Abstract
Aims
Women with evidence of ischaemia but no obstructive coronary artery disease (INOCA) often have coronary microvascular dysfunction (CMD). Although invasively measured coronary flow reserve (CFR) is useful for the diagnosis of CMD, intermediate CFR values are often found of uncertain significance. We investigated myocardial flow reserve and left ventricular (LV) structural and functional remodelling in women with suspected INOCA and intermediate CFR.
Methods and results
Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study participants who had invasively measured intermediate CFR of 2.0≤ CFR ≤3.0 (n = 125) were included for this analysis. LV strain, peak filling rate (PFR) and myocardial perfusion reserve index (MPRI) were obtained by cardiac magnetic resonance imaging. Participants were divided: (i) Group 1 (n = 66) high MPRI ≥ 1.8, and (ii) Group 2 (n = 59) low MPRI < 1.8. The mean age was 54 ± 12 years and CFR was 2.46 ± 0.27. MPRI was significantly different but CFR did not differ between groups. LV relative wall thickness (RWT) trended higher in Group 2 and circumferential peak systolic strain and early diastolic strain rate were lower (P = 0.039 and P = 0.035, respectively), despite a similar LV ejection fraction and LV mass. PFR was higher in Group 1 and LV RWT was negatively related to PFR (r = −0.296, P = 0.001).
Conclusions
In women with suspected INOCA and intermediate CFR, those with lower MPRI had a trend towards more adverse remodelling and impaired diastolic LV function compared with those with higher MPRI. CFR was similar between the two groups. These findings provide evidence that both coronary microvessel vasomotion and structural and functional myocardial remodelling contribute to CMD.
Keywords: coronary microvascular dysfunction, women, coronary flow reserve, cardiac magnetic resonance, remodelling
Introduction
More than half of women with evidence of ischaemia but no obstructive coronary artery disease (INOCA).1,2 Studies have shown that these patients often have coronary microvascular dysfunction (CMD) indicated by a reduced coronary flow reserve (CFR) of <2.5 in response to adenosine, which is the lower limit of normal flow reserve in coronary arteries without obstructive coronary artery disease (CAD).3,4 Although invasively measured CFR is useful for the diagnosis of CMD, this can provide intermediate values of uncertain significance in women with objective evidence of ischaemia. In these cases, additional information to inform clinical decision-making is needed; however, it remains unclear what additional information should be gathered. Moreover, CMD may result from not only functional coronary microvessel vasomotion but also the microvasculature structure which includes the stroma and myocardium. We have previously demonstrated that left ventricular (LV) diastolic function is impaired in women with signs and symptoms of ischaemia in the absence of obstructive CAD.5
Cardiac magnetic resonance imaging (CMRI) is a powerful, non-invasive tool, capable of providing objective, quantitative evaluation of LV morphology, function,6–8 and myocardial perfusion abnormalities.9–11 Therefore, CMRI would provide the additional insight necessary to differentiate women with suspected INOCA and intermediate CFR.
We investigated myocardial flow reserve and LV structural and functional remodelling assessed by CMRI in women with suspected INOCA and intermediate CFR to understand the potential contribution of myocardial remodelling to CMD.
Methods
This analysis includes women enrolled in the National Heart, Lung, and Blood Institute-sponsored prospective multicenter Women’s Ischemia Syndrome Evaluation–Coronary Vascular Dysfunction (WISE-CVD) study. All studies were performed at Cedars-Sinai Medical Center or the University of Florida, Gainesville, between January 2009 and June 2012 where institutional review boards approved the project and all participants provided written informed consent. Exclusion criteria were acute myocardial infarction within 30 days, percutaneous coronary intervention, coronary artery bypass grafting, or valve surgery subsequent to baseline qualifying coronary angiogram and any conditions that precluded accurate or safe testing or follow-up, specifically: obstructive CAD ≥50% luminal diameter stenosis in ≥1 epicardial coronary artery, acute coronary syndrome, primary valvular heart disease with need for valve repair or replacement, concurrent cardiogenic shock, prior non-cardiac illness with estimated life expectancy <4 years, chest pain with known non-ischaemic pathogenesis (e.g. pericarditis, pneumonia, oesophageal spasm), contraindications to CMRI (pacemaker, other electronic device, severe claustrophobia), severe asthma (vasodilator stress contraindicated), severe renal impairment (gadolinium contrast contraindicated).
