Abstract
Purpose
Internal mammary node recurrence after definitive breast cancer treatment is poorly characterized, with limited data to guide clinical management. The aim of this study was to analyze the outcomes of patients with recurrent breast cancer involving internal mammary nodes to understand their natural history and determine prognostic factors associated with improved overall survival.
Methods and Materials
We performed a retrospective analysis of 553 patients with recurrent breast cancer and identified 161 patients with radiographic evidence of locoregional recurrence as a first event. A total of 67 patients (42%) were identified with internal mammary involvement. Median follow-up times were 76 months from date of initial diagnosis and 30 months from date of recurrence.
Results
Of the 67 patients identified with internal mammary node failures, 10 (15%) presented with isolated recurrence, 14 (21%) presented with other sites of locoregional disease, and 43 (64%) presented with concomitant distant metastases. Median overall survival was 2.5 years and significantly associated with extent of disease (P < .0001). On multivariable analysis, concomitant distant metastases, inflammatory breast cancer, and triple negative histologic type were associated with worse overall survival, whereas salvage radiation therapy was associated with improved overall survival. Among the 10 patients with isolated internal mammary node failures, median progression-free survival was 6.0 years and salvage therapy with surgery, radiation, and chemotherapy were associated with the best outcomes.
Conclusions
Patients with isolated internal mammary node recurrences achieved long-term survival with aggressive therapies, and salvage radiation therapy was associated with improved survival.
Introduction
Breast cancer is the most commonly diagnosed malignancy and leading cause of death in women worldwide. Most patients in developed countries are diagnosed with early-stage disease and undergo mastectomy or breast conservation therapy. Adjuvant radiation and systemic therapies have improved the efficacy of these primary treatments; however, patients with locoregional failures have poor survival1, 2, 3 with limited data to guide salvage therapies.
Multiple randomized trials and large meta-analyses have found that, compared with local failures in the ipsilateral breast or chest wall, other locoregional recurrences are rare but portend worse prognosis. The 5-year overall survival rates among patients with axillary, supraclavicular, and internal mammary lymph node (IMN) recurrences are limited to 24% to 35%.1, 2 IMN involvement has been reported to comprise only 1% of patients with locoregional failures.4, 5 Advances in radiographic imaging, however, suggest that IMN failures may have been underdetected because FDG (fludeoxyglucose)–positron emission tomography and magnetic resonance imaging improve the detection of IMN metastases not identified on conventional chest imaging.6, 7
Because of anatomic proximity to the heart, IMN treatment is difficult to manage. Two large randomized studies suggest a benefit of radiation treatment to regional lymph nodes and have trended toward including IMN nodes within radiation fields.8, 9 Despite these data, internal mammary node radiation remains controversial.10 Many radiation oncologists remain hesitant to treat IMN nodes because of concerns regarding lung and cardiac toxicity,11, 12, 13 and IMN recurrences may be perceived by many oncologists as incurable.14
Given the significant advancements in radiographic detection and treatment paradigms for breast cancer, we sought to reexamine the natural history of internal mammary failures to understand prognostic factors associated with improved survival and guide salvage therapies.
Methods and Materials
This study was approved by the institutional review board and analyzed a single-institution database of patients with nonmetastatic breast cancer who underwent treatment between 1998 and 2013. Eligibility criteria included biopsy-proven recurrence and radiographic imaging of locoregional relapse. A total of 553 patients were identified, with pathologic and radiographic evidence of locoregional recurrence. A total of 161 patients presented with locoregional recurrence as a first event, with or without distant metastases identified within 2 months. Imaging was individually reviewed to identify patients with recurrent disease involving the internal mammary nodes.
The Kaplan-Meier method was employed to estimate overall survival after locoregional failure based on time until death. There were 49 events; 18 patients were censored. Survival curves were compared for statistical significance using the log-rank test. All events were defined from time of locoregional recurrence. Data were last updated May 21, 2018, and median follow-up times were 76 months from date of initial diagnosis and 30 months from date of recurrence. Multivariable analysis was conducted using IBM SPSS version 25 (IBM Corp., Armonk, NY).
