Abstract
Purpose
The radiation dose to specific substructures of the heart may be more critical than the dose to the whole heart. Yet, these substructures are sensitive to intrafractional motion from breathing and cardiac motion, which can affect their dose-volume histograms. We sought to investigate intrafractional motion of the heart and its substructures among free-breathing patients undergoing radiation for mediastinal lymphoma or lung cancer.
Methods and materials
After institutional review board approval, the medical records of 20 patients (12 with mediastinal lymphoma; 8 with lung cancer) were retrospectively reviewed. Patients underwent 4-dimensional computed tomography simulation and a contrasted scan for treatment planning. Using MIMVista software, the heart, coronary arteries, chambers, and valves were contoured on the 50% phase, and these contours were propagated to the other phases and edited. Each substructure was graded on the basis of its ease of contouring across all phases (1 = no difficulty; 2 = minor difficulty; 3 = moderate difficulty; and 4 = very difficult). The centroid position and volume of each substructure for all phases were exported to Excel to calculate basic statistics and the independent t test.
Results
The heart, 4 chambers, and atrioventricular valves were easily identified with a mean score of 1 to 1.2, and the pulmonic valve, left anterior descending artery, aortic valve, circumflex, and right coronary artery were minor-to-moderately difficult with a mean score of 2.1 to 3.2. The smallest centroid displacement was seen in the 4 chambers and mitral and pulmonic valves (0.7-1.1 cm). Greater displacement was seen in the coronary vessels and tricuspid and aortic valves (1.2-1.5 cm). The greatest displacement was in the Z direction (craniocaudal) for all substructures; however, the displacement was significantly greater among patients with lymphoma for the right ventricle, aortic valve, and left anterior descending artery (P < .05). However, patients with lung cancer had more displacement in the X and Y directions, which was statistically significant for the right atrium, tricuspid valve, right ventricle, and heart. When calculating overall displacement, no statistically significant difference was observed between patients with lymphoma and patients with lung cancer.
Conclusions
Intrafractional motion of the cardiac substructures ranged from 0.7 to 1.5 cm, mostly in the Z direction. Further investigation of the respiratory motion effect on the dose-volume histogram of the substructures is needed for patients treated with contemporary radiation techniques.
Introduction
Evidence has existed for years that unintentional radiation to the heart is associated with late cardiac toxicities among survivors of Hodgkin lymphoma (HL) and breast cancer decades after treatment.1, 2, 3 Recently, data have emerged that demonstrate a relationship between the mean radiation dose to the heart and the risk of cardiac morbidity, cardiac mortality, and overall mortality among patients with HL, breast cancer, or lung cancer.4, 5, 6
Radiation therapy to the heart is associated with various cardiac problems, including arrhythmias, cardiomyopathy, valvular disease, coronary artery disease/myocardial infarction, and pericarditis in cancer survivors.2, 7 Consequently, understanding the radiation dose to specific substructures of the heart might demonstrate a more meaningful relationship between radiation treatment and cardiac damage, which could inform our use of conformal radiation technologies. Data are already emerging that demonstrate that an increased radiation dose to the cardiac valves and left ventricle are associated with an increased risk of valvular disease and congestive heart failure.8, 9
The Michigan Heart Atlas was developed to aid radiation oncologists in contouring substructures of the heart to better understand the radiation dose delivered to these structures, with the caveat that treatment be delivered using the breath-hold technique.10 Unfortunately, many patients are not treated with breath-hold; thus, significant breath and cardiac motion have been observed. When considering the use of highly conformal radiation techniques (eg, intensity modulated radiation therapy, volumetric modulated arc therapy, and proton therapy), even small movements can have a large impact on the radiation dose to the heart.11 Such errors affect smaller structures such as coronary vessels much more than large ones such as the cardiac chambers.12
The purpose of this study was to investigate the magnitude of the intrafractional motion of the heart and its substructures among free-breathing patients treated with proton therapy for mediastinal lymphoma or lung cancer.
