Abstract
Background
Interdisciplinary antiretroviral stewardship teams, comprising a human immunodeficiency virus pharmacist specialist, an infectious diseases physician, and associated learners, have the ability to assist in identification and correction of inpatient antiretroviral-related errors.
Methods
Electronic medical records of patients with antiretroviral orders admitted to our hospital were evaluated for the number of interventions made by the stewardship team, number of admissions with errors identified, risk factors for occurrence of errors, and cost savings. Risk factors were analyzed by means of multivariable logistic regression. Cost savings were estimated by the documentation system Clinical Measures.
Results
A total of 567 admissions were included for analysis in a 1-year study period. Forty-three percent of admissions (245 of 567) had ≥1 intervention, with 336 interventions in total. The following were identified as risk factors for error: multitablet inpatient regimen (odds ratio, 1.834; 95% confidence interval, 1.160–2.899; P = .009), admission to the intensive care unit (2.803; 1.280–6.136; P = .01), care provided by a surgery service (1.762; 1.082–2.868; P = .02), increased number of days reviewed (1.061; 1.008–1.117; P = .02), and noninstitutional outpatient provider (1.375; .972–1.946; P = .07). The 1-year cost savings were estimated to be $263 428.
Conclusions
Antiretroviral stewardship teams optimize patient care through identification and correction of antiretroviral-related errors. Errors may be more common in patients with multitablet inpatient regimens, admission to the intensive care unit, care provided by a surgery service, and increased number of hospital days reviewed. Once antiretroviral-related errors are identified, the ability to correct them provides cost savings.
Keywords: antiretroviral, HIV, stewardship
Interdisciplinary antiretroviral stewardship teams optimize patient care and provide institutional cost savings. At the highest risk for errors are patients with multitablet inpatient regimens, intensive care unit admission, care provided by a surgery service, and increased number of days reviewed.
Hospitalizations involving human immunodeficiency virus (HIV)–infected patients are at high risk for antiretroviral related errors, with the reported incidence as high as 70% [1–3]. Of these errors, the most commonly reported include (1) drug-drug interactions, (2) incorrect doses, (3) incomplete antiretroviral regimens, and (4) a lack of dosage adjustments based on renal function [1–8]. Despite pharmacists taking on expanding roles within the healthcare team, there is a paucity of data quantifying the effects of collaboration among physicians and pharmacists for the management of inpatient antiretroviral therapy.
As of December 2017, there are 19 199 known persons living with HIV in Philadelphia, Pennsylvania, equating to a prevalence rate of 1256 persons per 100 000 [9]. Given this high prevalence of infection and likelihood for servicing this local community on hospitalization, a specialized antiretroviral stewardship team was formed at Temple University Hospital (TUH) in 2015, comprising an HIV pharmacist specialist, an infectious diseases attending physician, and associated learners. This team evaluates each patient admitted to the hospital with active orders for antiretroviral therapy, enters a standardized note into the patient record comprising medication reconciliation and follow-up recommendations, and subsequently performs daily profile review during hospitalization. Patients are identified by a generated report in the electronic health record (EHR) and are evaluated within 24 hours of prescribing. The goals of this team are to identify and correct medication errors, identify risk factors for error occurrence, identify the prevalence of specific types of errors, and estimate potential cost savings to the institution.
METHODS
Setting and Design
This retrospective cohort study was approved by the Institutional Review Board at TUH. Hospitalized patients with an active order for an antiretroviral between 1 July 2017 and 30 June 2018 were identified by the EHR (EPIC; Epic Systems). Inclusion criteria comprised all admissions of patients ≥18 years of age with ≥1 antiretroviral medication indicated for the treatment of HIV-1 infection. Admissions were excluded if the patient received antiretroviral medications for an indication other than HIV infection, such as hepatitis B or preexposure or postexposure prophylaxis.
Outcome Measures
The primary objective was to determine the effectiveness of an antiretroviral stewardship team in identifying and correcting antiretroviral-related medication errors. The primary outcome was defined as the number of interventions made by the antiretroviral stewardship team, as quantified by the total number of interventions made in the intervention documentation system used by the TUH Department of Pharmacy, Clinical Measures (MedKeeper).