Breast cancer subtypes were defined as follows: hormone receptor (HR) positive (estrogen-receptor and/or progesterone-receptor positive, HER2 negative), HER2 positive, and triple negative (TN, estrogen-receptor, progesterone-receptor, and HER2 negative).
Results
IMNs were commonly involved in patients with locoregionally recurrent breast cancer. Of the 161 patients with radiographic evidence of locoregional recurrence as a first event, 67 of 161 patients (42%) presented with internal mammary node failures. Baseline characteristics of these patients with recurrent IMN disease are summarized in Table 1. All patients had documented pathologic tumor and nodal stages. Median tumor size was 2.2 cm. Forty-two of 67 patients (63%) underwent mastectomy, of whom, 20 patients (48%) received postmastectomy radiation therapy. The most common subtype was TN, comprising 36 of 67 patients (54%). Eight of 67 patients (12%) presented with inflammatory breast cancer, 43 patients (64%) had nodal involvement, and 35 patients (52%) presented with lymphovascular invasion. Median interval from initial diagnosis to locoregional recurrence was 2.7 years (Table 2).
Table 1.
Patient, tumor, and treatment characteristics at initial presentation
| No. of patients | % | |
|---|---|---|
| Age (y) | ||
| Median | 49 | |
| Range | 23-78 | |
| Primary tumor | ||
| Left | 33 | 49 |
| Right | 34 | 51 |
| Tumor location | ||
| Medial | 32 | 48 |
| Central | 13 | 19 |
| Outer | 22 | 33 |
| Multicentric | ||
| Yes | 22 | 33 |
| No | 45 | 67 |
| Tumor stage | ||
| pT1 | 30 | 45 |
| pT2 | 28 | 42 |
| pT3 | 6 | 9 |
| pT4 | 3 | 4 |
| Nodal stage | ||
| pN0 | 24 | 36 |
| pN1 | 29 | 43 |
| pN2 | 4 | 6 |
| pN3 | 10 | 15 |
| Breast cancer subtype | ||
| HR positive | 27 | 40 |
| HER2 positive | 4 | 6 |
| Triple negative | 36 | 54 |
| Inflammatory | ||
| Yes | 8 | 12 |
| No | 59 | 88 |
| LVI | ||
| Absent | 32 | 48 |
| Present | 35 | 52 |
| Initial surgery | ||
| Lumpectomy | 25 | 37 |
| Mastectomy | 42 | 63 |
| Chemotherapy | ||
| None | 6 | 9 |
| Neoadjuvant | 20 | 30 |
| Adjuvant | 41 | 61 |
| Adjuvant RT | ||
| Postlumpectomy | 23 | 92 |
| Postmastectomy | 20 | 48 |
Abbreviations: HR = hormone receptor; LVI = lymphovascular invasion; RT = radiation therapy.
Table 2.
Patient and tumor characteristics of recurrent disease
| No. of patients | % | |
|---|---|---|
| Age at recurrence (y) | ||
| Median | 53 | |
| Range | 24-82 | |
| Interval to recurrence (y) | ||
| Median | 2.7 | |
| Range | 0.2-12.8 | |
| Molecular subtype of recurrence | ||
| HR positive | 18 | 33 |
| HER2 positive | 4 | 7 |
| Triple negative | 32 | 59 |
| Conversion | 12 | 22 |
| Salvage RT | ||
| Yes | 20 | 30 |
| No | 47 | 70 |
Abbreviations: HR = hormone receptor; RT = radiation therapy.
