Abstract
Purpose
The incidence of brain metastases is increasing as a result of more routine diagnostic imaging and improved extracranial systemic treatment strategies. As noted in recent consensus guidelines, postoperative stereotactic radiosurgery (SRS) to the resection cavity has lower rates of local control than whole brain radiation therapy but improved cognitive outcomes. Further analyses are needed to improve local control and minimize toxicity.
Methods and materials
Patients receiving SRS to a resection cavity between 2006 and 2016 were retrospectively analyzed. Presurgical variables, including tumor location, diameter, dural/meningeal contact, and histology, were collected, as were SRS treatment parameters. Patients had routine follow-up with magnetic resonance imaging, and those noted to have local failure were further assessed for the recurrence location, distance from the target volume, and dosimetric characteristics.
Results
Overall, 82 patients and 85 resection cavities underwent postoperative SRS during the study period. Of these, 58 patients with 60 resection cavities with available follow-up magnetic resonance imaging scans were included in this analysis. With a median follow-up of 19.8 months, local recurrence occurred in 12 of the resection cavities for a 15% 1-year and 18% 2-year local recurrence rate. Pretreatment tumor volume contacted the dura/meninges in 100% of cavities with recurrence versus 67% of controlled cavities (P = .025). A total of 5 infield, 5 marginal, and 4 out-of-field recurrences were found, with a median distance to the centroid from the target volume of 3 mm. The addition of a 10-mm dural margin increased the target volume overlap with the recurrence contours for 10 of the 14 recurrences.
Conclusions
Dural contact was associated with an increased rate of recurrence for patients who received SRS to a surgical cavity, and the median distance of marginal recurrences from the target volume was 3 mm. These results provide evidence in support of recent consensus guidelines suggesting that additional dural margin on SRS volumes may benefit local control.
Introduction
Between 9% and 17% of patients with cancer will develop brain metastases, and the incidence of secondary central nervous system tumors is increasing as detection methods and treatment strategies for systemic metastases improve.1 Current management of brain metastases uses a multidisciplinary approach, including neurosurgery, radiosurgery, radiation therapy, and, in some scenarios, targeted systemic agents or intrathecal chemotherapy.2 Patients with single brain metastases represent up to 50% of cases and are often managed with upfront surgical resection, followed by adjuvant radiosurgery.1, 3
Surgical resection provides both immediate decompression and tissue for pathologic diagnosis. For solitary or single metastases, surgical resection has been shown to improve local control and overall survival when compared with fractionated radiation therapy alone.4 After resection or debulking, adjuvant radiation, most commonly with whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS), is needed to reduce the risk of rapid local recurrence.5, 6, 7 Both adjuvant SRS and WBRT have been demonstrated to improve the rate of local failure at the resection cavity, although neither improves overall survival outcomes. Compared with adjuvant SRS, WBRT reduces the risk for distant brain failure and the need for salvage therapy, but it does so at the cost of significant neurotoxicity.6, 8, 9
The primary advantage of SRS over WBRT is the ability to generate steep dose gradients such that a high, ablative dose of radiation can be delivered to residual microscopic disease while sparing uninvolved brain tissue. Adjuvant WBRT has been employed for several decades and is well studied, but more focal approaches have only recently become more widely adapted.7, 10 This has been driven largely by an increasing appreciation for radiation-induced neurotoxicity, improved systemic agents leading to long-term survival in selected patients, and technical advances in target delineation and delivery.8 The steep dose gradients that are a hallmark of SRS place an increased emphasis on accurate target delineation and the need for optimized target delineation.