Methods and Materials
After institutional review board approval, 20 patients were selected for the project, including 8 patients with stage II or III non-small cell lung cancer (including those with prior myocardial infarction [n = 3], congestive heart failure [n = 2], and coronary artery disease [n = 4]) and 12 patients with mediastinal lymphoma without a history of cardiac events. These 2 populations balance each other because patients with lymphoma are much younger than patients with lung cancer who, because of their age and smoking habits, frequently have comorbidities and other factors that can affect thoracic motion. The patients with lung cancer were selected because they had undergone 4-dimensional computed tomography (CT) simulation with contrast. The 12 patients with mediastinal lymphoma had previously been studied and had a noncontrast 4-dimensional CT simulation with a subsequent 3-dimensional CT scan with contrast.13 The resolution was 1.2 × 1.2 × 1 mm for the 3-dimensional CT and 1.2 × 1.2 × 2 mm for the 4-dimensional CT.
Using MIMVista software (MIM Software Inc., Cleveland, OH), the cardiac structures were contoured per the Michigan Heart Atlas guidelines by a medical student with guidance and reviewed by a radiation oncologist with expertise in the management of mediastinal malignancies. The structures contoured included the whole heart, the left and right atria and ventricles, the left anterior descending artery (LAD), the right coronary artery (RCA), the left circumflex artery (LC), and the 4 valves (mitral, tricuspid, aortic, and pulmonic). The LAD contour included the left main stem and left coronary artery because the distinction between the 2 is difficult to pinpoint. The structures were initially contoured on 1 of the 50% phases of the 4-dimensional CT scan, and those contours were later propagated onto the other 9 phases. The propagated contours were then reviewed and corrected to match the quality of the contour on the initial phase because the software has limited accuracy owing to difficulty in identifying and tracking the motion of each substructure. For the patients with lymphoma, the 3-dimensional CT scan with contrast was fused to the noncontrast 4-dimensional scan to help contour the cardiac substructures.
For each patient, each structure was first graded by a medical student based on its ease of contouring across the 10 phases of the study and then was reviewed by an experienced radiation oncologist. The following numerical grading scale was used: 1 = no difficulty; 2 = minor difficulty; 3 = moderate difficulty; and 4 = very difficult. The centroid position of each of the cardiac substructures for all 10 phases was exported from MIMVista into Excel (Microsoft Corporation, Redmond, WA) where basic statistics, including mean and standard deviations across the cohort in the X, Y, and Z directions (left-right lateral, anteroposterior, and superoinferior, respectively) were calculated. The volumes of each substructure and the whole heart were also exported and analyzed. Using Excel, an independent t test was performed to evaluate differences between the lung and lymphoma cohort, and a P-value of < .05 was considered significant.
Results
Ease of identification
Table 1 shows the difficulty of grading contouring of the different cardiac substructures for each patient cohort. Overall, the heart, both atria, both ventricles, the mitral valve, and the tricuspid valve were the easiest of the cardiac substructures to contour, as identified on the 10 phases of the 4-dimensional CT scan, with a mean contour score of 1 to 1.2. Conversely, the pulmonic valve and LAD were more difficult to contour (mean, 1.6-2.3), and the aortic valve, LC, and RCA were the most difficult to contour (mean, 2.3-3.2). The coronary vessels and valves were more difficult to contour on patients with HL than on those with lung cancer, possibly because of the visible age-related calcification in the older cohort or the contrasted 4-dimensional scan that patients with lung cancer undergo. Nevertheless, the chambers were contoured with similar difficulty between the 2 patient populations.
Table 1.