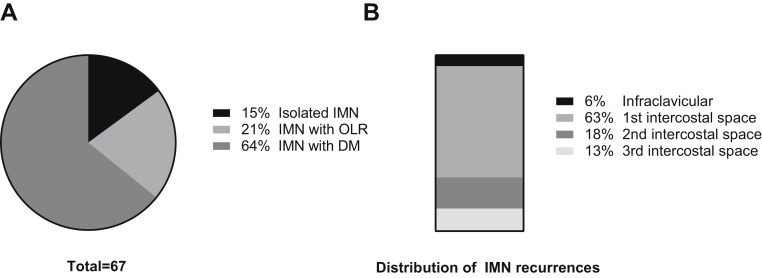
Ten of 67 patients (15%) presented with isolated IMN failures, 14 patients (21%) presented with other locoregional recurrence, and 43 patients (64%) presented with concomitant distant metastases (Fig 1A). Most recurrences occurred within the first intercostal space (63%; Fig 1B). A total of 18% of patients experience a recurrence within the second intercostal space and 13% within the third intercostal space. Four patients (6%) presented with infraclavicular failures, and no IMN recurrences were identified caudal to the third intercostal space.
Figure 1.
Overview of IMN failures. (A) Extent of disease. A total of 67 patients were identified with IMN failures as a first event. Ten patients (15%) presented with isolated IMN failures, 14 patients (21%) presented with other locoregional recurrence (IMN with OLR), and 43 patients (64%) presented with concomitant distant metastases (IMN with DM). (B) Anatomic distribution. IMN failures were mapped to intercostal locations; 6% of failures appeared in the infraclavicular space (between clavicle and first rib); 63% of failures mapped to the first intercostal space (between first and second rib); 18% mapped to the second intercostal space; 13% mapped to the third intercostal space; and no failures were identified caudal to the third intercostal space. Abbreviations: DM = distant metastases; IMN = internal mammary lymph node; OLR = other locoregional recurrence.
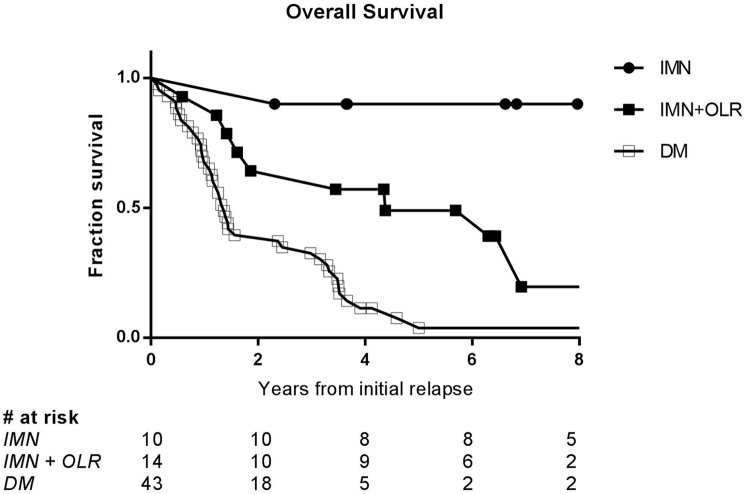
Among the 67 patients with recurrent disease involving internal mammary nodes, median overall survival was 2.5 years and significantly associated with extent of disease (P < .0001, Fig 2). At 5 years, overall survival was 28%. For the 43 patients (64% of 67) who presented with concomitant distant metastases, median overall survival was limited to 1.4 years. Fourteen patients (21%) presented with other locoregional recurrence (IMN with OLR) and had median overall survival of 4.4 years. For the 10 patients (15%) with isolated IMN failures, median overall survival was not reached.
Figure 2.
Overall survival significantly associated with extent of disease. Median overall survival for entire cohort of 67 patients with IMN recurrences as a first event was 2.5 years, with 5-year overall survival 28%. Among 43 patients with concomitant distant metastases, median overall survival was 1.4 years, compared with 4.4 years for patients with other locoregional recurrence. Median overall survival was not reached for the 10 patients with isolated IMN failures. On log-rank test, difference between the 3 groups was highly significant, with P < .0001. Abbreviations: DM = distant metastases; IMN = internal mammary lymph node; OLR = other locoregional recurrence.