The optimal definition of the appropriate target volume in the postoperative setting is controversial and may depend on intraoperative findings, treatment delivery platform, and the technique and timing of pre- and posttreatment imaging. A consensus guideline for postoperative radiosurgery was recently published; however, data from patterns-of-failure studies are limited.11 Investigations have identified an inverse correlation between the conformality index and local failure rates, suggesting that at-risk regions may extend beyond the immediate confines of the resection cavity target volume.12, 13 Additionally, a recent prospective trial of postoperative, cavity-directed SRS for completely resected brain metastases identified a higher risk of failure among tumors ≥3 cm and those that came in contact with the meninges.14
In our practice, delineation of the target volume did not include additional margin and was not systematically modified to account for differences in the surgical tract or preoperative dural contact. Therefore, we retrospectively reviewed our experience with adjuvant SRS for treatment of completely resected brain metastases to describe the outcomes, patterns of failure, and risk factors for recurrence.
Methods and Materials
Patients
Patients treated with postoperative SRS at a single institution for a brain metastasis from 2006 through 2016 were retrospectively analyzed with institutional review board approval. Patients included in this analysis underwent surgical resection to a dominant brain metastasis with postoperative SRS to the surgical cavity and any additional metastatic foci present on the day of radiation treatment. Patients who did not undergo surgical resection or underwent biopsy only were excluded from the analysis. After resection, the decision to undergo single-fraction SRS, stereotactic body radiation therapy or external beam radiation therapy was made by a multidisciplinary institutional panel of neurologic surgeons, radiation oncologists, and neuroradiologists with expertise in radiosurgical treatment. Patients who underwent WBRT at the time of SRS or before local recurrence were excluded from this analysis, as were patients treated with stereotactic body radiation therapy. Factors such as performance status, tumor size, and proximity to critical structures were considered in the decision regarding the patient's treatment modality.
Patient characteristics, including age at time of surgery, sex, tumor histology, date of surgery, preresection tumor location (supra/infratentorial), and meningeal/dural contact were retrospectively collected for analysis. These data were subsequently used for analysis of tumor recurrence, time to recurrence, clinical follow-up, and overall survival.
Treatment and follow-up
Decision for resection was left to the discretion of the multidisciplinary team and based on the tumor location, size, performance status, and symptoms at the time of presentation. Patients were included if they had multiple resections at 2 independent times and were analyzed on a per-lesion basis. Data on the extent of surgery, time from surgery to SRS, and postoperative magnetic resonance imaging (MRI) were collected.
In practice, the goal was to perform radiosurgery 1 month after surgical resection of the dominant brain tumor. Radiosurgery was delivered with a single-session, frame-fixed technique (Gamma Knife 4C from 2006 through October 2007 and Gamma Knife Perfexion beginning in November 2007). On the day of treatment, pretreatment MRI was performed for radiosurgery planning. Contours were created to include the enhancing margin of the resection cavity and any residual disease without additional planning target volume expansion by the treating physicians using Gamma Plan software. Any additional brain metastases noted at the time of treatment were included in the radiosurgery plan.
Treatment-specific data points, including target volume (TV); planned isodose volume (PIV); target volume in PIV (TIV); prescribed dose (in gray); prescribed isodose line; and the tumor's lateral, anteroposterior, and superoinferior (Dx, Dy, Dz respectively) dimensions were collected for analysis. Calculations of the conformity index (CI; PIV/TV), modified CI ([PIV × TIV]/[TV2]), and quadratic mean diameter (QMD = √[Dx2 + Dy2 + Dz2]/3]) were performed retrospectively and were not used at the time of treatment.12, 15, 16, 17, 18 Inclusion of the QMD reflects prior institutional analysis demonstrating the metric to have a high significance in modeling tumor control.
Routine follow-up MRI at intervals of 3 or 4 months were recommended for all patients and used in this analysis. Additional patient data, including date of resection cavity recurrence, date of adverse radiation effect (ARE), date of last imaging, date of last follow-up, date of death, and date of hospice were collected. Assessment of distant brain failure and leptomeningeal disease was performed by review of MRI reports generated by experienced neuroradiologists. ARE was assessed using previously established institutional criteria with T1 weighted post-gadolinium MRI and serial imaging to determine the evolution of the lesion size and differentiate local recurrence and radiation effect.19 The records of patients who had follow-up with local providers were not automatically requested by treating providers, but recommendations for ongoing surveillance were the same for all patients. At the time of the analysis, attempts were made to retrieve missing records for patients followed by local providers within the constraints of standard institutional review board practices. For patients who were lost to follow-up or followed up with local providers, social security databases were queried for death information and included in this analysis.
