not restricted in the type of child or adolescent cancer (i.e., diagnosis prior to age 18). Neuroimaging studies related to pre-surgical planning or differential diagnosis (e.g., low- vs high-grade brain tumors) were not reviewed.
The literature search identified 65 studies that examined brain structure or function in child or adolescent cancer patients/survivors (see Table 1). The majority (83%) of studies examined structural neurobiological changes during or following children’s treatments for cancer, including variation in regional gray matter volume or cortical thickness, as measured by structural MRI, or white matter macrostructure (e.g., fiber density, axonal diameter, and myelination), as measured by diffusion tensor imaging (DTI) MRI. Two studies used perfusion MRI (such as arterial spin labelling, ASL), which measures relative changes in cerebral blood volume and blood flow without the need for contrast administration (Hartkamp et al. 2013). Ten pediatric neuroimaging studies used blood-oxygen level-dependent (BOLD) fMRI to measure brain function via the hemodynamic response, with nine measuring fMRI response during a neuropsychological task (e.g., working memory), and one measuring resting-state functional connectivity (rsFC). RsFC measures spontaneous fluctuations in the BOLD signal, and correlations in activity are thought to reflect the baseline functional intrinsic architecture of the brain (Fox & Raichle 2007). Connectivity patterns observed during the resting-state have been shown to predict individual differences in fMRI response across a wide range of neuropsychological tasks (Tavor et al. 2016). The number of patients/survivors ranged widely in studies, with 2–197 for structural imaging studies, and 8–218 for functional neuroimaging studies. Forty-five percent of studies included patients with leukemia, and 63% with brain tumors, particularly in the posterior fossa, the most common site of pediatric CNS tumors. The majority (69%) of neuroimaging studies linked neurobiological changes to cognitive outcome measures (e.g., IQ, working memory, processing speed), and only five studies linked neurobiological changes to emotional outcomes (e.g., internalizing symptoms, emotional adjustment) or quality of life (see Table 1).
Table 1.
Review of neuroimaging studies in child and adolescent cancer survivors
| First author | Year of publication | Journal | Sample size (N) | Patients/Survivors (n) | Typically-developing controls (n) | Type of cancer | Survivor age at time of study in years (mean, SD, [range]) | Age at diagnosis/treatment in years (mean, SD, [range]) | Time since diagnosis/treatment conclusion in years (mean, SD, [range]) |
|---|---|---|---|---|---|---|---|---|---|
| 1 Li et al. | 2017 | The Journal of Pediatrics | 103 | 39 | 64 | Brain tumor (n = 21 MB, n = 18 PA). | MB: 14.7 [6.9–20.4], PA: 12.2 [5.0–18.3] | MB: 6.6 [1.2–15.7], PA: 7.7 [1.4–16.2] | MB: 7.0 [0.3–15.0], PA: 3.4 [1.0–8.6] |
| 2 Baron Nelson et al. | 2016 | Journal of Pediatric Oncology Nursing | 17 | 8 | 9 | Brain tumor | 8.5 (1.3) [5–13] | 2.65 (1.38) [1.17–4.58] | 5.4 (2.9) [2.5–11.4] |
| 3 Cheung et al. | 2016 | The Lancet Haematology | 190 | 190 | __ | ALL | 10–18 | 4.9 [2–10] | 7.35 [6–9] |
| 4 Kesler et al. | 2016 | Brain Connectivity | 70 | 31 | 39 | ALL | 11 (3.4) | 5.4 (3.7) [2–14] | 2.92 (2.58) [0.5–9.25] |
| 5 Krull et al. | 2016 | Journal of Clinical Oncology | 218 | 218 | __ | ALL | 13.8 (4.8) | 6.6 (4.5) | 7.7 (1.7) |
| 6 McEvoy et al. | 2016 | Neuroimage Clinical | 47 | 47 | __ | Cerebellar tumor | 9.7 (4.8) | >2 | [0–1] |
| 7 Oh et al. | 2017 | Journal of Neuro-Oncology | 39 | 19 | 20 | Brain tumor: PA (n = 10), and MB (n = 9) | 12.9 (5.1), [6.5–25.4] | 9.3 (4.5) | 3.6 (2.1) |
| 8 Scantlebury et al. | 2016 | Neuropsychology | 96 | 59 (n = 29 surgery with or without focal radiation, n = 30 cranial-spinal radiation) | 37 | Brain tumor (posterior fossa; n = 17 astrocytoma, n = 8 ependymoma, n = 31 MB n = 1 , ganglioglioma, n = 1 germinoma, n = 1 choroid plexus papilloma) | Surgery group: 11.2 (3.6), CSR group: 11.6 (3.5) | Surgery group: 7.1 (3.9) [0.2–15.6], CSR group: 8.1 (2.8) [4.3–15.2] | Surgery group: 4.1 (3.0) [0.3–10.5], CSR group: 3.4 (3.4) [0.0–11.4] |
| 9 Zou et al. | 2016 | Brain Imaging and Behavior | 61 | 40 | 21 | MB | n = 19 reading-- intervention: 11.7 (0.6) and n = 21 standard-of-care: 12.1 (0.6) | reading-intervention: 10 (0.6), standard-of-care: 9.5 (0.6) | Time since tumor treatment, 2.5 [1.2–5.4] and since reading intervention, 2.9 [1.6–5.9] |
| 10 Conklin et al. | 2015 | Journal of Clinical Oncology | 68 | 68 (n = 34 intervention group, n = 34 wait-list controls) | __ | ALL (n = 47), brain tumor (n = 21; 4 ependymoma, n = 2 glioma, n = 15 MB/PNET) | Intervention: 12.21 (2.47), Control: 11.82 (2.42) | Intervention: 5.15 (2.92), Control: 4.62 (2.68) | Intervention: 4.97 (3.02), Control: 5.04 (2.41) |
| 11 Khajuria et al. | 2015 | Child’s Nervous System | 34 | 34 | __ | Cerebellar tumor (n = 17 pilocytic astrocytoma and n = 17 MB) | MB patients: 13.2[7.8–20.6], PA patients: 13.1 [9.2–17.5] | MB patients: 7.6 [2.2–16.6], PA patients: 6.7 [0.9–12.2] | MB patients: 5.6 (3.2), PA patients: 6.3 (2.6) |
| 12 Liu et al. | 2015 | Neuro-oncology | 64 | 32 | 32 | LGG (subtentorial n = 19, supratentorial n = 13) | 13.99 (3.03) [8.42–19.12] | subtentorial: 7.22 (3.12) [1.60–12.33], supratentorial: 3.86 (2.26) [1.05–8.41] | subtentorial: 5.58 (3.95) [0.59–13.26], supratentorial: 11.89 (2.52) [8.82–16.76] |
| 13 Robinson et al. | 2015 | Child Neurosychology | 32 | 17 | 15 | Brain tumor (n = 9 pilocytic astrocytoma, n = 4 posterior fossa medulloblastoma, n = 3 dysembryoplastic neuroepithelial tumor, and n = 1 craniopharyngioma) | 12.60 (2.48) [8–16] | 6.94 (2.41) [2.06–11.62] | 5.29 (2.83) [2.13–10.92] |
| 14 Rueckriegel et al. | 2015 | Pediatric Blood and Cancer | 32 | 32 | __ | MB (n = 18), PA (n = 14) | MB: 15.2 (4.9), PA: 12.6 (5.0) | MB: 11.2 (3.7), PA: 9.9 (4.4) | MB: 3.8 (2.5), PA: 2.6 (2.1) |
| 15 Bhojwani et al. | 2014 | Journal of Clinical Oncology | 369 | 369 | __ | ALL | [1–18] | Not stated | Not stated |
| 16 Duffner et al. | 2014 | Journal of Pediatric Hematology-Oncology | 66 | 66 | __ | ALL | Not stated | P9201: 4.1 [1.1–7.5], P9605: 4.9 [2.3–9.8] | P9201: 5.3 [2.6–7.1], P9605: 5.3 [2.7–7.7] |
| 17 ElAlfy et al. | 2014 | Pediatric Hematology and Oncology | 122 | 62 | 60 | ALL | 6–18 | CCG protocol: 5.27 (2.38). Modified BFM 90 protocol: 5.6 (3.19). Modified BFM 83 protocol: 6.33 (3.8) | CCG protocol: 2.72 (0.61). Modified BFM 90 protocol: 4.19 (1.44). Modified BFM 83protocol: 7.96 (1.98) |
| 18 Horska et al. | 2014 | Child’s Nervous System | 18 | 9 | 9 | Brain tumor (n = 8), T-cell ALL (n = 1) | 11.8 (3.7) [5.5–18.6] | Not stated | 0–27 months post-CRT |
| 19 Jacola et al. | 2014 | Journal of Neuro-Oncology | 50 | 50 | __ | Brain tumor (n = 22 ependymoma, n = 16 craniopharyngioma, n = 12 LGG) | 13.13 (2.88) [8–18] | 6.34 (3.43) | 5.77 (2.27) |
| 20 Kesler et al. | 2014 | Pediatric Blood and Cancer | 29 | 15 | 14 | ALL | 11.5 (2.0) [8.9–15.9] | 4.4 (1.8) [1.5–8] | 3.65 (2.45) [0.75–9.17] |
| 21 Riggs et al. | 2014 | Journal of the International Neuropsychological Society | 33 | 20 | 13 | Posterior fossa brain tumor (n = 9 MB, n = 1 astrocytoma) | 12.4 [7.2–17.2] | 7.2 [4.3–12.8] | 5.1 [1.1–11.6] |
| 22 Robinson et al. | 2014 | Neuropsychology | 32 | 17 | 15 | Brain tumor: pilocytic astrocytoma (n = 9), posterior fossa MB (n = 4), dysembryoplastic neuroepithelial tumor (n = 3), and craniopharyngioma (n = 1) | 12.60 (2.48) [8–16] | 6.94 (2.41) [2.06–11.62] | 5.29 (2.83) [2.13–10.92] |
| 23 Badr et al. | 2013 | Oncology letters | 25 | 25 | __ | ALL | 12.9 (3.2) [8.5–20] | 6.9 (3.04) [2.5–13] | >5 |
| 24 Genschaft et al. | 2013 | PloS One | 54 | 27 | 27 | ALL | 17.9 (2.4) [14.9–22.8] | 5.6 (2.5) [1.1–10.2] | 12.4 (3.0) [6.1–18.5] |
| 25 Kuper et al. | 2013 | The Cerebellum | 23 | 12 | 11 | Cerebellar tumor | 11.1 [6–17] | Not stated | [0–1] |
| 26 Wolfe et al. | 2013 | Pediatric Blood and Cancer | 9 | 9 | __ | Posterior fossa brain tumor | 14.89 (1.9) [11.50–17.25] | 5.00 (2.7) [1.33–8.00] | 9.88 (3.4) [6.41–15.35] |
| 27 Hosseini et al. | 2012 | PloS One | 59 | 28 | 31 | ALL | 12.0 (4.6) [5.0–19.8] | Not stated Not s+ O13tated | 4.19 (2.55) [0.5–10.5] |
| 28 Zou et al. | 2012 | Archives of Clinical Neuropsychology | 42 | 14 | 28 | n = 7 ALL, n = 7 brain tumor (astrocytoma, ependymoma, MB, suprasella germinoma, suprasella craniopharyngioma) | 12.02 (0.09) [6–17] | 5.91 | > 1 |
| 29 Ashford et al. | 2010 | Cancer | 97 | 97 | __ | ALL | 10.84 (3.93) [6.02–21] | 8.22 (3.93) [3.46–18.45] | 2 (years after end of treatment, time of neurocognitive assessment) |
| 30 Ficek et al. | 2010 | Brain Edema XIV | 45 | 45 | __ | ALL | [4–17] | Not stated | [6–12] |
| 31 Kesler et al. | 2010 | Brain Imaging and Behavior | 59 | 28 | 31 | ALL | 12.0 (4.6) [5.0–19.8] | Not stated | 4.19 (2.55) [0.5–10.5] |
| 32 Robinson et al. | 2010 | Pediatric Blood and Cancer | 15 | 8 | 7 | ALL | 14.07 (2.32) | 4.94 | 6.46 |
| 33 Aukema et al. | 2009 | International Journal of Radiation Oncology Biology Physics | 34 | 17 | 17 | ALL (n = 11) and MB (n = 6) | 14 (2.5) [8.9–16.9) | 5.2 (3.1) [2.0–13.2] | 8.4 (3.5) [2.7–13.6] |
| 34 De Smet et al. | 2009 | Neuropsychology | 8 | 8 | __ | Posterior fossa brain tumor | [3–15] | Not stated | Began at average of 2 months post-surgery, longitudinal follow-up to average of 2 years later |
| 35 Reddick et al. | 2009 | American Journal of Neuroradiology | 197 | 197 | __ | ALL | [1.0–18.9] | 5.3 | N/A (active treatment). Average time between two MRI scans, 0.325 (.067) |
| 36 Carey et al. | 2008 | American Journal of Neuroradiology | 23 | 9 | 14 | ALL | 15.17 (5.48) [7.75–25.76] | 5.17 (2.96) [1.43–9.36] | 9.95 (5.13) [3.48–16.96] |
| 37 Kirschen et al. | 2008 | Behavioural Neurology | 24 | 12 | 12 | Cerebellar PA | 12.5 (4.1) [6–19] | Not stated | 5.5 (3.1) |
| 38 Zhang et al. | 2008 | Neuroimage | 27 | 13 | 14 | Posterior fossa brain tumor | 12.3 (3.1) [7–17] | Not stated | Not stated |
| 39 Qiu et al. | 2007 | International Journal of Radiation Oncology Biology Physics | 44 | 22 | 22 | MB | 12.1 (4.6) | 8.1 (4.6) | 3.9 (2.9) |
| 40 Khong et al. | 2006 | Journal of Clinical Oncology | 85 | 30 | 55 | ALL (n = 18), MB (n = 12) | 13.1 [6.0–22.1] | ALL w/out RT: 6.68 (6.32); ALL w/ RT: 6.47 (4.35); MB: 8.52 (3.57) | ALL w/out RT: 6.38 (4.29); ALL w/ RT: 8.39 (4.74); MB: 3.25 (2.26) |
| 41 Mabbott et al. | 2006 | Neuro-oncology | 16 | 8 | 8 | MB | 9.98 (2.90) | 7.48 (3.87) | 2.5 (0.72) |
| 42 Qiu et al. | 2006 | Neuroimage | 4 | 2 (Patient A female, Patient B male) | 2 (males, aged 23 years and 33 years) | MB | Patient A: 10.7, Patient B: 9.4 | Patient A: 10.7, Patient B: 9.5 | [0–1] |
| 43 Reddick et al. | 2006 | Cancer | 145 | 112 | 33 siblings | ALL | 9.8 (3.1) | 4.1 (2.6) | 6.0 (3.5) |
| 44 Shan et al. | 2006 | Magnetic Resonance Imaging | 58 | 58 (Group A decreased NAWM volume n = 39, Group B increased NAWM volume n = | __ | MB | Not stated | Group A: 8.26 (0.7) [3.2–20.18], Group B: 8.50 (0.8) [3.13–17.97] | [0–2] |
| 45 Konczak et al. | 2005 | Brain | 36 | 22 | 14 | Cerebellar tumor | [10–28] | [1–17] | 8.2 [>3] |
| 46 Reddickck et al. | 2005a, b | Neuro-oncology | 52 | 52 | 26 | MB | Not stated | 8.3 [3.4–20.0] | 2.5 [0.2–7.9] |
| 47 Reddick et al. | 2005a, b | American Journal of Neuroradiology | 45 | 45 | __ | ALL | [1.5–18.6] | Low risk: 5.0 (2.7), Standard/high risk: 9.2 (4.8) | Began at week 6 of remission induction and ended at week 120 of continuation treatment |
| 48 Reddick et al. | 2005a, b | American Journal of Neuroradiology | 45 | 45 | __ | ALL | [1.5–18.6] | Low risk: 5.0 (2.7), Standard/high risk: 9.2 (4.8) | Began at week 6 of remission induction and ended at week 120 of continuation treatment |
| 49 Zou et al. | 2005 | Neuroimage | 43 | 16 | 16 adults (ages 20–35), 11 siblings (ages 8–16) | n = 8 ALL, n = 8 brain tumor (glioma, astrocytoma, ependymoma, MB | 13.2 (2.4) [9–17] | 6.27 | 5.4 (2.3) [2–9] |
| 50 Hill et al. | 2004 | Pediatric Blood and Cancer | 20 | 10 | 10 | ALL | [6–14] | [3–5] | >3 |
| 51 Leung et al. | 2004 | Neuroimage | 32 | 16 | 16 | MB | 11.1 (4.2) [3.2–18.6] | 8.8 (4.6) [2.7–17] | 3.1 (1.8) [0.8–6.3] |
| 52 Mulhern et al. | 2004 | Journal of the International Neuropsychological Society | 37 | 37 | __ | Brain tumor (n = 17 MB, n = 7 astrocytoma, n = 5 ependymoma, n = 4 PNET, n = 2 germinoma, n = 1 oligodendroglioma, n = 1 craniopharyngioma) | Not stated | 6.5 [1.7–14.8] | 5.7 [2.6–15.3] |
| 53 Nagel et al. | 2004 | American Journal of Neuroradiology | 25 | 25 | __ | MB | 4–12 | 8.27 | 0.31 |
| 54 Chu et Al. | 2003 | Radiology | 23 | 23 | __ | ALL | Not stated | 1–14 | N/A (active treatment) |
| 55 Khong et al. | 2003 | American Journal of Neuroradiology | 18 | 9 | 9 | MB | 10.8 [3–19] | 7.8 [3–14] | 3.6 [1–6] |
| 56 Pääkkö et al. | 2003 | Pediatric Blood and Cancer | 19 | 19 | __ | ALL | 11.3 [4.6–20.1] | 6.0 [2.1–14.8] | Immediately after end of treatment (n = 9) or 4–8 years after end of treatment (n = 10) |
| 57 Reddick et al. | 2003 | Cancer | 40 | 40 | __ | Brain tumor (n = 18 MB n = 8 astrocytoma, n = 6 ependymoma, n = 4 primitive neuroectodermal tumors, n = 2 germinoma, n = 1 oligodendroglioma, n = | 12.8 [7.1–18.8] | 6.5 [1.7–14.8] | 5.7 [2.6–15.3] |
| 58 Palmer et al. | 2002 | American Journal of Neuroradiology | 35 | 35 | __ | MB | At most recent MRI: 9.61 (3.77) | 7.68 (3.25) [3.2–17.2] | At most recent MRI: 1.92 (0.97) |
| 59 Mulhern et al. | 2001 | Journal of Clinical Oncology | 42 | 42 | __ | MB | 12.6 (4.2) | 8.2 (3.8) | 4.0 (2.7) |
| 60 Levisohn et al. | 2000 | Brain | 19 | 19 | __ | Cerebellar tumors (n = 1 MB, n = 7 astrocytoma, n = 1 ependymoma) | [3.67–16.5] | 8.17 [3.25–14.83] | 0.43 (0.53) [0.08–1.83] |
| 61 Paakko et al. | 2000 | Pediatric Blood and Cancer | 33 | 33 | __ | ALL | Not stated | 6.2 [2.1–15.0] | N/A (active treatment) |
| 62 Reddick et al. | 2000 | Magnetic Resonance Imaging | 26 | 26 | __ | MB | Not explicitly stated - each patient had at least 4 MR examinations over at least of 7 months following CSI | 7.3 [3.2–17.2] | 1.56 [0.75–2.53] |
| 63 Mulhern et al. | 1999 | Annals of Neurology | 36 | 36 | __ | Brain tumor (n = 18 MB, n = 18 low-grade PF tumors) | Not stated | < 21 | MB: 3.8 (2.6), PF: 2.6(2.1) |
| 64 Harila-Saari et al. | 1998 | Cancer | 32 | 32 | __ | ALL | 13.2 [8–24] | 5.3 (3.5) | 5.0 (0.4) |
| 65 Reddick et al. | 1998 | Magnetic Resonance Imaging | 94 | 77 | 17 | Brain tumor | 5–21 | 6.9 [3.3–15.6] | 5.1 [1.2–10.6] |
| First author | Type of treatment | Neuroimaging modalities | Type of study | Physical and physiological outcome measures | Cognitive and behavioral outcome measures | Emotional and quality of life measures | Main findings | ||
| 1 Li et al. | MB: Surgical resection, CSI, and CT, PA: Surgical resection alone | Perfusion MRI (ASL); DWI | Cross-sectional | __ | IQ (n = 12 survivors) | __ | Young MB survivors had significantly reduced global CBF compared to controls, but PA survivors had normal CBF. Diffusion abnormalities (lower ADC) were apparent in the hippocampus and amygdala of MB survivors and in the amygdala of PA survivors. In n = 12 patients with IQ assessments, increased regional ADC was correlated with higher IQ- where-as CBF was not related to IQ. | ||
| 2 Baron Nelson et al. | CT | DTI | Cross-sectional | __ | Executive function, memory | Quality of life | Compared to control subjects, brain tumor patients exhibited significantly lower psychosocial and school functioning and overall quality of life. Indices of gray and white matter injury (elevated mean diffusivity and decreased FA) were apparent in memory and executive functioning areas in patients. Particularly, low inhibition scores correlated with heightened mean diffusivity in prefrontal areas in patients. | ||
| 3 Cheung et al. | CT | DTI | Longitudinal | Fine motor dexterity | IQ, executive function, processing speed, attention, memory | __ | Childhood ALL survivors had more problems with working memory, organization, initiation, and planning in addition to reduced memory span, processing speed, and executive function compared to population norms. Compared to ALL survivors with no history of leukoencephalopathy, ALL survivors with a history of leukoencephalopathy had more problems with organization and initiation, and showed decreased white matter integrity in the frontostriatal tract at long-term follow-up (at least 5 years post--diagnosis). | ||
| 4 Kesler et al. | Intrathecal CT standard dose (n = 23) and high dose (n = 9) | DTI | Cross-sectional | __ | Coding/processing speed, vocabulary, working memory, perceptual reasoning, visual and verbal learning | __ | Altered white matter connectome properties (lower small-worldness and network clustering coefficient) and greater cognitive impairment was found in the ALL group compared to controls. Atypical clustered connectivity was apparent in parietal, frontal, hippocampal, amygdalar, thalamic, and occipital regions in the ALL group. Decreased connectivity within neighboring brain regions in young survivors of ALL may be related to reductions in local information processing efficiency. | ||
| 5 Krull et al. | CT | Task-based fMRI (executive function “attention network task”), structural MRI, DTI | Longitudinal | Fine motor dexterity | IQ, executive function (cognitive flexibility, verbal fluency, working memory, organization, problem solving abilities), processing speed, attention, memory | __ | Though measures of executive function, processing speed, and memory were decreased in ALL survivors relative to population norms, intelligence was unimpaired. Increased plasma concentration of methotrexate was related to decreased executive function. Higher plasma concentration of methotrexate and greater neurocognitive impairment was associated with increased fMRI activation during an attentional task, increased cortical thickness in dorsolateral prefrontal brain regions, and with alterations in frontostriatal white matter microstructure. | ||
| 6 McEvoy et al. | Surgical resection | DTI (MRI scans at pre-op, post-op, and 1 year post-op) | Longitudinal | __ | Language functioning post-resection, 3 groups: intact (N = 19), mild deficit (N = 19), and posterior fossa syndrome (N = 9) | __ | Following tumor resection, patients with posterior fossa syndrome showed reduced FA in the left and right superior cerebellar peduncle compared to patients who did not develop language deficits. While language disturbances in posterior fossa syndrome patients resolved within months of surgery, white matter deficits in the superior cerebellar peduncle were still evident at one year post-surgery. | ||
| 7 Oh et al. | Surgical resection (100%), adjuvant CT (MB patients) | Structural MRI, DTI | Cross-sectional | Ataxia, fine motor function | __ | __ | Ataxia ratings were significantly higher in MB patients than in PA patients. Greater ataxia and fine motor function impairments correlated with volume loss of Cerebello-Thalamo-Cerebral (CTC) white matter pathway in MB patients, but not in PA patients. Cerebro-Ponto-Cerebellar (CPC) pathway white matter volume was significantly reduced in PA patients, but not in MB patients. Neither relationship was observed between the CPC pathway and ataxia or fine motor function. Patients with pediatric post-operative cerebellar mutism syndrome had greater ataxia and showed greater loss of volume in the CTC pathway. | ||
| 8 Scantlebury et al. | CSI (n = 30), surgery with or without focal radiation (n = 29) | DTI | Cross-sectional | __ | Processing speed | __ | Compared to controls and children treated with surgery/focal radiation, children with brain tumor treated with cranial-spinal radiation (CSR) show reduced integrity, indicated by significantly lower FA and greater RD, of multiple white matter tracts- particularly the optic radiations, inferior longitudinal fasciculi, and the inferior fronto-occipital fasciculi. Children treated with CSR also demonstrated lower information processing speed scores compared to healthy controls, which was related to reduced integrity of the right optic radiations. | ||
| 9 Zou et al. | Surgical resection, CRT, and CT | Task-based fMRI (5 tasks probing reading-related neural activation); 3 fMRI and reading evaluations at 1 year intervals. | Longitudinal | __ | Reading abilities, continuous processing | __ | Relative to standard-of-care, medulloblastoma patients receiving a reading intervention demonstrated altered brain activation during reading-related fMRI tasks in key areas involved in reading and language processing: left inferior frontal, temporal, ventral occipitotemporal, and subcortical regions. Improved sound awareness scores and an evident normative trend in patterns of brain activation during reading-related tasks in the intervention group highlight the potential neural and behavioral effects of preventative interventions given during medulloblastoma treatment in youth. | ||
| 10 Conklin et al. | CT only (n = 42), CSI and CT (n = 15), CRT and CT (n = 6), CT+ BMT and TBI (n = 5) | Task-based fMRI (grid-based spatial working memory) | Longitudinal | __ | IQ, working memory, spatial span backward, attention and processing speed and showed greater reductions in reported executive dysfunction | __ | Cancer survivors who completed the cognitive training intervention had greater improvement than wait-listed survivors on measures of working memory, spatial span backward, attention and processing speed and showed greater improvements in executive function. In the intervention group, post-intervention activation of left lateral prefrontal and bilateral medial frontal areas was reduced compared to pre-intervention activation. | ||
| 11 Khajuria et al. | Surgical resection, CRT, and CT | Structural MRI (post-resection) | Cross-sectional | __ | IQ, attention, working memory, and visual motor coordination | Health-related quality of life | In cerebellar tumor survivors, the quantity and extent of brain lesions after tumor resection was associated with cognitive impairments including intelligence and attention. These cognitive impairments were more apparent in MB survivors compared to PA survivors. In both groups, the extent of brain injury and related neurocognitive deficits did not impact health-related quality of life. | ||
| 12 Liu et al. | Neurosurgery (n = 25), CT (n = 10) | DTI | Cross-sectional | __ | IQ, verbal reasoning, nonverbal/visual reasoning, attention, working memory, processing speed | __ | Deficits in IQ and compromised white matter were evident in LGG patients compared to healthy controls. The effect of treatment for LGG on IQ was mediated by compromise of supratentorial white matter. Increased white matter compromise was observed in patients who presented without multiple symptoms, were not treated with surgery, were diagnosed at younger age, and whose parents had lower levels of education. | ||