Secondary objectives included (1) assessing prevalence of antiretroviral-related errors among hospitalizations, (2) identifying risk factors for error, (3) quantifying the acceptance rate of interventions made by the stewardship team, and (4) estimating the financial impact of interventions made. Medication errors were defined in the categories of: drug interactions (per drug information resources such as the University of Liverpool and tertiary pharmacy databases in conjunction with clinical judgment), incorrect regimen (medication reconciliation error), opportunistic infection (OI) prophylaxis–related error, renal dosage adjustment required (per US-approved labeling in conjunction with clinical judgment), additional laboratory values required, medication not included in hospital formulary, and wrong dose or route of administration.
Risk factors were identified as age, sex, noninstitutional (non-TUH) outpatient provider, admission to the intensive care unit, care provided by surgery service, multitablet inpatient regimen, multitablet home regimen, antiretroviral class (nonnucleoside reverse-transcriptase inhibitors, protease inhibitors, integrase strand transfer inhibitors [INSTIs], or nucleoside reverse-transcriptase inhibitor–sparing regimens), CD4 cell count <200/µL, change in renal function requiring renal dosage adjustment, and number of hospital days reviewed. The intervention acceptance rate and estimated cost savings were quantified using the clinical documentation system, Clinical Measures.
Statistical Analysis
Descriptive statistics were used for the primary outcome and to characterize the secondary outcomes related to prevalence of errors, acceptance of interventions, and financial impact. Continuous parametric data are presented as means and standard deviations and analyzed using the Student t test. Continuous nonparametric data were analyzed using the Mann-Whitney U test and presented as medians with interquartile ranges. Nominal data were analyzed using either χ 2 or Fisher’s exact tests. To determine risk factors for antiretroviral-related errors, a univariate analysis and Pearson correlation matrix were conducted to identify variables for inclusion into the final model.
After adjustment for multicollinearity, binary logistic regression was developed to determine risk factors for HIV medication errors. All variables from the univariate analysis with P values < .10 were considered statistically significant and were included in a backward stepwise multivariable logistic regression. Results are reported as adjusted odds ratios with corresponding 95% confidence intervals. Two-tailed statistical tests were used, and differences were considered statistically significant at P < .05. All data were analyzed using IBM SPSS Statistics for Windows software, version 22.0 (IBM).
Cost Savings
Clinical Measures is a Web-based documentation system that uses a proprietary formula to provide standardized monetary savings by identifying select direct costs associated with preventing potential adverse outcomes. Each intervention logged requires the team to input the following variables: patient demographics (medical record number, bed location, attending physician), type of intervention, associated medication, and status (accepted or rejected).
RESULTS
Baseline Characteristics
During the study period, 606 admissions with active antiretroviral orders were screened. Thirty-nine admissions were excluded for hepatitis B treatment (n = 27) and preexposure prophylaxis (n = 12), with 567 admissions included for analysis. Baseline characteristics are summarized in Table 1. The majority of patients were 45–64 years old, were treated by an internal medicine service and with a multitablet inpatient regimen, had an INSTI as part of their antiretroviral regimen, and had a CD4 cell count ≥200/µL. Approximately half of the patients admitted had institutional outpatient HIV providers from whom the patient’s corresponding outpatient EHR was readily accessible.
Table 1.
Baseline Patient Characteristics
| Characteristic | Patients, No. (%) (N = 567) |
|---|---|
| Age, y | |
| 18–24 | 3 (0.5) |
| 25–34 | 41 (7.2) |
| 35–44 | 55 (9.7) |
| 45–54 | 206 (36.3) |
| 55–64 | 181 (31.9) |
| 65–74 | 71 (12.5) |
| ≥75 | 9 (1.6) |
| Male sex | 312 (55.0) |
| Institutional outpatient provider | 284 (50.1) |
| Treatment service | |
| Intensive care unit | 34 (6.0) |
| Internal medicine | 427 (75.3) |
| Surgery | 81 (14.3) |
| Obstetrics and gynecology | 16 (2.8) |
| Other (ENT, orthopedics) | 8 (1.4) |
| Multitablet home regimen | 353 (62.3) |
| Multitablet inpatient regimen | 458 (80.8) |
| Antiretroviral classa | |
| NNRTI | 106 (18.7) |
| PI | 118 (20.8) |
| INSTI | 438 (77.3) |
| NRTI-sparing | 25 (4.4) |
| CD4, cells/µL | |
| ≥200 | 453 (79.9) |
| 100–199 | 55 (9.7) |
| 50–99 | 22 (3.9) |
| <50 | 27 (4.8) |
| Unknown | 9 (1.6) |
Abbreviations: ENT, ear, nose, and throat; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aSome patients received >1 class.