Analysis of recurrence
Patients identified as having a recurrence at the site of the resection cavity from serial imaging review were further analyzed. T1 axial post-gadolinium images from the MRI demonstrating recurrence were co-registered to T1 axial post-gadolinium images from the day of initial radiosurgery to ensure good anatomic alignment in the area(s) of recurrence.
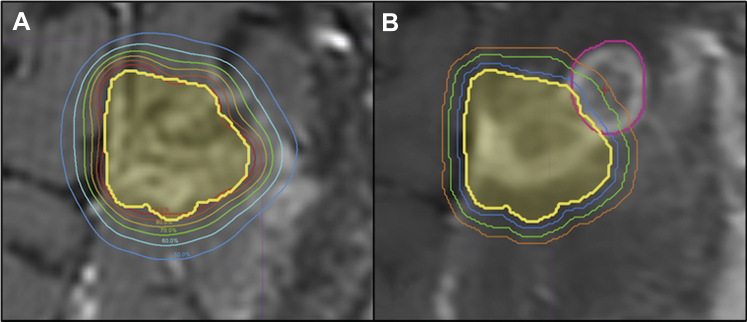
The contoured resection cavity, dose structures, and targets were imported from Gamma Plan (Elekta Leksell 2017) into MIM contouring software (MIM Maestro 2017) for comparison. The recurrence was contoured using just the T1 axial post-gadolinium imaging for its delineation, and a recurrence centroid for this volume was calculated by the contouring software. Use of the centroid is not meant to represent the most likely nidus of the recurrent tumor, but instead is employed as a standardized methodology for reviewing patterns of recurrence and distance from the surgical cavity. The initial cavity contour volume and dosimetric data then were overlaid on the recurrence MRI to classify the recurrences as infield, marginal, or out of field. Infield recurrences were defined as having the centroid of the recurrence volume within the contoured resection cavity volume. Marginal recurrence centroids were outside of the contoured cavity and fell between the prescription isodose line and the line corresponding to 50% of the prescription dose (Fig 1). Out-of-field recurrence centroids were outside of the contoured cavity and fell between 50% of the prescription dose and the line corresponding to 20% of the prescription dose. These institutional cutoffs were chosen because of the steep dose falloff of SRS plans and lack of prospective studies or guidelines to provide a consistent definition of marginal and out-of-field recurrences.
Figure 1.
Representative resection cavity (A) as outlined on the day of stereotactic radiosurgery (yellow) with isodose lines from 100% (red) to 50% (blue); (B) contour of a resection cavity recurrence with centroid (pink with red point marker) and serial 1 mm isotropic expansions of the resection cavity (blue, green, orange) created from the day of stereotactic radiosurgery contour (yellow). This was classified as a marginal recurrence because it fell in the zone receiving between 50% and 100% of the prescription dose, with the centroid 3 mm from the edge of the resection cavity.
For a separate exploratory analysis of the utility of adding dural margin to resection cavities for tumors with preoperative dural contact, the initially contoured cavities were retrospectively modified to include a 10 mm dural margin to assess for overlap with the recurrence volumes. This was done with a 4-mm brush starting at the intersection of the cavity and dura and moving along the dural surface for 10 mm in all directions. If this new contour overlapped the contour of the recurrence, it was considered to have likely increased coverage of the nidus of the recurrence.
The centroids of the contoured recurrences then were assessed for distance from the surgical cavity contour by creating a series of isotropic expansions of the previously contoured resection cavity. The values analyzed represent the smallest expansion that would encompass the centroid within the newly created volume. A representation of the resection cavity delineation and isodose lines and of the isotropic expansion used is shown in Figure 1. Additionally, dose-volume histograms were reviewed within the region of the recurrence to evaluate centroid dose and D20, D50, and D80 (dose encompassing 20%, 50%, and 80% of the recurrence volume, respectively).