| 13 Robinson et al. | Surgical resection (n = 17), CT and CRT (n = 5) | Task-based fMRI (letter N-back working memory) | Cross-sectional | __ | Executive function, attention problems | Parent- and self-reports of social, behavioral/internalizing problems, coping responses to stressful interpersonal and peer relationships | Compared to healthy controls and normative data, brain tumor survivors had higher levels of psychosocial and behavioral/emotional problems. Increased activation in prefrontal and other anterior regions in response to a working memory task were correlated with higher psychosocial functioning, use of engagement coping strategies, and less use of disengagement coping strategies in patients. Use of positive coping strategies partially explained the association between behavioral/emotional functioning and brain activation. | ||
| 14 Rueckriegel et al. | MB: Surgical resection, CSI and CT, PA: Surgical resection | DTI | Cross-sectional | Fine motor function/ataxia | IQ, executive function/processing speed | __ | In young survivors of posterior fossa tumors, significant associations were found between fronto-cerebellar tractography and intelligence as well as measures of motor function and executive function (i.e. processing speed, shifting attention). The degree of impaired fronto-cerebellar connectivity seems to underlie the extent of ataxia, fine motor dysfunction, and neurocognitive dysfuntion in pediatric posterior fossa tumor survivors. | ||
| 15 Bhojwani et al. | CT (5 courses of high-dose MTX and 13–25 doses of triple intrathecal therapy) | Structural MRI | Longitudinal | __ | __ | __ | High MTX exposure was associated with increased risk of leukoencephalopathy. Leuekoencephalopathy was evident in all symptomatic patients and 1 in 5 asymptomatic patients, and persisted in 58% of symptomatic and 74% of asymptomatic patients at the end of therapy. Concurrent genome-wide association study (GWAS) revealed that polymorphisms involved in neurogenesis may contribute to susceptibility to MTX-related neurotoxicity. Leukoencephalopathy persisted in nearly 3 of 4 asymptomatic and over half of symptomatic patients at the end of therapy. | ||
| 16 Duffner et al. | 2 CT protocols: P9201 (fewer CNS-directed treatment days during intensive consolidation, n = 24) and P9605 (intense CNS-directed therapy, n = 35) | Structural MRI (at least 2.6 years after the end of treatment) | Cross-sectional | __ | IQ, perceptual organization, processing speed, visuomotor integration, attention, continuous performance | __ | While patients in both treatment groups showed significant neurocognitive deficits, significantly more P9605 patients developed leukoencephalopathy and scored below average on more neurocognitive measures. Leukoencephalopathy was detected in survivors as late as 7.7 years after end of treatment, suggesting that treatment-related white matter changes in survivors are lasting and not simply transient. | ||
| 17 ElAlfy et al. | CT protocols: modified BFM 83, BFM 90, or CCG. | DTI | Cross-sectional | __ | IQ, visual perception/memory, attention, task-switching | __ | Relative to controls, ALL survivors treated with modified CCG protocol performed significantly lower on all cognitive measures and survivors treated with BFM 90 protocol had lower IQ and task- switching ability. While survivors in the BFM 90 group also had lower IQ and more executive function impairments than those in the BFM 83 group, no difference was found in cognitive test performance between controls and survivors treated with BFM 83. Frontal lobe FA was significantly reduced in the BFM 90 and BFM 83 groups compared to controls, but lower in the CCG group compared to all other groups. | ||
| 18 Horska et al. | CRT (n = 5 with neurosurgery, n = 2 with neurosurgery and CT, n = 1 with CT, n = 1 with CRT alone) | DTI (Baseline after surgery and before CRT, 6-month, 15-month and 27-month follow--ups after completion of CRT) | Longitudinal | __ | Working memory, motor speed | __ | Survivors had higher overall mean ADC (i.e., more diffusion) in the hippocampus compared with controls, indicating changes in deep gray matter microstructure. Survivors also showed heightened ADC at baseline and at the 27-month follow-up, however they showed normal verbal memory performance. Visual-spatial working memory performance in survivors decreased over time compared to controls. In both groups, decreased motor speed was associated with increased ADC in the globus pallidus and putamen. | ||
| 19 Jacola et al. | Surgical resection and CRT (n = 50), Pre-CRT CT (n = 6) | Structural MRI | Cross-sectional | __ | Q, working memory (behavioral measures), attention, executive function (parent ratings) | Parent reports of behavior and emotion regulation | Better working memory performance (longer digit span backwards and forwards) was positively associated with right frontal and right and left posterior NAWM volumes among brain tumor survivors. Tumor location and gender was also related to NAWM volumes. Participants with infratentorial tumors had significantly greater mean NAWM volume than those with supertentorial tumors in both right and left frontal areas. Overall, males had greater mean NAWM volume compared to females. No association was found between NAWM volumes and parent ratings. | ||
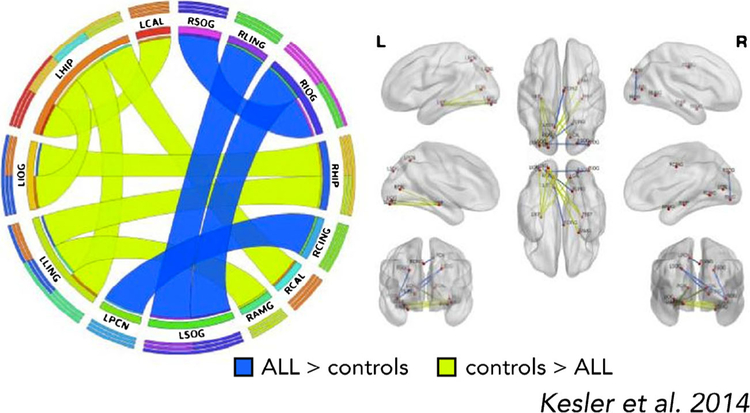
| 20 Kesler et al. | Intrathecal CT | Resting-state fMRI | Cross-sectional | __ | IQ, verbal learning, memory, reading and math fluency, executive function | __ | Compared to controls, ALL survivors showed reduced resting-state functional connectivity between bilateral hippocampus, left inferior occipital, left lingual gyrus, bilateral calcarine sulcus, and right amygdala. The ALL group showed regions of functional hyperconnectivity including right lingual gyrus, precuneus, bilateral superior occipital lobe, and right inferior occipital lobe. In the ALL group, impaired cognitive funtion and younger age at diagnosis were associated with functional hyperconnectivity. | ||
| 21 Riggs et al. | CT, surgical resection, CSI | Structural MRI, DTI | __ | Learning/memory | __ | Compared to controls, the brain tumor survivor group showed reduced white matter volume, damage to the uncinated fasciculus, and a smaller right hippocampus. The hippocampus may be particularly vulnerable to the effects of treatment, as reduced hippocampal volume was not related to brain volume differences. Among the survivors who also underwent memory testing (n =10), memory performance was associated with measures of hippocampal volume and uncinate fasciculus. | |||
| 22 Robinson et al. | Surgical resection (n = 17), CT and CRT (n = 5) | Task-based fMRI (letter N-back work ing memory) | Cross-sectional | __ | Executive function, attention problems, IQ, working memory, processing speed | __ | Survivors of pediatric brain tumors performed lower than controls on measures of general cognitive ability, attention, and executive function, and demonstrated altered brain activity during an fMRI working memory task. Survivors’ neurocognitve deficits were associated with lower activation in bilateral frontal regions associated with sustained attention and greater activation in left cingulate regions associated with problem-solving and performance monitoring during a working memory task. | ||
| 23 Badr et al. | CT (n = 25), CT and CRT (n = 4) | Structural MRI | Cross-sectional | __ | __ | __ | Brain abnormalities (i.e., leukoencephalopathy, brain atrophy, old infarcts or hemorrhages) were detected in 24% of childhood ALL survivors, with higher incidences of abnormalities occuring in survivors who received cranial radiation, had diagnoses involving the CNS, or were classified as high-risk. | ||
| 24 Genschaft et al. | CT | Structural MRI, DTI | Cross-sectional | Olfaction | Memory, executive function, attention, IQ | __ | Relative to controls, the ALL group showed smaller gray matter volumes of the left hippocampus, amygdala, thalamus, nucleus accumbens, left calcarine gyrus, bilateral lingual gyri and the left precuneus. ALL survivors had lower hippocampus-dependent memory scores, and lower memory scores correlated with reduced hippocampal volumes. No evidence of white matter pathology was found. | ||
| 25 Kuper et al. | Surgical resection | Structural MRI (and behavioral tests) at 3 time points: (1) within the first days, (2) 3 months, and (3) 1 year after surgery. | Longitudinal | Ataxia, balance, upper limb motor function | __ | __ | Lesion location in pediatric cerebellar tumor patients, particularly the involvement of the deep cerebellar nuclei, was the chief predictor of later functional recovery. Cerebellar lesion volumes were significantly reduced by disappearing edema within the first 3 months post-surgery, though behavioral improvements continued for up to a year. Permanent lesions of the inferior vermis and the deep cerebellar nuclei were associated with long-lasting impairments in balance and upper limb function. | ||
| 26 Wolfe et al. | Surgical resection, CRT, and CT | Task-based fMRI (letter N-back working memory) | Cross-sectional | __ | IQ | __ | Survivors of posterior fossa tumors showed typical activation patterns (during a working memory task) associated with working memory in the frontal-parietal network. Higher cardiorespiratory fitness was associated with better working memory performance (behavioral) as well as more efficient neural functioning. | ||
| 27 Hosseini et al. | Intrathecal CT | Structural MRI | Cross-sectional | __ | __ | __ | Relative to controls, ALL survivors show altered structural organization of large-scale brain networks, indicative of pervasive neurobiological damage. Compared to the control group, the ALL group showed significantly decreased small-world index- suggesting that brain network organization is less optimal, more standardized, and more vulnerable to failure in ALL survivors. | ||
| 28 Zou et al. | CRT, CT, or both | Task-based fMRI (continuous performance) | Longitudinal | __ | IQ, attention, academic achievement, memory, verbal learning, self-esteem | __ | Compared to healthy controls, survivors performed significantly lower on a measure of intellectual functioning and parent ratings indicated cognitive problems, innattention, and hyperactivity. In survivors, brain activation in ventral visual areas, cerebellum, supplementary motor area, and left inferior frontal cortex during a continuous performance task was diminished at baseline, and was increased at the end of a 20-session cognitive remediation program and at 6-month follow-up. Survivors participating in the cognitive remediation program also showed improvements between baseline and the 6-month follow-up in cognitive problems/-inattention. | ||
| 29 Ashford et al. | CT | Structural MRI (at end of treatment) | Longitudinal | __ | IQ, attention, working memory | __ | Working memory was impaired compared to norms for the total sample and the standard-/high-risk group. Leukoencephalopathy in survivors was predictive of lower total digit span, an important indicator of working memory. | ||
| 30 Ficek et al. | CT alone (n = 15), CT and CRT (n = 30) | Structural MRI and SPECT | Cross-sectional | __ | __ | __ | White matter changes were detected by MRI in 7% (n = 3) of ALL survivors, who had all received CRT. MR SPECT revealed changes in 1H-MRS metabolite ratios in 29% (n = 13) of survivors and decreased mean NAA/Cr ratio in survivors who received CRT. | ||
| 31 Kesler et al. | Intrathecal CT | Structural MRI | Cross-sectional | __ | IQ, verbal comprehension, working memory, processing speed, perceptual reasoning, executive function, verbal and visual declarative memory, visual-spatial processing, visual attention | __ | ALL survivors had significantly reduced white matter volume compared to controls, however, the groups did not differ on measures of gray matter or whole brain volumes. Observed white matter differences were particularly evident in the left caudate/left corpus callosum, right caudate, bilateral thalamus, fornix and bilateral superior fronto-occipital fasciculus. ALL survivors performed significantly lower than controls on neurocognitive measures of processing speed, working memory, and verbal memory. These cognitive performance deficits were not related to any regional nor whole brain volume differences. | ||
| 32 Robinson et al. | Intrathecal CT | Task-based fMRI (letter N-back working memory) | Cross-sectional | __ | IQ, working memory, processing speed, verbal and nonverbal executive functioning | __ | Compared to controls, ALL survivors performed less accurately on a working memory task and displayed greater activation in brain areas associated with working memory (dorso- and ventro-lateral prefrontal cortex) and error monitoring (anterior cingulate cortex). These findings align with the theory of compensatory activation in relevant brain regions, suggesting that increased cognitive effort is required to complete tasks in pediatric ALL survivors. | ||
| 33 Aukema et al. | MB (n = 6): Surgical resection, CSI and CT. ALL: CT (n = 5 high-dose MTX, n = 6 low- dose MTX) | DTI | Cross-sectional | Motor speed | IQ, verbal comprehension, perceptual organization, processing speed | __ | In survivors of childhood ALL and medulloblastoma, mean white matter FA was significantly reduced compared to controls, and specifically within the right inferior fronto-occipital fasciliculus (IFO) and genu of the corpus callosum (gCC). Processing speed was correlated with white matter FA in the splenium (sCC) and body of the corpus callosum (bCC); motor speed was related to white matter FA in the right IFO. | ||
| 34 De Smet et al. | Surgical resection (n = 8), CT and CRT (n = 3), CRT (n = 1) | Structural MRI, SPECT | Longitudinal | __ | IQ, executive function, language, verbal fluency, concentration, memory, praxis | Emotional coping, social adjustment | Following brain tumor resection, patients developed language and motor deficits, neurocognitive problems with executive function, concentration, and visuo-spatial attention, and behavioral and affective disturbances. Post-surgerical mutism was linked to perfusional deficits in supratentorial regions involved in language dynamics, syntax, naming, executive functioning, affective regulation, and behavior. | ||
| 35 Reddick et al. | CT | Structural MRI (at baseline after induction therapy and after end of consolidation therapy) | Longitudinal | __ | __ | __ | During treatment for ALL, patients developed WM hyperintensities involving the anterior, posterior, and superior corona radiata and superior longitudinal fasciculus fiber tracts. T2 signal intensity in these regions was greater on the second examination for all patients, with greater increases evident in older patients, who were treated with more intense CT. | ||
| 36 Carey et al. | CT only | Structural MRI | Cross-sectional | __ | IQ, language, attention, memory, processing speed, executive function, academic achievement, visual-constructional skills | __ | Compared to controls, ALL survivors showed reduced white matter in the right frontal lobes. Also relative to controls, survivors showed lower performances on tests of attention, visual-constructional skills, mental flexibility, and math achievement. While no regional gray matter volume differences were found between the groups, decreased performance on neuropsychological measures was related to reduced regional white matter volumes in survivors. | ||
| 37 Kirschen et al. | Surgical resection | Structural MRI (post-resection) | Cross-sectional | __ | IQ, continuous performance, phonological processing, verbal fluency, fine motor coordination, verbal working memory | __ | Cerebellar pilocytic astrocytoma patients did not differ from controls on neuropsychological tests of verbal fluency, animal naming, attention, phonological processing, or fine motor control- but did have significantly lower IQ scores. Damage to left hemispheral cerebellum lobule VIII was significantly correlated with reduced digit span for auditory (but not visual) stimuli in patients, who scored lower on these measures compared with controls. In patients, damage to the vermis and hemispheral lobule IV/V bilaterally was associated with decreased effects of articulatory suppression. | ||
| 38 Zhang et al. | CRT and CT | Structural MRI | Cross-sectional | __ | __ | __ | Brain tumor survivors showed reduced gray matter density in the thalamus and entorhinal cortex and reduced white matter density in the internal capsule, hypothalamus, corpus callosum, and cuneus of the occipital lobe, compared to healthy sibling controls. | ||
| 39 Qiu et al. | Neurosurgery, CSI, CT | DTI | Cross-sectional | __ | __ | __ | Compared with controls, frontal lobe and parietal lobe white matter FA were significantly less in MB survivors - with a larger difference in frontal lobe FA compared with the parietal lobe. This difference suggests that compared to the parietal lobe, frontal lobe white matter may be more sensitive to effects of cranial irradiation treatment. | ||
| 40 Khong et al. | ALL: CT (n = 18), CT and CSI (n = 9) MB: Surgical resection, CSI, CT | DTI | Cross-sectional | __ | IQ (full-scale, verbal, performance) | __ | There were no significant differences in IQ scores across patient groups. Perecent of difference in white matter FA for each patient was compared with the age-matched control group. Within survivors, the FA difference score was significantly correlated with all three measures of IQ (full-scale, verbal, performance), even after adjusting for age at treatment, irradiation dose, and time since completion of treatment. | ||
| 41 Mabbott et al. | Surgical resection, CSR, and adjuvant CT | DTI | Cross-sectional | __ | IQ | __ | In MB patients, reduced IQ was associated with reduced white matter integrity (increased apparent diffusion coefficient decreased FA). Altered white matter was evident in the CSR group compared to controls- with in creased apparent diffusion coefficient in all regions and lower FA in the genu of the corpus callosum, the anterior and posterior regions of the internal capsule, and inferior and high frontal white matter. | ||
| 42 Qiu et al. | Surgical resection, CT, CSI + posterior fossa boost (total dose 56 Gy) | DTI before the end of radiotherapy and at 3 months, 6 months and 1 year after completion of radiotherapy. | Longitudinal | __ | __ | __ | Across scans, increasing reduction in mean ΔFA over treatment for medulloblastoma was correlated with increasing radiation dose up to 45 Gy-at which point this trend reversed and mean FA approached baseline value. In both patients, more severe mean FA reduction was evident in the frontal lobes compared to the parietal lobes despite both brain regions being exposed to the same radiation dose. After cranial radiation, mean FA increase in the brainstem was also shown in both patients. | ||
| 43 Reddick et al. | CT only (n = 84), CT and cranial irradiation (n = 28) | Structural MRI | Cross-sectional | __ | IQ, attention, academic achievement | __ | ALL survivors performed significantly lower on most neurocognitive measures compared to normative test scores, with cranial irradiation-treated survivors performing lower than those treated with CT only. Both survivor groups had significantly reduced white matter volumes compared to sibling controls, and survivors treated with cranial irradiation had significantly smaller white matter volumes than survivors treated with CT alone. Additionally, smaller white matter volumes in ALL survivors were related to greater deficits in intelligence, attention and academic achievement. | ||
| 44 Shan et al. | CRT | Structural MRI (at start of therapy and again 2 years later) | Longitudinal | __ | __ | __ | Compared to medulloblastoma patients with increased NAWM volume following treatment, those with decreased NAWM volume showed significantly increased fractal features and NAWM boundary irregularities. In patients with decreased NAWM volume, fractal features were highly correlated with NAWM volume after treatment. | ||
| 45 Konczak et al. | Surgical resection (n = 24); some also had CRT alone, CT alone, or CRT and CT | Structural MRI | Cross-sectional | __ | Motor and cognitive performance, postural control, working memory | __ | Overall, cerebellar tumor patients did not differ from controls on cognitive measures; working memory was only impaired in patients who had received CT or RT after surgical resection. Patients with abnormal posture who did not receive CT or RT had brain lesions containing the fastigial and interposed nuclei (NF and NI), whereas patients with normal posture did not have lesions containing these nuclei. Age at surgery, time since surgery or lesion volume were not significant predictors of motor or cognitive recovery. | ||
| 46 Reddickck et al. | Surgical resection and CSI (n = 52), adjuvant CT (n = 38) | Structural MRI | Longitudinal | __ | __ | __ | Patients treated for MB at younger ages demonstrated reduced development of NAWM volume, compared to healthy controls. Younger age at irradiation and placement of a shunt were significantly associated with reduced NAWM volume in patients. Over a period of 4–5 years, differences in NAWM and CSF volume between patients who had shunts and those without, resolved. | ||
| 47 Reddick et al. | CT (IV-MXT) | Structural MRI (4 times throughout treatment) | Longitudinal | __ | __ | __ | With additional courses of CT, the amount of white matter impacted by treatment and the severity of leukoencephalopathy increased in both (low- and standard/high-risk) ALL groups. Importantly, both the severity and extent of leukoencephalopathy significantly decreased 1.5 years after completion of treatment. | ||
| 48 Reddick et al. | CT (IV-MXT) | Structural MRI (4 times throughout treatment) | Longitudinal | __ | __ | __ | Increasing exposure to CT (increased dose, additional courses) was associated with increased severity of leukoencephalopathy in ALL. The prevalence of leukoencephalopathy. was significantly reduced ~1.5 years after the completion of CT. | ||
| 49 Zou et al. | Surgical resection (n = 16); CRT alone (n = 3), CT alone (n = 7), CRT and CT (n = 5) | Task-based fMRI (visual stimulation) | Cross-sectional | __ | __ | __ | During a visual stimulation task, childhood cancer survivors showed smaller activation volume in the primary visual cortex relative to healthy controls. Brain tumor survivors showed significantly smaller activation volume compared to both ALL survivors and healthy controls. While these results indicate that BOLD fMRI is a feasible method to investigate brain function in childhood cancer survivors, future functional neuroimaging studies should account for effects of quantitative differences in the BOLD responses of survivors as compared to healthy subjects. | ||