Biological predictors of survival included inflammatory breast cancer and TN subtype. Median overall survival for patients with inflammatory breast cancer was 0.9 years compared with 3.3 years for noninflammatory breast cancer (P = .0001). TN biological subtype was also associated with poor prognosis. A total of 36 patients (54%) with recurrent IMN disease initially presented with TN subtype. Median overall survival for these TN patients was 1.4 years compared with 3.7 years for other subtypes (P = .01).
Fifty-four patients (81%) underwent hormone receptor profiling of recurrent disease (Table 2). Twelve patients had conversion to a different hormonal subtype. Median time to recurrence in this subset was 4.4 years (range, 1.0-8.9 years) compared with 3.1 years (range, 0.1-12.8 years) for patients who retained the same molecular subtype. A total of 32 patients (59%) had TN profiles, 18 patients had HR-positive disease, and 4 patients had HER2-positive disease. In 7 patients, HR-positive disease transformed into TN subtypes at time of recurrence. Median survival in this subgroup was 1.4 years. Median time to from initial presentation to IMN recurrence was 4.6 years.
Of the 20 patients (30%) who underwent radiation for recurrent IMN disease, 6 patients had concomitant distant metastases, 7 patients had additional sites of other locoregional recurrence, and 7 patients had isolated IMN failures. Patients who underwent salvage radiation therapy had a median overall survival of 6.9 years versus 1.6 years for patients who did not undergo salvage radiation. On multivariable analysis with inflammatory breast cancer, TN subtype, and distant metastases, radiation was associated with significantly improved survival (odds ratio [OR], 0.4; P = .04). Concomitant distant metastases had the greatest impact on decreasing survival (OR, 6.71; P < .001), followed by inflammatory breast disease (OR, 6.65; P < .001) and TN subtype (OR, 2.66; P = .002).
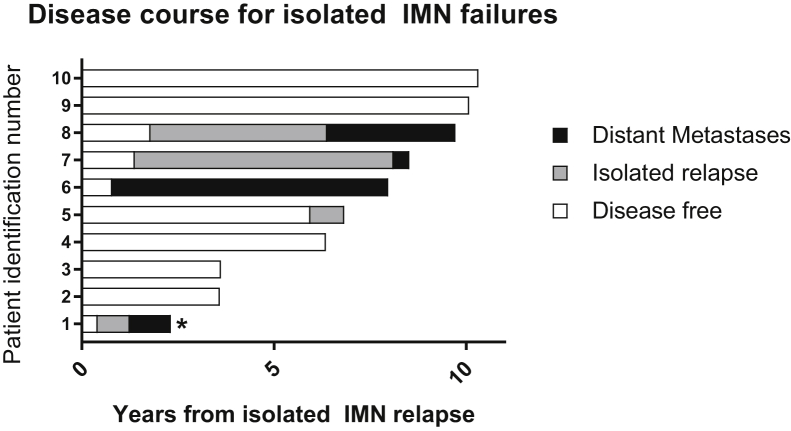
Isolated IMN recurrence was a rare event, identified in only 10 patients (6%) who presented with locoregional recurrence as a first event. Seven patients were detected through incidental findings on routine follow-up imaging (breast magnetic resonance imaging), 2 patients presented with elevated carcinoembryonic antigen prompting further imaging, and 1 patient presented with back pain. Initial treatment for these 10 patients consisted of breast conservation therapy for 2 patients, mastectomy for 8 patients, and postmastectomy radiation for 6 patients. Eight patients underwent radiation as part of initial therapy, of whom 7 had radiation records available for review. Within this cohort of 10 patients, a single patient (patient 1) presented with inflammatory disease, 4 patients (3, 5, 7, and 8) were HR positive, and 6 patients (1, 2, 4, 6, 9, and 10) had triple negative disease (Fig 3). Most isolated IMN failures (5 of 9 patients [56%] with reviewable imaging) occurred outside initial radiation field (Table 3).
