Abstract
Purpose
To report 15-year outcomes for dose-escalated intensity modulated radiation therapy (IMRT) for localized prostate cancer (PC) by evaluating biochemical relapse, distant metastases, cancer-specific survival, and long-term toxicity.
Methods and materials
A database search was conducted for the first cohort of patients treated at this institution with 81 or 86.4 Gy between 1996 and 1998 using IMRT. Toxicity data were scored according to the Common Terminology Criteria for Adverse Events version 3.0. Median follow-up was 11.6 years (range, 5-21 years).
Results
In the study, 301 patients were treated with 81 Gy (n = 269, 89%) or 86.4 Gy (n = 32, 11%). Patients were analyzed by National Comprehensive Cancer Network risk group, with 29% low risk (LR), 49% intermediate risk (IR), and 22% high risk (HR). Late grade 3 gastrointestinal (GI) toxicity was seen in 3 patients (1.0%). No grade 4 GI toxicity events occurred. Median time from radiation therapy to late grade 3 GI toxicity was 2.9 years. One event occurred after 10 years. Late grade 3 and 4 genitourinary (GU) toxicity was seen in 6 (2.0%) and 1 (0.3%) patient, respectively. Median time to late grade 3+ GU toxicity was 5.5 years. Two events occurred after 10 years. In addition, 38 (12.6%) developed second primary malignancies (SPMs), 8 of which were in-field malignancies. Median time from radiation therapy to all SPM and in-field SPM was 10 years. The 15-year relapse-free survival was 76%, 65%, and 55% in the LR, IR, and HR groups, respectively. Distant metastases-free survival was 88%, 75%, and 63% for LR, IR, and HR patients, respectively. PC-specific mortality was 1.9%, 7.1%, and 12.2% for LR, IR, and HR patients.
Conclusions
This report represents the longest follow-up data set to our knowledge of patients treated with high-dose IMRT for PC. Our findings indicate that it is well tolerated with 1.0% and 2.3% incidence of long-term grade 3+ GI and GU toxicity, respectively. The cohort had excellent PC-specific survival.
Introduction
The introduction of intensity modulated radiation therapy (IMRT) for the treatment of clinically localized prostate cancer has enabled the safer delivery of higher radiation dose levels to the prostate. Dose escalation studies showed that higher doses of external beam radiation therapy were associated with improved tumor control outcomes in patients with localized disease.1, 2, 3, 4, 5, 6, 7, 8 These studies reported low rates of gastrointestinal (GI) and genitourinary (GU) toxicity within the first 5 to 10 years after treatment, demonstrating that higher doses were not only effective but also safe. Our institution has published extensively on its experience using 81 Gy and 86.4 Gy delivered with IMRT in the treatment of localized prostate cancer, with up to 10 years of follow-up.2, 3, 8, 9, 10, 11, 12 However, the longer-term rate of toxicities and second primary malignancies among these patients, as well as tumor control outcomes, have not yet been reported. This study reports our 15-year outcomes for patients treated to 81 Gy or 86.4 Gy from 1996 to 1998, examining biochemical relapse, rate of distant metastasis, incidence of late GI and GU toxicity, and incidence of second primary malignancies.
Methods and Materials
A database search was conducted, yielding 301 patients with clinically localized prostate definitively treated between 1996 and 1998 with IMRT to 81 Gy (n = 269, 89%) or 86.4 Gy (n = 32, 11%). All patients had histologic confirmation of prostate adenocarcinoma from transrectal biopsy reviewed by a urologic pathologist at our institution. Pretreatment diagnostic evaluations were performed as previously described.8, 11 Standard follow-up was every 3 months from the time of completion of treatment for the first year, followed by every 6 months for the next 5 years, and annually thereafter, with a prostate-specific antigen (PSA) measurement obtained at each follow-up. The median follow-up was 12 years, and the median follow-up for those alive at last follow-up was 13.8 years, with an interquartile range of 10.7 to 17.9 years. Those with <5 years of follow-up were excluded (30 patients). Of those 30, 19 had died in that timeframe, only 4 of whom of prostate cancer.
Baseline demographic and clinical characteristics for the cohort are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | No. | % |
|---|---|---|
| Median age (y) | 69 | |
| Tumor stage | ||
| ≤T2a | 200 | 66 |
| T2b-T2c | 87 | 29 |
| ≥T3a | 14 | 5 |
| Gleason score | ||
| ≤6 | 161 | 53 |
| 7 | 107 | 36 |
| ≥8 | 33 | 11 |
| PSA | ||
| ≤10 | 200 | 66 |
| ≥10 | 101 | 34 |
| Risk group | ||
| Low | 95 | 32 |
| Intermediate | 140 | 46 |
| High | 66 | 22 |
| ADT use | ||
| Yes | 165 | 55 |
| No | 136 | 45 |
Abbreviations: ADT = androgen deprivation therapy; PSA = prostate-specific antigen.