| 50 Hill et al. | CT | Structural MRI | Cross-sectional | __ | Visual and verbal long-term memory | __ | ALL survivors did not differ from controls on measures hippocampal volume nor long-term memory performance, and hippocampal volumes were not related to measures of long-term memory. | ||
| 51 Leung et al. | Surgical resection, whole brain irradiation, CT | DTI | Cross-sectional | __ | __ | __ | Compared to controls, MB survivors displayed significantly reduced FA in temporal, parietal, and occipital periventricular white matter, corpus callosum, and corona radiata. | ||
| 52 Mulhern et al. | Surgical resection and CSI (n = 37), CT (n = 18) | Structural MRI | Cross-sectional | __ | IQ, continuous performance/attention | __ | Compared to norms, child brain tumor survivors showed intellectual and related attentional deficits. Greater attentional deficits were associated with reduced NAWM, particularly within the frontal lobe/prefrontal area and cingulate gyrus. | ||
| 53 Nagel et al. | Neurosurgery, CRT, and CT | Structural MRI (mean num. scans per patient = 6, up to 5 years after treatment) | Longitudinal | __ | __ | __ | Both right and left hippocampal volumes continually decreased after medulloblastoma treatment until approximately 2–3 years after diagnosis, when hippocampal volumes resumed a normal positive growth pattern. Hippocampal volume loss occurred mainly in the posterior regions, and was associated with female sex, low parental education, shunt placement, and positive seizure history. | ||
| 54 Chu et Al. | CT alone (n = 18), CT and CRT (n = 4), CT and whole-body irradiation (n = 1) | Structural MRI and SPECT (0, 8, and 20 weeks and 1, 2, and 3 years after diagnosis) | Longitudinal | __ | __ | __ | Metabolite changes in the brain after treatment of childhood ALL were detected, although simultaneous structural white matter abnormalities were not observed: 81% of patients showed metabolite changes while only 23% showed white matter changes at 20 weeks. | ||
| 55 Khong et al. | Surgical resection, CSI, CT | DTI | Cross-sectional | __ | Intellectual outcome (school performance) | __ | With the exception of frontal periventrical white matter, FA was significantly reduced in medulloblastoma patients compared to controls in all anatomical sites (including posterior fossa and supratentorial white matter). Decreased supratentorial white matter FA was associated with younger age at treatment, longer interval since treatment, and decline in school performance. | ||
| 56 Pääkkö et al. | CT (n = 19), and CSI (n = 9)? | Perfusion MRI at end of treatment (n = 19), and SPECT (n = 17) | Cross-sectional | __ | __ | __ | In children treated for ALL, small brain defects were detected by SPECT in 29% of children in the left basal, frontal or temporal areas, whereas perfusion MRI showed no focal perfusion defects. | ||
| 57 Reddick et al. | Surgical resection (n = 40), whole–brain irradiation (n = 24), local irradiation only (n = 16), CT (n = 18) | Structural MRI | Cross-sectional | __ | IQ, attention, memory, academic achievement | __ | Brain tumor demonstrated impaired neurocognitive test performance on all measures. NAWM volumes were associated with both attentional abilities and IQ, with a significant amount of the relationship between NAWM and IQ explained by attentional ability. These results suggest that reduced NAWM among pediatric brain tumor patients contributes to declining IQ and academic achievement because of its detrimental effect on attention. | ||
| 58 Palmer et al. | Neurosurgery, CSI, CT | Structural MRI (multiple times over 4-yr period) | Longitudinal | __ | __ | __ | In contrast to normal development, the total midsagittal corpus callosum area of medulloblastoma patients decreased as time since cranial-spinal radiation increased. Additional declines in area were also observed in the genu, rostral body, anterior midbody, posterior midbody, isthmus and splenium- with the greatest deviations from typical development occuring the isthmus and the splenium. These subregions of the corpus collosum, which normally have a high rate of growth in childhood, are impacted by the high dose of irradiation that they are exposed to in the treatment of pediatric MB. | ||
| 59 Mulhern et al. | Surgical resection and CRT (n = 42), CT (n = 29) | Structural MRI | Cross-sectional | __ | IQ, verbal memory, and sustained attention | __ | Neurocognitive performance in MB survivors was below age-related norms. Younger age at CRT was related to lower performance on all neurocognitive tests with the exception of verbal memory, while increased time since completion of CRT was correlated with lower performance on all neurocognitive tests except sustained attention. A significant amount of the association between age at CRT and neurocognitive measures (IQ, factual knowledge, verbal and nonverbal thinking) was accounted for by NAWM. | ||
| 60 Levisohn et al. | Surgical resection | Structural MRI (post-resection) | Cross-sectional | __ | Executive function (incl. planning and sequencing), visual–spatial function, expressive language, verbal memory | Parent and clinician report of behavioral problems, regulation of affect | Cerebellar tumor survivors exhibited impaired executive function, visual–spatial function, expressive language, verbal memory and modulation of affect. Particularly, lesions of the vermis were associated with dysregulation of affect. Older survivors showed more behavioral deficits than younger survivors. | ||
| 61 Paakko et al. | CT (n = 33), CSI (n = 15) | Structural MRI (At least 4 scans from beginning to end of treatment, n = 26) | Longitudinal | __ | Attention, language, motor and sensory functions, visuospatial function, memory and learning, IQ, concentration, inhibition and control | __ | Transient white matter hyperintensities (prominent in frontal lobes) were noted in patients during treatment for ALL with CT only (n = 3), who were significantly younger than those without highintensity white matter changes. Except for deficits of attention and functions directly dependent upon frontal areas, white matter changes were not correlatedwith neuropsychological tests. | ||
| 62 Reddick et al. | CSI (conventional or reduced dose) and CT | Structural MRI | Longitudinal | __ | __ | __ | MB patients treated with cranial-spinal radiation have significant loss of NAWM volume. There were no significant differences in the rate of NAWM volume loss based on age at cranial-spinal radiation, however, the rate of NAWM volume loss was significantly slower in children receiving reduced-dose cranial-spinal radiation | ||
| 63 Mulhern et al. | MB: Surgical resection and CSI (n = 9), surgical resection, CSI and CT (n = 9), PF: surgery alone | Structural MRI (T1, T2, PD [proton density]) | Cross-sectional | __ | IQ (full-scale, verbal, performance) | __ | Childhood MB survivors treated with cranial radiation (with or without CT) had significantly reduced NAWM and lower full-scale IQ scores compared to PF survivors treated with surgery alone. Further, decreased NAWM in MB survivors following was associated with lower full-scale IQ. | ||
| 64 Harila-Saari et al. | CT alone (n = 15), CT and CRT (n = 17) | Structural MRI (first at end of treatment, second 5 years later) | Longitudinal | __ | IQ, attention, language, motor/sensory/visuospatial function, memory | __ | Overall, treatment-related brain abnormalities (e.g. high-intensity white matter changes, cortical atrophy, calcifications) were detected in 25% of ALL patients post-treatment. Neuropsychologic test results did not significantly differ between patients with brain abnormalities and patients with normal MRI findings, however patients with persistent white matter changes (n = 2) had reductions in verbal function. | ||
| 65 Reddick et al. | Surgical resection, CRT and/or CT | Structural MRI | Cross-sectional | __ | __ | __ | Within brain tumor survivors, brain parenchyma and white matter volumes significantly decreased as atrophy increased (as graded by radiologists). Gray matter volumes had no relationship with atrophy. Patients who received surgery, irradiation, and chemotherapy did not show differences in brain parenchyma, white matter, and gray matter volumes relative to patients treated with surgery and iraddiation alone. Patients who received surgery and irradiation demonstrated reduced white matter volumes relative to patients treated with surgery alone. | ||
Abbreviations: ALL, acute lymphoblastic leukemia; PA, pilocytic astrocytoma; MB, medulloblastoma; PNET, primitive neuroectodermal tumor; WM, white matter; NAWM, normal appearing white mater; LGG, low-grade glioma; CT, chemotherapy; CRT; cranial radiation therapy; IV-MTX, intravenous methotrexate; CSI, craniospinal irradiation; BMT, bone marrow transplant; DTI, diffusion tensor imaging; fMRI, functional magnetic resonance imaging; SPECT, single-photon emission computed tomography; DWI, diffusion weighted imaging; ASL, arterial spin labelling; IQ, intelligence quotient; FA, fractional anisotropy; ADC, apparent diffusion coefficient; CBF, cerebral blood flow.
Structural or diffusion MRI studies
The majority (63%) of the 55 structural MRI or DTI studies examined CNS tumor patients and survivors, and the remaining 37% examined ALL survivors exclusively. Several studies in brain tumor patients and survivors identify damage to brain areas following surgical tumor resection, for e.g., the cerebellum. Given the critical role of the cerebellum in motor control and balance, several studies have linked cerebellar lesions to deficits in motor functioning (Küper et al. 2013; Khajuria et al. 2015; Konczak et al. 2005). However, the cerebellum is increasingly recognized for its role in high-order non-motor processes such as learning, memory, and emotion, and several studies link damage in particular locations with neurocognitive difficulties (i.e., left hemisphere lobule VIII; Kirschen et al. 2008) and parent-reported affect dysregulation (i.e., vermis; Levisohn et al. 2000).
Several studies also suggest that brain tumor and its resection disrupt white matter pathways connecting cerebellum with prefrontal, superior temporal, and limbic regions, and reduced white matter is frequently associated with poorer neurocognitive functioning (e.g., processing speed, IQ, memory). Reductions in white matter volume are also reported in five studies of young ALL survivors (Kesler et al. 2010; Ashford et al. 2010; ElAlfy et al. 2014; Cheung et al. 2016; Aukema et al. 2009), including in the corpus callosum, striatum, and thalamus, and these reductions have also been linked to deficits in cognitive functioning (see Table 1). Several studies report greater reductions in white matter volume or indicators of macrostructure with younger age at treatment, greater time since treatment, and greater CNS treatment intensity (see Table 1). Taken together, existing studies indicate white matter damage in children and adolescents, irrespective of cancer type or treatment received. However, there is some evidence suggesting that degree of white matter damage is more severe following cranial radiation relative to chemotherapy only, and in brain tumor survivors relative to ALL survivors, potentially because radiation is a more primary component of treatment for childhood CNS tumors relative to ALL. The relation between white matter integrity and neurocognitive functioning has prompted several investigators to assert that altered white matter integrity may serve as a biomarker for identifying risk for neurocognitive impairment. The cause of injury to white matter is unknown, but may involve treatment-induced damage to newly synthesized and less stable myelin, glial cells, oligodendrocyte precursors, and microvascular structure (Hopewell et al. 1993; Krull et al. 2013a, b; Monje & Dietrich 2012), and/or microglial activation associated with oxidative and nitrosative stress (Lull & Block 2010).
In addition, many patients develop chronic or transient leukoencephalopathy (i.e., white matter lesions) during treatment, a more overt marker of white matter neurotoxicity. In fact, as many as 80% of patients treated for ALL without irradiation may develop leukoencephalopathy (Reddick et al. 2005a, b), leaving them at high risk for severe neurologic morbidity. A recent longitudinal neuroimaging study demonstrates that nearly a quarter of children treated for ALL developed asymptomatic leukoencephalopathy during active chemotherapy (Bhojwani et al. 2014), and these children displayed more parent-rated cognitive problems (i.e., poorer working memory [capacity to manipulate information in one’s mind], organization [ability to organize information to achieve a goal or organize one’s environment], and initiation, [ability to get started on activities]) at follow-up more than 5 years after diagnosis than did survivors who did not display leukoencephalopathy (Cheung et al. 2016). In addition, leukoencephalopathy during chemotherapy treatment predicted reduced white matter integrity in the frontostriatal tract at follow-up, suggesting further white matter damage (Cheung et al. 2016). These findings demonstrate that changes in the brain, even in the absence of current symptoms or overt behavioral changes, can predict later neurocognitive outcomes (e.g., executive functioning).
Several studies examine gray matter structure. Three studies report reductions in hippocampal volume following treatment of brain tumor or ALL (see Fig. 2a; Riggs et al. 2014; Nagel et al. 2004; Genschaft et al. 2013), which corresponded with poorer performance on hippocampal-dependent memory tasks (Genschaft et al. 2013; Riggs et al. 2014). In these studies, hippocampal damage was largely attributed to cancer treatment-induced inhibition of hippocampal neurogenesis. However, one study comparing 10 ALL survivors (ages 7–14) with 10 matched controls failed to find significant differences in hippocampal volume, or in long-term memory (Hill et al. 2004), which may suggest significant inter-individual variability in hippocampal damage, or may be related to specific study characteristics (e.g., patients, sample size, methodology for measuring hippocampus). Riggs et al. (2014) also found reductions in indicators of white matter macrostructure among brain tumor survivors within the uncinate fasciculus, the major white matter pathway connecting hippocampus and amygdala with frontal regions, including the prefrontal cortex (PFC) and anterior cingulate cortex (ACC). The uncinate fasciculus plays a significant role in the development and support of memory processes (Ghetti & Bunge 2012), and shows a protracted maturational course into adulthood (Simmonds et al. 2014). Thus, early cancer-related disruptions to this pathway may underlie deficits in later-emerging processes that rely on interactions between temporal and prefrontal regions (e.g., memory, emotion regulation).
Fig. 2.

Altered brain structure and function in young survivors of pediatric cancer. a Reduced hippocampal volume in n = 27 ALL survivors (ages 15–22 years; in remission for 6–18 years) treated with chemotherapy-only, relative to n = 27 matched controls (Genschaft et al. 2013). b Regional increases (warm colors) and decrease (cool colors) in white matter clustered connectivity, as measured using graph theoretical analysis of DTI data in n = 31 young ALL survivors (ages 5–19 years, 6–111 months off treatment) relative to n = 39 matched controls. Of note, increased nodal clustering was noted in the ALL group in hippocampus and insula, a core SEN region, and decreased clustering in amygdala, considered a part of the SEN (Kesler et al. 2016). Abbreviations: ALL, acute lymphoblastic leukemia; SEN, salience and emotion network; DTI, diffusion tensor imaging. Image for panel B provided courtesy of Dr. Shelli Kesler. All images are adapted with permission
In addition to alterations in the hippocampus, Genschaft et al. (2013) reported reduced volumes of the amygdala, thalamus, and nucleus accumbens in ALL survivors relative to controls. These regions are considered part of the “salience and emotion network” (SEN), a large-scale neurocognitive network involved in attentional awareness, emotion processing, and regulation (Seeley et al. 2007). Altered organization of large-scale structural brain networks has been reported in two studies. Hosseini et al. (2012) applied graph theoretical analysis to structural MRI data, and observed a significant reduction in small-world characteristics (a measure of information processing efficiency) in young ALL survivors relative to controls, consistent with widespread neurobiological injury. Similarly, Kesler et al. (2016) found lower small-worldness in young ALL survivors relative to controls, using graph theoretical analysis and DTI data. In this study, regional differences in nodal clustering was observed in several regions of the SEN, including amygdala and insula, and also hippocampus (see Fig. 2b; Kesler et al. 2016). Together, these findings suggest more widespread alterations in brain structure following pediatric cancer, even without CNS disease or cranial radiation therapy.
Functional MRI studies
Of the 10 existing task-based fMRI studies (see Table 1), five examined neural functioning during a working memory task (Robinson et al. 2010; Conklin et al. 2015; Wolfe et al. 2013; Robinson et al. 2014; Robinson et al. 2015), two during an attention task (continuous performance task, “attention network task”; Krull et al. 2016; Zou et al. 2012), one during a visual stimulation task (Zou et al. 2005), and one during several fMRI tasks probing reading-related neural activation (Zou et al. 2016). Four studies (Zou et al. 2016; Robinson et al. 2014; Robinson et al. 2015; Wolfe et al. 2013) examined brain tumor patients/survivors exclusively, two (Krull et al. 2016; Robinson et al. 2010) examined ALL survivors exclusively, and three examined a mix of ALL and CNS tumor survivors (Zou et al. 2012; Zou et al. 2005; Conklin et al. 2015). The three studies by Robinson et al. (2010, 2014, 2015) demonstrate a similar pattern of increased neural response in areas of the PFC, considered part of the “central executive network” (CEN), and the ACC (a SEN region) during an n-back working memory paradigm among survivors of brain tumor or ALL relative to healthy controls (see Fig. 3a). Given the critical role of PFC and ACC in working memory and executive functioning more broadly, the authors propose that greater neural response in these regions reflects a compensatory neural mechanism in survivors that helps to maintain behavioral performance following brain insult. In line with this interpretation, survivors of ALL did not differ in behavioral performance from controls (Robinson et al. 2010). However, pediatric brain tumor survivors performed significantly less accurately than controls, suggesting an ineffective compensatory neural mechanism in this group (Robinson et al. 2014). In fact, higher response in ACC was associated with poorer task accuracy, suggesting that increased engagement of the SEN did not facilitate better performance (Robinson et al. 2014). Together, these findings are interesting and important because they uncover similar neurobehavioral changes in a sample of brain tumor survivors, treated primarily (71%) with surgical resection and cranial radiation (without chemotherapy), and a sample of ALL survivors, treated exclusively with chemotherapy (without surgery and cranial radiation). Further, Robinson and colleagues reported that chemotherapy and radiation dosage were not associated with behavioral performance during the working memory task (Robinson et al. 2014). Altogether, these findings raise the possibility that specific brain regions, particularly those showing a protracted developmental trajectory (e.g., PFC, ACC; Gogtay & Thompson 2010; Tiemeier et al. 2010) are sensitive to various CNS-directed cancer therapies during childhood (e.g., radiation therapy, chemotherapy), or, that there are shared aspects of the childhood cancer experience (e.g., adversity) that may imprint on brain development. As we outlined in the beginning, these are not mutually exclusive.
Fig. 3.
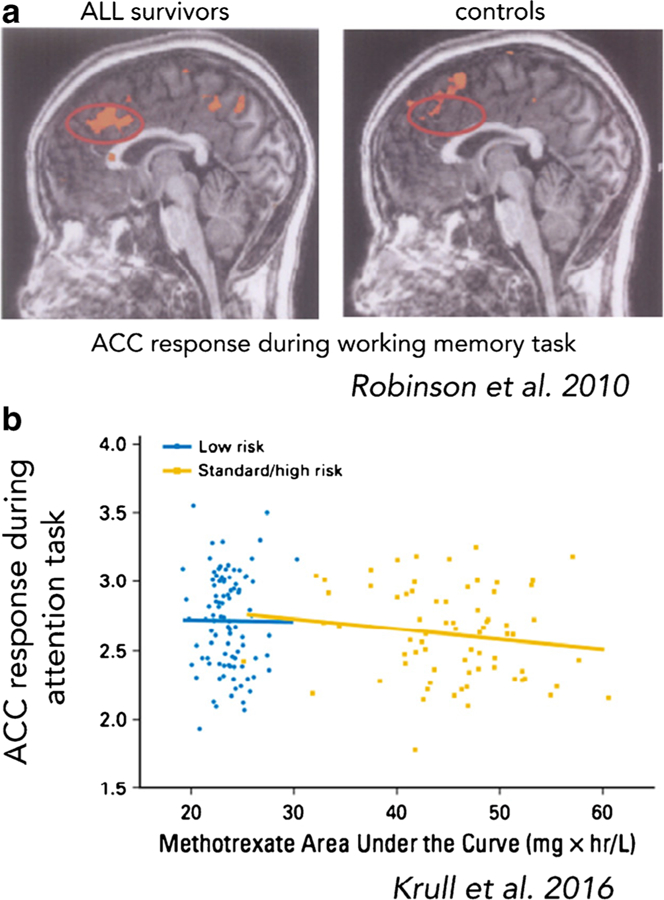
Altered brain function in young survivors of pediatric cancer. a Significant increases in response in ACC, a core SEN region, in n = 8 young ALL survivors (m = 14.54 years, SD = 2.47; 4–12 years after treatment) relative to matched controls, during a working memory (n-back) paradigm (2-back condition). No significant differences in behavioral performance (i.e., accuracy, reaction time) were observed between groups (Robinson et al. 2010). b Higher plasma methotrexate exposure during treatment for ALL is associated with lower response in ACC, a core SEN, during an attention task at more than five years (m= 7.7, SD = 1.7) post-diagnosis (n = 147; m age at scan = 13.8 years, SD = 4.8; Krull et al. 2016). Abbreviations: ALL, acute lymphoblastic leukemia; ACC, anterior cingulate cortex; SEN, salience and emotion network. All images are adapted with permission
Robinson et al.’s 2014 study was also the only fMRI study that linked measures of neural function with measures of psychosocial or emotional wellbeing. Specifically, the authors reported elevated psychosocial and behavioral/emotional difficulties (e.g., reduced self- and parent-reports of social competence) in survivors relative to controls and normative data, and that, both within and across groups, children who showed higher PFC response also reported better psychological functioning (i.e. lower symptoms of anxiety and depression). Further, self-reported engagement of secondary control coping strategies to social stress (i.e., acceptance, cognitive restructuring, positive thinking, distraction) accounted for a significant portion of the association between brain activation and psychological functioning, suggesting that engagement of the PFC is adaptive in this context. The link between psychological wellbeing and PFC response during working memory is not surprising given that areas of the PFC are also implicated in socioemotional processes (Etkin et al. 2011). These data are important because they are the first to link altered neural functioning with psychosocial and emotional wellbeing in survivors. As we will discuss later, it will be critical to expand our understanding of neurobehavioral correlates of psychological and emotional wellbeing in young survivors, and interrogate proximal processes that may be relevant for social and emotional functioning (e.g., emotion regulation, fear- and extinction-learning).