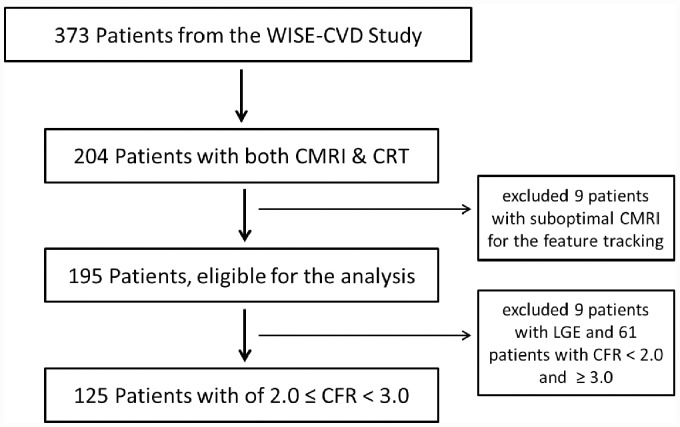
Stress CMRI was performed in 373 women per protocol. A subset (n = 204) underwent clinically indicated invasive coronary reactivity testing (CRT). The mean time difference between the CRT and CMRI was 35 ± 28 days, with no changes in medications and testing conditions per protocol. Eleven women were excluded from this analysis due to suboptimal CMRI for the feature tracking, and an additional nine women were excluded due to presence of myocardial scar detected by CMRI late gadolinium enhancement. Finally, 125 women had an intermediate CFR of 2.0 ≤ CFR≤3.0 and were included in this analysis (Figure 1).
Figure 1.
Subject flow chart. CFR, coronary flow reserve; CMRI, cardiac magnetic resonance imaging; CRT, coronary reactivity testing; LGE, late gadolinium enhancement.
Long-acting nitrates, short-acting calcium-channel blockers, α-blockers, β-blockers, and angiotensin-converting enzyme-I/angiotensin-II receptor antagonists were withdrawn 24 h, and long-acting calcium-channel blockers were held for 48 h before CRT and CMRI testing. Sublingual nitroglycerine was not taken within 4 h before testing, and participants were caffeine-free and nicotine-free for 24 h before vasodilator stress.
Invasive coronary flow reserve measurement
CFR was measured using a standardized protocol.12 A Doppler guidewire was placed in the proximal left anterior descending (LAD), and assessed coronary flow, with intracoronary adenosine (18 and 36 μg) used to achieve hyperaemia. CFR was derived from the ratio of the average peak velocity of blood flow at maximal hyperaemia and average peak velocity at rest as previously described. We have previously shown that this ratio closely approximates volumetric CFR in similar women enrolled in WISE.13 Graded doses of intracoronary acetylcholine (0.182 and 18.2 μg/mL) were infused to determine % epicardial coronary artery diameter change (ΔAch) and % coronary blood flow change (ΔCBF) in response to acetylcholine, as previously described.12,14
Cardiac magnetic resonance imaging
A standardized CMRI protocol and equipment were used (1.5 T Magnetom Avanto; Siemens Healthcare, Erlangen, Germany).11 Stress and rest first-pass perfusion imaging were performed using gadolinium contrast of 0.05 mM/kg each (Gadodiamide, Omniscan, Amersham, Piscataway, NJ). For adenosine studies, the standard protocol of the WISE-CVD study was used with adenosine (140 mcg/kg/min). Regadenoson was employed as the coronary vasodilator if patients had a history of mild-moderate asthma or had prior intolerance to adenosine. LV function and delayed enhancement imaging were performed using a standardized approach, as previously described.5 LV mass and volumes were assessed by manually tracing the epicardial and endocardial borders of short-axis cine images using CAAS MRV 3.3 software (Pie Medical Imaging B.V., Netherlands). Volume–time curves were used to derive indices of diastolic dysfunction, including early peak filling rate (PFR) and time-to-peak-filling rate (tPFR). LV end-diastolic dimension, septal wall, and inferolateral wall thickness were measured in end-diastole from a short-axis frame immediately basal to the tip of papillary muscle tips.15,16 Relative wall thickness (RWT) was calculated as [septal wall thickness + inferolateral wall thickness]/LV end-diastolic dimension.
Myocardial perfusion reserve index (MPRI), the CMRO-derived semi-quantitative index of CFR was defined as MPRI = Relative upslope_stress/Relative upslope_rest.11 An American Heart Association 16-segment model was used (true apex not imaged); mean MPRI was the average of 16 segments. An A-MPRI was defined as the mean MPRI from nine LAD coronary artery territory segments to relate to the invasively CFR and CBF measures obtained in the LAD. Based on MPRI values,11,17 participants were divided into two groups for the analysis: (i) Group 1, MPRI (n = 66), high MPRI ≥ 1.8, and (ii) Group 2, MPRI (n = 59), low MPRI < 1.8.