Interventions
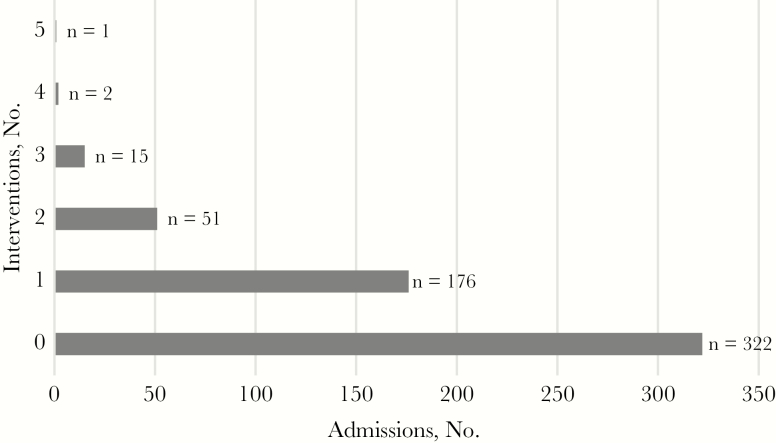
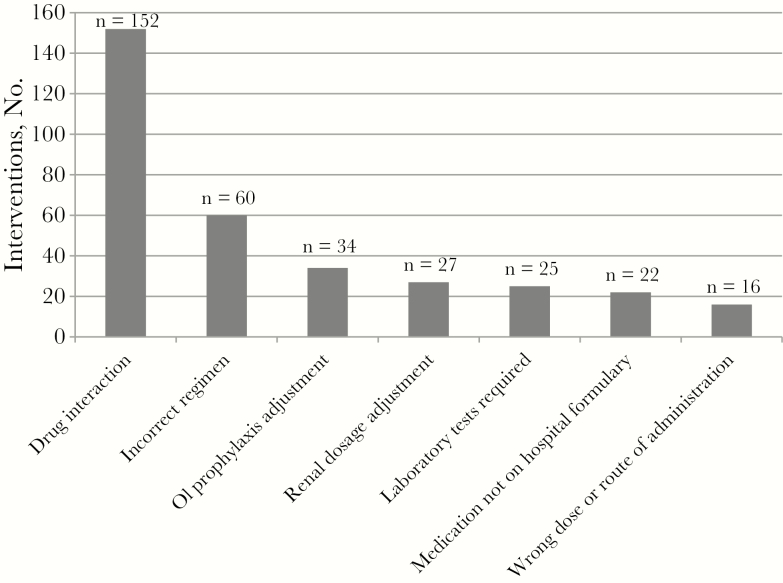
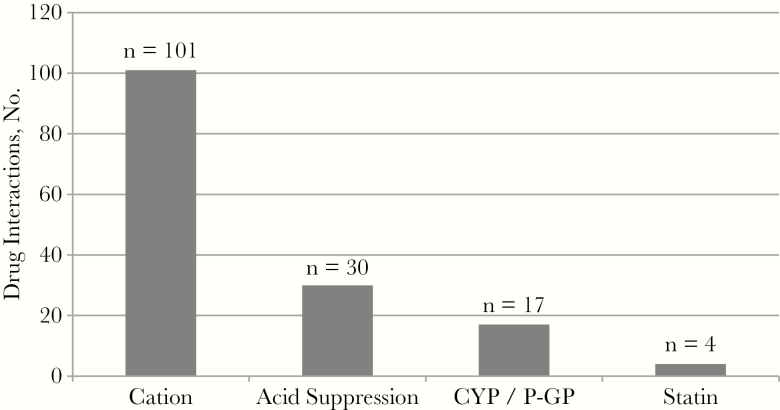
The stewardship team made 336 interventions. Figure 1 illustrates the number of interventions per admission, with 43.2% of admissions (245 of 567) requiring ≥1 intervention. Figure 2 depicts the number of interventions made by category. The highest occurring intervention categories were drug interactions (45.2%), followed by incorrect regimen (17.9%) and OI prophylaxis–related error (10.1%). Among the drug interactions identified (Figure 3), polyvalent-cation and INSTI coadministration had the highest frequency (66.5%), followed by acid-suppressing medication coadministered with rilpivirine or atazanavir.
Figure 1.
Number of interventions per admission (N = 567).
Figure 2.
Interventions by category (N = 336).
Figure 3.