A recent fMRI study by Krull et al. (2016) demonstrated the potential utility of neuroimaging for identifying markers or potential drivers of neurodevelopmental change following pediatric cancer. In this study, the authors identified negative associations between SEN response (in ACC) during an attention task and prior methotrexate exposure during cancer treatment in ALL survivors (see Fig. 3b). Identification of markers of adverse neurodevelopmental outcomes may help to not only identify individuals who may benefit from intervention, but also lead to new avenues for the development of neuro-protective techniques to be given as an adjuvant during treatment (e.g., folate therapy and adjunctive pyridoxine and cobalamin supplementation).
The fMRI studies by Conklin et al. (2015) and Zou et al. (2012, 2016) coupled fMRI with a cognitive training intervention in cancer patients/survivors, to identify neural mechanisms underlying therapeutic change. For instance, Conklin et al. (2015) examined the short-term efficacy of a 25-session internet-based cognitive computerized intervention (Cogmed, www.cogmed.com) and neural correlates of cognitive changes in children who received CNS-directed therapy for ALL or brain tumor (ages 8–16 years). Survivors were randomly assigned to intervention or waitlist, and fMRI was used to examine neural responses during a spatial working memory task at pre- and post-intervention time points. They found that, relative to waitlist controls, survivors completing the intervention demonstrated greater improvements in working memory, attention, and processing speed. In a follow-up study, these cognitive benefits were even maintained six months later (Conklin et al. 2016). FMRI scanning revealed that improvements in behavioral performance were accompanied by a significant pre- to post-intervention reduction in activation of frontal regions that are considered part of the SEN (i.e., ACC) and CEN (i.e., lateral PFC), during a spatial working memory task (see Fig. 4, top and lower left). These results suggest greater neural efficiency in brain areas known to support working memory, following a computerized cognitive training intervention. In addition, lower pre-intervention responses in dorsolateral PFC, a region of the CEN, were predictive of positive intervention response (Fig. 4, lower right; Conklin et al. 2016). These exciting results suggest that changes in CEN and SEN functioning may represent the neural bases of training-based behavioral change. They also demonstrate the potential utility of baseline neuroimaging for predicting intervention response, and guiding the selection of personalized interventions.
Fig. 4.
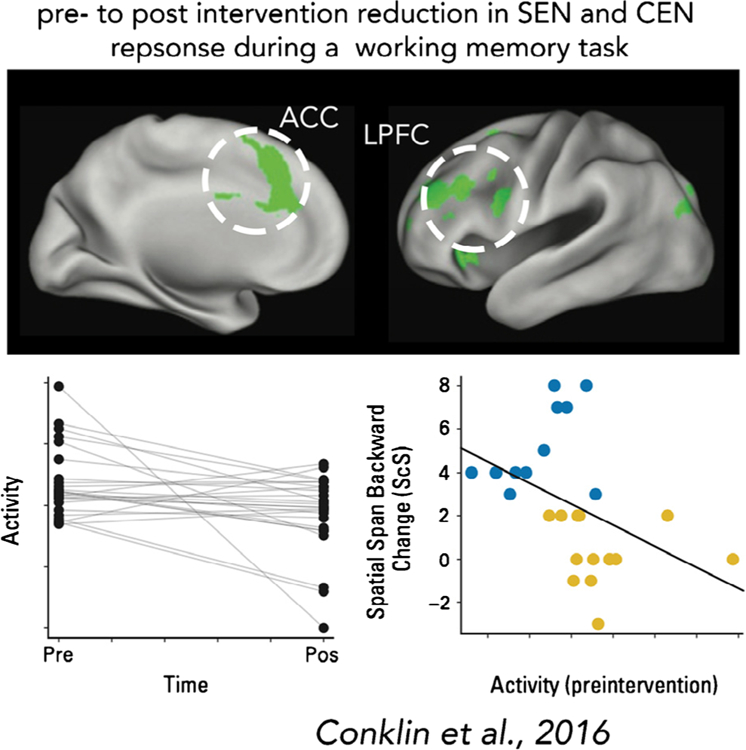
Function of the salience and emotion network (SEN) and other large-scale neurocognitive networks represent key targets for psychosocial and behavioral interventions. 30 survivors of ALL or brain tumor who completed a 25-week computerized cognitive training intervention exhibited increased behavioral performance in several cognitive domains (Conklin et al. 2016). This improvement in behavioral performance was accompanied by a pre- to post-intervention reduction in SEN (anterior cingulate cortex, ACC) and CEN (lateral prefrontal cortex, LPFC) activity during a working memory task (top and bottom left panels). Additionally, lower pre-intervention activity in CEN (dorsolateral prefrontal cortex) during a working memory task predicted positive treatment response, i.e., greater positive change in working memory performance (i.e., spatial span backward; bottom right panel). Abbreviations: ALL, acute lymphoblastic leukemia; CEN, central executive network; SEN, salience and emotion network
Only one published fMRI study has evaluated rsFC in young cancer survivors (15 ALL survivors [ages 8–15] relative to 14 matched controls; Kesler et al. 2014). In this study, rsFC between several brain regions was altered, including reduced rsFC of hippocampus and amygdala (part of the SEN) with several regions involved in attention and visual processing (e.g., occipital areas) in survivors relative to controls (see Figs. 5 and 6). Although no group differences in cognitive functioning were observed between survivors and controls, lower amygdalar and hippocampal rsFC was associated with poorer cognitive functioning (i.e., IQ, Color Naming; Kesler et al. 2014). This study was the first to identify disruptions in intrinsic brain connectivity in pediatric cancer survivors, and suggests that alterations in rsFC may be detected even when objective cognitive functioning seems normal. Similar to other neuroimaging studies in survivors (e.g., Kesler et al. 2016), time since treatment and treatment intensity were not related to rsFC. These findings suggest that neuroimaging correlates of pediatric cancer can be observed as early as six months off treatment, and may reflect disruptions in more global patterns of brain organization following a developmental insult (i.e., CNS-directed cancer treatment or childhood adversity). In addition, lower rsFC was related to younger age at diagnosis in the ALL group, suggesting that younger children are more vulnerable to neurobiological changes, and therefore adverse cognitive, behavioral, and emotional consequences, following cancer. This is consistent with observations from behavioral and psychological research, for e.g., higher treatment intensity, younger age at diagnosis, and female gender are associated with poorer emotional health (e.g., higher PTSS; Bruce 2006) and poorer performance on a range of neuropsychological tasks (e.g., sustained attention, visuo-motor control; Smibert et al. 1996; Buizer et al. 2005a; Buizer et al. 2005b).
Fig. 5.
Altered rsFC in young survivors of pediatric cancer. Regional increases (blue) and decreases (green-yellow) in rsFC in n = 15 young ALL (ages 8–15 years, 9–110 months off treatment) and n = 14 matched controls. In particular, ALL survivors showed reduced rsFC of amygdala (‘RAMG’) and hippocampus (‘LHIP’,’RHIP’) with attention and visual regions (e.g., occipital; Kesler et al. 2014). Abbreviations: ALL, acute lymphoblastic leukemia; rsFC, resting-state functional connectivity. All images are adapted with permission
Fig. 6.
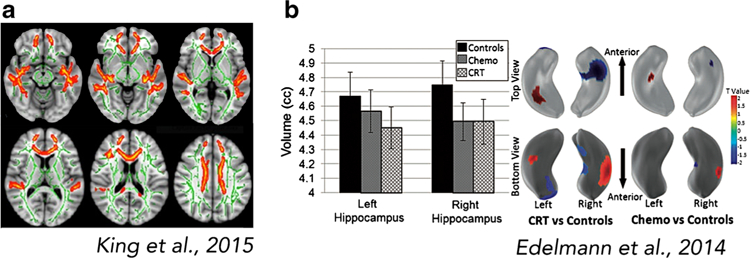
Altered brain structure in adult survivors of pediatric cancer. a Relative to matched controls (n = 27), adult survivors of childhood brain tumor (n = 27, ages 18–32; average of 13.7 years [SD = 5.37] since diagnosis) demonstrate reductions in indicators of white matter integrity in frontal and temporal areas. White matter integrity in frontal areas was positively correlated with IQ (King et al. 2015b). b Altered hippocampal volume and shape in adult survivors of pediatric ALL treated with CRT (n = 39, mean age = 26.7 years [SD = 3.4]; average of 23.9 years [SD = 3.1] since diagnosis) or CT only (n = 36, mean age = 24.9 years [SD = 3.6]; average of 15 years [SD = 1.7] since diagnosis) relative to controls (n = 23, mean age = 23.1 years; Edelmann et al. 2014). Abbreviations: ALL, acute lymphoblastic leukemia; CRT, cranial radiation therapy; CT, chemotherapy. All images are adapted with permission
Neuroimaging studies in adult survivors of childhood cancer
An analogous literature search identified 16 studies of brain structure or brain function in adult (i.e., ages ≥ 18) survivors of childhood cancer (Table 2). Most studies used structural MRI or DTI to measure brain structure, and only three studies (19%) used fMRI to examine task-based neural activity (n = 2) or rsFC (n = 1). Just over half (56%) of the identified studies included ALL survivors, 37% included CNS tumors, and one study (6%) examined Hodgkin Lymphoma survivors. The majority (81%) of the 16 studies linked structural or functional neural variation to measures of cognitive or behavioral functioning (e.g., working memory). None linked neural variation to physical (e.g., ataxia), emotional (e.g., anxiety), or quality of life outcome measures.
Table 2.
Review of neuroimaging studies in adult survivors of pediatric cancer
| First author | Year of publication | Journal | Sample size (N) | Survivors (n) | Healthy controls (n) | Age at assessment (years) | Age at diagnosis in years (mean, SD, [range]) | Time since diagnosis/treatme nt in years (mean, SD, | Type of cancer |
|---|---|---|---|---|---|---|---|---|---|
| 1 Chen et al. | 2016 | Neuroimage Clinical | 32 | 16 | 16 | 22.5 (5.2) [1734] | 7.6 (5.1) | 14.9 (7.3) | Cerebellar tumor |
| 2 Tamnes et al. | 2015 | Pediatric Blood and Cancer | 260 | 130 | 130 | 29.3 (7.3) [18.6–46.5] | 6.2 (4.0) [0.3–16.0] | 23.0 (7.7) [7.4–40.0] | ALL |
| 3 Jayakar et al. | 2015 | Neuropsychology | 94 | 35 | 59 | 24.10 (4.93) [17–36] | 8.17 (4.43) [117] | 15.38 (5.34) [524] | Brain tumor (posterior fossa, pituitary, frontal, temporal, other) |
| 4 King et al. | 2015b | PLoS ONE | 54 | 27 | 27 | 22.7 (4.5) [18–32] | 9.0 (5.14) | 13.7 (5.37) | Brain tumor (posterior fossa, temporal, occipital, hypothalamic, third ventricle) |
| 5 King et al. | 2015a | Journal of the International Neuropsychological Society | 34 | 17 | 17 | 23.2 (5.9) [1735] | 7.65 (4.90) [117] | 15.5 (7.6) [4.5–30] | Posterior fossa brain tumor |
| 6 Smith et. al. | 2014 | Neuropsychology | 37 | 18 | 19 | 24.19 (4.51) [19–40] | 7.22 (4.57) [117] | 17.13 (5.43) [525] | Brain tumor (n = 6 MB n = 1 pineoblastoma, n = 9 astrocytoma, n = 1 ganglioglioma, and n = 1 craniopharyngioma). Location of tumor: n = 13 in posterior fossa, n = 1 pareital lobe, n = 1 occipital |
| 7 Edelmann et al. | 2014 | Brain | 98 | 75 | 23 | CT alone: 24.9 (3.6), CRT: 26.7 (3.4) | CT alone: 9.97 (3.99), CRT: 2.81 (1.73) | CT alone: 15.0 (1.7), CRT: 23.9 (3.1) | ALL |
| 8 Schuite ma et al | 2013 | Journal of Clinical Oncology | 142 | 93 | 49 | CRT and CT: 31.2 (4.8), CT: 26.7 (5.1) | CRT and CT: 5.7 (3.7), CT: 5.3 (3.5) | CRT and CT: 25.4 (3.2), CT: 21.4 (2.9) | ALL, lymphoma |
| 9 Zeller et al. | 2013 | Journal of Clinical Oncology | 260 | 130 | 130 | 28.4 [18.646.5] | 5.3 [0.3–15.9] | 22.5 [7.4–40.0] | ALL |
| 10 Edelmann et al. | 2013 | Pediatric Blood and Cancer | 38 | 38 | __ | Without dex: 24.6 [20.432.4], with dex: 24.6 [19.731.2] | Without dex: 8.7 [3.8–16.9], with dex: 11.8 [5.818.6] | Without dex: 15.9 [14.8–17.9], with dex: 13.3 [12.015.1] | ALL |
| 11 Monje et al. | 2013 | Pediatric Blood and Cancer | 20 | 10 | 10 | 30.8 [22–40] | 10.2 [1.83–17.0] | Not stated | ALL |
| 12 Armstrong et al. | 2013 | Journal of the National Cancer Institute | 85 | 85 | __ | 36.7 (6.4) | 6.6 (4.1) | 30.1 (6.2) | ALL |
| 13 Krull et al. | 2012 | Journal of Clinical Oncology | 62 | 62 | __ | 42.2 (4.77) [1855] | 15.1 (3.30) | >= 15 | Hodgkin lymphoma |
| 14 Brinkman et al. | 2012 | Neuro-oncology | 20 | 20 | __ | 29 [21–36] | [2–17] | 18 [12–25] | MB |
| 15 Dellani et al. | 2008 | Journal of Magnetic Resonance Imaging | 27 | 13 | 14 | 17–37 | 2–16 | 16–23 | ALL |
| 16 Porto et al. | 2008 | European radiology | 41 | 20 (n=10 male, n=10 female) | 21 | Male: 22.0 (3.2) [18–28], Female: 23.2 (3.5) [18–27] | Male: 8.1 (3.8) [2.2–13.7], Female: 8.1 (3.8) [3.0–13.3] | Not stated | |
| First author | Type of treatment | Neuroimaging modalities | Type of study | Physical and physiological outcome measures | Cognitive and behavioral outcome measures | Emotional and quality of life measures | Main findings | ||
| 1 Chen et al. | Surgical resection (n = 16), CT (n = 7), CRT (n = 8) | Resting-state fMRI | Cross-sectional | __ | __ | __ | Pediatric brain tumor survivors showed altered rsFC in frontal functional networks (executive control, salience, default mode) compared to controls. Even at rest, survivors’ hyperconnectivity in frontal functional networks may reflect recruitment of more brain regions due to continuous needs of a higher level of cognitive effort (“all hands on deck” approach). | ||
| 2 Tamnes et al. | CT (n=130), CT and CRT (n=18), CT and stem-cell transplantation (n=3) | Structural MRI | Cross-sectional | __ | Executive functioning | __ | ALL survivors had smaller surface area in several cortical regions but reduced cortical thickness in only one region, compared to controls. Cortical surface area/thickness in these regions was not associated with disease or treatment variables. In survivors, reduced cortical surface area in prefrontal regions was associated with more self-reported problems in executive functioning (particularly difficulties in emotional control and self-monitoring). | ||
| 3 Jayakar et al. | Surgical resection (n=35), CT (n=12), CRT (n=16) | Structural MRI | Cross-sectional | __ | Verbal memory, IQ | __ | Compared to controls, brain tumor survivors had lower hippocampal, putamen, and whole brain volumes as well as lower verbal memory scores (auditory attention list span and final list learning). Hippocampal volume in survivors was significantly correlated with auditory attention. In both groups, hippocampal and putamen volumes were significantly correlated with each other but not with total brain volume. | ||
| 4 King et al. | CRT with CT (n=11), CRT without CT (n=3), no CRT (n=12), CT and no CRT (n=1) | Structural MRI, DTI | Cross-sectional | __ | IQ | __ | Brain tumor survivors treated with CRT had lower IQ and a lower level of white matter integrity relative to survivors without CRT and healthy controls. Non-CRT treated survivors had lower mean FA compared with healthy controls. IQ and cumulative neurological factors were related to white matter disruption in the CRT-treated group of survivors. | ||
| 5 King et al. | Neurosurgery (n=17), CT (n=8), CRT (n=9) | Task-based fMRI (N-back paradigm) | Cross-sectional | __ | Working memory, IQ | __ | Survivors of childhood posterior fossa brain tumors had lower IQ scores and working memory performance compared with controls. Among survivors, increased prefrontal activation (left superior/middle frontal gyri) during a working memory task was associated with increased working memory demands and re duced working memory performance. | ||
| 6 Smith et. al. | Surgical resection (n = 18), CT and CRT (n = 8), CT (n = 1), and CRT (n = 1) | DTI | Cross-sectional | __ | Reading achievement, processing speed, skilled motor speed | __ | Among brain tumor survivors and controls, white matter FA values of the parietotemporal-occipitotemoral (PT-OT) tract were associated with word reading, and FA values in the inferior fronto-occipital fasciculus (IFOF) were associated with reading in survivors only. Among survivors only, processing speed mediated the relation between white matter FA (in PTOT and IFOF) and word reading skill. | ||
| 7 Edelmann et al. | CT alone (n=36), CT and CRT (n=39) | Structural MRI, DTI | Cross-sectional | __ | IQ, academic performance, attention, memory, processing speed, executive function | __ | CRT-treated ALL survivors performed lower than survivors treated with CT alone on only 3 of 20 neurocognitive measures. Compared to healthy controls, ALL survivors had lower neurocognitive performance, reduced gray and white matter, and higher FA in fibre tracts within the left hemisphere. Frontal and temporal lobe volumes correlated with vocabulary and academic ability; frontal, parietal, and temporal white matter volumes were associated with memory. Higher FA in the left longitudinal fasciculus and left uncinate fasciculus were associated with lower memory and learning performance. Increased FA in the left sagittal stratum was associated with better sustained attention | ||
| 8 Schuite ma et al | CT (n=49), CT and CRT (n=44) | DTI | Cross-sectional | __ | IQ, speed and accuracy of information processing, attention, working memory | __ | Compared with controls, ALL and lymphoma survivors treated with CRT showed decreased FA in frontal, parietal, and temporal WM tracts. Decreased FA was asociated with poorer neuropsychological performance. Trends for lower WM FA were seen in the CT-treated survivors. Similarly, CRT-treated survivors performed significantly lower on all neuropsychological measures compared to controls whereas survivors treated with CT alone did not differ significantly from controls. Reduced WM integrity in CRT-treated survivors was associated with younger age at CRT and higher dosage. | ||
| 9 Zeller et al. | CT (n=130), CT and CRT (n=18), CT and stem-cell transplantation (n=3) | Structural MRI | Cross-sectional | __ | IQ, processing speed, executive function, verbal learning/memory | __ | ALL survivors showed smaller volumes of cortical gray matter, cerebral white matter, amygdala, caudate, hippocampus, thalamus, and intracranial volume compared with controls – with the strongest effect found in the caudate. These neuroanatomic volumes were not effected by age at diagnosis nor treatment variables. Survivors also showed reduced processing speed, executive function, and verbal learning/memory in survivors compared with controls. Reduced neurocognitive performance correlated with smaller volumes of cortical gray matter, caudate, and thalamus and intracranial volume in survivors | ||
| 10 Edelmann et al. | CT (n=20 with prednisone and no dexamethasone, n=18 with dexamethasone) | Structural MRI, task-based fMRI (memory recognition tasks: word & face) | Cross-sectional | __ | IQ, academic performance, memory | __ | Compared to survivors treated with only prednisone (without dexamethasone), ALL survivors treated with dexamethasone had lower performance on several memory-dependent measures in cluding story memory and word recognition. Decreased neural activation in the left retrosplenial brain region was associated with dexamethasone treatment; story memory was associated with altered activation in left inferior frontal-temporal brain regions. | ||
| 11 Monje et al. | CT and CRT (n=10) | Structural MRI; task-based fMRI (memory encoding task) | Cross-sectional | __ | Recognition memory | __ | Compared to healthy controls, ALL survivors demonstrated lower recognition memory, greater hippocampal atrophy and altered memory-related hippocampal activation. Survivors showed increased neural activation in several brain regions during unsuccessful memory encoding, which may reflect ineffective compensatory neural recruitment. | ||
| 12 Armstrong et al. | CT (n=85), 24 Gy CRT (n=36), 18 Gy CRT (n=49) | Task-based fMRI (memory task), DTI | Cross-sectional | __ | Memory, IQ, cognitive status | __ | ALL survivors who received 24 Gy (but not 18 Gy) CRT had reduced cognitive status and memory performance. The mean score for long-term narrative memory among survivors who received 24 Gy CRT was equivalent to that for individuals older than 69 years. Memory impairments were associated with smaller temporal lobe white matter volumes, thinner parietal and frontal cortices, increased radial diffusivity (inverse measure of WM integrity) in parietal and temporal lobes, and reduced hippocampal volume. Neural activation during memory retrieval in the left anterior hippocampus was correlated with design memory impairment in all survivors, and was driven by the the 24 Gy rather than the 18 Gy group. Neural activation during | ||
| 13 Krull et al. | CT and thoracic radiation | Structural MRI | Cross-sectional | __ | IQ, attention, memory, processing speed, executive function | __ | Lymphoma survivors showed lower performance on sustained attention, short-term memory, long-term memory, working memory, naming speed, and cognitive fluency compared with age-adjusted norms. Leukoencephalopathy was present in 53% of survivors, who demonstrated lower cognitive fluency than those without leukoencephalopathy. Evidence of cerebrovascular injury was present in 37% of survivors. | ||
| 14 Brinkman et al. | CRT (n=20), CT (n=15) | Structural MRI, DTI | Cross-sectional | __ | IQ, academic skills, memory, attention, processing speed, motor function, executive function | __ | IQ in medulloblastoma survivors was lower than population norms, and 75% of survivors showed executive function impairment. Lower performance on executive function tasks was correlated with reduced white matter intergrity in multiple brain regions. Radial diffusivity in the frontal lobes was correlated with shifting attention and cognitive flexibility, whereas volume and cortical thickness were not correlated with neurocognitive function. Neurocognitive impairment was common and involved many domains. | ||
| 15 Dellani et al. | Total brain radiation (18–24 Gy) and CT | DTI | Cross-sectional | __ | __ | __ | ALL survivors had significantly reduced white matter FA values in the temporal lobes, hippocampi, and thalami along with significant white matter volume loss. Although survivors did not show reductions in gray matter, they did show decreased total brain volume and intracranial volume compared to controls. Concerning structural white matter changes (as indexed by global and frontal WM mean diffusivity), adult survivors of childhood ALL show the same age dependence as controls - no age dependence of radiation damage was found. | ||
| 16 Porto et al. | CT (n = 10), CT and CRT (n = 10) | Structural MRI, DTI | Cross-sectional | __ | __ | __ | Compared to controls, ALL survivors showed reduced white matter FA, with the most severe effects apparent in those who had received both CRT and CT (compared to CT alone). Survivors treated with CRT had reduced WM volumes and gray matter concentration within the caudate nucleus and thalamus. | ||
Abbreviations: ALL, acute lymphoblastic leukemia; PA, pilocytic astrocytoma; MB, medulloblastoma; PNET, primitive neuroectodermal tumor; WM, white matter; NAWM, normal appearing white mater; LGG, low-grade glioma; CT, chemotherapy; CRT; cranial radiation therapy; IV-MTX, intravenous methotrexate; CSI, craniospinal irradiation; BMT, bone marrow transplant; DTI, diffusion tensor imaging; fMRI, functional magnetic resonance imaging; SPECT, single-photon emission computed tomography; DWI, diffusion weighted imaging; ASL, arterial spin labelling; IQ, intelligence quotient; FA, fractional anisotropy,
Structural or diffusion MRI studies
Similar to structural neuroimaging studies in children, neuroimaging studies in adult survivors of childhood cancer commonly report reductions in volume or indicators of white matter macrostructure, including frontal, parietal, and temporal areas implicated in memory, affect, and executive functioning (see Fig. 7a; King et al. 2015a; Brinkman et al. 2012; Schuitema et al. 2013; Dellani et al. 2008; Porto et al. 2008). Of note, one study reported no difference in white matter macrostructure among adult survivors of pediatric ALL treated with chemotherapy only and matched controls (Genschaft et al. 2013), while another study reported regional increases in indicators of white matter macrostructure within several pathways (e.g., uncinate fasciculus, cingulum bundle) in adult survivors of childhood ALL, regardless of treatment (chemotherapy, radiation), which also corresponded with poorer neurocognitive functioning (e.g., memory span; Edelmann et al. 2014). Edelmann et al. (2014) speculated that increased white matter macrostructure among survivors may reflect glial scarring or white matter compaction. Also in agreement with studies in children, white matter alterations are observed irrespective of location of the tumor and treatment, although white matter alterations appear to be exacerbated among adults who received cranial radiation therapy relative to chemotherapy alone as children. Younger age at treatment is related to worse neuroanatomical outcomes among adult survivors (see Table 2). Taken together, these findings indicate persistent disruptions in white matter development among adult survivors of childhood cancer.