CMRI myocardial strain analysis
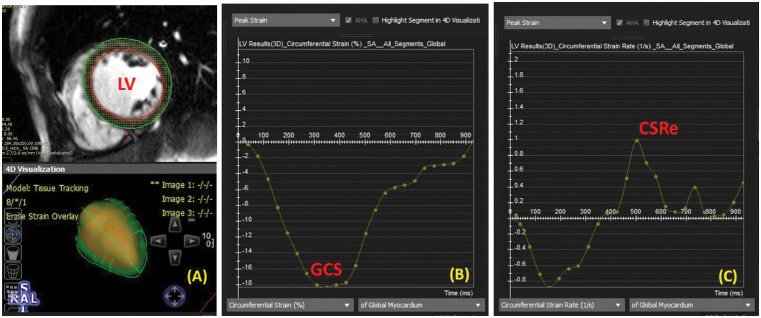
Myocardial tissue tracking was performed on resting CMRI offline using previously acquired steady-state free precession cine images, and dedicated software (cvi42; Circle Cardiovascular Imaging, Inc., Calgary, AB, Canada).7 A series of short-axis CMRI images were chosen, spanning the left ventricle (base to apex). We obtained global circumferential strain (GCS), circumferential early diastolic strain rate (CSRe), global radial strain (GRS), and radial early diastolic strain rate (RSRe) from the strain analysis curves (Figure 2). Our in-lab intra-rater variability, expressed as a coefficient of variation, for each of the primary endpoints is as follows [mean ± standard deviation (SD)]: GCS, 1% ± 1%; GRS, 3% ± 2%; CSRe, 6% ± 5%; and RSRe, 7% ± 5%.7
Figure 2.
Myocardial tissue feature tracking in a representative ventricular short-axis cine image. (A) Contours are drawn on the endocardial and epicardial boarders at a single phase of the cardiac cycle. (B) Global circumferential strain (GCS) curve from basal to apical all segments is shown throughout the cardiac cycle. (C) Circumferential early diastolic strain rate (CSRe) was automatically calculated as the time derivative of circumferential strain from (B).
Statistical analysis
Values are expressed as mean ± standard deviation or percentages as indicated. The t-test or chi-square test was used to evaluate differences in Group 1 vs. Group 2. The correlation between MPRI and other cardiac parameters assessed by CMRI was evaluated with the correlation analysis, and the significance of correlation between MPRI and PFR and other cardiac function was determined by multivariate linear regression analysis. After univariate screening, any candidate variable with a value of P < 0.10 was forced to enter a multivariate model. SPSS software (version 20.0) was used for statistical analysis. P-value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
Among the 373 WISE-CVD women who underwent stress CMRI, 204 women underwent clinically indicated invasive CRT and were included in this analysis. Nine women were identified as having presence of myocardial scar detected by CMRI late gadolinium enhancement. Because this was determined to be their likely primary aetiology, these women were not included in the remaining analysis. Of the women with clinically indicated CFR, 125 women had an intermediate CFR of 2.0 ≤ CFR < 3.0 and were included in this analysis (Figure 1).
Baseline characteristics are summarized in Table 1. Of 125 women, 66 (52.8%) subjects had MPRI ≥1.8 (Group 1) and 59 (47.2%) subjects had MPRI <1.8 (Group 2). The mean age of study participants was 54 ± 12 years and CFR was 2.46 ± 0.27. Group 2 participants were older (P < 0.008), but otherwise there were no significant group difference baseline demographics, clinical risk variables, or medication (P = NS in all). Despite the older age in Group 2, CFR, ΔAch, and ΔCBF were not different between two groups (Table 1).
Table 1.
Baseline characteristics of women by MPRI
| Total subjects (n = 125) | Group 1 High MPRI (MPRI ≥1.8, n = 66) | Group 2 Low MPRI (MPRI <1.8, n = 59) | P-value | |
|---|---|---|---|---|
| Age, years | 54.3 ± 11.7 | 51.3 ± 9.9 | 56.9 ± 12.6 | 0.008 |
| Systolic BP, mmHg | 127 ± 19 | 124 ± 20 | 130 ± 20 | 0.10 |
| Diastolic BP, mmHg | 70 ± 12 | 70 ± 11 | 70 ± 12 | 0.57 |
| Heart rate, rpm | 66 ± 10 | 66 ± 10 | 65 ± 10 | 0.66 |
| Body mass index, kg/m2 | 29.4 ± 7.2 | 29.2 ± 6.7 | 29.6 ± 7.6 | 0.74 |
| Hypertension, n (%) | 45 (38.5) | 20 (37.0) | 25 (39.7) | 0.85 |
| Diabetes mellitus, n (%) | 13 (10.5) | 7 (10.8) | 6 (10.2) | 0.77 |
| Dyslipidaemia, n (%) | 15 (12) | 6 (9.1) | 9 (15.3) | 0.54 |
| Current smoker, n (%) | 6 (4.8) | 4 (6.1) | 2 (3.4) | 0.34 |
| Medication | ||||
| Beta blockers, n (%) | 28 (22.4) | 14 (21.2) | 14 (23.7) | 0.84 |
| CCB, n (%) | 23 (18.4) | 9 (13.6) | 14 (23.7) | 0.33 |
| Nitrate, n (%) | 35 (28) | 17 (25.6) | 18 (30.5) | 0.98 |
| ACEI or ARB, n (%) | 26 (20.8) | 11 (16.7) | 15 (25.4) | 0.61 |
| Aspirin, n (%) | 3 (2.4) | 3 (3.6) | 0 (0) | 0.66 |
| Statins, n (%) | 44 (35.2) | 17 (25.8) | 27 (45.8) | 0.16 |
| Coronary severity score | 9.9 ± 4.4 | 9.8 ± 4.5 | 10.0 ± 4.3 | 0.67 |
| Coronary reactivity test | ||||
| CFR | 2.46 ± 0.27 | 2.43 ± 0.26 | 2.49 ± 0.27 | 0.16 |
| ΔAch, % | 0.2 ± 12.6 | 1.5 ± 13.1 | −1.1 ± 12.1 | 0.29 |
| ΔCBF | 64.4 ± 70.8 | 64.0 ± 73.2 | 61.0 ± 68.3 | 0.83 |
| LVEDP, mmHg | 14.2 ± 4.9 | 14.8 ± 5.2 | 13.7 ± 4.6 | 0.21 |
ΔAch, change in epicardial coronary artery diameter in response to acetylcholine; ΔCBF, change in coronary blood flow (CBF) in response to acetylcholine; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; BP, blood pressure; CCB, calcium-channel blockers; CFR, coronary flow reserve; LVEDP, left ventricular end-diastolic pressure.