Statistical analysis
Statistical analysis was performed using R Studio (Rstudio 2015). The local recurrence rate was calculated cumulatively and presented as an actuarial rate. Because of the small sample size and uncertain distribution, nonparametric measures were used for analysis of the data. A comparison of the categorical variables was performed with Fisher's exact test or χ2 depending on the number of observations, and continuous variables were compared with Wilcoxon rank sum (P-value for significance was .05). An assessment of the dose-volume histograms was done using the mean dose of all recurrences by decile stratified in accordance with the recurrence classification.
Results
A total of 82 patients and 85 resection cavities had postoperative SRS after subtotal, near total, or gross total resection during the study period. Ten of the excluded patients could not be analyzed because of lack of follow-up MRI, 8 had incomplete institutional records or resection at an outside facility and could not be analyzed, 4 patients were treated with fractionated SRS, 1 had preoperative SRS, and 1 patient underwent I-131 brachytherapy at the time of surgery. All attempts were made to obtain follow-up information or complete missing data. For the remaining 58 patients included in the study, the median clinical follow-up was 19.8 months (interquartile range [IQR], 9.3-31.3) with a median MRI follow-up time of 19.0 months (IQR, 11.0-32.7). Over the time period of this retrospective analysis, 12 patients had resection site recurrence with a total of 14 distinct lesions, 19 patients died, and the median overall survival was 19.8 months from the date of postoperative radiosurgery.
The median age of patients was 59 years (IQR, 50-68 years) without a significant difference between those who developed recurrence and those who did not (P = .09). Within the cohort, 29 patients were men and 29 were women. The recurrences spanned a broad range of histologies, including non-small cell lung cancer, breast cancer, colorectal cancer, melanoma, and renal cell carcinoma among others. Further baseline characteristics are summarized in Table 1 comparing cavities without versus with resection cavity recurrence.
Table 1.
Tumor characteristics
| Resected tumor characteristics |
P-value | ||
|---|---|---|---|
| No recurrence (n = 48) |
Recurrence (n = 12) |
||
| Age | 60 | 52 | .09 |
| Sex | .747 | ||
| Male | 25 | 5 | |
| Female | 23 | 7 | |
| Histology | .03 | ||
| Non-small cell lung cancer | 18 | 3 | |
| Breast | 9 | 3 | |
| Melanoma | 9 | 1 | |
| Colorectal | 0 | 3 | |
| RCC | 5 | 0 | |
| Other | 7 | 2 | |
| Tumor location | .59 | ||
| Supratentorial | 44 | 10 | |
| Infratentorial | 4 | 2 | |
| Dural/meningeal contact | .025 | ||
| Yes | 32 | 12 | |
| No | 16 | 0 | |
| Surgical resection | .33 | ||
| Gross total resection | 42 | 12 | |
| Sub/near total resection | 6 | 0 | |
Abbreviation: RCC = renal cell carcinoma.
Among the 58 patients, there were 60 surgically resected lesions. The median time from surgery to SRS was 4.0 weeks (IQR, 3.3-5.6 weeks; range, 2-14 weeks). All patients were treated with single-fraction radiosurgery based on a same-day planning stereotactic MRI of the brain with a median dose of 17 Gy (IQR, 16-18 Gy) prescribed to the 50% isodose line (range, 50%-80%) and encompassing the resection cavity rim including any enhancement, without additional margin. Overall, the median target volume was 5.68 cm3 (IQR, 3.47-8.78 cm3) and the median PIV was 7.77 cm3 (IQR, 4.77-10.76 cm3). The increase in volume from TV to PIV is a consequence of treatment planning and the irregular shape of resection cavities and was not modified to add additional margin.