Fig. 7.
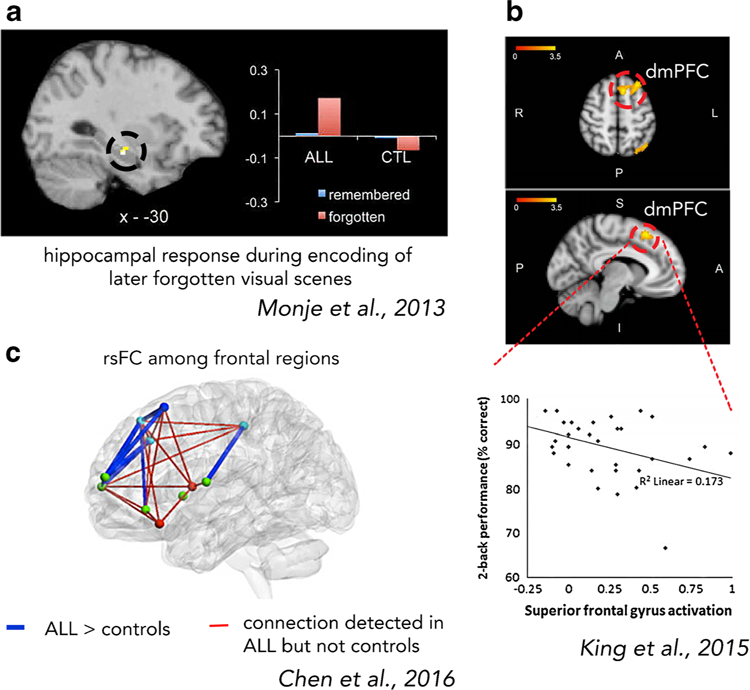
Altered brain function in adult survivors of pediatric cancer. a Abnormal elevation in hippocampal activity during encoding of later forgotten items in n = 10 adult survivors of pediatric ALL (average age = 30.8 years; 20–30 years after treatment) relative to n = 10 matched controls (“CTL”, Monje et al. 2013). Functional neural changes were accompanied by poorer recognition memory and hippocampal atrophy in the ALL relative to control group. b Adult survivors of pediatric brain tumor (n = 17, average age = 23.39 [SD = 4.46]; 15.5 years (SD = 7.6) post diagnosis) exhibited greater activation in dmPFC, a SEN region, during a working memory paradigm (2-back condition) relative to matched controls (n = 17). Higher dmPFC activation was associated with poorer performance, suggesting an ineffective compensatory neural mechanism – similar to studies in children (Fig. 3a; King et al. 2015a). (c) Increased rsFC among frontal regions in n = 16 adult survivors of childhood cerebellar tumors (ages 17–34 years; average of 14.0 years [SD = 7.3] since diagnosis) relative to n = 16 matched controls (Chen et al. 2016). Abbreviations: ALL, acute lymphoblastic leukemia; SEN, salience and emotion network; rsFC, resting-state functional connectivity; dmPFC, dorsomedial prefrontal cortex. All images are adapted with permission
Five studies reported reduced volume or shape alterations of the hippocampus among adult survivors of pediatric ALL (Monje et al. 2013; see Fig. 7b; Edelmann et al. 2014; Zeller et al. 2013; Armstrong et al. 2013) or brain tumor (Nagel et al. 2004; see Table 2; Jayakar et al. 2015). Several studies linked hippocampal atrophy to poorer neurocognitive functioning (e.g., episodic memory; see Table 2). In addition to the hippocampus, reductions in caudate, putamen, and SEN regions (amygdala, thalamus) are reported among adult survivors of ALL and brain tumor (Jayakar et al. 2015; Zeller et al. 2013; Porto et al. 2008). Reductions in these areas were also reported in young survivors of childhood cancer (see Table 1), indicating persistent disruptions in subcortical development.
Functional MRI studies
Four studies used fMRI to examine neural correlates of pediatric cancer in adult survivors. In addition to hippocampal atrophy, Monje et al. (2013) report altered hippocampal activation during memory encoding in 10 adult survivors of childhood ALL relative to 10 matched controls (see Fig. 7a). Whereas controls recruited hippocampus during the encoding of items that were later remembered, ALL survivors showed the reverse—they recruited the hippocampus during the encoding of items that were later forgotten, which the authors interpreted as reflecting ineffective neural recruitment following pediatric cancer (Monje et al. 2013). Neurobehavioral responses to working memory have also been examined among adult survivors of childhood cancer. Consistent with studies in youth, adult survivors of childhood cancer (i.e., brain tumor) demonstrate increased neural response in fronto-parietal areas during higher load (i.e., 2-back) conditions, relative to controls (King et al. 2015a; see Fig.7b). Similarly, performance was worse for higher load (i.e., 2- and 3-back) but not lower load conditions (i.e., 0- and 1-back) in survivors relative to controls, and higher BOLD response in frontal regions was associated with poorer working memory performance during the 2-back condition. Taken together, these findings suggest that adult survivors of childhood cancer engage greater neural resources in response to increased demands of working memory relative to controls. The pattern of increased neural response and poorer performance for higher- but not lower-demanding conditions is consistent with an ineffective compensatory neural mechanism that has been observed in working memory studies of young brain tumor survivors (e.g., Robinson et al. 2014). In other words, survivors display a ramping up of cognitive and neural resources in the context of poorer behavioral performance to more demanding memory conditions (King et al. 2015a), and that this neurobehavioral dysfunction persists well into adulthood.
To date, only one study has examined rsFC among adult survivors of pediatric cancer. Chen et al. (2016) observed increased rsFC in frontal regions of the CEN, SEN, and DMN (see Fig. 7c). Consistent with the notion of (ineffective) compensatory neural functioning, the authors speculated that increased rsFC among frontal regions may reflect a need for a higher level of cognitive effort among survivors that requires recruitment of additional higher-order brain regions (Chen et al. 2016). Although we identified only two studies that examined functional regional interactions in child, adolescent, or adult survivors (Chen et al. 2016; Kesler et al. 2014), these studies are important because they suggest that well-documented regional changes (for e.g., in hippocampus) are accompanied by changes in neural organization at the network or whole-brain level. Examining neural network dynamics may provide better mechanistic understanding of cognitive or affective disruptions following pediatric cancer, as brain regions do not operate in isolation, and cognitive and affective processes rely on interactions between a distributed set of brain regions and networks.
Summary and gaps in current literature
In summary, our literature review identified 65 neuroimaging studies in child or adolescent patients or survivors, and 16 in adult survivors of childhood cancer. Together, most studies (83%) used structural MRI or DTI to examine brain structure (e.g., volume, thickness, or integrity of gray or white matter). Fewer studies used fMRI, with 14 task-related fMRI studies, and two rsFC studies. Studies in adults are critical because they demonstrate that neurological consequences of pediatric cancer are lasting, and can be detected years and even decades following the conclusion of treatment. Evidence of persistent neurobiological changes following pediatric cancer underscores the potency of this developmental experience, and motivates early prevention and intervention efforts that are capable of moving individuals off a harmful neurodevelopmental trajectory and on to a healthier one. Intervening early during brain development capitalizes on the inherent plasticity of the developing brain that may allow for more enduring positive effects. We assert that research in childhood, proximal to the time of brain injury and insult, will be essential for identifying (1) age-appropriate targets for such interventions, and (2) developmental periods of sensitivity to insult and intervention.
We identify at least two major gaps in our current understanding. First, while most studies link neural changes with cognitive dysfunction, only five studies linked neural changes with emotion-related psychological outcomes (e.g., internalizing symptoms, emotional adjustment) or quality of life measures (see Table 1). This is surprising, given that psychosocial and emotional difficulties are well documented in survivors of pediatric cancer, and many brain regions found to be consistently altered in survivors (e.g., amygdala, hippocampus, insula, PFC, ACC) also play a pivotal role in emotion processing and regulation (Etkin et al. 2015; Etkin et al. 2011; Phelps & LeDoux 2005). Alterations in these regions are centrally implicated in the development and expression of emotional disorders, including anxiety, depression, and PTSD (see meta-analyses by Etkin & Wager 2007; Hamilton et al. 2012). To our knowledge, no neuroimaging studies to date have used task-based fMRI to evaluate neurobehavioral processes associated with active emotion processing or regulation in pediatric cancer survivors.
Second, observed neurobiological changes are largely attributed to neurotoxic effects of cancer treatments on brain development – with several studies demonstrating dose-dependent effects of cancer treatment intensity (i.e., dosage, modality) on brain structure and function (e.g., Qiu et al. 2006; Reddick et al. 2005a, b; see Tables 1 and 2). The role of childhood adversity, in the form of threat exposure, in pediatric cancer has been largely ignored in the existing neuroscientific literature, despite the presence of significant threat to life and physical integrity associated with the disease and its invasive medical procedures. The disconnect, both regarding the measurement of emotion-related psychological outcomes in studies of neural development and the potential drivers of change (i.e., threat exposure, cancer treatment), constitutes a critical barrier to identifying pathways through which childhood cancer impacts neural development, and ultimately, psychological wellbeing. The field lacks a neurodevelopmental framework that considers the joint effects of early threat and cancer treatments, and could provide a more integrative understanding of cancer-related changes across cognitive, behavioral, and emotional domains.
An integrated neurodevelopmental framework
To address this gap in the literature, we propose a novel neurodevelopmental framework that considers childhood cancer as a type of childhood adversity, specifically an early threat exposure, and considers the joint impact of early threat and cancer treatments on specific sensitive brain systems (see Fig. 1). We argue that this framework may be helpful for identifying pathways through which childhood cancer impacts neural development, and ultimately, cognitive, behavioral, and emotional sequela. We build the framework of childhood cancer as a type of early threat exposure based on the existing literature on neurodevelopmental consequences of other more commonly-studied threat-related childhood adversities (e.g., violence, abuse) and integrate these neurodevelopmental consequences with research on psychosocial and neurocognitive consequences of childhood cancer.
Childhood cancer as a form of early threat exposure
Existing neuroscientific research on childhood adversity indicates that different types of exposures have distinct effects on brain development. A recent neurobiological framework proposed by McLaughlin et al. (2014) differentiates between deprivation (e.g., neglect) and threat (e.g., community/domestic violence, physical/sexual abuse, assault) as core dimensions of childhood adversity that have strong and distinct effects on neural development. Although experiences of violence and abuse differ in many ways from childhood cancer (e.g., context, nature), we assert that these experiences share a common element of early threat that is thought to lead to certain neurodevelopmental adaptations in violence and abuse, and may also contribute to similar neurodevelopmental changes following pediatric cancer.
The ‘active ingredient’ in threat exposure is the presence of unexpected inputs that represent significant threats to the physical integrity or wellbeing of the child (McLaughlin et al. 2014). This is largely consistent with the cancer experience. Childhood cancer involves the presence of a life-threatening disease that evokes a great sense of uncertainty about the future that remains throughout the lifespan. At the same time, families are confronted with an unpredictable and uncontrollable course of cancer treatment, including intrusive, unfamiliar, and sometimes painful medical procedures that may leave the patient and caregivers to feel immense helplessness. Together, these distressing experiences contribute to high rates of PTSS among patients and their family members, which may take the form of intrusive thoughts, avoidant behaviors, hyperarousal, dissociation, and negative changes in mood or cognition (American Psychiatric Association 2013). As many as 75% of young adult survivors of childhood cancer report re-experiencing traumatic parts of cancer, and nearly 50% report increased physiological responses when reminded of cancer (Rourke & Kazak 2005; Price et al., 2016b). Given these data, PTSS has emerged as one of the most important psychological consequences of childhood cancer (Kazak et al. 2001; Erickson & Steiner 2001). Consistent with our conceptualization of childhood cancer as a form of threat exposure, PTSS is more commonly linked to experiences of life threat rather than deprivation (e.g., neglect) (Sullivan et al. 2006). This is also reflected by diagnostic criteria for PTSD in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as the ‘trauma’ must involve exposure to actual or threatened serious injury, violence, or death (American Psychiatric Association 2013; see Kangas 2013 for discussion of diagnostic criteria in the context of cancer).
Of note, although we propose that the early threat exposure dimension is a useful starting point for understanding the effects of childhood cancer on neural development, there are many important differences between childhood cancer and other more commonly-studied forms of early (i.e., physical/sexual abuse, assault, domestic violence, community violence). That is, while these experiences share an element of early threat, they are not the same in other important ways (e.g., family functioning, chaotic environment). For instance, violence and abuse can be systemic, encompass the whole of a child’s ecological environment, and is often perpetrated by a family member or another trusted individual. Childhood cancer, in contrast, can be very specific and focal, and does not necessarily pose a threat to the primary attachment relationships. However, cancer may have the additional burden of chronic impairments and physical disability, which can also represent early adversity for some children. These factors will be considered in more detail, below.
Sensitivity of the hippocampus and regions of the salience and emotion network (SEN) to pediatric cancer and its treatment
Changes in the brain are a proximal indicator of the biological embedding of cancer-related change during development, and should reveal mechanisms by which individual differences or environmental factors modulate cognitive, behavioral, and emotional outcomes (see Fig. 1). Due to the heterochronous nature of brain development, certain brain areas may be particularly vulnerable to developmental insults related to threat exposure or CNS-directed cancer treatments during periods of brain development such as during childhood. Brain areas that play a central role in threat-related processing, learning, or memory are also likely to be altered in response to significant threat to life or physical integrity. The model proposed here considers the vulnerability of the hippocampus and regions of the “salience and emotion network” (SEN) to developmental insult, and aims to link the diverse functions of these brain systems (e.g., memory, attention, emotion processing and regulation, cognitive control) to the array of cognitive, behavioral, and emotional disruptions observed among patients and survivors of childhood cancer. Sensitivity of the hippocampus and SEN to pediatric cancer is supported by our literature review. We found several studies that reported structural and functional changes in the hippocampus and various SEN regions in childhood cancer patients and survivors, including amygdala, thalamus, nucleus accumbens, insula, and ACC (see Tables 1 and 2, Figs. 2, 3, 4 and 5). Although we assert that the hippocampus and regions of the SEN are likely vulnerable to developmental insults, we are not arguing that these are the only brain systems affected. Altered development of those areas and their connections may lead to disrupted integration into larger-scale brain systems, or compensatory effects in other brain areas or systems (e.g., PFC). These processes likely evolve over time and over the course of brain development.
The SEN is involved in a wide range of cognitive and affective functions by detecting and orienting attention to biologically or cognitively relevant internal and external stimuli (Seeley et al., 2007), and is thus critical to understanding cancer as a threat exposure. The SEN is anchored in the insula and ACC, but also encompasses several subcortical regions that allow for integration of salient affective and motivationally-relevant cues into processing. Subcortical areas include: the amygdala—involved in emotional learning and expression; the thalamus—involved in emotional attention and awareness; the ventral striatum (including nucleus accumbens)—involved in reward evaluation and incentive-based learning; and the substantia nigra/ventral tegmental area (VTA)—a midbrain region home to dopamine cells that signal motivational value or salience (e.g., rewards or threats) and prime the rapid detection of potentially significant cues (Seeley et al., 2007; Berridge 2007; Smith et al. 2011; Pessoa & Adolphs 2010). Given its position at the interface of cognitive, homeostatic, motivational, and affective systems of the brain, the SEN is well positioned to adaptively guide behavior. Although the SEN plays a central role in socioemotional processing, it contributes to a variety of complex brain functions through the integration of sensory, emotional, and cognitive information. Once a salient event has been detected, the SEN facilitates access to attention and working memory resources. This distinctive role underscores the potential for profound disruptions in cognitive and affective functioning should development of the SEN be altered (see review by Menon 2011).
Separate lines of evidence converge on the hippocampus as a brain area that is sensitive to neurotoxic effects of chemo- and radiation-therapy, but also neurotoxic effects of threat exposures during sensitive periods of brain development (Heim & Binder 2012). Broadly, the hippocampus is involved in spatial navigation within an environment and forming long-term memories for events that occur within an environment (see review by Buzsáki and Moser 2013). Although the hippocampus is typically not considered to be part of the SEN, it is densely interconnected with various SEN regions, including amygdala, ACC, insula, VTA, and ventral striatum (Witter 2010), and participates in emotional processing, and the transfer of salient events (including emotional events) into long-term memory. Thus, salient emotional events, such as those associated with significant threat to life or physical integrity, are likely to be strongly held in memory, and may thus alter functional and structural development of the hippocampus. Another factor that may contribute to the sensitivity of the hippocampus to pediatric cancer is that it is one of few brain areas that shows active postnatal neurogenesis (Kohman & Rhodes 2013), which may render it more sensitive to developmental insults. Indeed, animal models demonstrate adversity-related suppression in hippocampal neurogenesis, and remodeling of dendritic and spine morphology (Joëls et al. 2007). Likewise, radiation and cytostatic drugs are shown to induce long-lasting and progressive neurotoxic effects by damaging neural progenitor cell populations (Monje & Dietrich 2012; Dietrich et al. 2015). The contribution of each of these mechanistic processes to changes in neural development, and ultimately, cognitive, behavioral, and emotional outcomes in children is unknown.
Importantly, many neurodevelopmental changes observed in patients and survivors of childhood cancer are similar to those reported in individuals exposed to other forms of threat, including violence and abuse (see Fig. 8). For instance, individuals exposed to early threat demonstrate reduced hippocampal volume (Fig. 8a; for a meta-analysis see, Riem et al. 2015); reductions in frontal and temporal white matter volume or macrostructure (e.g., uncinate fasciculus FA; Hanson et al. 2015; Huang et al. 2012); impairments in working and episodic memory (see Pechtel & Pizzagalli 2011 for a review); increased compensatory SEN response during an executive control task (Fig. 8b); altered amygdala rsFC with temporo-occipital regions (Fig. 8c); altered SEN rsFC and task-related activation (Fig. 8d); and variation in whole brain structural organization (Fig. 8e). The convergence of findings from neurobehavioral studies in pediatric cancer survivors and individuals exposed to interpersonal threat during childhood raises the notion that certain brain areas, particularly hippocampus and SEN regions, are vulnerable to different types of developmental insults in early life (i.e., neural adaptations following early threat or neurotoxicity related to CNS-directed cancer treatments). Examining the potential commonalities and differences among these childhood threat exposures may provide new understanding of neurodevelopmental consequences of childhood adversity, and neurobiological substrates of adversity-related cognitive and affective impairments that are frequently observed in the wake of both pediatric cancer and interpersonal threat exposure.
Fig. 8.