Patients were categorized according to the National Comprehensive Cancer Network risk group classification, with 29% low risk, 49% intermediate risk, and 22% high risk. The median age was 69 years. Toxicity data were scored according to the Common Terminology Criteria for Adverse Events version 3.0.
All patients were treated with a 5- to 7-field IMRT plan with 15-MV photons. The details of this technique have been described previously.8, 11, 13, 14, 15 Briefly, patients were simulated in the prone position with a customized thermoplastic mold for immobilization. A computed tomography scan was obtained at the time of simulation, and images were then transferred to the treatment planning system. A planning target volume (PTV) was contoured, consisting of the prostate and seminal vesicles with a 1-cm margin except posteriorly at the prostate-rectal interface, where the margin was reduced to 0.6 cm. Bowel, rectal wall, and bladder wall were contoured as critical normal tissue structures. Hot spots in the center of the gland were avoided in the region of the urethra. An in-house treatment planning system and optimization algorithm were used to design the IMRT plan. The standard beam arrangement was posterior (0), right posterior oblique (75), right anterior oblique (135), left anterior oblique (225), and left posterior oblique field (285). When required to meet normal tissue constraints and target dose criteria, 2 additional posterior obliques were added at 37 degrees and 323 degrees to create 7 field plans. Treatment plans were then optimized with an inverse optimization algorithm.16, 17 Optimization was performed using dose or dose-volume constraints and penalties to control the PTV dose (with separate constraints applied to areas of rectal/PTV overlap), homogeneity within the PTV, and doses to critical structures. Dose constraints included that no more than 30% of the rectal wall volume should receive >75.6 Gy (V75.6 Gy <30) and that no more than 53% of the rectal and bladder walls should receive >47 Gy (V47 <53). In the overlap region between the PTV and these critical organs, the constraint was set at 88% of the prescription dose for the rectum and at 98% for the bladder.
During this time period, prostate localization techniques were not used, and patient position was verified with weekly port films. A policy of routine fiducial marker placement and daily 2D-kV imaging for prostate localization was later instituted for all patients receiving prostate IMRT.
Androgen deprivation therapy (ADT) was added at the discretion of the treating physician. Indications for ADT included those patients with high-risk features or patients who had a prostate volume >70 mL, which required pretreatment cytoreduction. In general, patients received 3 months of neoadjuvant ADT, which was maintained during the course of radiation. A total of 172 patients (57%) were treated with ADT in our cohort. ADT use by risk group was 36 (38%), 78 (56%), and 58 (88%) for low-, intermediate-, and high-risk groups, respectively. Median ADT duration by risk group was 6.0, 6.8, and 6.9 months for low-, intermediate-, and high-risk groups, respectively. The agents used were GnRH agonists with an antiandrogen, typically leuprolide acetate with bicalutamide.
Toxicity was scored according to the National Cancer Institute–designated Common Terminology Criteria for Adverse Events version 3.0. Grade 1 was defined as minimal side effects not affecting activities of daily living; grade 2 was defined as side effects requiring medication for symptom management (or increase in dosage of pre-existing medication) with symptoms affecting activities of daily living; grade 3 side effects consisted of severe or medically significant but not life-threatening toxicities or those necessitating procedures; grade 4 included life-threatening side effects requiring urgent intervention. Urinary symptoms were monitored with the International Prostate Symptom Score and quality of life questionnaire before treatment and at each follow-up visit.
Disease status was determined at the time of analysis in February 2017. Biochemical failure was defined using the Phoenix consensus definition of nadir PSA concentration plus 2 ng/mL. Cause of death was recorded for all patients who died during the analysis period, when available.