There was no significant difference of symptoms between chest pain and shortness of breath according to MPRI, CFR, and LV function. However, there was a trend towards higher frequency of chest pain episodes in low MPRI group vs. high MPRI group (P = 0.065), although angina symptoms (location, duration, triggering factors, and response to nitroglycerin) did not differ between two subgroups.
Assessment of left ventricular structural and functional remodelling by CMRI
Overall, MPRI was weakly related to age (r = −0.173, P = 0.056), but CFR was not (r = 0.045, P = 0.624). Comparison of LV myocardial function by MPRI group is shown in Table 2. By design, mean MPRI was significantly higher in Group 1. Furthermore, A-MPRI which specifically evaluated the same LAD coronary artery territory with that of invasive CFR, was also higher in Group 1. Among this subgroup of WISE-CVD, there was no significant correlation between CFR and MPRI (r = −0.048, P = 0.603, with adjusted by age). LV RWT trended higher in Group 2, despite a similar LV mass index, compared with Group 1. GCS and CSRe were lower (P = 0.039 and P = 0.035, respectively) in Group 2, but LV ejection fraction was not different between two groups. GRS was higher in Group 2, but RSRe was not different between the groups.
Table 2.
Comparison of LV myocardial structure and function by MPRI groups
| Group 1 High MPRI (MPRI ≥ 1.8, n = 66) | Group 2 Low MPRI (MPRI < 1.8, n = 59) | P-value | |
|---|---|---|---|
| MPRI | 2.24 ± 0.34 | 1.52 ± 0.23 | <0.001 |
| A-MPRI | 2.21 ± 0.39 | 1.50 ± 0.25 | <0.001 |
| LV mass index, g/m2 | 51.8 ± 5.8 | 51.3 ± 8.1 | 0.66 |
| LV RWT | 0.33 ± 0.06 | 0.36 ± 0.09 | 0.08 |
| LV ejection fraction, % | 66 ± 6 | 67 ± 7 | 0.52 |
| PFR, mL/s | 374.1 ± 104.4 | 335.1 ± 99.5 | 0.035 |
| Time to PFR, ms | 199.6 ± 75.0 | 198.5 ± 51.3 | 0.92 |
| GRS (%) | 48.7 ± 7.7 | 52.0 ± 9.7 | 0.046 |
| GCS (%) | −23.7 ± 2.2 | −22.7 ± 3.1 | 0.039 |
| RSRe (1/s) | −3.23 ± 0.74 | −3.28 ± 0.98 | 0.75 |
| CSRe (1/s) | 1.47 ± 0.35 | 1.34 ± 0.32 | 0.037 |
A-MPRI, MPRI corresponding left anterior descending coronary artery; CSRe, circumferential early diastolic strain rate; GCS, global circumferential strain; GRS, global radial strain; LV, left ventricular; MPRI, myocardial perfusion reserve index; PFR, peak filling rate; RSRe, radial early diastolic strain rate; RWT, relative wall thickness.
Univariate analysis of the relationship between groups to other cardiac parameters is depicted in Table 3. Low MPRI was related to older age, low PFR, GCS, GRS, and CSRe; however, age-adjusted analysis demonstrated that only low GCS remained negatively related to low MPRI (r = −0.22, P = 0.02). In multivariate analysis, GRS and GCS were related to MPRI group (Table 3).
Table 3.