Types of drug interactions (N = 152). Abbreviations: CYP, Cytochrome P450; P-GP, P-glycoprotein.
Risk Factors
Univariate analysis (Table 2) found significant effects for noninstitutional outpatient provider, intensive care unit admission, internal medicine service, surgical service, change in glomerular filtration rate, multitablet home regimen, multitablet inpatient regimen, CD4 cell count <200/µL, and number of hospital days reviewed (P < .10 for all). Multivariable logistic regression (Table 3) demonstrated multitablet inpatient regimen (P = .009), admission to the intensive care unit (P = .01), care provided by a surgery service (P = .02), increased number of days reviewed (P = .02), and noninstitutional outpatient provider (P = .07) as significant risk factors. Model fit analysis resulted in a Hosmer and Lemeshow goodness of fit of 0.853 and an area under the receiver operating characteristic curve of 0.624.
Table 2.
Risk Factors for Antiretroviral-Related Medication Errors
| Variable | Patients, No. (%)a | P Value | |
|---|---|---|---|
| No Intervention (n = 322) | Intervention (n = 245) | ||
| Noninstitutional outpatient provider | 150 (46.6) | 132 (53.9) | .09 |
| Male sex | 168 (52.2) | 144 (58.8) | .13 |
| Age ≥45 y | 269 (83.5) | 198 (80.8) | .35 |
| Intensive care unit admission | 10 (3.1) | 24 (9.8) | .001 |
| Internal medicine service | 259 (80.4) | 168 (68.6) | .001 |
| Surgery service | 38 (11.8) | 43 (17.6) | .05 |
| Change in GFR | 27 (8.4) | 32 (13.1) | .07 |
| NNRTI | 62 (19.3) | 44 (18.0) | .68 |
| PI | 60 (18.6) | 58 (23.7) | .15 |
| INSTI | 244 (75.8) | 194 (79.2) | .37 |
| NRTI-sparing | 16 (5.0) | 9 (3.7) | .45 |
| Multitablet home regimen | 188 (58.4) | 165 (67.3) | .03 |
| Multitablet inpatient regimen | 248 (77.0) | 210 (85.7) | .01 |
| CD4 cell count <200/µL | 50 (15.5) | 54 (22.0) | .049 |
| Days reviewed, median (IQR), no. | 2 (1–4)b | 3 (1–5)b | .002 |
Abbreviations: GFR, glomerular filtration rate; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aData represent no. (%) of patients unless otherwise specified.
Table 3.
Multivariable Logistic Regression
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Multitablet inpatient regimen | 1.834 (1.160–2.899) | .009 |
| Intensive care unit admission | 2.803 (1.280–6.136) | .01 |
| Surgery service | 1.762 (1.082–2.868) | .02 |
| No. of days reviewed | 1.061 (1.008–1.117) | .02 |
| Noninstitutional outpatient provider | 1.375 (.972–1.946) | .07 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Cost Savings
The intervention acceptance rate was 96.4% (324 of 336), with an associated cost savings of $263 428. The mean cost savings per intervention was $813, with a mean monthly savings of $21 952.
DISCUSSION
The Infectious Diseases Society of America defines stewardship as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration” [10]. Having a team in place to specifically review antiretroviral medications allows for optimization of HIV care in the inpatient setting. Despite EHR implementation designed for patient safety, inpatient antiretroviral errors have been routinely reported to exceed 50% of admissions (with HIV pharmacist intervention being a validated approach to timely correction) [1–3, 11, 12].
Providing education, having dedicated personnel (eg, HIV pharmacist specialists, infectious diseases physicians) to evaluate HIV-infected patients, optimizing formulary selections, and medication reconciliation have also been identified as ways to decrease antiretroviral-related error rates [13]. The antiretroviral stewardship team described herein not only contacts the outpatient HIV provider to confirm medication regimens but also relays all information to the primary team and provides education to prevent such errors from recurring.
Although many studies look at pharmacy review or changes to computer entry and education to prevent or correct antiretroviral-related errors, there are limited data focused on interdisciplinary patient review as the primary intervention [11, 14, 15]. The approach discussed here differs from that in the previous literature, because patients are both reviewed on admission (with a standardized note placed in the patient record) and then reviewed and documented upon throughout hospitalization. The additional measurement of acceptance rates and cost savings via Clinical Measures adds to existing data. Although previous studies have documented intervention acceptance rates typically ranging from 90% to 100% (coinciding with our acceptance rate of 96.4%), no other study to our knowledge has extrapolated these data to determine the financial impact of such an initiative [1, 3, 4, 8, 15–17]. Using Clinical Measures, our study is limited by its retrospective nature and its dependence on accurate documentation for data analysis.