Actuarial likelihood estimates were determined using the Kaplan-Meier method for biochemical relapse-free survival (bRFS), distant metastasis-free survival (DMFS), and GI/GU toxicity and were compared using the log-rank test. Prostate cancer–specific mortality (PCSM) was estimated using the cumulative incidence method with competing risk analysis. Cox proportional hazards regression model was used to determine the effect of covariates, and a stepwise model selection tool was used to construct the final multivariate model. Statistical analysis was performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Biochemical control
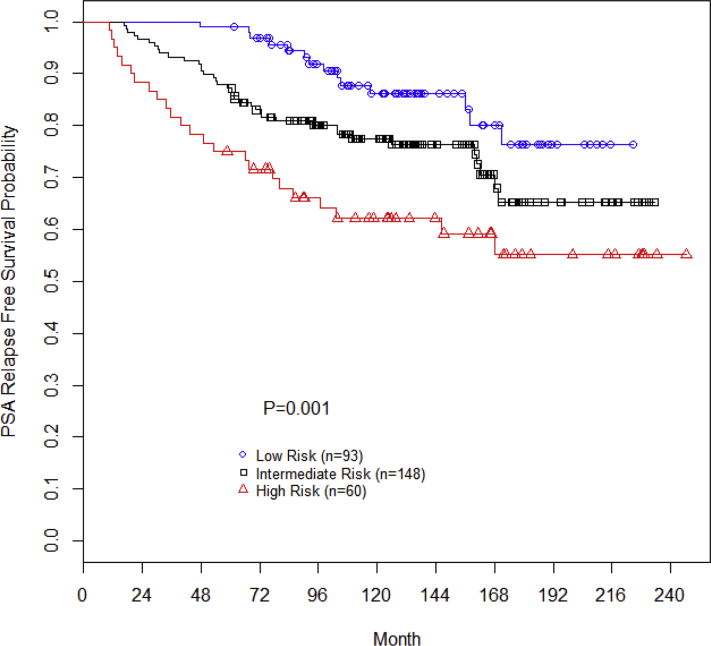
The 15-year bRFS was 76%, 65%, and 55% for the low-, intermediate-, and high-risk patients, respectively (P = .001; Fig 1). On multivariate Cox regression analysis, pretreatment PSA (P = .001; hazard ratio [HR], 1.03; 95% confidence interval [CI], 1.01-1.05), Gleason score (P = .004; HR, 1.50; 95% CI, 1.14-1.97), T stage (P < .001; HR, 1.40; 95% CI, 1.21-1.62), and use of neoadjuvant/concurrent/adjuvant ADT (P = .004; HR, 0.46; 95% CI, 0.27-0.78) predicted for biochemical progression-free survival. Age was not associated with biochemical progression-free survival (P = .64).
Figure 1.
Biochemical recurrence-free survival stratified by National Comprehensive Cancer Network risk group.
Distant metastasis-free survival
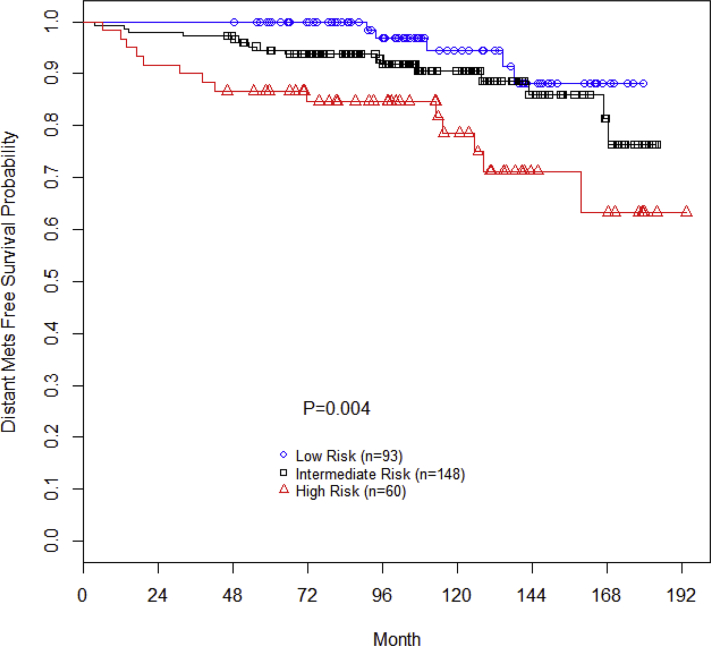
The 15-year DMFS was 88%, 75%, and 63% for low-, intermediate-, and high-risk patients, respectively (P = .004; Fig 2). On multivariate Cox regression analysis, Gleason score (P = .004; HR, 1.82; 95% CI, 1.21-2.72) and T stage (P < .001; HR, 1.60; 95% CI, 1.32-1.94) predicted for DMFS. Pretreatment PSA (P = .174); use of neoadjuvant, concurrent, or adjuvant ADT (P = .110); and age (P = .957) were not associated with DMFS.
Figure 2.
Distant metastases-free survival stratified by National Comprehensive Cancer Network risk group.
