Abstract
Cryopreservation is an important tool for long-term storage of plant germplasm that is currently used for plant germplasm storage at many institutes worldwide. Recently, novel cryogenic procedures (V and D cryo-plate methods) have been developed. In this study, the most suitable conditions for preserving blueberry shoot tips derived from in vitro and current shoots using the D cryo-plate method were investigated. The D cryo-plate method has advantages such as higher regrowth after cryopreservation and a more user-friendly process compared with conventional cryogenic methods. The optimum duration of desiccation for regrowth of shoot tips from each shoot type was 1 h. To induce dehydration tolerance for the shoot tips, the effects of two cryoprotection treatments (sucrose preculture and loading solution [LS] treatment) on shoot regrowth after cryopreservation were investigated. The combined effect of both treatments significantly increased percentage regrowth (approximately 90%). No regrowth of shoot tips was attained without the two treatments. Thus, preculture and LS treatment were effective to induce dehydration tolerance for cryopreservation of blueberry shoot tips. The optimized conditions for blueberry shoot tips using the D cryo-plate technique were: preculture with 0.3 M sucrose for 1 day, LS treatment (2 M glycerol +0.4–1.0 M sucrose) for 30 min, and air dehydration for 1 h. This optimized procedure was applied to additional blueberry cultivars shoot tips derived from in vitro shoots (regrowth 46.7–100%) and current shoots (regrowth 17.2–62.7%). Furthermore, in vitro shoot tips were suitable material for the D cryo-plate method in blueberry.
Keywords: blueberry, cryopreservation, current shoot, D cryo-plate, in vitro shoot
Introduction
Blueberry (Vaccinium L. spp.; Ericaceae) is one of the most commercially important fruits grown worldwide. Blueberry contains anthocyanin, a functional component that is important for eye health. Eighteen Vaccinium species are cultivated in Japan as table fruit and to provide raw material for processed foods; among these, highbush and rabbiteye cultivars are predominantly grown. Recently, the loss of Vaccinium genetic resources has become an issue of concern because of commercial and residential development (Ballington 2001). The conservation of blueberry species and cultivars is mainly achieved through the establishment of field genebanks. That is, blueberry genetic resources of blueberry are mainly preserved in field genebanks. The repository of the United States Department of Agriculture, Agricultural Research Service in Covallis, Oregon preserves about 1,500 accessions of Vaccinium species and strains (Boches et al. 2006). However, preservation in a field genebank is susceptible to a number of problems, such as loss of accessions as a result of a natural disaster or damage from pests or disease (Rao 2011). Furthermore, field genebanks require an adequate area of land and continuous maintenance (Niino and Arizaga 2015).
To overcome these problems, in vitro preservation is used for many plant species (Niino and Arizaga 2015). However, this storage method has certain disadvantages, such as the risk of losing accessions because of contamination or human error and the possibility of genetic changes during long-term storage (Kaviani 2011). Cryopreservation, a method of storing structurally intact cells and tissues at extremely low temperatures, such as in liquid nitrogen (LN), has been developed as a long-term conservation method for plant germplasm and is used in genebanks of many countries (Sakai and Engelmann 2007). Cryopreservation has unique attributes that minimize space and maintenance requirements without causing genetic alterations (Sakai 1997). Recently, two novel cryogenic protocols using aluminum cryo-plates were developed, the osmo-dehydration method (V cryo-plate; Sekizawa et al. 2011; Yamamoto et al. 2011, 2012a, 2012b) and the air-dehydration method (D cryo-plate; Niino et al. 2013). The major advantages of the cryo-plate methods are their user-friendly procedures, minimization of shoot injury, and rapid cooling and warming, which results in higher recovery (Matsumoto et al. 2015; Niino et al. 2013, 2014; Salma et al. 2014). Furthermore, the D cryo-plate method can be used with larger samples that are sensitive to physical damage and toxicity from certain cryoprotectants, such as PVS2 (Niino et al. 2014). In this study, we investigated the most suitable conditions for the D cryo-plate technique to develop an efficient cryopreservation protocol for blueberry shoot tips derived from in vitro and current shoots.