Analysis of the tumor and treatment characteristics of tumors without or with resection cavity recurrence is shown in Table 2. Review of the presurgical imaging of the tumors revealed that in all 12 tumors with recurrences (100%), the tumor was in contact with the dura/meninges, but only 67% (32 of 48 tumors) without recurrence had dural contact (P = .025). The median QMD was 2.4 cm (IQR, 2.1-3.0 cm), and 14 tumors had a QMD >3 cm. There was no significant association between QMD and preoperative dural/meningeal contact or resection cavity recurrence (P = .87 and 1.00, respectively).
Table 2.
Median treatment parameters with interquartile range
| Treatment parameters | No recurrence | Recurrence | P-value |
|---|---|---|---|
| Target volume (cm3) | 5.17 (3.43-8.77) | 6.65 (5.83-9.52) | .24 |
| Prescription isodose volume (cm3) | 7.02 (4.44-10.72) | 8.53 (7.78-12.01) | .26 |
| Quadratic mean diameter (cm) | 2.50 (2.08-2.96) | 2.37 (2.35-3.05) | .41 |
| Conformity index | 1.31 (1.20-1.41) | 1.26 (1.18-1.43) | .80 |
| Modified conformity index | 1.28 (1.18-1.40) | 1.22 (1.15-1.43) | .59 |
| Dose (Gy) | 17 (16-18) | 16.5 (16-17) | .13 |
The cumulative 1- and 2-year failure rates at the resection cavity were 15% and 18%, respectively, and 1 patient failed after 2 years. Two cavities had 2 separate sites of failure, so there were 14 failure centroids, including 5 that were infield and 9 outside of the target volume. Of those outside of the target volume, 5 were classified as marginal (between the prescription isodose line and 50% of prescription dose) and 4 as out of field (ie, between 20% and 50% of the prescription dose). Of the marginal recurrences, the median distance from the target volume was 3 mm (range, 2-5 mm); for out-of-field recurrences, the distance was 5.5 mm (range, 4-8 mm). Additional characteristics of the recurrence centroid distance from the target volume are shown in Table 3. The retrospectively modified cavity contours, including a 10 mm dural margin, were found to increase the target volume overlap with the recurrence contours for 10 of the 14 recurrences.
Table 3.
Mean D20, D50, D80, and D100 for infield, marginal, and out-of-field recurrences
| Recurrence characteristics∗ |
||||||
|---|---|---|---|---|---|---|
| D20 | D50 | D80 | D100 | Median centroid distance (mm) | Range (mm) | |
| Infield (n = 5) | 21.2 | 19.7 | 17.8 | 13.4 | -- | -- |
| Marginal (n = 5) | 15.4 | 9.1 | 6.3 | 3.5 | 3 | 2-5 |
| Out-of-field (n = 4) | 8.4 | 5.1 | 3.6 | 2.2 | 5.5 | 4-8 |
Abbreviation: Dx = dose that encompasses x% of the recurrence volume.
Two of 12 cavities with recurrence had 2 separate foci of recurrence.