Prostate cancer–specific mortality
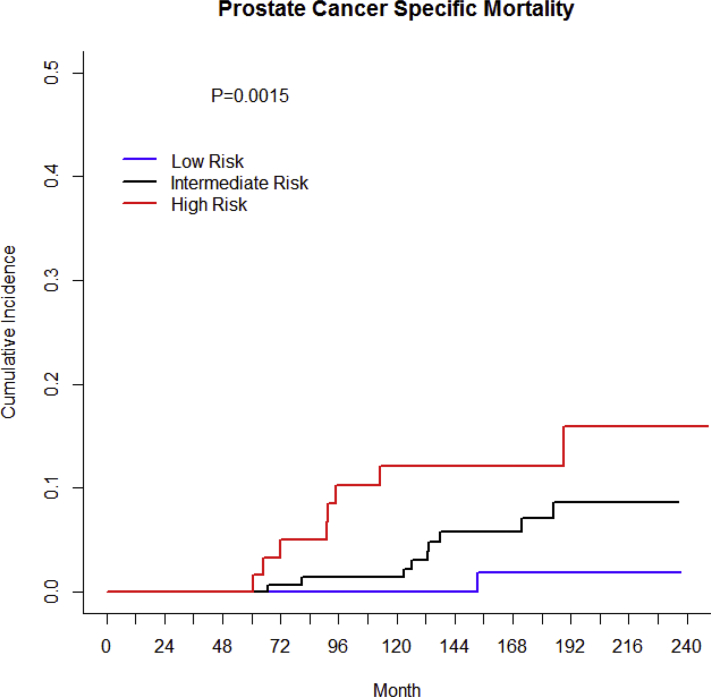
The 15-year PCSM using a competing risk analysis was 1.9%, 7.1%, and 12.2% for low-, intermediate-, and high-risk patients, respectively (P = .0015; Fig 3). On multivariate Cox regression analysis, Gleason score (P = .025; HR, 1.64; 95% CI, 1.06-2.54) and T stage (P = .003; HR, 1.56; 95% CI, 1.17-2.07) predicted for PCSM. Pretreatment PSA (P = .110); use of neoadjuvant, concurrent, and adjuvant ADT (P = .810); and age (P = .780) were not associated with PCSM.
Figure 3.
Cancer-specific mortality stratified by National Comprehensive Cancer Network risk group.
Late toxicity
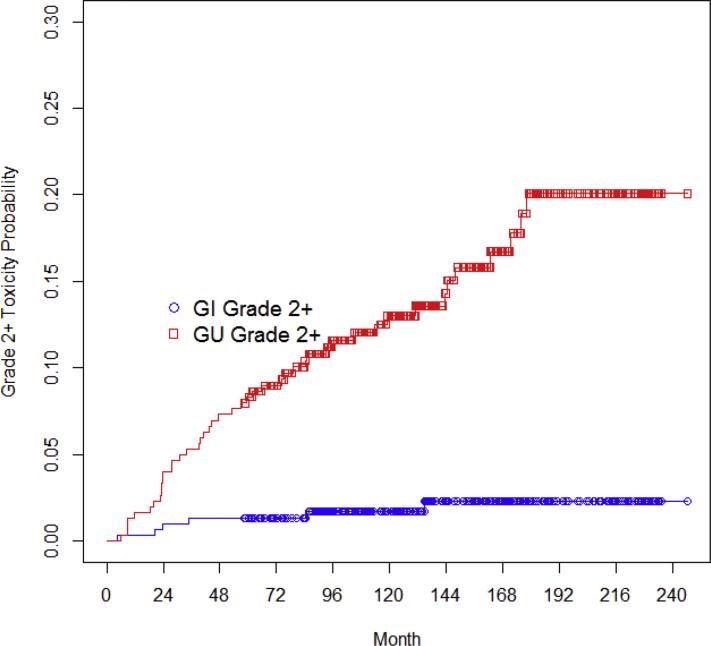
Late grade 2 GI toxicity was seen in 3 patients (1.0%), as was late grade 3 GI toxicity (3; 1.0%). All 3 grade 3 cases were rectal bleeding requiring transfusion, and 1 required embolization. No grade 4 GI toxicity events occurred. Median time from RT to late grade 2+ GI toxicity was 1.6 years, with 1 event occurring after 10 years; 83% of late GI toxicities resolved, and the median time for toxicity resolution was 10 months.
Late grade 2, 3, and 4 GU toxicity was observed in 25 (8.3%), 6 (2.0%), and 1 (0.3%) patient, respectively. Grade 3 GU toxicities included the development of a urethral stricture requiring a dilatation procedure or hematuria/cystitis requiring bladder irrigation/debridement. The 1 patient who developed grade 4 urinary toxicity experienced persistent hematuria ultimately requiring cystectomy. The median time to late grade 2+ GU toxicity was 2.9 years, with 8 events (25%) occurring after 10 years; 47% of late GU toxicities resolved, and median time to toxicity resolution was 4 months. Figure 4 demonstrates the probability of late grade 2 and higher GI and GU toxicity for this patient cohort.