Our antiretroviral-related admission rate averaged 47 admissions per month during the study period and exceeds that of other published studies [1, 3–8, 11, 12, 15–20]. It is a limitation, however, that patients were identified through antiretroviral ordering alone, because patients who did not disclose their HIV status or had medications held on admission were not identified or included. Thus, the true number of HIV-infected patients admitted to TUH may exceed the numbers presented here. The capabilities of the stewardship team were also limited in that reviews were conducted only during the standard workweek, so patients who were hospitalized only on the weekend were not screened or included. Infectious diseases consultation was available for assistance during such times when the stewardship team was unavailable.
Our study found an antiretroviral error rate of 43.2%, aligning with established literature [1–3, 11, 12, 14, 15, 17, 18, 21, 22]. Studies at The Brooklyn Hospital Center in New York, The Johns Hopkins Hospital in Maryland, and Hahnemann University Hospital in Pennsylvania (all large, inner-city hospitals serving substantial HIV-infected populations) have shown significant reductions in medication errors after the implementation of directed antiretroviral stewardship programs [5, 15, 21]. Data from The Johns Hopkins Hospital demonstrated that 12 of 22 errors occurring on day 1 were not corrected by the next day (with another 10 errors occurring since review) [21]. In the approach described here, continuous review and documentation throughout the admission were able to track acceptance and rejection rates in real time and ensure patient safety.
Common antiretroviral-related errors described in previous trials include medication reconciliation errors, dosing and scheduling errors, and drug-drug interactions [1–8, 11–22]. Although many types of errors occurred, our data concur with the literature, because drug-drug interaction and medication reconciliation errors formed the majority of interventions made. As an added opportunity for identifying errors, this program describes multiple errors related to OI prophylaxis medications, a subject that has been reviewed only minimally [5, 12, 17]. No specific antiretroviral drug class was identified as statistically significant to error predisposition in this study; however, a trend was seen with INSTIs and concomitant polyvalent-cation administration. Most of the data in this area were obtained before widespread usage of this class, so this is among the first reports to quantify inpatient INSTI drug errors [15]. Our data demonstrate that despite widespread use of a class of medications deemed to have less potential for error, stewardship is still required.
The multivariable logistic regression analysis resulted multiple statistically significant risk factors for medication-related errors. These include multitablet inpatient regimens, increased number of days reviewed, admission to the intensive care unit, and care provided by a surgery service. These data expand on and add new findings to previous literature beyond traditionally identified risk factors (renal impairment and use of protease inhibitors) and may be of benefit to evaluate in future studies [1, 3–5, 7, 8, 11, 14, 16, 19–22]. Although risk for error increases as patients require more medications and length of hospitalization increases, it is also important to consider the level of acuity of care as well as concurrent procedures that may occur. Critically ill patients present unique barriers to complete medication reconciliation including, but not limited to, inability to communicate, changes in enteral status, and labile renal function. In addition, patients may need single-tablet home regimens to be converted to a multitablet regimen if particular drugs are not available on the hospital formulary.
This is the first study to report cost savings associated with an antiretroviral stewardship program. The documentation program that we used (Clinical Measures) allows for a standardized approach to evaluate monetary savings, and although a formulaic estimation, it demonstrates the financial value of antiretroviral stewardship. Although our institution uses Clinical Measures, other financial estimation monitoring tools are available for use. Antiretroviral-related errors tend to be multifactorial and complex, making them more difficult for computer systems to detect [13]. Lapses in therapy, drug interactions, and inappropriate dosing of antiretroviral medications may lead to HIV-related disease or death, so it can be proposed that the per-patient savings presented here may be underestimated, when compared with loss of virologic control and related complications.
In conclusion, an antiretroviral stewardship team allows for interprofessional communication to optimize patient care and provide cost savings through prevention of medication errors. Recognizing risk factors such as multitablet inpatient regimens, admission to the intensive care unit, care provided by a surgery service, and increased number of days reviewed may help prioritize HIV-infected patients at higher risk for error.
Acknowledgments
Financial support. No external or internal funding was received for this study.