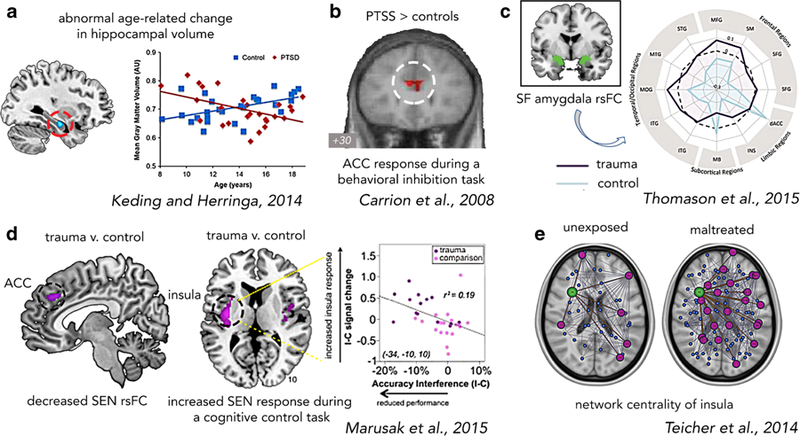
Common alterations in brain structure and function in individuals exposed to more commonly-studied childhood experiences that involve similar elements of early threat (e.g., interpersonal violence, abuse), suggesting a role for neurotoxic effects of early threat exposure in neurodevelopmental consequences of pediatric cancer. a Age-related decreases in hippocampal volume of 27 threat-exposed youth with PTSD (mean age = 14.2 years, SD = 2.7) relative to 27 matched controls (Keding & Herringa 2015). b Increased response in ACC, a core SEN, during a behavioral inhibition task (Go/No-Go) in 16 youth with interpersonal trauma-related PTSS (ages 10–16 years) relative to 14 matched controls (Carrion et al. 2008). No group differences in task performance were observed, suggesting a compensatory neural mechanism that is also observed in pediatric cancer survivors (see Fig. 3a). c Relative to 21 unexposed matched controls, 21 youth exposed to interpersonal threat (e.g., violence, abuse) demonstrated altered rsFC between superficial (‘SF’) amygdala and various regions of SEN (e.g., ACC, insula), prefrontal cortex, and temporal and occipital lobes. Similar to a study of young survivors of pediatric ALL (see Fig. 5), threat-exposed youth demonstrate reduced (negative) rsFC between amygdala and temporo-occitiptal studies, involved in attention and visual processing (Thomason et al. 2015). d Relative to 19 matched controls, 14 youth exposed to interpersonal threat (M age = 12.61 years, SD = 2.11) demonstrate reduced SEN rsFC within the ACC, and increased response in insula, a core SEN region, during a cognitive control (conflict) task. Higher insula response was associated with poorer task performance (Marusak et al. 2015a). e Increased network centrality of the insula, a core SEN region, in 145 18–25 year-olds with histories of maltreatment exposure relative to 123 matched controls (Teicher et al. 2014). Increased prominence of the insula within a whole-brain network is similar to a report in young ALL survivors (Kesler et al. 2014; see Fig. 5). Network centrality was measured using graph theoretical analysis of structural MRI data. Abbreviations: ALL, acute lymphoblastic leukemia; ACC, anterior cingulate cortex; SEN, salience and emotion network; rsFC, resting-state functional connectivity; PTSS, posttraumatic stress symptoms. All images are adapted with permission
It is also important to consider potential areas of divergence, and salient areas of intact functioning between pediatric cancer survivors and individuals exposed to more commonly-studied forms of early threat (e.g., domestic violence, physical and sexual abuse). For example, white matter lesions are commonly reported in the context of pediatric cancer and other forms of brain injury (e.g., traumatic brain injury), but not in studies of interpersonal threat exposure during childhood. In addition, while processing speed, attention, and memory appear to be impaired in both populations, auditory-linguistic domains (e.g., vocabulary, language comprehension) may be relatively spared (Kavanaugh et al. 2017; Riccio et al. 2010). Evaluating the areas of divergence and common areas of intact functioning may provide clues about the role of these environmental experiences in shaping development of specific neural systems.
Given the broad and integral role of the hippocampus and SEN in an array of cognitive and affective processes, evaluation of hippocampal and SEN development may provide a more integrative framework for understanding cognitive, behavioral, and emotional consequences of pediatric cancer. A more integrative network-oriented framework may help to link the existing studies reporting disruptions in localized brain areas (e.g., ACC) or processes (e.g., working memory) with initial reports of altered interactions within and between large-scale neural networks (e.g., DMN-SEN). For instance, we have previously shown disruptions in localized SEN regions (e.g., insula) in youth exposed to interpersonal threat (e.g., violence, abuse) that relate to altered neurocognitive functioning (e.g., conflict interference) and connectivity within the SEN, and between SEN and DMN (Marusak et al. 2015a). Given that the SEN undergoes dramatic reorganization throughout childhood that span both within- and across-network links (Marusak et al., 2017a, b; Uddin et al. 2011), evaluating how pediatric cancer affects the construction and developmental trajectories of the SEN and other neurocognitive “core” networks (i.e., CEN, DMN) may provide unique insights into neurodevelopmental consequences.
Emotional learning and memory
Together with the hippocampus, the SEN plays a key role in emotional learning and memory, and particularly relevant in the context of threat exposures such as pediatric cancer—threat-related learning. Central in this system is the amygdala, which detects whether a stimulus, person, or event is threatening (LeDoux 2003). The role of the hippocampus is to encode contextual features surrounding the aversive stimulus or threatening event, and to regulate the expression or suppression of fear across different contexts (see review by Maren et al. 2013). Emerging research demonstrates that exposure to threatening circumstances early in life can alter fear learning and memory and the underlying neural structures. In one recent study (McLaughlin et al. 2016), a group of children and adolescents exposed to interpersonal threat (physical abuse, sexual abuse, or domestic violence), ages 6–18, and a matched group of unexposed youth underwent a cued fear conditioning paradigm during which blue or yellow cartoon bells (conditioned stimulus, CS) were paired or unpaired with a startling alarm noise (unconditioned stimulus, US). Threat-exposed youth demonstrated blunted physiologic (skin conductance) responses to the threatening cue (i.e., CS+) and showed a lower ability to discriminate between threat (CS+) and safety (CS-) cues. Lower physiologic response to the threatening cue was associated with lower volume of the amygdala and hippocampus among threat-exposed youth. These findings are important because they demonstrate that exposure to threat in early life may fundamentally change neurobehavioral mechanisms of emotional learning, which may lead to disrupted maturation of the SEN and interconnected systems, and aberrant social, affective, and cognitive development. Neurobehavioral mechanisms underlying threat, emotional learning, and memory have yet to be tested in childhood cancer survivors, and may contribute to problems associated with emotional learning among childhood cancer patients and survivors, including PTSS, treatment-related phobia, social anxiety, and school avoidance (e.g., Bessell 2001; Kazak et al. 2001).
Distorted perception of threat and safety may alter a child’s ability to learn and interact with others in social circumstances. Thus, evaluation of emotional learning mechanisms may shed new light onto social difficulties observed in many pediatric cancer survivors, including diminished social competence (e.g., Schulte & Barrera 2010), poor social skills, and difficulties with peer relationships (e.g., Mahajan & Jenney 2004). These difficulties are likely compounded by chronic impairment and physical disability, and feeling like they are different from other children, which can lead to loneliness, social isolation, exacerbation of internalizing problems, and poor academic achievement due to both neural and psychological consequences. The ability to interpret, regulate, and respond appropriately to social cues relies on the hippocampus and the SEN. It is not surprising, then, that these areas are centrally implicated in the pathophysiology of anxiety and depressive disorders, which show a sharp increase in incidence during the transition into adolescence (Kessler et al. 2005). This increase further motivates interventions that target hippocampus, SEN, and emotional learning mechanisms as a means of improving social and emotional adjustment among survivors.
Another neural mechanism for emotional learning depends on the connections between the hippocampus and the VTA, which are considered critical for the gating of novel or salient information into long-term memory (Lisman & Grace 2005). We have recently shown reduced rsFC in hippocampal-VTA circuitry in children and adolescents exposed to interpersonal threat relative to unexposed youth (Marusak et al., 2017b). Lower connectivity in this circuitry suggests a novel mechanism that may serve to adaptively prevent the overwriting of a previously stored trauma memory, but at the same time contribute to the broad range of cognitive, behavioral, and emotional difficulties linked to early threat exposure. It is possible that this circuitry is also altered in pediatric cancer patients and survivors.
Together with the hippocampus, SEN regions are key targets of the mesocorticolimbic dopamine pathway, involved in reward and motivation processing. Deficits in reward processing and motivation are clinical and neurobiological hallmarks of depressive disorders (see meta-analysis by Zhang et al. 2013; see Bogdan et al. 2013). Stress-related alterations within the mesocorticolimbic pathway, particularly while the system is still developing, may contribute to the development of affective disorders. In line with this hypothesis, we and others have found deficits in brain and behavioral measures of reward sensitivity among youths exposed to interpersonal violence (e.g., Marusak et al. 2015b; Marusak et al. 2015a; Guyer et al. 2006) and youth at elevated risk for the development of depressive disorders (e.g., Norman et al. 2012). Thus, reward and motivation neurobehavioral processes represent an open area of inquiry for understanding mechanisms underlying affective dysfunction in childhood cancer survivors.
The role of the SEN in cancer-related pain and cognitive dysfunction
The SEN is involved in a diversity of homeostatic functions beyond socioemotional processing. Of relevance in the context of pediatric cancer is pain expectation, perception, and distress. Pain related to medical procedures, treatment side effects, or the cancer itself is a major source of compromised quality of life among children with cancer and their families (Zebrack & Chesler 2002). Recent longitudinal studies indicate that the burden of cancer-related pain does not end when treatment concludes: many survivors of childhood cancer report cancer-related pain well into adulthood (Lu et al. 2011). Given the central role of the SEN in pain perception, altered development of the SEN may represent a neurobiological target for understanding and treating cancer-related pain. Interestingly, neuroimaging work in adults suggests that the SEN is not only involved in first-hand experiencing of pain, but also complex social emotions, including empathetic responses to emotional distress and perceiving pain in others (Singer et al. 2004). Engagement of the SEN to more vicarious instances of pain and distress may have important implications for adverse cognitive, behavioral, and emotional outcomes in family members, such as parents or siblings, who often witness patients receiving medical treatments and exhibit PTSS in rates that frequently exceed those reported by the patients themselves (e.g., Alderfer et al. 2003).
Another core function of the SEN is controlling engagement of other large-scale neural networks that facilitate access to working memory and attentional resources (e.g., CEN). Thus, characterizing development of the SEN following cancer may shed new light into mechanisms underlying cognitive dysfunction. For instance, altered SEN development may contribute to attentional difficulties, which remain extremely prevalent (up to 67%) among ALL survivors following contemporary chemotherapy-only treatment (Conklin et al., 2012a, b; see meta-analysis by Iyer et al. 2015). Evaluation of the SEN may also provide new insights into potential protective mechanisms and interventional approaches, given research showing that attentional control plays an important role in children’s immediate and longer-term responses to cancer-related medical procedures (Trentacosta et al. 2016).
To summarize, our integrated neurodevelopmental framework considers childhood cancer as a type of childhood adversity, specifically an early threat exposure, and considers the joint impact of threat and cancer treatments on neural development (see Fig. 1). We suggest that changes in the brain may confer alterations in core cognitive and affective processes (e.g., elevated threat processing, decreased attentional control) that increase risk for cognitive, behavioral, and emotional problems in some youth. We also suggest that brain areas that are sensitive to (1) developmental insults during childhood (e.g., ongoing development or active postnatal neurogenesis), or (2) that are centrally involved in threat-related processing, may be critical for identifying pathways through which childhood cancer impacts neural development, and ultimately, psychological outcomes. We offer the hippocampus and the SEN as key brain systems of interest, based on existing neuroimaging studies in pediatric cancer survivors and in children exposed to other forms of early threat (e.g., violence, abuse).
Directions for future research
Consideration of other external and individual difference factors
Although we focus on threat here, given that the threat to life and treatments represent significant dangers to the physical integrity or wellbeing of the child and are also reported to be the most distressing aspects of the experience (Alderfer & Kazak 2006), we acknowledge that there may be other external factors that cause some children to be on a different playing field before ever being diagnosed with cancer. These factors may include exposure to deprivation, other types of threat exposures or unstable home environments, neighborhood quality, race, or socioeconomic status (SES; see Fig. 1; e.g., Aber et al. 1997; Chen & Miller 2013; Hackman & Farah 2009; Lucas et al. 2017; Saban et al. 2014; Stepanikova et al. 2017). These factors may play an important role in moderating neurodevelopmental outcomes. For example, families of a child with cancer and lower SES might have fewer resources to deal with stress or have reduced access to high quality healthcare, thus exacerbating negative outcomes. This notion is supported by empirical data showing that socio demographic risk factors (e.g., single parenting, lower annual family income, caregiver education level) predict cognitive and behavioral outcomes following childhood cancer (e.g., Bemis et al. 2015; Uphold et al. 2013). In addition, national survey data indicate that over 70% of children will experience at least one type of interpersonal threat (e.g., abuse, bullying, witnessing violence) before age 18, many of which are linked to dysfunctional family life (Finkelhor et al. 2009). Given the known links between interpersonal threat exposures and psychological and neurodevelopmental outcomes, a more comprehensive and diverse approach to measuring environmental exposures should aid our understanding of mechanisms and outcomes following pediatric cancer. Given also that low SES and minority populations are more likely to suffer from adverse cancer-related effects (Meeske et al. 2007; Uphold et al. 2013; Zeltzer et al., 2009b) and are more likely to experience other forms of adversity (e.g., violence, discrimination; Gillespie et al. 2009; Penner et al. 2016; Taylor & Turner 2002; Kessler et al. 2010), research focusing on high sociodemographic risk populations may help to reduce health disparities. For example, our research is centrally located in Detroit, Michigan, a low-resource minority city that is disproportionately burdened by adversity and associated physical and mental health problems. Despite increased risk, minorities are underserved in healthcare and underrepresented in medical research, and therefore more research in minority communities is needed to address these disparities.
An interesting question for future research is how neurodevelopmental consequences of childhood cancer differ from, or are similar to, those related to more commonly-studied forms of early threat (e.g., violence, abuse). There may also be converging areas of intact function in individuals exposed to childhood cancer and those exposed to interpersonal threat. Identifying the potential relational, emotional, and contextual factors that are operating in childhood cancer vs. interpersonal threat may be critical for understanding developmental outcomes. For instance, the source of the threat is conceptually different; in violence and abuse the threat is interpersonal, often involving someone that the child trusted, whereas in cancer it is the disease itself or related medical procedures. These differences may be relevant for how children process and cope with these threats, and the form and quality of family and social support. We have heard firsthand from the families that participate in our studies that these differences matter. One mother had suffered childhood abuse and recently had a child complete treatment for cancer. She relayed that childhood abuse or other types of family violence are often “undercover” and rarely talked about. The child often feels that he/she has no one to turn to for comfort and security - especially if the caregiver is the perpetrator. Attachment research has shown that such safe and protective early relationships are critical for long-term psychological wellbeing (Ainsworth 1979; Sroufe et al. 2005). In contrast to the isolating experience of childhood violence or abuse, childhood cancer is “out in the open” and has been called a “family disease”, as families frequently report feeling closer in the wake of this experience (Duran, 2013a, b). Many individuals, including medical staff and members of the community, often rally support around the child and their family. Further, unlike common interpersonal threat exposures, childhood cancer does not necessarily pose a threat to the primary attachment relationships. Childhood cancer is, however, associated with chronic impairment and physical disability that is not typically associated with violence or abuse, and may too have long-lasting effects.
These factors may influence whether children deploy maladaptive (e.g., rumination, “I have to go through this alone because I have no one to turn to or who cares”) or adaptive (e.g., reappraisal, “I am strong, I survived this”) cognitive coping strategies following stress. In support of this, children with cancer are more likely to evidence a pattern of resilience and positive growth when referring to a cancer event compared to a non-cancer event (Sharp et al. 2016). These discrepancies are thought to arise from differences in family support, and may help to explain individual differences in psychological outcomes. For example, previous work by our group and others has found that parents’ behavior before and during their child’s painful cancer-related medical procedures influences children’s distress levels (e.g., Cline et al. 2006). Taken together, considering how the early cancer experience is similar to and diverges from other forms of early stress, as well as identifying potential converging areas of intact function, may provide clues into factors that mediate outcomes. This will have important implications for how we diagnose, treat, and support families, and for identifying children at highest risk for adverse outcomes and at greatest need of resources.
Emerging evidence suggests that adversity-related changes in the brain may be independent of the presence or absence of psychopathology, suggesting that individuals are sensitive to neural adaption but may not be sensitive to psychological consequences (see reviews by McCrory et al. 2017; Teicher et al. 2016). Therefore, many adversity-related changes in the brain are thought to reflect a latent vulnerability to psychological problems, rather than an expression of those problems (McCrory et al. 2017). Such adversity-related neural changes are important to characterize among childhood cancer survivors, as not all children will experience psychological consequences (e.g., Trentacosta et al. 2016), but - as we saw in the reviewed literature - many will experience neurological consequences. We argue that understanding neurodevelopmental consequences of pediatric cancer is a necessary first step towards understanding the significant variability in responses and outcomes. It follows, then, that factors that correspond with a greater or lesser degree of neurological change may increase or decrease a child’s risk for the development of psychological negative outcomes, respectively. Several existing neuroimaging studies in pediatric cancer patients/survivors have identified several risk factors for greater degree of neurobiological change, including younger age at diagnosis, tumor location, female gender, cranial irradiation, and increased intensity of CNS-directed treatment (see Tables 1 and 2). Many of these risk factors have also been linked to cognitive impairment in cancer survivors, and many neuroimaging studies link observed neurobiological changes to behavioral or cognitive functioning. As we saw above, however, very few studies have linked neurologic measures to changes in emotion-related psychological functioning.
There are several characteristics of the child (i.e., individual differences) that may play an important role in psychological adjustment among young survivors (see Fig. 1). Researchers have found that neuroticism, defensiveness, conscientiousness, and effortful control are attributes relevant for children coping with cancer (Phipps et al. 2006; De Clercq et al. 2004; Harper et al. 2014a). An important question for future research is whether these attributes relate to brain structural or functional variation observed in pediatric cancer patients or survivors. Such research may critically advance existing psychosocial models by providing new mechanistic insights into individual differences in outcomes.
Genes are individual difference factors that also likely account for substantial variability in outcomes following pediatric cancer. Indeed, emerging research has linked specific genetic polymorphisms to cognitive and behavioral outcomes among pediatric cancer survivors (Cole et al. 2015; Krull et al., 2013a, b). These studies suggest a role for genes related to oxidative stress and neuroinflammation in contributing to chemotherapy-associated neurocognitive decline among pediatric cancer survivors (Cole et al. 2015; Krull et al., 2013a, b). However, to our knowledge genetic factors have yet to be explored as they relate to emotional or neurodevelopmental outcomes in this population. “Imaging genetic” studies demonstrate that common genetic polymorphisms (e.g., in genes encoding brain-derived neurotropic factor [BDNF] or the oxytocin receptor [OXTR]) modulate structure and function of the SEN and other systems of the brain in children without cancer (Marusak et al. 2016), and that they play an important role in modulating cognitive, psychological, and emotional outcomes in individuals exposed to childhood adversity (see review by McCrory et al. 2010). Thus, genetic polymorphisms may be an area of future study.
Although the prevailing view is that childhood adversity is bad for the developing brain, it is important to consider that some of the adversity-related changes in neurobiological or psychological domains may be adaptive. Adaptive changes in nervous system organization prepare the child to avoid or deal with future threats. However, changes that are adaptive in the short-term may be maladaptive later in life (e.g., when re-integrating into normal family, school, and social life, or years down the road), which underscores the need for longitudinal research.
Among the possible changes following cancer, most survivors of pediatric cancer report positive psychological changes, including posttraumatic growth (PTG; Gianinazzi et al. 2016; Duran, 2013a, b). PTG is the idea that struggling with and overcoming a significant challenge, such as childhood cancer, can lead to greater self-awareness or appreciation for life, more meaningful interpersonal relationships, or increased sense of personal strength (Tedeschi & Calhoun 1995). PTG may directly relate to positive adjustment outcomes and buffer against negative psychological effects and may be related to the dispositional attribute of “ego-resilience”, which reflects the degree to which a person can endure and “bounce back” after a stressful experience (Eisenberg et al. 2000; Harper et al. 2007). As research on aftereffects of stressful experiences has traditionally emphasized negative outcomes, it is not surprising that there has been little research on neural mechanisms underlying PTG. In fact, we are only aware of one study using MRI to examine the neural correlates of PTG. In this study of healthy adults reporting a range of adversities (e.g., interpersonal conflicts, death of family/close friend, academic failure; Fujisawa et al. 2015), PTG was positively associated with rsFC between the superior parietal lobe (a CEN region) and the supramarginal gyrus - a brain region involved in memory and social functioning. A better understanding of the neural basis of PTG may help establish methods for augmenting positive change for the subset of survivors who do not report some form of PTG (Duran, 2013a, b).
Resolving neurodevelopmental effects of early threat exposure vs. cancer treatments
A major challenge in research on neurodevelopmental consequences of childhood cancer is disentangling effects of adversity from cancer treatment-induced neuronal damage (see Fig. 1). These are not mutually exclusive and are difficult to disentangle. These may also have unique relationships with external and individual difference variables, contributing to individual differences in outcomes. To our knowledge, no neuroimaging studies have examined both, highlighting a critical gap in our understanding of mechanisms of neurodevelopmental change. Variation in the brain may provide early clues about underlying etiological causes or potential protective mechanisms. Although more research is needed to understand how to better resolve these, we offer some recommendations here. One approach would be to simultaneously measure cancer drug exposure and adversity, and test how these variables relate to neural and behavioral measures. Although no neuroimaging studies have measured both, several have demonstrated dose-dependent effects of cancer treatment intensity (i.e., dosage or modality) on brain structure and function. However, results are inconsistent. This may be since drug dosage is widely used as a surrogate for drug exposure, which may introduce measurement error and compromise the accuracy of results. Plasma drug exposure and assessment or central (i.e., neural) and peripheral biomarkers may provide better precision in identifying the source of long-term neurodevelopmental and psychological outcomes.
A recent pioneering study by Krull et al. (2016) measured plasma drug exposure (high-dose methotrexate) and subsequent biomarker response (plasma homocysteine), and linked those measures to neurocognitive and brain imaging outcomes in 218 long-term survivors of childhood ALL (mean age = 13.8 years, SD = 4.8) treated with chemotherapy only. They found that higher plasma methotrexate and homocysteine levels were associated with poorer scores on various cognitive measures. Neuroimaging data suggested that higher drug and peripheral biomarker concentrations predicted increased neural response in SEN (e.g., ACC) and CEN regions (e.g., dorsolateral PFC) during a task that measures sustained attention and executive functioning (see Fig. 3b). In addition, higher dexamethasone was associated with a thinner cortex in the ACC and increased axial diffusivity in frontal areas, as measured by structural MRI and DTI, respectively. These gray and white matter changes were associated with neurocognitive impairment (Krull et al. 2016). This study is the first to link plasma drug exposure to neural and cognitive changes following childhood cancer, and provides novel mechanistic insight into pathways leading to psychological late effects. Results are also in line with the notion that regions of the SEN are particularly susceptible to cancer-related drug exposure while the brain is still developing.