The relationship between MPRI group and other cardiac parameters
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| R | P-value | β | P-value | 95% CI | |
| Age | −0.237 | 0.008 | −1.04 | 0.063 | −0.998 to 1.082 |
| Systolic blood pressure | −0.172 | 0.057 | |||
| LV mass index | 0.040 | 0.658 | |||
| LV RWT | −0.155 | 0.096 | |||
| PFR | 0.189 | 0.035 | |||
| GRS | −0.187 | 0.049 | −1.19 | 0.024 | −1.372 to 1.035 |
| GCS | −0.196 | 0.039 | −1.99 | 0.013 | −3.448 to 1.153 |
| CSRe | 0.199 | 0.037 | |||
CSRe, circumferential early diastolic strain rate; GCS, global circumferential strain; GRS, global radial strain; LV, left ventricle; MPRI, myocardial perfusion reserve index; PFR, peak filling rate; RWT, relative wall thickness.
PFR was related to LV RWT, GRS, RSRe, and CSRe. Systolic BP and LVMI were weakly related to PFR (Table 4). Regarding the relation to concentric remodelling, PFR was negatively related to LV RWT (r = −0.296, P = 0.001) and this correlation was more apparent with adjusted by age (r = −0.336, P < 0.001, Table 4). By multivariate analysis, LV RWT was the most independent parameter related to PFR (Table 4).
Table 4.
The relationship between PFR and other cardiac parameters
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| R | P-value | β | P-value | 95% CI | |
| Systolic blood pressure | 0.159 | 0.089 | 0.126 | 0.17 | −3.968 to 0.701 |
| LV mass index | 0.165 | 0.089 | 0.196 | 0.048 | 0.192 to 34.482 |
| LV RWT | −0.336 | <0.001 | −0.307 | <0.001 | −0.005 to −0.001 |
| MPRI | 0.014 | 0.882 | |||
| GRS | 0.256 | 0.008 | −0.138 | 0.17 | −0.267 to 1.531 |
| GCS | −0.149 | 0.124 | |||
| RSRe | −0.418 | <0.001 | −0.455 | 0.001 | −0.044 to −0.012 |
| CSRe | 0.262 | 0.006 | 0.104 | 0.39 | −0.024 to 0.010 |
Regression analysis was performed with Low MPRI and other cardiac parameters, and adjusted for age. CSRe, circumferential early diastolic strain rate; GCS, global circumferential strain; GRS, global radial strain; LV, left ventricle; MPRI, myocardial perfusion reserve index; PFR, peak filling rate; RWT, relative wall thickness.
Discussion
In women with suspected INOCA (evidence of ischaemia but no obstructive CAD) and intermediate CFR, those with lower MPRI had more LV concentric modelling and impaired diastolic LV function compared with those with higher MPRI, despite having similar CFR. Impaired LV circumferential strain values in women with lower MPRI may indicate greater subendocardial dysfunction. These results support the hypothesis that LV concentric remodelling and LV diastolic dysfunction may contribute to CMD, in addition to impairment of vasomotion, by progressive fibrosis from chronic repetitive coronary microvascular ischaemia,18 although causality cannot be inferred by our cross-sectional design.
The LV wall consists of endocardium, myocardium, and epicardium. The endocardium is the most vulnerable to the afterload or ischaemia and the function of endocardium is the most easily impaired in various cardiac diseases. Circumferential function is mainly determined by the endocardial layer, whereas radial function is an action of myocardial layer.19 In our study, GCS and CSRe were both lower in patients with low MPRI although LV ejection fraction was preserved. Our results combined with our prior work5,20 demonstrate that women with suspected INOCA have impaired LV endocardial function and diastolic dysfunction, which supports aetiological relations among endocardial impairment, LV diastolic dysfunction, and CMD, although directional causality cannot be not determined.
Notably, in our study of women with intermediate CFR, vasomotion represented by invasively determined CFR was similar in patients with high MPRI and with low MPRI, suggesting that MPRI may more accurately reflect CMD-related ischaemia due to contribution from the myocardium. Abnormalities in MPRI but without impairment in invasive CFR may reflect early or mild CMD.21 We previously demonstrated weak correlation between CFR and MPRI in women with suspected angina but no obstructive CAD.11 Therefore, factors other than coronary vasomotion appear to be important in the physiology of microvascular ischaemia, such as LV concentric remodelling and dysfunction. Indeed, the low MPRI group demonstrated more LV concentric remodelling and impaired LV function than the high MPRI group.
MPRI was inversely related to LV concentric remodelling, with no increase in LV mass index in this population. LV concentric remodelling can occur due to an increase in afterload such as hypertension; however, in our study, the prevalence of hypertension was not different between the two groups, although systolic BP was slightly higher in the low MPRI group. Cardiovascular risk factors, such as hypertension, diabetes, and smoking contribute to a minority of CMD variance in WISE participants,22 thus it is not surprising that there were no significant differences in cardiovascular risk factor prevalence in the two MPRI groups.