Mean structure contouring difficulty across the full 10 phases of a 4-dimensional computed tomography planning scan
| Structure | Contouring difficulty |
|||
|---|---|---|---|---|
| Hodgkin lymphoma |
Lung cancer |
|||
| Mean | Standard deviation | Mean | Standard deviation | |
| Heart | 1 | 0 | 1 | 0 |
| Left atrium | 1.1 | 0.29 | 1 | 0 |
| Right atrium | 1.1 | 0.29 | 1.2 | 0.42 |
| Left ventricle | 1.1 | 0.29 | 1.1 | 0.32 |
| Right ventricle | 1.1 | 0.29 | 1.2 | 0.42 |
| Tricuspid valve | 1.1 | 0.29 | 1.2 | 0.42 |
| Mitral valve | 1.1 | 0.29 | 1.1 | 0.32 |
| Pulmonic valve | 2.1 | 0.67 | 2.3 | 0.67 |
| Aortic valve | 2.3 | 0.65 | 3.1 | 0.88 |
| Left anterior descending artery | 1.6 | 0.67 | 2.3 | 0.95 |
| Circumflex | 2.3 | 0.49 | 3 | 1.15 |
| Right coronary artery | 2.8 | 0.97 | 3.2 | 0.92 |
Displacement
The median centroid displacement of each substructure and the standard deviation for the cohort is reported in Table 2 for the X, Y, and Z directions and for the total displacement. The smallest total centroid displacement was seen in both atria, both ventricles, and the mitral and pulmonic valves (0.7-1.1 cm). Greater total displacement was seen with the tricuspid and aortic valve and the coronary vessels (1.2-1.5 cm). The displacements in the X and Y directions were comparable, but the greatest displacement was seen in the Z direction for all substructures, peaking at 1.13 cm for the LAD, correlating with direction of respiratory motion.
Table 2.
Mean centroid displacement of cardiac substructures across the full 10 phases of a 4-dimensional computed tomography planning scan
| Structure | Centroid X (cm) value |
Centroid Y (cm) value |
Centroid Z (cm) value |
Centroid (cm) displacement |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HL |
Lung |
HL |
Lung |
HL |
Lung |
HL |
Lung |
|||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Heart | 0.13 | 0.08 | 0.22 | 0.13 | 0.15 | 0.06 | 0.25 | 0.12 | 0.55 | 0.21 | 0.46 | 0.19 | 0.6 | 0.2 | 0.6 | 0.2 |
| Left atrium | 0.28 | 0.14 | 0.29 | 0.19 | 0.21 | 0.07 | 0.23 | 0.09 | 0.65 | 0.31 | 0.59 | 0.1 | 0.7 | 0.3 | 0.7 | 0.2 |
| Right atrium | 0.21 | 0.13 | 0.39 | 0.15 | 0.38 | 0.19 | 0.57 | 0.31 | 0.68 | 0.3 | 0.65 | 0.22 | 0.8 | 0.3 | 1 | 0.2 |
| Left ventricle | 0.25 | 0.12 | 0.29 | 0.18 | 0.25 | 0.13 | 0.32 | 0.11 | 0.7 | 0.28 | 0.55 | 0.23 | 0.8 | 0.3 | 0.7 | 0.3 |
| Right ventricle | 0.33 | 0.2 | 0.51 | 0.24 | 0.19 | 0.09 | 0.37 | 0.14 | 0.74 | 0.23 | 0.58 | 0.1 | 0.9 | 0.3 | 0.9 | 0.2 |
| Tricuspid valve | 0.61 | 0.3 | 0.82 | 0.15 | 0.56 | 0.26 | 1.03 | 0.48 | 0.87 | 0.5 | 0.64 | 0.32 | 1.2 | 0.5 | 1.5 | 0.4 |
| Mitral valve | 0.56 | 0.31 | 0.58 | 0.34 | 0.41 | 0.24 | 0.47 | 0.14 | 0.77 | 0.41 | 0.54 | 0.14 | 1.1 | 0.5 | 1 | 0.2 |
| Pulmonic valve | 0.41 | 0.18 | 0.57 | 0.29 | 0.5 | 0.24 | 0.53 | 0.17 | 0.63 | 0.21 | 0.57 | 0.23 | 0.9 | 0.3 | 1 | 0.3 |
| Aortic valve | 0.43 | 0.25 | 0.41 | 0.15 | 0.42 | 0.28 | 0.46 | 0.12 | 0.95 | 0.5 | 0.55 | 0.32 | 1.2 | 0.6 | 0.9 | 0.3 |
| LAD | 0.46 | 0.21 | 0.63 | 0.22 | 0.77 | 0.38 | 0.77 | 0.27 | 1.13 | 0.33 | 0.75 | 0.27 | 1.5 | 0.5 | 1.3 | 0.4 |
| Circumflex | 0.7 | 0.43 | 0.62 | 0.36 | 0.5 | 0.23 | 0.62 | 0.36 | 1.07 | 0.29 | 0.95 | 0.31 | 1.4 | 0.4 | 1.3 | 0.5 |
| RCA | 0.66 | 0.28 | 0.79 | 0.23 | 0.64 | 0.3 | 0.75 | 0.27 | 1.04 | 0.33 | 1.06 | 0.28 | 1.4 | 0.3 | 1.5 | 0.4 |
Abbreviations: HL = Hodgkin lymphoma; LAD = left anterior descending artery; RCA = right coronary artery; SD = standard deviation.