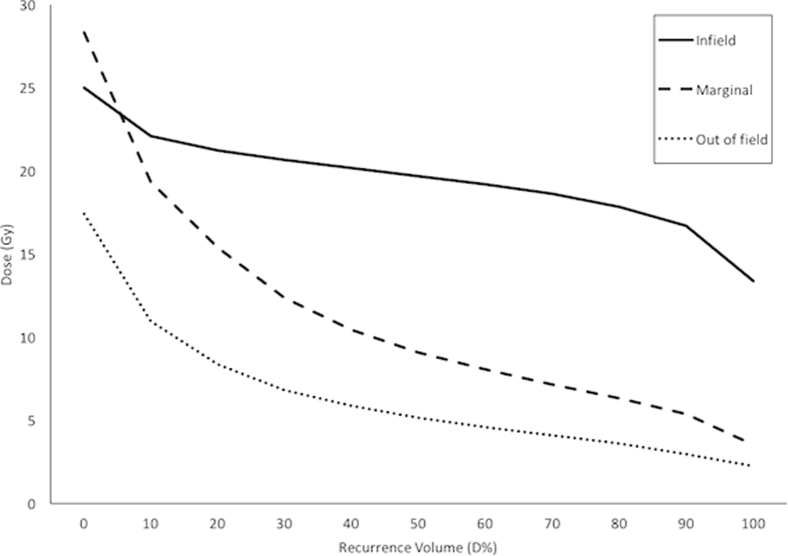
Dosimetric data for the retrospectively contoured recurrences are shown in Table 3. The mean D20, D50, and D80 for the infield recurrences were 21.2, 19.7, and 17.8 Gy. For the marginal and out-of-field recurrences, the mean D20, D50, and D80 were 15.4, 9.1, and 6.3 and 8.4, 5.1, and 3.6 Gy, respectively. The mean dose volume curves for the infield, marginal, and out-of-field recurrences are shown in Figure 2.
Figure 2.
Dose-volume histogram of the mean dose received by the region of the contoured recurrence.
Over the follow-up period, 42 patients (72%) experienced a distant brain failure, but only a single patient developed leptomeningeal disease on MRI. Seven patients developed radiographic ARE within the follow-up period. This represents 12% of patients and cavities in our cohort and had no significant association with PIV, TV, or recurrence. Of these patients who developed ARE, 5 were within 1 year of treatment, accounting for a rate of 8%.
Discussion
Surgical cavity recurrence after resection of a brain metastasis remains an ongoing challenge for radiation oncologists. Balancing the desire for local control and minimization of toxicity is especially important because reirradiation has an increased risk of necrosis.20, 21 This study sought to further understand the characteristics of surgical cavity recurrences after resection and SRS to optimize this treatment for future patients. In this series, we noted 1- and 2-year resection site recurrence rates of 15% and 18%, respectively, which is consistent with the results of prior series.14, 22, 23
Of the patients who developed local recurrence, there was a significant association with preoperative dural/meningeal contact. Of the 14 sites of recurrence in 12 resection cavities in this study, 10 would have had an increased overlap between the target volume and the volume of recurrence had a 10-mm dural margin been added. Additionally, and unique to this study for which the target volume included resection cavity without additional margin, we demonstrated that 64% of recurrences occur outside of the surgical cavity target volume with a median marginal centroid distance from the resection cavity of 3 mm.
Historically, WBRT has shown a local control rate of 70% to 90%; however, only 1 prospective series has directly compared modern stereotactic approaches with WBRT.5, 6, 7 In study N107C/CEC, 3 patients were randomized to either WBRT or SRS with initial SRS treatment using a 1 mm margin and subsequently expanded to 2 mm during the course of the trial. The study demonstrated a local control rate in the SRS group that was inferior to that of the WBRT group, with 6-month control rates of 80.4% and 87.1%, respectively (P = .00068), with the benefit of improved cognitive outcomes in patients receiving SRS. The lower rates of local control could be related to conservative prescription doses or insufficient cavity margins to treat possible foci of recurrence. As demonstrated in this series, even a 2 mm expansion may not be sufficient to encompass all areas of recurrence, and increased isotropic expansion may predispose patients to increased rates of ARE. Therefore, consideration of targeted expansions that minimize dose to areas of brain parenchyma at lower risk of recurrence is warranted.
Two possible alternative techniques to improve local control and decrease ARE are preoperative and fractionated SRS. Reports of preoperative SRS have demonstrated its ability to aid in the certainty of target delineation and decrease the risk of ARE.24 When compared retrospectively with postoperative SRS, the technique has noted equivalent local control and decreased rates of leptomeningeal disease (16.6% vs 3.2%) and ARE (16.4% vs 4.9%).25
Ultimately, in the current series, the rates of radiographic ARE were modest at 8%. Only 1 case of leptomeningeal disease was found after treatment; however, this may be artificially low because of the inclusion criteria and the retrospective nature of the study. An alternative that could be explored further is the use of fractionated SRS, which has the potential to decrease toxicity and allow for targeted expansions of CTV to include dural and meningeal areas at risk for recurrence.