Figure 3.
Disease course for isolated IMN failures. Timeline of disease progression for the 10 patients who presented with isolated IMN failures. Bars indicate length of disease-free interval (white), isolated relapse (gray), and survival with distant metastases (black). Five patients had repeat IMN relapse after salvage treatments (1, 5, 6, 7, and 8). Four patients developed distant metastases (1, 6, 7, and 8), and there was 1 patient death (1, starred). The 3 patients with the longest overall survival (patients 8, 9, and 10) all received chemotherapy, surgery, and radiation for salvage treatment. Abbreviation: IMN = internal mammary lymph node.
Table 3.
Characteristics of isolated IMN failures
| No. of patients | % | |
|---|---|---|
| Primary tumor location | ||
| Medial | 2 | 20 |
| Central | 3 | 30 |
| Outer | 5 | 50 |
| Multicentric | ||
| yes | 3 | 30 |
| No | 7 | 70 |
| Relation to RT field | ||
| Out of field | 5 | 56 |
| In field | 2 | 22 |
| No RT | 2 | 20 |
| Events | ||
| Death | 1 | 10 |
| Repeat IMN failure | 5 | 50 |
| DM | 4 | 40 |
| Salvage therapy | ||
| Surgery | 5 | 50 |
| Chemo | 4 | 40 |
| Radiation | 7 | 70 |
| Hormonal therapy | 3 | 30 |
| Combination of Surgery, chemotherapy, and radiation | ||
| Monotherapy | 3 | 30 |
| Bimodality | 3 | 30 |
| Trimodality | 3 | 30 |
Abbreviations: DM = distant metastases; IMN = internal mammary lymph node; RT = radiation therapy.
Over a median follow-up of 7.4 years, 5 of 10 patients with isolated IMN recurrence (50%) experienced repeat IMN failures (Fig 3). Median progression-free survival was 6.0 years. Eighty percent of patients who failed salvage therapy for isolated IMN failures (n = 4) then progressed to distant metastases, with a median time to progression of 2.7 years. There was 1 patient death (Fig 3, patient identification number 1). This patient presented with inflammatory disease and was treated with salvage surgery and capecitabine; developed IMN relapse after 5 months and lung metastases 10 months later; and passed away 2.3 years after initial IMN failure.
Salvage treatments for isolated IMN failures included hormonal therapy, chemotherapy, surgical resection, and radiation therapy (Table 3). For the 7 of 10 patients (70%) who received salvage radiation therapy, 5 underwent radiation with initial treatment (2 with IMNs included within radiation fields) and 2 did not previously receive radiation. After salvage radiation therapy, 3 of 7 patients (43%) experienced repeat IMN relapse and subsequent distant metastases. There were no patient deaths. The median dose in 2 Gy equivalents (assuming α/β 10) was 45 Gy (range, 40-50) for the 3 patients who experienced repeat IMN relapse and 47 Gy (range, 44-66) for the 4 patients with no subsequent disease progression. Most patients received a combination of treatments. One patient received hormonal therapy alone. Three patients received monotherapy with surgery, radiation, or chemotherapy. Another 3 patients received bimodality treatment (combination of surgery, radiation, and chemotherapy). The 3 patients with the longest survival (patients 8-10) received trimodality salvage with surgery, chemotherapy, and radiation.