When comparing the HL cohort with the lung cohort, a greater displacement in the X direction for patients with lung cancer was observed, which was statistically significant only for the tricuspid valve and right atrium (Table 3). Similarly, greater displacement in the Y direction was observed for the lung cohort, which was statistically significant for the heart, right ventricle, and tricuspid valve. These displacements contrasted with the Z direction, for which patients with HL had consistently greater displacement for all structures (except the RCA), and these displacements were statistically significantly different for the right ventricle, aortic valve, and LAD.
Table 3.
Results of t test comparing the Hodgkin lymphoma with the lung cancer cohort (P < .05 is significant)
| Structure | Centroid X (cm) P-value | Centroid Y (cm) P-value | Centroid Z (cm) P-value | Centroid (cm) displacement | Volume (ml) P-value | Volume % P-value |
|---|---|---|---|---|---|---|
| Heart | .10 | .048 | .33 | .92 | .02 | .30 |
| Left atrium | .92 | .59 | .57 | .78 | .15 | .30 |
| Right atrium | .01 | .15 | .82 | .20 | .00 | .06 |
| Left ventricle | .53 | .20 | .20 | .54 | .91 | .03 |
| Right ventricle | .09 | .01 | .05 | .78 | .12 | .25 |
| Tricuspid valve | .048 | .03 | .22 | .19 | .19 | .44 |
| Mitral valve | .89 | .49 | .10 | .58 | .27 | .12 |
| Pulmonic valve | .19 | .79 | .61 | .70 | .33 | .91 |
| Aortic valve | .81 | .68 | .04 | .13 | .03 | .30 |
| LAD | .10 | .98 | .01 | .28 | .96 | .42 |
| Circumflex | .64 | .29 | .40 | .61 | .07 | .12 |
| RCA | .27 | .42 | .91 | .57 | .34 | .61 |
Abbreviations: LAD = left anterior descending artery; RCA = right coronary artery.
Volume change
Substructure volumes across the 10 phases are reported in Table 4. Compared with the HL cohort, the lung cancer cohort had a consistently greater change in volume in the cardiac substructures. Indeed, all total volume changes were greater in the lung cancer cohort, with the exception of the left ventricular volume. The change was statistically significant in milliters for the heart, right atrium, and aortic valve (Table 3). Yet, when measuring the volume difference as a percent change in volume, only left the ventricle volume was statistically significant, with a difference of 14.6% versus 8.4% in patients with HL; this is because patients in the lung cancer cohort had larger hearts overall.
Table 4.
Mean volume change of cardiac substructures across the full 10 phases of a 4-dimensional computed tomography planning scan
| Structure | Volume (ml) value |
Volume (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| HL |
Lung |
HL |
Lung |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Heart | 34.38 | 15.92 | 52.9 | 15.63 | 5.86 | 2.64 | 4.98 | 0.84 |
| Left atrium | 14.18 | 13.58 | 23.49 | 13.44 | 22.17 | 18.91 | 15.72 | 6.94 |
| Right atrium | 15.51 | 9.37 | 35.66 | 12.1 | 17.96 | 8.03 | 29.03 | 13.52 |
| Left ventricle | 27.34 | 15.54 | 26.62 | 11.23 | 14.59 | 7.17 | 8.41 | 2.73 |
| Right ventricle | 26.29 | 16.98 | 44.2 | 26.99 | 19.01 | 9.08 | 24.1 | 9.48 |
Abbreviations: HL = Hodgkin lymphoma; SD = standard deviation.