Materials and methods
Plant material
The rabbiteye blueberry cultivar ‘Tifblue’ (Vaccinium ashei J. M. Reade) was mainly used in this study. In vitro blueberry shoots were cultured in solidified 1/4MS medium (Murashige and Skoog 1962) (1/4 strength KNO3 and NH4NO3, 1/2 strength other macro elements) supplemented with 1 mg l−1 zeatin, 3% (w/v) sucrose and 0.2% (w/v) gellan gum. The cultures were incubated at 25°C with a 16 h photoperiod under white fluorescent light (50 µmol m−1 s−2) and subcultured every month. Current shoots with axillary buds were collected between May and July 2014 from the Honjo experimental farm of Shimane University (Matsue, Japan). The shoot sections (approximately 5 cm long), with one to three axillary buds each, were washed with tap water and then sterilized in 70% ethanol for 30 s and in 1% sodium hypochlorite solution containing 0.01% Tween 20 for 15 min. The shoot sections were then rinsed four times in sterile distilled water, and kept in sterilized distilled water for about 30 min. Subsequently, the shoot tips (1.5 mm size) were dissected from the axillary buds and used for the following experiments.
Cryopreservation procedure for shoot tips using the D cryo-plate procedure
The D cryo-plate technique developed by Niino et al. (2013, 2014) was performed with some modifications (Matsumoto et al. 2015). The cryo-plates (Yamamoto et al. 2011) used were obtained from the Genetic Resources Center, National Agriculture and Food Research Organization (Tsukuba, Japan).
Excised shoot tips derived from in vitro or current shoots were precultured on solidified 1/2MS medium with sucrose at 25°C, and plated on wells of aluminum cryo-plate with approximately 2.0 µl of 2% (w/w) Na-alginate solution supplemented with 0.4 M sucrose in 1/2MS medium. Then, 100 mM CaCl2 solution supplemented with 0.4 M sucrose in 1/2MS was poured on the aluminum plates with shoot tips and left for 15 min for polymerization. After removing the CaCl2 solution, the cryo-plate attached shoot tips was treated with LS solution (2 M glycerol+sucrose) in a Petri dish for 30 min at 25°C, and dehydrated in a laminar flow cabinet (Sanyo Electric Co., Ltd., Osaka, Japan). In the cabinet, the air current velocity was approximately 0.6 m s−1, the temperature was 25°C and relative humidity was about 32%. The cryo-plates with dehydrated shoot tips were transferred to uncapped 2 ml cryotubes held on a cryo-cane and directly plunged into LN for at least 30 min. For plant regeneration, cryo-plates with shoot tips in LN were transferred to 1.2 M sucrose solution with 1/2MS in Petri dishes for 15 min at 25°C for rapid warming and unloading. Then, shoot tips were plated on solidified 1/4MS medium supplemented with 3% sucrose and 1 mg l−1 zeatin, and incubated at 25°C under white fluorescent light (50 µmol s−1 m−2) and a 16 h photoperiod.
Optimization of the D cryo-plate technique with preculture and LS treatment
The effects of sucrose preculture and LS treatment on regrowth were investigated. In a preliminary experiment using the D cryo-plate method, osmoprotection treatment including preculture (0.3 M sucrose) for 1 day and LS treatment (2 M glycerol +0.6 M sucrose) for 30 min resulted in high regrowth. We investigated the effects of preculture and LS treatment with the above conditions on the regrowth of in vitro blueberry shoot tips cryopreserved using the D cryo-plate method. To optimize the D cryo-plate method protocol for blueberry in vitro shoot tips, we evaluated the suitability of osmoprotection treatments, including preculture (sucrose concentration, 0.3 to 0.7 M; 1–3 days duration) at 25°C and LS treatment (sucrose concentration, 0.4 to 1.2 M) for 30 min at 25°C, for shoot tip regrowth.
Application of the protocol for other cultivars/species
To compare regrowth after cryopreservation using the optimized D cryo-plate technique, we used shoot tips derived from in vitro and current shoots from an additional seven cultivars.
Data analysis
Post-rewarming regrowth (normal shoot formation) was evaluated after 1 month of reculture. The shoot tips that produced normal shoots after 1 month of reculture were recorded as regrowth shoots. Three replicates of approximately 10 shoot tips were tested in each experiment. Statistical analyses were performed using Student’s t test or Tukey’s test after one-way ANOVA.