Potential conflicts of interest. D. E. K. has served on medical advisory panels for ViiV Healthcare and Gilead Sciences. He has also received investigator-initiated research funding from Gilead Sciences. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pastakia SD, Corbett AH, Raasch RH, et al. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother 2008; 42:491–7. [DOI] [PubMed] [Google Scholar]
- 2. Daniels LM, Raasch RH, Corbett AH. Implementation of targeted interventions to decrease antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm 2012; 69:422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garey KW, Teichner P. Pharmacist intervention program for hospitalized patients with HIV infection. Am J Health Syst Pharm 2000; 57:2283–4. [DOI] [PubMed] [Google Scholar]
- 4. Carcelero E, Tuset M, Martin M, et al. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med 2011; 12:494–9. [DOI] [PubMed] [Google Scholar]
- 5. Billedo JA, Berkowitz LB, Cha A. Evaluating the impact of a pharmacist-led antiretroviral stewardship program on reducing drug interactions in HIV-infected patients. J Int Assoc Provid AIDS Care 2016; 15:84–8. [DOI] [PubMed] [Google Scholar]
- 6. Heelon M, Skiest D, Tereso G, et al. Effect of a clinical pharmacist’s interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm 2007; 64:2064–8. [DOI] [PubMed] [Google Scholar]
- 7. Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis 2006; 43:933–8. [DOI] [PubMed] [Google Scholar]
- 8. Bias TE, Venugopalan V, Berkowitz L, Cha A. Incidence of antiretroviral drug interactions during hospital course: the role of a pharmacist-led antiretroviral stewardship program. J Pharm Technol 2014; 30:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philadelphia Department of Public Health, AIDS Activities Coordinating Office. Surveillance Report, 2017. Philadelphia, PA: City of Philadelphia; 2018.
- 10. Fishman N. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33:322–7. [DOI] [PubMed] [Google Scholar]
- 11. Sanders J, Pallotta A, Bauer S, et al. Antimicrobial stewardship program to reduce antiretroviral medication errors in hospitalized patients with human immunodeficiency virus infection. Infect Control Hosp Epidemiol 2014; 35:272–7. [DOI] [PubMed] [Google Scholar]
- 12. Eginger KH, Yarborough LL, Inge LD, et al. Medication errors in HIV-infected hospitalized patients: a pharmacist’s impact. Ann Pharmacother 2013; 47:953–60. [DOI] [PubMed] [Google Scholar]
- 13. Li EH, Foisy MM. Antiretroviral and medication errors in hospitalized HIV-positive patients. Ann Pharmacother 2014; 48:998–1010. [DOI] [PubMed] [Google Scholar]
- 14. Zucker J, Mittal J, Jen SP, et al. Impact of stewardship interventions on antiretroviral medication errors in an urban medical center: a 3-year, multiphase study. Pharmacotherapy 2016; 36:245–51. [DOI] [PubMed] [Google Scholar]
- 15. Nimarko K, Bandali A, Bias T, Mindel S. Impact of an antimicrobial stewardship (ASP) initiative evaluating antiretroviral regimens for HIV-positive patients. Open Forum Infect Dis 2019; 5:S217. [Google Scholar]
- 16. Batra R, Wolbach-Lowes J, Swindells S, et al. Impact of an electronic medical record on the incidence of antiretroviral prescription errors and HIV pharmacist reconciliation on error correction among hospitalized HIV-infected patients. Antivir Ther 2015; 20:555–9. [DOI] [PubMed] [Google Scholar]
- 17. Chiampas TD, Kim H, Badowski M. Evaluation of the occurrence and type of antiretroviral and opportunistic infection medication errors within the inpatient setting. Pharm Pract (Granada) 2015; 13:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Commers T, Swindells S, Sayles H, et al. Antiretroviral medication prescribing errors are common with hospitalization of HIV-infected patients. J Antimicrob Chemother 2014; 69:262–7. [DOI] [PubMed] [Google Scholar]
- 19. Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother 2000; 34:833–8. [DOI] [PubMed] [Google Scholar]
- 20. Shea KM, Hobbs AL, Shumake JD, et al. Impact of an antiretroviral stewardship strategy on medication error rates. Am J Health Syst Pharm 2018; 75:876–85. [DOI] [PubMed] [Google Scholar]
- 21. Yehia BR, Mehta JM, Ciuffetelli D, et al. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis 2012; 55:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo Y, Chung P, Weiss C, et al. Customized order-entry sets can prevent antiretroviral prescribing errors: a novel opportunity for antimicrobial stewardship. P T 2015; 40:353–60. [PMC free article] [PubMed] [Google Scholar]