Discussion
Although respiratory motion obviously can cause displacement of the cardiac structures, the present study provides actual measurements of the displacement and volume of the specific cardiac substructures for 2 distinct patient cohorts that represent opposite ends of the patient spectrum: young patients with lymphoma who are relatively free of comorbidities versus older patients with lung cancer who have more comorbidities and a history of heavy smoking. We found that both cohorts experienced large displacements of the cardiac substructures.
Cardiac substructure delineation in this study demonstrated that the largest structures and atrioventricular valves were the easiest to contour, but the coronary arteries were more difficult. Furthermore, the vessels and valves were more difficult to contour on patients with HL than on patients with lung cancer, likely because these structures in lung patients were frequently calcified and easier to identify. It also appeared that the cardiac substructures moved less in patients with lung cancer throughout the 4-dimensional CT scan, which made those structures more difficult to trace in patients with HL.
These findings are limited by the subjectivity of the 2 individuals who contoured and reviewed the plans, but similar studies with different methodologies have drawn the same conclusions. Investigators of the Michigan Heart Atlas study found that, after implementing the atlas, the interobserver contour overlap for the whole heart and left ventricle were >90%, but the overlap was only 24% for the RCA.10 The investigators also found that the right atrium moves significantly and that the size of the chamber varies greatly between slices on a noncardiac gated CT scan, which complicates the contouring of structures, such as the right coronary artery, which sits in the right atrioventricular groove.10
Zhou et al found similar results after implementing their cardiac atlas.14 The researchers observed much less interobserver variability for structures such as the heart and 4 chambers than for the coronary arteries. They posit that imperfect fusion of cardiac slices in noncardiac gated CT scans affects smaller structure contours more so than larger structures, and they highlight limitations such as subjective structure boundaries and poor image resolution, which is relevant to the present study.
We observed that intrafractional motion of the cardiac substructures ranges from 0.7 to 1.5 cm, and most of the motion is in the Z direction. Patients with HL showed consistently greater displacement in the Z direction than patients with lung cancer, likely because patients with lung cancer have smoking-related comorbidities that lead to hyperinflated lungs, thereby limiting the mediastinal motion in the Z direction. However, greater motion was seen in the X and Y direction among patients with lung cancer.
The displacement of the cardiac substructures is not solely due to respiratory motion. Cardiac contractile motion accounts for changes in substructure volume throughout the 4-dimensional CT scan, which also causes substructure displacement. The cardiac contractions may have caused more displacement of the cardiac substructures in the X and Y direction in patients with lung cancer because there was less motion in the Z direction than in patients with HL. This finding suggests different breathing and cardiac motion in 2 very different patient cohorts. Despite these differences, when looking at the overall displacement , there was no remarkable difference in cardiac substructure displacement between patients with lymphoma and lung cancer.
Other studies have evaluated cardiac motion and assessed cardiac substructure motion. Kataria et al15 found that coronary artery displacement is predominantly craniocaudal because of respiration, that radial displacement of the coronary arteries is attributable to cardiac motion (with more motion in the anteroposterior direction than the left-right direction), and that patients with lung cancer experience less craniocaudal displacement owing to restricted lung movement, subclinical cardiac comorbidities, or poor pulmonary function.
Wang et al16 observed the displacement of the cardiac substructures during cardiac motion alone with deep-inspiration breath-hold and found that most motion occurred in the heart's posterior.16 When focusing on the LAD specifically, which moved an average of 2.6 mm and 2.3 mm in the X and Y directions, respectively, they noted vast variability in the extent of LAD motion both between patients and within individual patients.