Other retrospective series have demonstrated that, as SRS plans became less conformal and CI increases, an improved rate of local control (100% in the least conformal quartile) was noted.12 When treating the PIV and TV in these plans as spheres and examining the marginal increase in radius using 4/3πr3, the least conformal quartile had the equivalent of a 2.4 mm expansion. In the current study, in which no marginal expansion was used, we found no association with local recurrence and PIV, TV, or CI. Using the same methodology as Soltys et al,12 if we were to retroactively add a 2 mm margin to our cohort, the median TV would increase from 5.62 cm3 to 9.27 cm3; if we were to expand to 3 mm margins, the increase would be to 11.57 cm3. When considering that the median distance of the centroid of a marginal recurrence was 3 mm, this additional margin may not be sufficient to prevent many recurrences, and the large increase in TV has the potential to increase the risk of toxicity without any benefit to overall survival.23, 26 With an ARE rate of 12% in our study, increasing the margin in an isotropic manner and maintaining an ablative dose of radiation would likely lead to a further increase in ARE that may impinge on the benefit provided by improved local control.19
The ongoing difficulty with surgical cavity SRS is the size of the target volume and concerns over decreased local control and increased toxicity. Numerous studies have examined this issue with conflicting results.22, 27 In a prospective series, Mahajan et al reported 1-year rates of local control of only 44% in tumors measuring >3 cm, whereas tumors <3 cm had a 74% rate of local control (P = .0078).27 However, a retrospective analysis by Zhong et al noted 1-year local recurrence rates of 12.3% for 90 tumors ≤4 cm and 16.0% for 27 tumors >4 cm (P = .60).22 As noted, we found no significant association between local control and TV or PIV; however, our cohort was treated without margin, so this effect may be difficult to assess. In examining our results, one method of balancing local control and ARE would be to increase the dural margin of the SRS volume while maintaining a smaller margin of parenchymal tissue, or to consider fractionated treatment of the resection cavities.
An understanding of the likely reasons for infield, marginal, and out-of-field recurrences will help further refine SRS as a treatment technique. Within this series, infield recurrences received a dose nearly equal to the prescription dose with a mean D90 of 16.7 Gy, which raises the potential issue that the prescribed dose was insufficient for local control but not clearly histology dependent. Marginal and out-of-field recurrences received a mean D90 of only 5.0 Gy and 3.0 Gy, respectively; some of these recurrences may have been prevented by isotropic addition of margin and/or delineation of high-risk areas of recurrence. Pretreatment risk stratification of patients who had dural or meningeal contact of their presurgical tumor could also provide some guidance in the selection of patients who may benefit from higher prescribed doses and/or additional margins and identify patients at lower risk for local recurrence, for whom the benefits of higher dose and/or treating a larger volume may not outweigh the additional risk of ARE.
This study provides some initial evidence for consideration, prompted by the new consensus guidelines for SRS, of the addition of a dural margin to the SRS cavity.11 These guidelines make the recommendation to include a 5 to 10 mm expansion along the dura if the preresection tumor had dural contact. In our series, all 12 surgical cavities that developed a recurrence had presurgical dural contact of the tumor, and none of the resected tumors without dural contact were found to have developed local recurrence. This series supports the idea of an association between dural contact and local recurrence. Addition of a dural margin along the bone flap may increase local control rates by reducing the marginal and out-of-field recurrences and has lower risk of the development of ARE than large isotropic expansions into brain parenchyma.
Our study offers clear support for these recommendations, but further evaluation with larger cohorts will be needed to assess its potential effect.