Results and discussion
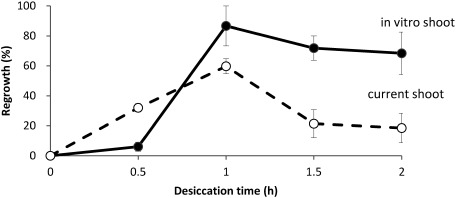
The effects of desiccation duration on blueberry shoot tips derived from in vitro and current shoots using the D cryo-plate method are shown in Figure 1. The highest percentage of regrowth was achieved with 1 h desiccation of shoot tips derived from in vitro shoots (86.7%) and shoot tips from current shoots (59.7%). The regrowth of in vitro shoots after cryopreservation using the D cryo-plate method was significantly higher than that of current shoots. Prolonged duration of desiccation resulted in decreased percentage regrowth. To induce osmo-tolerance for in vitro shoot tips, the effects of two cryoprotection treatments, 0.3 M sucrose preculture for 1 day and LS treatment with 2 M glycerol+0.6 M sucrose solution, on shoot regrowth after cryopreservation were investigated. A high percentage of regrowth (93.6%) was achieved following the two osmoprotection treatments (Table 1). Shoot tips that had been subjected to a single osmoprotection treatment displayed 19.4–36.4% regrowth. These results indicated that preculture and LS treatment were necessary to increase the percentage of regrowth of blueberry shoot tips with the D cryo-plate technique.
Figure 1. Effect of desiccation time on regrowth of cryopreserved blueberry shoot tips by D cryo-plate method. Blueberry (Vaccinium ashei var. Tifblue). Vertical bars: SE, current shoot: shoot tips derived from field grown shoots, in vitro shoot: shoot tips derived from in vitro grown shoots.
Table 1. Effect of preculture and LS treatments on the regrowth of in vitro blueberry shoot tips cryopreserved by D cryo-plate method.
| Preculture | LS treatment | Regrowth (%±SE) |
|---|---|---|
| + | + | 93.6±3.2 a |
| + | − | 19.4±2.8 c |
| − | + | 36.4±5.2 b |
| − | − | 0 d |
Blueberry (Vaccinium ashei var. Tifblue) shoot tips were precultured on 0.3 M sucrose for 1 day, LS treatment with 2 M glycerol+0.6 M sucrose for 30 min and desiccated for 1 h at 25°C. Regrowth: percent of shoot tips produced normal shoots after 4 weeks of plating. Approximately 10 shoot tips were tested for each of three replicates. Data followed by different letters are significantly different at p<0.05 (by Tukey’s test).
In vitro shoot tips were precultured on solidified 1/2MS medium supplemented with different concentrations (0.3, 0.5 and 0.7 M) of sucrose at 25°C for 1 day and treated with LS solution of 2 M glycerol+1 M sucrose, then dehydrated for 1 h prior to freezing in LN. The highest percentage regrowth (74.2%) was achieved with shoot tips precultured with 0.3 M sucrose (Table 2). The optimum preculture duration was also determined. As shown in Table 3, the highest percentage regrowth (79.5%) was attained by preculture with 0.3 M sucrose for 1 day. Thus, preculture with 0.3 M sucrose for 1 day was chosen as the optimum condition in the following experiments. Preculturing shoot tips on solidified medium with a high sucrose concentration is extremely effective at inducing osmo-tolerance for cryopreservation in many plant species (Engelmann et al. 2008; Sakai et al. 2008). Similarly, LS treatment is very effective at improving the regrowth rate after cryopreservation and is used for many plant species (Matsumoto et al. 1994).
Table 2. Effect of concentration of sucrose in preculture on the regrowth of cryopreserved in vitro shoot tips of blueberry by D cryo-plate method.
| Sucrose concentration | Regrowth (%±SE) |
|---|---|
| 0.3 M | 74.2±0.8 a |
| 0.5 M | 45.4±2.4 b |
| 0.7 M | 17.3±3.9 c |
Blueberry (Vaccinium ashei var. Tifblue) shoot tips were precultured for 1 day, LS treatment with 2 M glycerol+0.6 M sucrose for 30 min and desiccated for 1 h at 25°C. Regrowth: percent of shoot tips produced normal shoots after 1 month of plating. Approximately 10 shoot tips were tested for each of three replicates. Data followed by different letters are significantly different at p<0.05 (by Tukey’s test).
Table 3. Effect of preculture duration on the regrowth of cryopreserved blueberry in vitro shoot tips by D cryo-plate method.
| Preculture duration | Regrowth (%±SE) |
|---|---|
| 1 day | 79.5±2.3 a |
| 2 days | 45.2±2.9 b |
| 3 days | 35.4±4.7 c |
Blueberry (Vaccinium ashei var. Tifblue) shoot tips were precultured on 0.3 M sucrose, LS treatment with 2 M glycerol+0.6 M sucrose for 30 min and desiccated for 1 h at 25°C. Regrowth: percent of shoot tips produced normal shoots after 1 month of plating. Approximately 10 shoot tips were tested for each of three replicates. Data followed by different letters are significantly different at p<0.05 (by Tukey’s test).