Shechter et al compared the impact of respiratory and cardiac motion on the displacement of the coronary arteries.17 They concluded that the coronary arteries were displaced more from cardiac motion than from breathing motion. In the RCA, the displacements were 14.4 mm and 5 mm, respectively. The researchers also found that the arteries moved caudally during inspiration, but the X and Y motion varied.
In the present study, heart volume appeared to change more for patients with lung cancer than for those with lymphoma, likely owing to older age and comorbidities. Volume change in the heart is important to understand because it may have an impact on radiation planning, especially with proton therapy if beams traverse the entirety of the heart volume. Jan et al18 analyzed interfractional volume change in the whole heart and found that positional changes could alter lung volume and diaphragm position. They suggested that volume status can also play a role in volume changes, although this would be difficult to assess on noncardiac gated CT scans, which allow for a more average assessment of volume change.
Our study has several limitations and is relevant only to patients treated with free breathing because more sophisticated motion management strategies, such as breath hold and respiratory-gated radiation, are not addressed. Contouring is subjective and may have been graded as easier (or harder) by a radiologist or cardiologist. Furthermore, the Michigan Heart Atlas facilitates consistency, but it is also imperfect and was established using the breath-hold technique. Smaller structures are consistently more difficult to identify than larger structures. Structure boundaries are subjective, and erroneously delineated boarders are magnified in smaller structures, which is further affected by image resolution.
In our study, the 4-dimensional CT scans were noncardiac gated, and the heart contracted during the scan with imperfect fusion of cardiac structures between slices. This affected mobile structures, such as the RCA, the most. Some of the difficulty in contouring might be attributable to the vast individual variation in the location of coronary vessels, especially in patients with lung cancer and collateral vessels, which may have developed because of cardiac injury from smoking.19 Because patients with lung cancer had contrasted 4-dimensional CT scans and calcified vessels and valves that were thought to make contouring easier, they were contoured first. However, contouring patients with lymphoma yielded lower difficulty scores, and we presume this ease in contouring is likely attributable to the gained confidence and experience after contouring the cardiac substructures in patients with lung cancer.
Additionally, we did not use our results to develop planning organ-at-risk volume (PRV) margins. PRV margins can be helpful in treatment planning, but whether they accurately reflect the dose delivered to a patient is unclear. Consequently, once we obtain the dosimetric results to determine whether the PRV margin would be suitable for predicting dose, we can evaluate this issue.
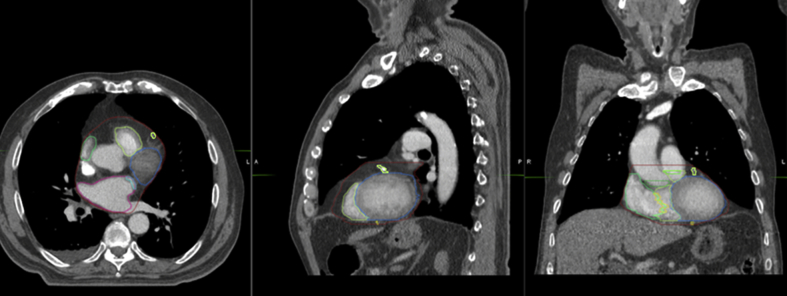
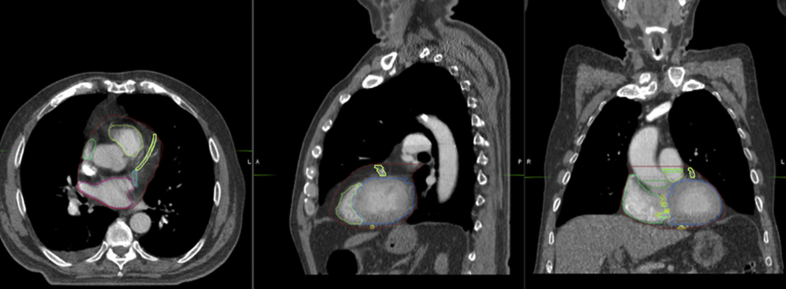
The future direction of this project is to evaluate the mean radiation dose to the different substructures when recalculated according to the dose to the different phases of the 4-dimensional CT scan. Once we determine the actual dose delivered to the cardiac substructures over the respiratory cycle, we can identify the best surrogate structure for determining this dose. The surrogate structure can be based on the structure drawn on 1 of the 10 phases of the 4-dimensional CT, such as the 50% (exhalation, Fig 1) or 0% (inhalation, Fig 2) phase, or on the average scan. Alternatively, the surrogate structure can be based on an expansion of the cardiac structure, such as a PRV margin.