We advocate that concurrent evaluation of drug exposure and threat/stress may help to more fully explain neurodevelopmental changes, and ultimately, cognitive, behavioral, and emotional consequences. While serum drug exposure provides a useful measure for drug exposure, the best approach to measure adversity is less clear. We and others have previously evaluated pediatric cancer patients’ distress and perceptions of life threat (or threat of bodily harm), and had multiple, independent raters assess children’s distress during cancer-related medical procedures via video-recordings (e.g., Trentacosta et al. 2016; Harper et al. 2014b; Harper et al. 2013). However, McLaughlin et al.’ (2014) neurodevelopmental model of childhood adversity emphasizes the environmental aspect of stress (i.e., the circumstances) rather than an individual child’s perceptions or responses, which are likely influenced by myriad other factors (e.g., family support, available coping strategies). Indeed, there are enormous individual differences in response to environmental circumstances, and two children experiencing the same event may have vastly different responses. While these different responses are certainty important for moderating outcomes (e.g., anxiety), the environmental experience itself sets a process of neurodevelopmental adaption in motion (McLaughlin, Personal Communication, March 8, 2017). Complicating the resolution of threat and cancer treatments in the study of pediatric cancer is that these are co-occurring; there are no patients experiencing threat without cancer treatments, and vice versa. However, borrowing from the existing literature on childhood adversity, one approach would be to assess frequency and severity of early threat within this group. For example, frequency (e.g., frequency of invasive cancer-related medical procedures, length of treatment, number of days of hospitalization/hospital visits) and severity (e.g., different treatment modalities, emergency hospitalizations, treatment side effects, deaths of other patients, relapse or reoccurrence) of cancer-related adversity could be measured. It is important to note that the literature on childhood adversity is still evolving, and there is not yet a consensus on the definition and measurement of childhood adversity (McLaughlin 2016). We expect that advances in research on childhood adversity will inform research in the area of pediatric cancer.
Broader application of the model
There are few early experiences as consequential as childhood cancer. Childhood cancer has a radical impact on a person’s life, redefining priorities, objectives, and perceptions (see Jim & Jacobsen 2008). As we have seen, following early adverse experiences, individuals experience the world with a fundamentally altered nervous system. Changes at the level of structural and functional neurobiology have been linked to increased risk of behavior problems and cognitive dysfunction. Future studies may also identify neurodevelopmental substrates underlying risk of emotion-related problems, including anxiety, depression, and PTSS among survivors, as well as positive changes, such as PTG. Although we focus on pediatric cancer here, the proposed neurodevelopmental model may have broader applications for understanding psychological and neurodevelopmental consequences for the millions of children who are living with other chronic- or life-threatening illnesses (e.g., hemophilia, sickle cell, HIV/AIDS, chronic pain), or who endure other intensive medical interventions (e.g., intensive care, accidents). For example, although there are many points of difference among these experiences (e.g., treatments, characteristics, onset, duration), there may also be some commonalities among experiences (e.g., invasive medical procedures, diagnosis of a life-threatening injury or illness, threat of reoccurrence or long-term complications) that may be associated with a similar range in child responses (e.g., PTSS; Price et al., 2016b; Kazak et al. 2005) and may also, as we argue, have similar effects on neural development.
Conclusions
Today, children are surviving pediatric cancer at unprecedented rates, making it one of modern medicine’s true success stories. However, we are increasingly becoming aware of the negative impact that the early cancer experience has on long-term cognitive, behavioral, and emotional functioning. These adverse effects have been largely attributed to the injurious effects of cancer treatments (e.g., chemotherapy, cranial irradiation) on brain development. We contend that the effects of pediatric cancer as an early threat experience are also important to consider when evaluating psychological and neurodevelopmental consequences. The role of childhood adversity in pediatric cancer – namely, the presence of a life-threatening disease and endurance of invasive medical procedures – has been largely ignored in the neuroscientific literature, despite compelling research by our group and others showing that exposure to other forms of childhood adversity (e.g., violence, abuse) strongly imprints on neural development, and that these neurobiological changes alter core cognitive and affective processes that are thought to increase risk for psychological problems. Here, we offer a novel neurodevelopmental framework that characterizes childhood cancer as a type of early threat exposure, and focuses on the sensitivity of the hippocampus and the “salience and emotion network” (SEN) to early threat and treatment-induced brain injury. This model may help to advance interventions and psycho-social models by identifying new pathways through which childhood cancer impacts neural development, and ultimately psychological outcomes.
Public significance statements.
It is estimated that nearly 500,000 survivors of pediatric cancer will be living in the US by the year 2020. A proportion of these survivors will experience cognitive, behavioral, or emotional difficulties that greatly compromise quality of life, disrupt everyday functioning, and may increase morbidity, mortality, and healthcare costs. Here, we review existing neuroimaging studies on brain mechanisms that may underlie cancer-related psychological problems, and provide a new integrative neurodevelopmental framework that considers pediatric cancer as a type of threat-related childhood adversity, and considers the joint impact of early threat and cancer treatments on specific sensitive brain systems.
Acknowledgements
We would like to thank the young cancer survivors and their families for participating in our research and more importantly, for sharing their stories about their experiences with us.
Funding None. Dr. MaCociety award 129368-PF-16–057-01-PCSM. Dr. Rabinak is supported by National Institute of Mental Health grants K01MH101123 and R61MH111935.
References
- Aber JL, Bennett NG, Conley DC, & Li J (1997). The effects of poverty on child health and development. Annual review of public health, 18(1), 463–483. [DOI] [PubMed] [Google Scholar]
- Ainsworth MS (1979). Infant–mother attachment. American psychologist, 34(10), 932. [DOI] [PubMed] [Google Scholar]
- Alderfer MA, & Kazak AE (2006). Family issues when a child is on treatment for cancer. In Comprehensive handbook of childhood cancer and sickle cell disease: A biopsychosocial approach (pp. 53–74). New York: Oxford University Press. [Google Scholar]
- Alderfer MA, Labay LE, & Kazak AE (2003). Brief report: does posttraumatic stress apply to siblings of childhood cancer survivors? Journal of pediatric psychology, 28(4), 281–286. [DOI] [PubMed] [Google Scholar]
- Anderson FS, & Kunin-Batson AS (2009). Neurocognitive late effects of chemotherapy in children: The past 10 years of research on brain structure and function. Pediatric Blood & Cancer, 52(2), 159–164. 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Reddick WE, Petersen RC, Santucci A, Zhang N, Srivastava D, et al. (2013). Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. Journal of the National Cancer Institute, 105(12), 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford J, Schoffstall C, Reddick WE, Leone C, Laningham FH, Glass JO, et al. (2010). Attention and Working Memory Abilities in Children Treated for Acute Lymphoblastic Leukemia. Cancer, 116(19), 4638–4645. 10.1002/cncr.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Trauma- and stressor-related disorders. In Diagnostic and statistical manual of mental disorders (5th ed.). Washington D.C.: American Psychiatric Publishing. [Google Scholar]
- Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, et al. (2009). White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International Journal of Radiation Oncology* Biology* Physics, 74(3), 837–843. [DOI] [PubMed] [Google Scholar]
- Badr MA, Hassan TH, El-Gerby KM, & Lamey ME (2013). Magnetic resonance imaging of the brain in survivors of childhood acute lymphoblastic leukemia. Oncology Letters, 5, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron Nelson M, Compton P, Macey PM, Patel SK, Jacob E, O’Neil S, … Harper RM (2016). Diffusion Tensor Imaging and Neurobehavioral Outcome in Children With Brain Tumors Treated With Chemotherapy. Journal of Pediatric Oncology Nursing, 33(2), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis H, Yarboi J, Gerhardt CA, Vannatta K, Desjardins L, Murphy LK, et al. (2015). Childhood cancer in context: sociodemographic factors, stress, and psychological distress among mothers and children. Journal of pediatric psychology, 40(8), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology, 191(3), 391–431. [DOI] [PubMed] [Google Scholar]
- Bessell AG (2001). Children surviving cancer: Psychosocial adjustment, quality of life, and school experiences. Exceptional Children, 67(3), 345–359. [Google Scholar]
- Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. (2014). Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. Journal of clinical oncology, 32(9), 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsko MJ, Cohen D, Dillon R, Harvey J, Krull K, & Klosky JL (2016). Psychosocial Late Effects in Pediatric Cancer Survivors: A Report From the Children’s Oncology Group. Pediatric Blood & Cancer, 63(2), 337–343. 10.1002/pbc.25773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, & Pizzagalli DA (2013). Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiology of disease, 52, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman TM, Reddick WE, Luxton J, Glass JO, Sabin ND, Srivastava DK, et al. (2012). Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro-Oncology, 14(suppl 4), iv25–iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M (2006). A systematic and conceptual review of posttraumatic stress in childhood cancer survivors and their parents. Clinical psychology review, 26(3), 233–256. [DOI] [PubMed] [Google Scholar]
- Buizer AI, De Sonneville LM, Van Den Heuvel-eibrink MM, Njiokiktjien C, & Veerman AJ (2005a). Visuomotor control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Journal of the International Neuropsychological Society, 11(05), 554–565. [DOI] [PubMed] [Google Scholar]
- Buizer AI, de Sonneville LM, van den HeuvelEibrink MM, & Veerman AJ (2005b). Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatric blood & cancer, 45(3), 281–290. [DOI] [PubMed] [Google Scholar]
- Butler RW, & Haser JK (2006). Neurocognitive effects of treatment for childhood cancer. Mental Retardation and Developmental Disabilities Research Reviews, 12(3), 184–191. 10.1002/mrdd.20110. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, & Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience, 16(2), 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M, Haut M, Reminger S, Hutter J, Theilmann R, & Kaemingk K (2008). Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. American Journal of Neuroradiology, 29(4), 792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, & Reiss AL (2008). Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and anxiety, 25(6), 514–526. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. (2014). The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clinical psychological science : a journal of the Association for Psychological Science, 2(2), 119–137. 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino SM, Ullrich NJ, Whelen MJ, & Lange BJ (2014). Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. Journal of the National Cancer Institute, 106(8), dju186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, & Miller GE (2013). Socioeconomic status and health: mediating and moderating factors. Annual Review of Clinical Psychology, 9, 723–749. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang L, King TZ, & Mao H (2016). Increased frontal functional networks in adult survivors of childhood brain tumors. NeuroImage: Clinical, 11, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, & Krull KR (2015). Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neuroscience & Biobehavioral Reviews, 53, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Sabin ND, Reddick WE, Bhojwani D, Liu W, Brinkman TM, et al. (2016). Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. The Lancet Haematology, 3(10), e456–e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WC, Chik K.-w., Chan Y.-l., Yeung DK, Roebuck DJ, Howard RG, … Metreweli C (2003). White matter and cerebral metabolite changes in children undergoing treatment for acute lymphoblastic leukemia: longitudinal study with MR imaging and 1H MR spectroscopy. Radiology, 229(3), 659–669. [DOI] [PubMed] [Google Scholar]
- Cline RJ, Harper FW, Penner LA, Peterson AM, Taub JW, & Albrecht TL (2006). Parent communication and child pain and distress during painful pediatric cancer treatments. Social science & medicine, 63(4), 883–898. [DOI] [PubMed] [Google Scholar]
- Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, et al. (2015). Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J Clin Oncol, 33(19), 2205–2211. 10.1200/jco.2014.59.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin H, Krull K, Reddick W, Pei D, Cheng C, & Pui C (2012a). Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. Journal of the National Cancer Institute, 104(18), 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, & Pui CH (2012b). Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst, 104(18), 1386–1395. 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Ogg RJ, Ashford JM, Scoggins MA, Zou P, Clark KN, et al. (2015). Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. Journal of clinical oncology, 33(33), 3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Ashford JM, Clark KN, Martin-Elbahesh K, Hardy KK, Merchant TE, et al. (2016). Long-term efficacy of computerized cognitive training among survivors of childhood cancer: A single-blind randomized controlled trial. Journal of Pediatric Psychology, 42, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, & Ahles TA (2008). Neurocognitive changes in cancer survivors. Cancer J, 14(6), 396–400. 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- De Clercq B, De Fruyt F, Koot HM, & Benoit Y (2004). Quality of life in children surviving cancer: a personality and multi-informant perspective. Journal of pediatric psychology, 29(8), 579–590. [DOI] [PubMed] [Google Scholar]
- Dellani PR, Eder S, Gawehn J, Vucurevic G, Fellgiebel A, Müller MJ, et al. (2008). Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. Journal of Magnetic Resonance Imaging, 27(6), 1250–1255. [DOI] [PubMed] [Google Scholar]
- De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Paquier PF, … Mariën P (2009). Long-term cognitive deficits following posterior fossa tumor resection: a neuropsychological and functional neuroimaging follow-up study. Neuropsychology, 23(6), 694. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Prust M, & Kaiser J (2015). Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience, 309, 224–232. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Armstrong FD, Chen L, Helton K, Brecher ML, Bell B, & Chauvenet AR (2014). Neurocognitive and neuroradiologic central nervous system late effects in children treated on Pediatric Oncology Group (POG) P9605 (standard risk) and P9201 (lesser risk) acute lymphoblastic leukemia protocols (ACCL0131): a methotrexate consequence? A report from the Children’s Oncology Group. Journal of Pediatric Hematology/Oncology, 36(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran B (2013a). Posttraumatic growth as experienced by childhood cancer survivors and their families: a narrative synthesis of qualitative and quantitative research. Journal of pediatric oncology nursing, 30(4), 179–197. [DOI] [PubMed] [Google Scholar]
- Duran B (2013b). Posttraumatic growth as experienced by childhood cancer survivors and their families: a narrative synthesis of qualitative and quantitative research. J Pediatr Oncol Nurs, 30(4), 179–197. 10.1177/1043454213487433. [DOI] [PubMed] [Google Scholar]
- Edelmann MN, Ogg RJ, Scoggins MA, Brinkman TM, Sabin ND, Pui CH, … Krull KR (2013). Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: A report from the SJLIFE cohort. Pediatric Blood & Cancer, 60(11), 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann MN, Krull KR, Liu W, Glass JO, Ji Q, Ogg RJ, et al. (2014). Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain, 137(11), 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt MJ, Sandlund JT, Zhang N, Liu W, Ness KK, Bhakta N, Chemaitilly W, et al. (2017) Late outcomes of adult survivors of childhood non-Hodgkin lymphoma: A report from the St. Jude life-time cohort study. Pediatric Blood & Cancer, 64 10.1002/pbc.26338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, & Reiser M (2000). Dispositional emotionality and regulation: their role in predicting quality of social functioning. Journal of personality and social psychology, 78(1), 136. [DOI] [PubMed] [Google Scholar]
- Eiser C, Hill JJ, & Vance YH (2000). Examining the psychological consequences of surviving childhood cancer: systematic review as a research method in pediatric psychology. Journal of pediatric psychology, 25(6), 449–460. [DOI] [PubMed] [Google Scholar]
- ElAlfy M, Ragab I, Azab I, Amin S, & Abdel-Maguid M (2014). Neurocognitive outcome and white matter anisotropy in childhood acute lymphoblastic leukemia survivors treated with different protocols. Pediatric hematology and oncology, 31(2), 194–204. [DOI] [PubMed] [Google Scholar]
- Erickson SJ, & Steiner H (2001). Trauma and personality correlates in long term pediatric cancer survivors. Child Psychiatry & Human Development, 31(3), 195–213. [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, & Gross JJ (2015). The neural bases of emotion regulation. Nature reviews neuroscience, 16(11), 693–700. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Ficek K, Blamek S, Syguła D, Miszczyk L, Sońta-Jakimczyk D, & Tarnawski R (2010). Evaluation of the late effects of CNS prophylactic treatment in childhood acute lymphoblastic leukemia (ALL) using magnetic resonance spectroscopy Brain Edema XIV (pp. 195–197): Springer. [DOI] [PubMed] [Google Scholar]
- Finkelhor D (2009). Children’s exposure to violence: A comprehensive national survey Washington, DC: U.S. Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention. [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews neuroscience, 8(9), 700–711. [DOI] [PubMed] [Google Scholar]
- Fujisawa TX, Jung M, Kojima M, Saito DN, Kosaka H, & Tomoda A (2015). Neural basis of psychological growth following adverse experiences: a resting-state functional MRI study. PloS one, 10(8), e0136427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschaft M, Huebner T, Plessow F, Ikonomidou VN, Abolmaali N, Krone F, et al. (2013). Impact of chemotherapy for childhood leukemia on brain morphology and function. PloS one, 8(11), e78599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Bunge SA (2012). Neural changes underlying the development of episodic memory during middle childhood. Developmental cognitive neuroscience, 2(4), 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi ME, Rueegg CS, Vetsch J, Luer S, Kuehni CE, & Michel G (2016). Cancer’s positive flip side: posttraumatic growth after childhood cancer. Support Care Cancer, 24(1), 195–203. 10.1007/s00520-015-2746-1. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General hospital psychiatry, 31(6), 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, & Thompson PM (2010). Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain and cognition, 72(1), 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, et al. (2006). Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1059–1067. [DOI] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in cognitive sciences, 13(2), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry, 169(7), 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, & Hariri AR (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Development and Psychopathology, 27(4pt2), 1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harila-Saari AH, Pääkkö EL, Vainionpää LK, Pyhtinen J, & Lanning BM (1998). A longitudinal magnetic resonance imaging study of the brain in survivors of childhood acute lymphoblastic leukemia. Cancer, 83(12), 2608–2617. [PubMed] [Google Scholar]
- Harper K, Felicity W, Schmidt JE, Beacham AO, Salsman JM, Averill AJ, et al. (2007). The role of social cognitive processing theory and optimism in positive psychosocial and physical behavior change after cancer diagnosis and treatment. Psycho-Oncology, 16(1), 79–91. [DOI] [PubMed] [Google Scholar]
- Harper FW, Peterson AM, Uphold H, Albrecht TL, Taub JW, Orom H, et al. (2013). Longitudinal study of parent caregiving self-efficacy and parent stress reactions with pediatric cancer treatment procedures. Psycho-Oncology, 22(7), 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper FW, Goodlett BD, Trentacosta CJ, Albrecht TL, Taub JW, Phipps S, et al. (2014a). Temperament, personality, and quality of life in pediatric cancer patients. Journal of pediatric psychology, 39(4), 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper FW, Peterson AM, Albrecht TL, Taub JW, Phipps S, & Penner LA (2014b). Posttraumatic stress symptoms in parents of pediatric cancer patients: A mediational analysis. Journal of Traumatic Stress Disorders & Treatment, 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper FW, Peterson AM, Albrecht TL, Taub JW, Phipps S, & Penner LA (2015). Satisfaction with support versus size of network: Differential effects of social support on psychological distress in parents of pediatric cancer patients. Psycho-Oncology, 25, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkamp NS, Petersen ET, De Vis JB, Bokkers RP, & Hendrikse J (2013). Mapping of cerebral perfusion territories using territorial arterial spin labeling: techniques and clinical application. NMR in Biomedicine, 26(8), 901–912. [DOI] [PubMed] [Google Scholar]
- Hedström M, Haglund K, Skolin I, & Von Essen L (2003). Distressing events for children and adolescents with cancer: Child, parent, an nurse perceptions. Journal of pediatric oncology nursing, 20(3), 120–132. [DOI] [PubMed] [Google Scholar]
- Heim C, & Binder EB (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental neurology, 233(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Hill DE, Ciesielski KT, Hart BL, & Jung RE (2004). MRI morphometric and neuropsychological correlates of long-term memory in survivors of childhood leukemia. Pediatric Blood & Cancer, 42(7), 611–617. [DOI] [PubMed] [Google Scholar]
- Hobbie WL, Ogle SK, Reilly M, Ginsberg JP, Rourke M, Ratcliffe S, et al. (2010). Identifying the educational needs of parents at the completion of their child’s cancer therapy. Journal of pediatric oncology nursing, 27(4), 190–195. [DOI] [PubMed] [Google Scholar]
- Hopewell J, Calvo W, Jaenke R, Reinhold H, Robbins M, & Whitehouse E (1993). Microvasculature and radiation damage. In Acute and Long-Term Side-Effects of Radiotherapy (pp. 1–16). Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- Horska A, Nidecker A, Intrapiromkul J, Tannazi F, Ardekani S, Brant LJ, Wharam M Jr., et al. (2014). Diffusion tensor imaging of deep gray matter in children treated for brain malignancies. Child’s Nervous System, 30, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SH, Hoeft F, & Kesler SR (2012). GAT: a graphtheoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PloS one, 7(7), e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Altekruse S, et al. (2016). SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; Bethesda. [Google Scholar]
- Huang H, Gundapuneedi T, & Rao U (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology, 37(12), 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NS, Balsamo LM, Bracken MB, & Kadan-Lottick NS (2015). Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood, 126(3), 346–353. [DOI] [PubMed] [Google Scholar]
- Jacola LM, Ashford JM, Reddick WE, Glass JO, Ogg RJ, Merchant TE, & Conklin HM (2014). The relationship between working memory and cerebral white matter volume in survivors of childhood brain tumors treated with conformal radiation therapy. Journal of Neuro-Oncology, 119, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakar R, King TZ, Morris R, & Na S (2015). Hippocampal volume and auditory attention on a verbal memory task with adult survivors of pediatric brain tumor. Neuropsychology, 29(2), 303. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre P, & McDonald BC (2016). Neuroepidemiology of cancer and treatment-related neurocognitive dysfunction in adult-onset cancer patients and survivors. Handb Clin Neurol, 138, 297–309. 10.1016/b978-0-12-802973-2.00017-3. [DOI] [PubMed] [Google Scholar]
- Jim HS, & Jacobsen PB (2008). Posttraumatic stress and posttraumatic growth in cancer survivorship: a review. The Cancer Journal, 14(6), 414–419. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, Krugers HJ, & Lucassen PJ (2007). Chronic stress: implications for neuronal morphology, function and neurogenesis. Frontiers in Neuroendocrinology, 28(2), 72–96. [DOI] [PubMed] [Google Scholar]
- Kangas M (2013). DSM-5 Trauma and Stress-Related Disorders: Implications for Screening for Cancer-Related Stress. Frontiers in Psychiatry, 4, 122 10.3389/fpsyt.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh BC, Dupont-Frechette JA, Jerskey BA, & Holler KA (2017). Neurocognitive deficits in children and adolescents following maltreatment: Neurodevelopmental consequences and neuropsychological implications of traumatic stress. Appl Neuropsychol Child, 6(1), 64–78. 10.1080/21622965.2015.1079712. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Barakat LP, Alderfer M, Rourke MT, Meeske K, Gallagher PR, et al. (2001). Posttraumatic stress in survivors of childhood cancer and mothers: Development and validation of the Impact of Traumatic Stressors Interview Schedule (ITSIS). Journal of Clinical Psychology in Medical Settings, 8(4), 307–323. [Google Scholar]