Discussion
Regional failures after definitive breast cancer therapy are associated with poor prognosis. Contrary to current biases considering IMN involvement to be especially aggressive and incurable,14 our 28% 5-year overall survival rates are similar to the 24% to 35% reported for other sites of locoregional recurrence.1, 2
As expected, aggressive underlying tumor biological characteristics were associated with poor prognosis. Inflammatory breast disease had the greatest impact on overall survival, followed by TN subtype. Previous studies analyzing locoregional breast tumor recurrence identified positive nodal status, early time to recurrence, larger tumor size, older age, and presence of lymphovascular invasion as prognostic factors associated with increased mortality.1, 15, 16 We also found decreased survival with these factors, although were not powered to test statistical significance in our limited cohort of patients.
Hormone receptor conversion was identified in 22% of patients. Seven patients converted from HR positive to TN subtype. These patients had a median overall survival of 1.4 years, which matched the median overall survival of the 36 patients who initially presented with TN disease. ER receptor status converted in 15% and PR receptor status converted in 33% of patients analyzed. These substantial rates of hormone receptor conversion are similar to conversion rates reported in distant metastases17, 18 and underscore the importance of recurrent lesion biopsy because hormone receptor status harbors significant implications for systemic salvage therapy.
Salvage therapy for isolated locoregional recurrence was examined in the CALOR (Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer) randomized trial. It was found that adjuvant chemotherapy after surgical excision of isolated locoregional recurrence improved overall survival in patients with ER-negative, but not ER-positive, disease.19 Radiation was mandated for patients with microscopically involved surgical margins and “recommended” for all patients who had not received radiation therapy as part of primary treatment. Salvage radiation was administered to only 37% of patients and remains to be explored for its role in optimizing outcomes for patients with recurrent disease.
Internal mammary nodes represent an anatomic site that oncologists appear to be especially reluctant to treat.14 Our institution mirrored these biases because only 30% of patients underwent salvage radiation therapy for recurrent IMN disease. Some of this may be attributable to referral patterns from other specialties. Multivariable analysis controlling for distant metastases, inflammatory breast cancer, and TN subtype identified significant improvement in overall survival with salvage radiation. Their improved outcomes are likely confounded by selection bias, because the patients who underwent radiation likely reflect a better prognostic group, and the limitations of retrospective analyses. Nonetheless, these data compel consideration of salvage radiation to improve overall survival.
Isolated IMN failures exhibited surprisingly excellent outcomes, especially when managed with aggressive salvage treatments. A study on isolated supraclavicular failures reported median overall survival rates of 2.6 and 1.2 years for patients treated with and without salvage radiation therapy, respectively.20 In contrast, we had only 1 failure in our cohort of 10 patients. The patients in our analysis were treated very aggressively, with 70% receiving salvage radiation. The 3 patients treated most aggressively, with a combination of surgery, radiation, and chemotherapy, had the best outcomes.
Potential drawbacks and biases of this report include the intrinsic limitations of retrospective analysis. Patients selected for salvage therapy are likely to be of better performance status with other confounding variables to explain their excellent outcomes. Despite these biases with patient selection, we believe our results compel the consideration of aggressive therapy for patients able to tolerate treatment.
Conclusions
In conclusion, we found the overall survival of patients with IMN recurrence to be similar to other sites of locoregional recurrence. Patients with isolated IMN recurrence may achieve long-term survival. Overall prognosis is driven by tumor biology rather than anatomic location, especially extent of disease, TN subtype, and inflammatory breast cancer. Salvage radiation therapy, often reserved for palliation of symptoms associated with local disease progression, including bleeding, pain, ulceration, and edema, should also be considered to enhance disease outcomes. Aggressive salvage treatment with chemotherapy, radiation, and surgery should be considered for isolated IMN failures.
Footnotes
Sources of support: This research was supported by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosure: Dr. Powell reports consulting fees from Varian and honorarium from VisionRT, outside the submitted work.