Concentric remodelling can lead to extravascular compression of the coronary microvessels and coronary blood flow occurs predominantly during diastole, it is conceivable that LV diastolic changes also play a role in CMD. In INOCA patients with LV concentric remodelling, CFR reduction may initiate a process of abnormal coronary microvascular perfusion paralleling increased metabolic demand caused by pressure overload and increased wall thickness. Myocardial ischaemia occurs when there is an imbalance between myocardial oxygen (MVO2) supply and demand; and the ischaemia is usually entirely or predominantly subendocardial. A previous WISE study showed that compared with the reference group, WISE participants with no obstructive CAD have changes in systolic wave reflections and diastolic timing that increase LV afterload and MVO2 demand with the potential to reduce coronary artery perfusion.23 They suggested that these alterations in cardiovascular function contribute to an undesirable mismatch in the MVO2 supply/demand that promotes ischaemia and chest pain. In addition, wave-intensity analysis has shed light on the impact of LV hypertrophy on coronary microvascular flow, suggesting a disruption between systolic compression of the microcirculation and the resultant diastolic decompression.24,25
The reduction in coronary microvascular perfusion can be also influenced by the increase in LV filling pressure, which enhances extravascular coronary resistance.26 Because most of the coronary filling occurs during LV active relaxation,27 the delay in LV relaxation can also contribute to reducing hyperaemic diastolic coronary flow.25,28 It is also possible that progressive subtle fibrosis caused by chronic repetitive coronary microvascular impairment results in impairment of mid-wall LV function. In our result, RSRe represented as mid-wall LV diastolic function was significantly related to PFR.
Given that coronary blood flow mainly occurs in diastole, abnormalities in this phase of the cardiac cycle can have a strong effect on myocardial perfusion. Nevertheless, an increase in systolic intra-myocardial and ventricular pressures, as typically occurs in primary and secondary LV hypertrophy, can also adversely affect myocardial perfusion.29–31 In contrast, myocardial ischaemia may produce LV diastolic alterations, even before the detection of wall motion and/or electrocardiographic ST segment changes. It is well known that LV diastolic dysfunction is detected in the early stage of reduced coronary blood flow to the myocardium. Specifically, elevations of LV end-diastolic pressure have long been recognized to occur during spontaneous or provoked ischaemia.32–34 Although we excluded participants with myocardial scar by CMRI late gadolinium enhancement, we cannot exclude subclinical uniformly and focal perivascular fibrosis or interstitial fibrosis caused by chronic myocardial ischaemia and leading to increased microvascular resistance.26,35 Therefore, in CMD, while gross LV systolic dysfunction may not be apparent, LV diastolic function impairment may be both contributing to and a consequence of repeated bouts of subendocardial ischaemia.
These findings have prognostic significance in women with INOCA, as these women have worse diastolic function compared with normal controls.5,20 Low CFR and low myocardial perfusion reserve in patients with INOCA are related to worse prognosis.36–38 In addition, LV concentric remodelling itself also has adverse prognostic significance.39,40 Long-term follow-up of women with INOCA demonstrated a three-fold higher risk of cardiovascular events in comparison to asymptomatic healthy women, with the most frequent event being heart failure hospitalization,41 which we have documented to be heart failure with preserved ejection fraction (HFpEF).42 These findings have led to the hypothesis that CMD is a contributor to the development of HFpEF.43 We propose that contributors to CMD are also known to favour hypertrophy and fibrosis contributing to diastolic dysfunction. Further long-term studies are needed to determine the prognostic role of LV concentric remodelling with diastolic impairment in patients with CMD, and to determine whether functional CMD has a similar or a different prognostic weight as compared with structural CMD.
Clinical implications
We have previously demonstrated that more than half of women with suspected INOCA have gold-standard evidence of myocardial ischaemia on magnetic resonance spectroscopy.44 These women also have relatively high rates of heart failure hospitalizations, repeated coronary angiography, treatment costs, and adverse cardiovascular events compared with asymptomatic women.41,45 Despite these findings, knowledge gaps remain regarding pathophysiology which could lead to treatment targets. Our results demonstrating that both functional coronary microvessel vasomotion and structural microvasculature remodelling contribute to low MPRI provide additional pathophysiological insight and potential novel treatment targets. For example, these results may explain why standard pharmacological anti-anginal treatment can sometimes be disappointing in controlling angina symptoms. Further, why focused treatment on ventricular remodelling can improve angina,46 and offers a putative treatment target to reduce progression to HFpEF.42 Accordingly, CMRI MPRI and tissue tracking may provide additive information to assess suspected INOCA participants with intermediate CFR. Moreover, clinical trials of strategies to modulate myocardial substrate and function rather than functional coronary vasomotion should be considered.