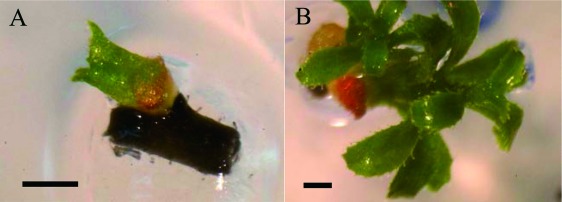
The optimum sucrose concentration for the LS solution was determined for in vitro blueberry shoot tips. As shown in Table 4, no significant difference was observed in concentrations of sucrose from 0.4 to 1 M. Thus, the optimized conditions for cryopreservation of blueberry shoot tips using the D cryo-plate technique were considered to be, as follows: preculture with 0.3 M sucrose for 1 day, LS treatment (2 M glycerol+0.4–1.0 M sucrose) for 30 min and dehydration for 1 h. Plant regrowth via callus after cryopreservation is not desirable because it may induce somaclonal variation (Seibert 1976). Successfully cryopreserved and warmed blueberry shoot tips resumed growth in about 10 days and developed shoots within 45 days without intermediary callus formation (Figure 2).
Table 4. Effect of sucrose concentration in LS treatment on the regrowth of cryopreserved in vitro shoot tips of blueberry using D cryo-plate method.
| Sucrose concentration | Regrowth (%±SE) |
|---|---|
| 0.4 M | 55.2±4.8 a |
| 0.6 M | 61.8±8.1 a |
| 0.8 M | 57.0±1.4 a |
| 1.0 M | 54.5±6.6 a |
Blueberry (Vaccinium ashei var. Tifblue) shoot tips were precultured for 1 day on 0.3 M sucrose, LS treatment for 30 min and desiccated for 1 h at 25°C. Regrowth: percent of shoot tips producing normal shoots after 1 month of plating. Approximately 10 shoot tips were tested for each of three replicates. Data followed by different letters are significantly different at p<0.05 (by Tukey’s test).
Figure 2. Shoots formed from cryopreserved in vitro blueberry shoot tips. Blueberry (Vaccinium ashei var. Tifblue). A: 20 days after reculture, B: 45 days after reculture. Bars=1 mm.
Shoot tips derived from in vitro and current shoots of seven additional cultivars were tested using the optimal conditions established above. The percentage regrowth of in vitro shoot tips and current shoot tips ranged 46.7–100% (average 78.4%) and 17.2–71.5% (50.7%), respectively (Table 5). Uchendu and Reed (2009) reported a high recovery (>80%) of cryopreserved in vitro-grown blueberry shoot tips using the encapsulation–dehydration technique. However, 7 h of desiccation time and a cold acclimation period of 22°C with 8 h low light/−1°C 16 h dark for 2 weeks were needed for the in vitro plant materials in that study. In contrast, the optimized D cryo-plate procedure we developed here does not require a cold acclimation period for the plant materials, and the desiccation time needed is only 1 h.
Table 5. Regrowth of different cultivars of cryopreserved blueberry shoot tips derived from in vitro and current shoots by D cryo-plate method.
| Cultivars | Regrowth (%±SE) | t-test | |
|---|---|---|---|
| In vitro shoots | Current shoots | ||
| Rebiteye | |||
| Festival | 96.9±3.0 | 56.7±8.8 | * |
| Climax | 68.8±2.7 | 61.9±11.9 | NS |
| Highbush | |||
| Magnolia | 68.5±4.2 | 61.1±8.4 | NS |
| Sharpblue | 77.5±4.5 | 57.9±4.1 | * |
| Bluegold | 100.0 | 17.2±9.6 | ** |
| Brigitta | 90.2±5.3 | 62.7±6.5 | * |
| Weymouth | 46.7±2.8 | 37.1±2.4 | NS |
| Average | 78.4 | 50.7 | |
Shoot tips were precultured on 0.3 M sucrose for 1 day, LS treatment with 2 M glycerol+0.6 M sucrose for 30 min and desiccated for 1 h at 25°C. Regrowth: percent of shoot tips produced normal shoots after 4 weeks of plating. Approximately 10 shoot tips were tested for each of three replicates.