Conclusions
Adjuvant SRS to the postoperative resection cavity has not been demonstrated to improve overall survival and as such should be an exercise in balancing the durability of local control and risk of development of toxicities. In our study, we found a significant association with dural and meningeal contact of the presurgery tumor volume and local recurrence, providing some evidence that the addition of a dural margin during surgical cavity SRS may provide improve local recurrence.
Additionally, the median centroid distance of marginal recurrences in this study was 3 mm from the SRS target volume, and any isotropic expansion to include this region could greatly increase the risk of ARE. To improve local control after surgical cavity SRS, tailored expansions may be needed to achieve acceptable rates of ARE while providing sufficient ablative doses of radiation.
Footnotes
Sources of support: This work was funded by the University of California – San Francisco, Department of Radiation Oncology.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Eichler A.F., Loeffler J.S. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 3.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Gonçalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 4.Patchell R.A., Tibbs P.A., Walsh J.W. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown P.D., Ballman K.V., Cerhan J.H. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patchell R.A., Tibbs P.A., Regine W.F. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 8.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 9.Kayama T., Sato S., Sakurada K. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): A phase III, noninferiority, randomized controlled trial. https://doi.org/10.1200/JCO.2018.78.6186 J Clin Oncol. [DOI] [PubMed]
- 10.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 11.Soliman H., Ruschin M., Angelov L. Consensus contouring guidelines for post-operative completely resected cavity stereotactic radiosurgery (SRS) for brain metastases. Int J Radiat Oncol. 2018;100:436–442. doi: 10.1016/j.ijrobp.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Soltys S.G., Adler J.R., Lipani J.D. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187–193. doi: 10.1016/j.ijrobp.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 13.Choi C.Y.H., Chang S.D., Gibbs I.C. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: Prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys. 2012;84:336–342. doi: 10.1016/j.ijrobp.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Brennan C., Yang T.J., Hilden P. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feuvret L., Noël G., Mazeron J.J., Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Lomax N.J., Scheib S.G. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 2003;55:1409–1419. doi: 10.1016/s0360-3016(02)04599-6. [DOI] [PubMed] [Google Scholar]
- 17.Shaw E., Kline R., Gillin M. Radiation therapy oncology group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 18.Ma L., Chiu J., McDermott M. Quadratic mean diameter is highly significant in predicting tumor control for stereotactic radiosurgery of brain metastases. J Radiosurg SBRT. 2015;3 [Google Scholar]
- 19.Sneed P.K., Mendez J., Vemer-van den Hoek J.G. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J Neurosurg. 2015;123:373–386. doi: 10.3171/2014.10.JNS141610. [DOI] [PubMed] [Google Scholar]
- 20.Rana N., Pendyala P., Cleary R.K. Long-term outcomes after salvage stereotactic radiosurgery (SRS) following in-field failure of initial SRS for brain metastases. Front Oncol. 2017;7:1–8. doi: 10.3389/fonc.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKay W.H., McTyre E.R., Okoukoni C. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J Neurosurg. 2017;127:148–156. doi: 10.3171/2016.5.JNS153051. [DOI] [PubMed] [Google Scholar]
- 22.Zhong J., Ferris M.J., Switchenko J. Postoperative stereotactic radiosurgery for resected brain metastases: A comparison of outcomes for large resection cavities. Pract Radiat Oncol. 2017;7:e419–e425. doi: 10.1016/j.prro.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel K.R., Prabhu R.S., Kandula S. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol. 2014;120:657–663. doi: 10.1007/s11060-014-1601-4. [DOI] [PubMed] [Google Scholar]
- 24.Asher A.L., Burri S.H., Wiggins W.F. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. doi: 10.1016/j.ijrobp.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Patel K.R., Burri S.H., Asher A.L. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: A multi-institutional analysis. Neurosurgery. 2016;79:279–285. doi: 10.1227/NEU.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 26.Blonigen B.J., Steinmetz R.D., Levin L., Lamba M.A., Warnick R.E., Breneman J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan A., Ahmed S., McAleer M.F. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]