References
- 1.Wapnir I.L., Anderson S.J., Mamounas E.P. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S.J., Wapnir I., Dignam J.J. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson P., Cole B.F., Chua B.H. Patterns and risk factors for locoregional failures after mastectomy for breast cancer: An International Breast Cancer Study Group report. Ann Oncol. 2012;23:2852–2858. doi: 10.1093/annonc/mds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R.C., Lin N.U., Golshan M. Internal mammary nodes in breast cancer: Diagnosis and implications for patient management—a systematic review. J Clin Oncol. 2008;26:4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 5.Zeichner S.B., Ambros T., Zaravinos J. Defining the survival benchmark for breast cancer patients with systemic relapse. Breast Cancer (Auckl) 2015;9:9–17. doi: 10.4137/BCBCR.S23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellon J.R., Livingston R.B., Eubank W.B. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC) Am J Clin Oncol. 2004;27:407–410. doi: 10.1097/01.coc.0000128869.19357.9b. [DOI] [PubMed] [Google Scholar]
- 7.Jochelson M.S., Lebron L., Jacobs S.S. Detection of internal mammary adenopathy in patients with breast cancer by PET/CT and MRI. AJR Am J Roentgenol. 2015;205:899–904. doi: 10.2214/AJR.14.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poortmans P.M., Struikmans H., Bartelink H. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:1879–1880. doi: 10.1056/NEJMc1510505. [DOI] [PubMed] [Google Scholar]
- 9.Whelan T.J., Olivotto I.A., Levine M.N. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:1878–1879. doi: 10.1056/NEJMc1510505. [DOI] [PubMed] [Google Scholar]
- 10.Verma V., Beriwal S. Internal mammary node radiation in light of the EORTC 22922 and MA.20 Trials—what have we really learned? JAMA Oncol. 2016;2:992–993. doi: 10.1001/jamaoncol.2015.5810. [DOI] [PubMed] [Google Scholar]
- 11.Haffty B.G., Whelan T., Poortmans P.M. Radiation of the internal mammary nodes: Is there a benefit? J Clin Oncol. 2016;34:297–299. doi: 10.1200/JCO.2015.64.7552. [DOI] [PubMed] [Google Scholar]
- 12.Hennequin C., Bossard N., Servagi-Vernat S. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2013;86:860–866. doi: 10.1016/j.ijrobp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 14.Clemons M., Hamilton T., Mansi J., Lockwood G., Goss P. Management of recurrent locoregional breast cancer: Oncologist survey. Breast. 2003;12:328–337. doi: 10.1016/s0960-9776(03)00107-3. [DOI] [PubMed] [Google Scholar]
- 15.Alpert T.E., Kuerer H.M., Arthur D.W., Lannin D.R., Haffty B.G. Ipsilateral breast tumor recurrence after breast conservation therapy: Outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys. 2005;63:845–851. doi: 10.1016/j.ijrobp.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 16.van Tienhoven G., Voogd A.C., Peterse J.L. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer. 1999;35:32–38. doi: 10.1016/s0959-8049(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 17.Hoefnagel L.D., Moelans C.B., Meijer S.L. Prognostic value of estrogen receptor α and progesterone receptor conversion in distant breast cancer metastases. Cancer. 2012;118:4929–4935. doi: 10.1002/cncr.27518. [DOI] [PubMed] [Google Scholar]
- 18.Hoefnagel L.D., van de Vijver M.J., van Slooten H.J. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wapnir I.L., Price K.N., Anderson S.J. Efficacy of chemotherapy for ER-negative and ER-positive isolated locoregional recurrence of breast cancer: Final analysis of the CALOR Trial. J Clin Oncol. 2018;36:1073–1079. doi: 10.1200/JCO.2017.76.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Sangen M.J., Coebergh J.W., Roumen R.M., Rutten H.J., Vreugdenhil G., Voogd A.C. Detection, treatment, and outcome of isolated supraclavicular recurrence in 42 patients with invasive breast carcinoma. Cancer. 2003;98:11–17. doi: 10.1002/cncr.11469. [DOI] [PubMed] [Google Scholar]