Figure 1.
Contour of the whole heart and its substructures during exhalation (50% phase).
Figure 2.
Contour of the whole heart and its substructures during inhalation (0% phase).
Conclusions
Modern radiation treatment planning for thoracic malignancies will require cardiac substructure contouring in an effort to better understand cardiac radiation dose and the subsequent risk of late toxicity. The present study demonstrates that cardiac substructures can be difficult to identify and can move dramatically during free breathing. This motion can differ depending on the specific cohort of patients. Future studies are needed to evaluate the impact of this motion on the dose to the cardiac substructures when using contemporary radiation techniques.
Acknowledgments
The authors thank Jessica Kirwan and Christopher Stich for their editorial assistance and Amanda Prince and the research nurses at the University of Florida Health Proton Therapy Institute for their administrative support. This research was made possible through the support of the James E Lockwood, Jr, Professorship.
Footnotes
Sources of support: Lidia Guzhva was funded by the Goodman Research Award.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Hancock S.L., Tucker M.A., Hoppe R.T. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 2.Hull M.C., Morris C.G., Pepine C.J. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 3.Rutqvist L.E., Lax I., Fornander T., Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–896. doi: 10.1016/0360-3016(92)90784-f. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Nimwegen F.A., Schaapveld M., Cutter D.J. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 6.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 7.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Nimwegen F.A., Ntentas G., Darby S.C. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–2265. doi: 10.1182/blood-2016-09-740332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutter D.J., Schaapveld M., Darby S.C. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107:djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck R.E., Kim L., Yue N.J., Haffty B.G., Khan A.J., Goyal S. Treatment techniques to reduce cardiac irradiation for breast cancer patients treated with breast-conserving surgery and radiation therapy: A review. Front Oncol. 2014;4:327. doi: 10.3389/fonc.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sherif O., Yu E., Xhaferllari I., Gaede S. Assessment of intrafraction breathing motion on left anterior descending artery dose during left-sided breast radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1075–1082. doi: 10.1016/j.ijrobp.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe B.S., Flampouri S., Zaiden R. Involved-node proton therapy in combined modality therapy for Hodgkin lymphoma: results of a phase 2 study. Int J Radiat Oncol Biol Phys. 2014;89:1053–1059. doi: 10.1016/j.ijrobp.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Zhou R., Liao Z., Pan T. Cardiac atlas development and validation for automatic segmentation of cardiac substructures. Radiother Oncol. 2017;122:66–71. doi: 10.1016/j.radonc.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataria T., Bisht S.S., Gupta D. Quantification of coronary artery motion and internal risk volume from ECG gated radiotherapy planning scans. Radiother Oncol. 2016;121:59–63. doi: 10.1016/j.radonc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Pan T., Pinnix C. Cardiac motion during deep-inspiration breath-hold: Implications for breast cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:708–714. doi: 10.1016/j.ijrobp.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shechter G., Resar J.R., McVeigh E.R. Displacement and velocity of the coronary arteries: Cardiac and respiratory motion. IEEE Trans Med Imaging. 2006;25:369–375. doi: 10.1109/TMI.2005.862752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jan N., Guy C., Reshko L.B., Hugo G.D., Weiss E. Lung and heart dose variability during radiation therapy of non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:683–690. doi: 10.1016/j.ijrobp.2017.02.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White B.M., Vennarini S., Lin L. Accuracy of routine treatment planning 4-dimensional and deep-inspiration breath-hold computed tomography delineation of the left anterior descending artery in radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91:825–831. doi: 10.1016/j.ijrobp.2014.11.036. [DOI] [PubMed] [Google Scholar]