Limitations
There are several limitations in this study. First, the invasive CFR and CMRI were performed on different days, potentially leading to day-to-day variability, although testing conditions were matched for the two measures. Secondly, we conducted this analysis from the WISE-CVD study which enrolled women only. While women are more likely to have no obstructive CAD on coronary angiography compared with men,47 and women have relatively more CMD than men,48 studies have been confounded by coronary angiography referral bias, and the relative prevalence of CMD in women and men is unknown. Thirdly, myocardial tissue feature tracking is a novel method to evaluate myocardial function by CMRI, is rapid and semi-automated, requires no additional scans and sequences, and reduces post-processing time compared with traditional myocardial tissue tagging.49,50 However, feature tracking imaging algorithms are dependent on image quality, frames, and endocardial border definition. The temporal resolution is lower than in echocardiography, which might be relevant for the assessment of strain rates for the diastolic function. Our feature tracking analyses were conducted on resting CMRI images, precluding our ability to understand direct links between CMD-related ischaemia and diastolic function.
Conclusions
In women with suspected INOCA and intermediate CFR, participants with lower MPRI had a trend towards more adverse concentric remodelling and impaired diastolic function compared with those with higher MPRI. Impaired LV strain values in women with lower MPRI may indicate greater subendocardial dysfunction despite similar CFR. These findings provide evidence that not only coronary microvessel vasomotion but also structural and functional myocardial remodelling may be contributors to CMD.
Funding
Research reported in this publication was supported by the National Heart, Lung and Blood Institute (NHLBI) under grant numbers N01HV68161, N01HV68162, N01HV68163, N01HV68164, U01HL64829, U01HL64914, U01HL64924, K23HL105787, T32HL69751, R01HL090957, R01HL33610, R01HL56921, and UM1HL087366; the National Institute on Aging (NIA) under grant number R03AG032631; the National Center for Research Resources (NCRR) under grant number M01RR000425; the National Center for Advancing Translational Sciences (NCATS) under grant numbers UL1TR000124, UL1TR000064, and UL1TR001427. This work was also supported by grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; The Society for Women’s Health Research (SWHR), Washington, DC; QMED, Inc., Laurence Harbor, NJ; The Women’s Guild of Cedars-Sinai, the Edythe L. Broad, the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, the Erika J. Glazer Women’s Heart Research Initiative, and The Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA; the Gatorade Trust and the PCORnet-One Florida Clinical Research Consortium CDRN-1501-26692, University of Florida, Gainesville, FL.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
References
- 1. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM. et al. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV. et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kothawade K, Bairey Merz CN.. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol 2011;36:291–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanza GA, Crea F.. Primary coronary microvascular dysfunction. Circulation 2010;121:2317.. [DOI] [PubMed] [Google Scholar]
- 5. Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B. et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014;7:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN.. Diastolic dysfunction: improved understanding using emerging imaging techniques. Am Heart J 2010;160:394–404. [DOI] [PubMed] [Google Scholar]
- 7. Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C. et al. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol 2017;40:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Geest RJ, Reiber JH.. Quantification in cardiac MRI. J Magn Reson Imaging 1999;10:602–8. [DOI] [PubMed] [Google Scholar]
- 9. Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K. et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation 2003;108:432–7. [DOI] [PubMed] [Google Scholar]
- 10. Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y. et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK. et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circulation Cardiovasc Imaging 2015;8:pii:e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD. et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interventions 2012;5:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ. et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 1999;33:1469–75. [DOI] [PubMed] [Google Scholar]
- 14. von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL. et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–5. [DOI] [PubMed] [Google Scholar]
- 15. Aurigemma GP, Gaasch WH.. Clinical practice. Diastolic heart failure. N Engl J Med 2004;351:1097–105. [DOI] [PubMed] [Google Scholar]
- 16. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J.. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012;60:1455–69. [DOI] [PubMed] [Google Scholar]