In this study, we demonstrated that the D cryo-plate technique led to high percentage regrowth of several blueberry cultivars/species using in vitro shoot tips. Additionally, we found that in vitro shoots were a more suitable material for using the D cryo-plate method than current shoots. The optimized protocol is likely to be applicable for the cryopreservation of other Ericaceae species and cultivars with some modifications.
Acknowledgments
This study was mainly supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics-based Technology for Agricultural Improvement, CRS-1001).
Abbreviations
- LN
liquid nitrogen
- LS treatment
loading solution treatment
- MS medium
Murashige and Skoog medium (1962)
References
- Ballington JR (2001) Collection, utilization, and preservation of genetic resources in Vaccinium. HortSci 36: 213–220 [Google Scholar]
- Boches P, Bassil NV, Rowland L (2006) Genetic diversity in the highbush blueberry evaluated with microsatellite markers. J Am Soc Hortic Sci 131: 674–686 [Google Scholar]
- Engelmann F, Gonzalez-Arnao MT, Wu Y, Escobar R (2008) The development of encapsulation dehydration. In: Reed BM (ed) Plant Cryopreservation: A Practical Guide. Springer, New York, pp 59–75
- Kaviani B (2011) Conservation of plant genetic resources by cryopreservation. Aust J Crop Sci 5: 778–800 [Google Scholar]
- Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13: 442–446 [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamamoto S, Fukui K, Rafique T, Engelmann F, Niino T (2015) Cryopreservation of persimmon shoot tips from dormant buds using the D Cryo-plate technique. The Hort J 84: 106–110 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Niino T, Arizaga MV (2015) Cryopreservation for preservation of potato genetic resources. Breed Sci 65: 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niino T, Wunna, Watanabe K, Nohara N, Rafique T, Yamamoto S, Fukui K, Arizaga MV, Martinez CRC, Matsumoto T, et al. (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotechnol 31: 281–287 [Google Scholar]
- Niino T, Yamamoto S, Fukui K, Castillo Martínez CR, Arizaga MV, Matsumoto T, Engelmann F (2013) Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. Cryo Letters 34: 549–560 [PubMed] [Google Scholar]
- Rao VR (2011) PGR conservation and use: Principles and concepts and complementary conservation strategy, A training module for the international course on the management and utilization of field genebanks and in vitro collections: 12–21
- Sakai A (1997) Potentially valuable cryogenic procedures for cryopreservation of cultured plant meristems. In: Razdan MK, Cocking EC (eds) Conservation of Plant Genetic Resources in Vitro. Science Publishers, New York, pp 53–66
- Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification. Cryo Letters 28: 151–172 [PubMed] [Google Scholar]
- Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification and encapsulation-vitrification protocols. In: Reed BM (ed) Plant Cryopreservation: A Practical Guide. Springer, New York, pp 33–58
- Salma M, Fki L, Engelmann-Sylvestre I, Niino T, Engelmann F (2014) Comparison of droplet-vitrification and D-cryoplate for cryopreservation of date palm (Phoenix dactylifera L.) polyembryonic masses. Sci Hortic 179: 91–97 [Google Scholar]
- Seibert M (1976) Shoot initiation from carnation shoot apices frozen to −196°C. Science 191: 1178–1179 [DOI] [PubMed] [Google Scholar]
- Sekizawa K, Yamamoto S, Rafique T, Fukui K, Niino T (2011) Cryopreservation of in vitro-grown shoot tips of carnation (Dianthus caryophyllus L.) by vitrification method using aluminium cryo-plates. Plant Biotechnol 28: 401–405 [Google Scholar]
- Uchendu EE, Reed B (2009) Desiccation tolerance and cryopreservation of in vitro grown blueberry and cranberry shoot tips. Acta Hortic 810: 567–574 [Google Scholar]
- Yamamoto S, Fukui K, Rafique T, Khan NI, Castillo Martinez CR, Sekizawa K, Matsumoto T, Niino T (2012a) Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminium cryo-plates. Plant Gen Res: Charact Utiliz 10: 14–19 [Google Scholar]
- Yamamoto S, Rafique T, Fukui K, Sekizawa K, Niino T (2012b) V-cryo-plate procedure as an effective protocol for cryobanks: Case study of Mint cryopreservation. Cryo Letters 33: 12–23 [PubMed] [Google Scholar]
- Yamamoto S, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium cryo-plates. Cryo Letters 32: 256–265 [PubMed] [Google Scholar]