- Kazak AE, Alderfer M, Rourke MT, Simms S, Streisand R, & Grossman JR (2004). Posttraumatic stress disorder (PTSD) and posttraumatic stress symptoms (PTSS) in families of adolescent childhood cancer survivors. Journal of pediatric psychology, 29(3), 211–219. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Kassam-Adams N, Schneider S, Zelikovsky N, Alderfer MA, & Rourke M (2005). An integrative model of pediatric medical traumatic stress. J Pediatr Psychol, 31(4), 343–355. [DOI] [PubMed] [Google Scholar]
- Keding TJ, & Herringa RJ (2015). Abnormal structure of fear circuitry in pediatric post-traumati c stress d is order. Neuropsychopharmacology, 40(3), 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Tanaka H, & Koovakkattu D (2010). Cognitive reserve and brain volumes in pediatric acute lymphoblastic leukemia. Brain imaging and behavior, 4(3–4), 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Pritchard-Berman M, Lee C, Kutner E, Hosseini S, et al. (2014). Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatric Blood & Cancer, 61(7), 1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Huston-Warren E, & Watson C (2016). Atypical structural connectome organization and cognitive impairment in young survivors of acute lymphoblastic leukemia. Brain connectivity, 6(4), 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, et al. (2012). Childhood maltreatment and the structure of common psychiatric disorders. Br J Psychiatry, 200(2), 107–115. 10.1192/bjp.bp.111.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajuria RK, Blankenburg F, Wuithschick I, Rueckriegel S, Thomale U-W, Mansour M, et al. (2015). Morphological brain lesions of pediatric cerebellar tumor survivors correlate with inferior neurocognitive function but do not affect health-related quality of life. Child’s Nervous System, 31(4), 569–580. [DOI] [PubMed] [Google Scholar]
- Khong P-L, Kwong DL, Chan GC, Sham JS, Chan F-L, & Ooi G-C (2003). Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. American Journal of Neuroradiology, 24(4), 734–740. [PMC free article] [PubMed] [Google Scholar]
- Khong P-L, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, … Chan GC (2006). White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. Journal of Clinical Oncology, 24(6), 884–890. [DOI] [PubMed] [Google Scholar]
- King TZ, Na S, & Mao H (2015a). Neural underpinnings of working memory in adult survivors of childhood brain tumors. Journal of the International Neuropsychological Society, 21(07), 494–505. [DOI] [PubMed] [Google Scholar]
- King TZ, Wang L, & Mao H (2015b). Disruption of white matter integrity in adult survivors of childhood brain tumors: correlates with long-term intellectual outcomes. PloS one, 10(7), e0131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Davis-Ratner MS, Milner MW, Chen S, Schraedley-Desmond P, Fisher PG, et al. (2008). Verbal memory impairments in children after cerebellar tumor resection. Behavioural neurology, 20(1–2), 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, & Rhodes JS (2013). Neurogenesis, inflammation and behavior. Brain, behavior, and immunity, 27, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Schoch B, Dimitrova A, Gizewski E, & Timmann D (2005). Functional recovery of children and adolescents after cerebellar tumour resection. Brain, 128(6), 1428–1441. [DOI] [PubMed] [Google Scholar]
- Krull KR, Sabin ND, Reddick WE, Zhu L, Armstrong GT, Green DM, … Robison LL (2012). Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. Journal of Clinical Oncology, 30(29), 3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Bhojwani D, Conklin HM, Pei D, Cheng C, Reddick WE, et al. (2013a). Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol, 31(17), 2182–2188. 10.1200/jco.2012.46.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Hockenberry MJ, Miketova P, Carey M, & Moore IM (2013b). Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leukemia & lymphoma, 54(3), 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Cheung YT, Liu W, Fellah S, Reddick WE, Brinkman TM, et al. (2016). Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Journal of clinical oncology, 34(22), 2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin-Batson AS, Lu X, Balsamo L, Graber K, Devidas M, Hunger SP, et al. (2016). Prevalence and predictors of anxiety and depression after completion of chemotherapy for childhood acute lymphoblastic leukemia: A prospective longitudinal study. Cancer, 122(10), 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M, Döring K, Spangenberg C, Konczak J, Gizewski E, Schoch B, et al. (2013). Location and restoration of function after cerebellar tumor removal—a longitudinal study of children and adolescents. The. Cerebellum, 12(1), 48–58. [DOI] [PubMed] [Google Scholar]
- Landolt MA, Ystrom E, Sennhauser FH, Gnehm HE, & Vollrath ME (2012). The mutual prospective influence of child and parental post-traumatic stress symptoms in pediatric patients. Journal of child psychology and psychiatry, and allied disciplines, 53(7), 767–774. 10.1111/j.1469-7610.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2003). The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology, 23(4–5), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LH, Ooi G-C, Kwong DL, Chan GC, Cao G, & Khong P-L (2004). White-matter diffusion anisotropy after chemo-irradiation: a statistical parametric mapping study and histogram analysis. NeuroImage, 21(1), 261–268. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, & Schmahmann JD (2000). Neuropsychological consequences of cerebellar tumour resection in children. Brain, 123(5), 1041–1050. [DOI] [PubMed] [Google Scholar]
- Li MD, Forkert ND, Kundu P, Ambler C, Lober RM, Burns TC, … Fisher PG (2017). Brain perfusion and diffusion abnormalities in children treated for posterior fossa brain tumors. The Journal of Pediatrics, 185, 173–180. e173. [DOI] [PubMed] [Google Scholar]
- Lisman JE, & Grace AA (2005). The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron, 46(5), 703–713. 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Scantlebury N, Tabori U, Bouffet E, Laughlin S, Strother D, … Brière M-E (2014). White matter compromise predicts poor intellectual outcome in survivors of pediatric low-grade glioma [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Krull KR, Leisenring W, Owen JE, Kawashima T, Tsao JC, et al. (2011). Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain, 152(11), 2616–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, & Granger DA (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79, 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, & Block ML (2010). Microglial activation and chronic neurodegeneration. Neurotherapeutics, 7(4), 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, & Laughlin S (2006). Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro-Oncology, 8(3), 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, & Jenney ME (2004). The survivors of childhood cancer. In Psychosocial aspects of pediatric oncology (pp. 265–278). Chichester: John Wiley & Sons, Ltd. [Google Scholar]
- Marcoux S, Drouin S, Laverdière C, Alos N, Andelfinger GU, Bertout L, et al. (2016). The PETALE study: Late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatric Blood & Cancer 10.1002/pbc.26361. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews neuroscience, 14(6), 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Etkin A, & Thomason ME (2015a). Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage: Clinical, 8, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, & Thomason ME (2015b). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40(5), 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Kuruvadi N, Vila AM, Shattuck DW, Joshi SH, Joshi AA, et al. (2016). Interactive effects of BDNF Val66Met genotype and trauma on limbic brain anatomy in childhood. European child & adolescent psychiatry, 25(5), 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, et al. (2017a). Dynamic functional connectivity of neurocognitive networks in children. Human brain mapping, 38(1), 97–108. 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Hatfield JRB, Thomason ME, & Rabinak CA (2017b). Reduced Ventral Tegmental Area–Hippocampal Connectivity in Children and Adolescents Exposed to Early Threat. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(2), 130–137. 10.1016/j.bpsc.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, & Viding E (2010). Research review: the neurobiology and genetics of maltreatment and adversity. Journal of child psychology and psychiatry, 51(10), 1079–1095. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Gerin MI, & Viding E (2017). Annual research review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry–the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry, 58, 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell GA, Salley CG, Barnett M, DeRosa AP, Werk RS, Hourani A, et al. (2017). Anxiety Among Adolescent Survivors of Pediatric Cancer. Journal of Adolescent Health 10.1016/j.jadohealth.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy SD, Lee A, Poliakov A, Friedman S, Shaw D, Browd SR, … Mac Donald CL (2016). Longitudinal cerebellar diffusion tensor imaging changes in posterior fossa syndrome. NeuroImage: Clinical, 12, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future Directions in Childhood Adversity and Youth Psychopathology. J Clin Child Adolesc Psychol, 45(3), 361–382. 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, et al. (2016). Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology, 41(8), 1956–1964. 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine I. o., & Council NR (2006). From Cancer Patient to Cancer Survivor: Lost in Transition Washington, DC: The National Academies Press. [Google Scholar]
- Meeske KA, Patel SK, Palmer SN, Nelson MB, & Parow AM (2007). Factors associated with health-related quality of life in pediatric cancer survivors. Pediatric Blood & Cancer, 49(3), 298–305. [DOI] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Monje M, & Dietrich J (2012). Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behavioural brain research, 227(2), 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Thomason ME, Rigolo L, Wang Y, Waber DP, Sallan SE, et al. (2013). Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatric Blood & Cancer, 60(2), 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern RK, & Butler RW (2004). Review Neurocognitive sequelae of childhood cancers and their treatment. Pediatric Rehabilitation, 7(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Reddick WE, Palmer SL, Glass JO, Elkin TD, Kun LE, … Gajjar A (1999). Neurocognitive deficits in medulloblastoma survivors and white matter loss. Annals of Neurology, 46(6), 834–841. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor J, Langston J, & Gajjar A (2001). Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. Journal of clinical oncology, 19(2), 472–479. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, et al. (2004). Abnormal hippocampal development in children with medulloblastoma treated with risk-adapted irradiation. American Journal of Neuroradiology, 25(9), 1575–1582. [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, & Vos T (2012). The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med, 9(11), e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea SC, Brinkman TM, Ness KK, Krull KR, Smith WA, Srivastava DK, et al. (2014). Emotional distress among adult survivors of childhood cancer. Journal of Cancer Survivorship, 8(2), 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell E, MacKenzie J, & Collins B (2013). Clearing the air: a review of our current understanding of “chemo fog”. Curr Oncol Rep, 15(3), 260–269. 10.1007/s11912-013-0307-7. [DOI] [PubMed] [Google Scholar]
- Oh ME, Driever PH, Khajuria RK, Rueckriegel SM, Koustenis E, Bruhn H, & Thomale U-W (2017). DTI fiber tractography of cerebro-cerebellar pathways and clinical evaluation of ataxia in childhood posterior fossa tumor survivors. Journal of NeuroOncology, 131(2), 267–276. [DOI] [PubMed] [Google Scholar]
- Paakko E, Harila-Saari A, Vanionpaa L, Himanen S, Pyhtinen J, & Lanning M (2000). White matter changes on MRI during treatment in children with acute lymphoblastic leukemia: correlation with neuropsychological findings. Medical and Pediatric Oncology, 35, 456–461. [DOI] [PubMed] [Google Scholar]
- Pääkkö E, Lehtinen S, Harila‐Saari A, Ahonen A, Jauhiainen J, Torniainen P, Pyhtinen J, & Lanning M (2003). Perfusion MRI and SPECT of brain after treatment for childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer, 40(2), 88–92. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, & Mulhern RK (2002). Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. American Journal of Neuroradiology, 23(7), 1088–1094. [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner LA, Dovidio JF, Gonzalez R, Albrecht TL, Chapman R, Foster T, et al. (2016). The effects of oncologist implicit racial bias in racially discordant oncology interactions. Journal of clinical oncology, 34(24), 2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, & Adolphs R (2010). Emotion processing and the amygdala: from a’low road’to’many roads’ of evaluating biological significance. Nature reviews neuroscience, 11(11), 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AM, Harper FWK, Albrecht TL, Taub JW, Orom H, Phipps S, et al. (2014). Parent Caregiver Self-Efficacy and Child Reactions to Pediatric Cancer Treatment Procedures. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses, 31(1), 18–27. 10.1177/1043454213514792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Phipps S, Larson S, Long A, & Rai SN (2006). Adaptive style and symptoms of posttraumatic stress in children with cancer and their parents. Journal of pediatric psychology, 31(3), 298–309. [DOI] [PubMed] [Google Scholar]
- Pogany L, Barr RD, Shaw A, Speechley KN, Barrera M, & Maunsell E (2006). Health Status in Survivors of Cancer in Childhood and Adolescence. Quality of Life Research, 15(1), 143–157. 10.1007/s11136-005-0198-7. [DOI] [PubMed] [Google Scholar]
- Porto L, Preibisch C, Hattingen E, Bartels M, Lehrnbecher T, Dewitz R, et al. (2008). Voxel-based morphometry and diffusion-tensor MR imaging of the brain in long-term survivors of childhood leukemia. European radiology, 18(11), 2691. [DOI] [PubMed] [Google Scholar]
- Price J, Kassam-Adams N, Alderfer MA, Christofferson J, & Kazak AE (2016). Systematic Review: A Reevaluation and Update of the Integrative (Trajectory) Model of Pediatric Medical Traumatic Stress. J Pediatr Psychol, 41(1), 86–97. 10.1093/jpepsy/jsv074. [DOI] [PubMed] [Google Scholar]
- PTSD, N. C. f. (2016) http://www.ptsd.va.gov/. Accessed 4/25/2016.
- Qiu D, Leung LH, Kwong DL, Chan GC, & Khong PL (2006). Mapping radiation dose distribution on the fractional anisotropy map: applications in the assessment of treatment-induced white matter injury. Neuroimage, 31(1), 109–115. 10.1016/j.neuroimage.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Qiu D, Kwong DL, Chan GC, Leung LH, & Khong P-L (2007). Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? International Journal of Radiation Oncology* Biology* Physics, 69(3), 846–851. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Mulhern RK, Elkin TD, Glass JO, Merchant TE, & Langston JW (1998). A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magnetic Resonance Imaging, 16(4), 413–421. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Russell JM, Glass JO, Xiong X, Mulhern RK, Langston JW, Merchant TE, et al. (2000). Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magnetic Resonance Imaging, 18, 787–793. [DOI] [PubMed] [Google Scholar]
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Leigh L, & Mulhern RK (2003). Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer, 97(10), 2512–2519. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Helton KJ, Langston JW, Li CS, & Pui CH (2005a). A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. AJNR Am J Neuroradiol, 26(9), 2371–2377. [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Helton KJ, Langston JW, Xiong X, Wu S, et al. (2005b). Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. American Journal of Neuroradiology, 26(5), 1263–1269. [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, Shan ZY, Glass JO, Helton S, Xiong X, Wu S, … Khan RB (2006). Smaller whitematter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer, 106(4), 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Johnson DP, Laningham FH, & Pui C-H (2009). Voxel-based analysis of T2 hyperintensities in white matter during treatment of childhood leukemia. American Journal of Neuroradiology, 30(10), 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Sullivan JR, & Cohen MJ (2010). Neuropsychological assessment and intervention for childhood and adolescent disorders Hoboken: John Wiley & Sons. [Google Scholar]
- Riem MM, Alink LR, Out D, Van Ijzendoorn MH, & Bakermans-Kranenburg MJ (2015). Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Development and psychopathology, 27(02), 507–520. [DOI] [PubMed] [Google Scholar]
- Riggs L, Bouffet E, Laughlin S, Laperriere N, Liu F, Skocic J, et al. (2014). Changes to memory structures in children treated for posterior fossa tumors. Journal of the International Neuropsychological Society, 20(02), 168–180. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Livesay KL, Campbell LK, Scaduto M, Cannistraci CJ, Anderson AW, et al. (2010). Working memory in survivors of childhood acute lymphocytic leukemia: functional neuroimaging analyses. Pediatric Blood & Cancer, 54(4), 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KE, Fraley CE, Pearson MM, Kuttesch JF, & Compas BE (2013). Neurocognitive late effects of pediatric brain tumors of the posterior fossa: A quantitative review. Journal of the International Neuropsychological Society, 19(01), 44–53. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF Jr, Wymer K, et al. (2014). Neuroimaging of executive function in survivors of pediatric brain tumors and healthy controls. Neuropsychology, 28(5), 791. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Pearson MM, Cannistraci CJ, Anderson AW, Kuttesch JF Jr, Wymer K, et al. (2015). Functional neuroimaging of working memory in survivors of childhood brain tumors and healthy children: Associations with coping and psychosocial outcomes. Child Neuropsychology, 21(6), 779–802. [DOI] [PubMed] [Google Scholar]
- Robison LL, & Hudson MM (2014). Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nature Reviews Cancer, 14(1), 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke MT, & Kazak AE (2005). Psychological aspects of long-term survivorship. In Survivors of childhood and adolescent cancer (pp. 295–304): Springer. [Google Scholar]
- Rueckriegel SM, Bruhn H, Thomale UW, & Hernáiz Driever P (2015). Cerebral white matter fractional anisotropy and tract volume as measured by MR imaging are associated with impaired cognitive and motor function in pediatric posterior fossa tumor survivors. Pediatric Blood & Cancer, 62(7), 1252–1258. [DOI] [PubMed] [Google Scholar]
- Saban KL, Matthews HL, DeVon HA, & Janusek LW (2014). Epigenetics and social context: implications for disparity in cardiovascular disease. Aging and disease, 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury N, Bouffet E, Laughlin S, Strother D, McConnell D, Hukin J, … Keene D (2016). White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology, 30(4), 425. [DOI] [PubMed] [Google Scholar]
- Schuitema I, Deprez S, Van Hecke W, Daams M, Uyttebroeck A, Sunaert S, et al. (2013). Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. Journal of clinical oncology, 31(27), 3378–3388. [DOI] [PubMed] [Google Scholar]
- Schulte F, & Barrera M (2010). Social competence in childhood brain tumor survivors: a comprehensive review. Supportive Care in Cancer, 18(12), 1499–1513. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan ZY, Liu JZ, Glass JO, Gajjar A, Li C-S, & Reddick WE (2006). Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magnetic Resonance Imaging, 24(8), 1015–1022. [DOI] [PubMed] [Google Scholar]
- Sharp KMH, Willard VW, Barnes S, Tillery R, Long A, & Phipps S (2016). Emotion socialization in the context of childhood cancer: Perceptions of parental support promotes posttraumatic growth. Journal of Pediatric Psychology, 42, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, & Luna B (2014). Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage, 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’doherty J, Kaube H, Dolan RJ, & Frith CD (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Smibert E, Anderson V, Godber T, & Ekert H (1996). Risk factors for intellectual and educational sequelae of cranial irradiation in childhood acute lymphoblastic leukaemia. British Journal of Cancer, 73(6), 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, & Aldridge JW (2011). Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences, 108(27), E255–E264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, King TZ, Jayakar R, & Morris RD (2014). Reading skill in adult survivors of childhood brain tumor: A theory-based neurocognitive model. Neuropsychology, 28(3), 448. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson EA, & Collins WA (2005). The Development of the Person: The Minnesota Study of Risk and Adaptation from Birth to Adulthood: Guilford Publications. [Google Scholar]
- Stein KD, Syrjala KL, & Andrykowski MA (2008). Physical and psychological long-term and late effects of cancer. Cancer, 112(S11), 2577–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, & Oates GR (2017). Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. American journal of preventive medicine, 52(1), S63–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber ML, Kazak AE, Meeske K, & Barakat L (1998). Is posttraumatic stress a viable model for understanding responses to childhood cancer? Child and Adolescent Psychiatric Clinics of North America, 7, 169–182. [PubMed] [Google Scholar]
- Sullivan TP, Fehon DC, Andres-Hyman RC, Lipschitz DS, & Grilo CM (2006). Differential relationships of childhood abuse and neglect subtypes to PTSD symptom clusters among adolescent inpatients. Journal of traumatic stress, 19(2), 229–239. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Zeller B, Amlien IK, Kanellopoulos A, Andersson S, Due-Tønnessen P, … Fjell AM (2015). Cortical surface area and thickness in adult survivors of pediatric acute lymphoblastic leukemia. Pediatric Blood & Cancer, 62(6), 1027–1034. [DOI] [PubMed] [Google Scholar]
- Tavor I, Jones OP, Mars R, Smith S, Behrens T, & Jbabdi S (2016). Task-free MRI predicts individual differences in brain activity during task performance. Science, 352(6282), 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, & Turner RJ (2002). Perceived discrimination, social stress, and depression in the transition to adulthood: Racial contrasts. Social Psychology Quarterly, 65, 213–225. [Google Scholar]
- Tedeschi RG, & Calhoun LG (1995). Trauma and Transformation: Growing in the Aftermath of Suffering: SAGE Publications. [Google Scholar]
- Teicher MH, & Samson JA (2016). Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. Journal of child Psychology and Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, & Polcari A (2014). Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biological psychiatry, 76(4), 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci, 17(10), 652–666. 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thomason ME, & Marusak HA (2016). Toward understanding the impact of trauma on the early developing human brain. Neuroscience 10.1016/j.neuroscience.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, & Rosenberg DR (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience, 10, 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, & Giedd JN (2010). Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage, 49(1), 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta CJ, Harper FW, Albrecht TL, Taub JW, Phipps S, & Penner LA (2016). Pediatric Cancer Patients’ Treatment-Related Distress and Longer-Term Anxiety: An Individual Differences Perspective. Journal of Developmental & Behavioral Pediatrics, 37(9), 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, & Menon V (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(50), 18578–18589. 10.1523/jneurosci.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphold H, Peterson A, Harper FW, Fox J, Foster T, Phipps S, et al. (2013). Basic needs of pediatric oncology patients, families, and their psychosocial adjustment. Journal of Clinical Oncology, 31, e20646 10.1200/jco.2013.31.15_suppl.e20646 [DOI] [Google Scholar]
- Witter MP (2010). Connectivity of the hippocampus. In Hippocampal microcircuits (pp. 5–26): Springer. [Google Scholar]
- Wolfe KR, Madan-Swain A, & Kana RK (2012). Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Developmental neuropsychology, 37(2), 153–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KR, Madan-Swain A, Hunter GR, Reddy AT, Baños J, & Kana RK (2013). An fMRI investigation of working memory and its relationship with cardiorespiratory fitness in pediatric posterior fossa tumor survivors who received cranial radiation therapy. Pediatric Blood & Cancer, 60(4), 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrack BJ, & Chesler MA (2002). Quality of life in childhood cancer survivors. Psycho-Oncology, 11(2), 132–141. [DOI] [PubMed] [Google Scholar]
- Zeller B, Tamnes CK, Kanellopoulos A, Amlien IK, Andersson S, Due-Tønnessen P, et al. (2013). Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. Journal of clinical oncology, 31(17), 2078–2085. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. (2009). Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology, 27(14), 2396–2404. 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zou P, Mulhern RK, Butler RW, Laningham FH, & Ogg RJ (2008). Brain structural abnormalities in survivors of pediatric posterior fossa brain tumors: a voxel-based morphometry study using free-form deformation. NeuroImage, 42(1), 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, & Wang J (2013). The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of affective disorders, 151(2), 531–539. [DOI] [PubMed] [Google Scholar]
- Zou P, Mulhern RK, Butler RW, Li C-S, Langston JW, & Ogg RJ (2005). BOLD responses to visual stimulation in survivors of childhood cancer. Neuroimage, 24(1), 61–69. [DOI] [PubMed] [Google Scholar]
- Zou P, Li Y, Conklin HM, Mulhern RK, Butler RW, & Ogg RJ (2012). Evidence of change in brain activity among childhood cancer survivors participating in a cognitive remediation program. Archives of clinical neuropsychology, 27(8), 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P, Conklin HM, Scoggins MA, Li Y, Li X, Jones MM, et al. (2016). Functional MRI in medulloblastoma survivors supports prophylactic reading intervention during tumor treatment. Brain imaging and behavior, 10(1), 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]