- 17. Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J. et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson MD, Wei J, Bairey Merz CN.. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J 2018;39:850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH.. Left ventricular fibre architecture in man. Br Heart J 1981;45:248–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE. et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol 2016;310:H14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu A, Wijesurendra RS, Liu JM, Forfar JC, Channon KM, Jerosch-Herold M. et al. Diagnosis of microvascular angina using cardiac magnetic resonance. J Am Coll Cardiol 2018;71:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Wessel TR, Arant CB, McGorray SP, Sharaf BL, Reis SE, Kerensky RA. et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin Cardiol 2007;30:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nichols WW, Denardo SJ, Johnson BD, Sharaf BL, Bairey Merz CN, Pepine CJ.. Increased wave reflection and ejection duration in women with chest pain and nonobstructive coronary artery disease: ancillary study from the Women’s Ischemia Syndrome Evaluation. J Hypertens 2013;31:1447–54; discussion 1454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broyd CJ, Davies JE, Escaned JE, Hughes A, Parker K.. Wave intensity analysis and its application to the coronary circulation. Glob Cardiol Sci Pract 2017;2017:e201705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies JE, Whinnett ZI, Francis DP, Manisty CH, Aguado-Sierra J, Willson K. et al. Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation 2006;113:1768–78. [DOI] [PubMed] [Google Scholar]
- 26. Voudris V, Manginas A, Vassilikos V, Koutelou M, Kantzis J, Cokkinos DV.. Coronary flow velocity changes after intravenous dipyridamole infusion: measurements using intravascular Doppler guide wire. A documentation of flow inhomogeneity. J Am Coll Cardiol 1996;27:1148–55. [DOI] [PubMed] [Google Scholar]
- 27. Jeremy RW, Hughes CF, Fletcher PJ.. Effects of left ventricular diastolic pressure on the pressure-flow relation of the coronary circulation during physiological vasodilatation. Cardiovasc Res 1986;20:922–30. [DOI] [PubMed] [Google Scholar]
- 28. Tadaoka S, Wada Y, Kimura A, Yada T, Tamura K, Hasegawa K. et al. Effect of left ventricular hypertrophy secondary to systemic hypertension on left coronary artery flow dynamics. Cardiovasc Res 1991;25:955–64. [DOI] [PubMed] [Google Scholar]
- 29. Choudhury L, Rosen SD, Patel D, Nihoyannopoulos P, Camici PG.. Coronary vasodilator reserve in primary and secondary left ventricular hypertrophy. A study with positron emission tomography. Eur Heart J 1997;18:108–16. [DOI] [PubMed] [Google Scholar]
- 30. Downey JM. Extravascular coronary resistance In: Sperelakis N, Kurachi Y, Terzic A, Cohen MV, eds.Physiology and Pathophysiology of the Heart. Ann Arbor, MI: Kluwer Academic Publishers; 1995. p1109–23. [Google Scholar]
- 31. Inoue K, Hamada M, Ohtsuka T, Hara Y, Shigematsu Y, Nakata S. et al. Myocardial microvascular abnormalities observed by intravenous myocardial contrast echocardiography in patients with hypertrophic cardiomyopathy. Am J Cardiol 2004;94:55–8. [DOI] [PubMed] [Google Scholar]
- 32. el-Said ES, Roelandt JR, Fioretti PM, McNeill AJ, Forster T, Boersma H. et al. Abnormal left ventricular early diastolic filling during dobutamine stress Doppler echocardiography is a sensitive indicator of significant coronary artery disease. J Am Coll Cardiol 1994;24:1618–24. [DOI] [PubMed] [Google Scholar]
- 33. Fujibayashi Y, Yamazaki S, Chang BL, Rajagopalan RE, Meerbaum S, Corday E.. Comparative echocardiographic study of recovery of diastolic versus systolic function after brief periods of coronary occlusion: differential effects of intravenous nifedipine administered before and during occlusion. J Am Coll Cardiol 1985;6:1289–98. [DOI] [PubMed] [Google Scholar]
- 34. Wijns W, Serruys PW, Slager CJ, Grimm J, Krayenbuehl HP, Hugenholtz PG. et al. Effect of coronary occlusion during percutaneous transluminal angioplasty in humans on left ventricular chamber stiffness and regional diastolic pressure-radius relations. J Am Coll Cardiol 1986;7:455–63. [DOI] [PubMed] [Google Scholar]
- 35. Camici PG, Crea F.. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 36. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM. et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G. et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balazs E, Pinter KS, Egyed A, Csanady M, Forster T, Nemes A.. The independent long-term prognostic value of coronary flow velocity reserve in female patients with chest pain and negative coronary angiograms (results from the SZEGED study). Int J Cardiol 2011;146:259–61. [DOI] [PubMed] [Google Scholar]
- 39. Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Cuccurullo F, Mezzetti A.. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens 2004;17:1035–9. [DOI] [PubMed] [Google Scholar]
- 40. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C. et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol 1995;25:871–8. [DOI] [PubMed] [Google Scholar]
- 41. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM. et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B. et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the women’s ischemia syndrome evaluation study. Int J Cardiol 2016;223:936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pepine CJ, Petersen JW, Bairey Merz CN.. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. JACC Cardiovasc Imaging 2014;7:362–5. [DOI] [PubMed] [Google Scholar]
- 44. Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N. et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- 45. Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN. et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–9. [DOI] [PubMed] [Google Scholar]
- 46. Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM. et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2011;162:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M. et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol 2015;66:1918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A.. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interventions 2015;8:1445–53. [DOI] [PubMed] [Google Scholar]
- 49. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R. et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 2010;3:144–51. [DOI] [PubMed] [Google Scholar]
- 50. Kempny A, Fernandez-Jimenez R, Orwat S, Schuler P, Bunck AC, Maintz D. et al. Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of Fallot and healthy controls. J Cardiovasc Magn Reson 2012;14:32.. [DOI] [PMC free article] [PubMed] [Google Scholar]