Figure 4.
Probability of late grade 2 and higher gastrointestinal (GI) and genitourinary (GU) toxicity.
SPM
In the study, 38 patients (12.6%) developed a second primary malignancy, 10 of whom were in-field (3.3%), including colorectal (n = 4) and bladder cancers (n = 6) and 1 case of pubic bone sarcoma, as shown in Table 2. Of note, 1 patient had both bladder and colon cancers. The median time from RT to all SPM was 9 years and to in-field SPM was 10 years. Bladder cancers were all stage I or less at diagnosis by TNM staging; 1 was of sarcomatous histology, developed 18 years after radiation therapy, and was cured with cystectomy. Colon cancers were stage I, I, and III at diagnosis. The pubic bone sarcoma was diagnosed 14 years after radiation therapy but could not be staged because diagnosis and treatment were at an outside institution without adequate information available. SPM was the cause of death in 7 patients, 1 of which was in-field (colon).
Table 2.
Type and time to development of second primary malignancy
| Type of second primary malignancy | Patients n (% by total cohort) | Median time to SM (y) |
|---|---|---|
| IF-SPM | ||
| Colorectal | 4 (1.0) | 8.5 |
| Bladder | 6 (2.0) | 8.5 |
| Bone sarcoma (pubic) | 1 (0.3) | 14.2 |
| Total | 11 (3.0)∗ | 10.4 |
| OOF-SPM | ||
| Lung | 6 (2.0) | 8.5 |
| Esophagogastric | 2 (0.6) | 15.6 |
| Pancreas | 4 (1.3) | 7.6 |
| Renal | 4 (1.3) | 9.8 |
| Thyroid | 1 (0.3) | 9.1 |
| Leukemia/lymphoma/myeloma | 6 (2.0) | 11.1 |
| Melanoma | 7 (2.3) | 8.4 |
| Bone sarcoma (mandible) | 1 (0.3) | 15.7 |
| Total | 31 (9.3)∗ | 8.6 |
| Total patients | 38 (12.6)∗ | 8.8 |
Abbreviations: IF-SPM = in-field second primary malignancy; OOF-SPM = out-of-field second primary malignancy.
Of note, 1 patient had 2 IF-SPM, 2 patients had both an IF- and OOF-SPM, 2 patients had 2 OOF-SPM, and 1 patient had an unknown SPM not categorized as IF or OOF.
Discussion
Prior studies that have reported outcomes of dose-escalated external beam radiation for prostate cancer with a 5- to 10-year median follow-up have shown improved biochemical and clinical cancer control, which have not translated into an overall survival benefit.7, 18, 19, 20, 21, 22 This retrospective report is the first with 15-year follow-up on a dose-escalated cohort treated with IMRT.
We note that patients continue to present with biochemical failure and distant metastases after 10 years after treatment. An earlier analysis of patients with prostate cancer receiving 86.4 Gy at this institution with 7-year follow-up showed bRFS of 99%, 86%, and 68% for low-, intermediate-, and high-risk patients, respectively. The ASCENDE-RT (Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy) trial reported a comparable 7-year bRFS of 75% among its intermediate- and high-risk patients who received 78 Gy external beam radiation therapy (EBRT), and Radiation Therapy Oncology Group 0126 reported an 8-year biochemical failure rate of 20% among its intermediate-risk cohort that received 79.2 Gy. We now report 15-year bRFS of 76%, 65%, and 55% for low-, intermediate-, and high-risk patients, showing continued decline over time. It is important to note that the standard of care has changed since this cohort, in that intermediate- and especially high-risk patients are now treated more consistently with longer courses of ADT, which has been shown to improve biochemical and distant metastatic outcomes.
We see similarly comparable distant metastatic outcomes in our retrospective analysis compared with prospective reports, with continued gradual decline over time. Our previously reported 7-year outcomes in patients receiving 86.4 Gy showed DMFS of 99%, 94%, and 82% for low-, intermediate-, and high-risk patients, respectively. The ASCENDE-RT trial reported a 7-year DMFS of 93% among its intermediate- and high-risk patients who received 78-Gy EBRT, and Radiation Therapy Oncology Group 0126 reported an 8-year distant metastasis rate of 4% among its intermediate-risk cohort that received 79.2 Gy. We now report a 15-year DMFS of 88%, 75%, and 63% in low-, intermediate-, and high-risk patients, respectively. These numbers show a consistent increase in distant spread with longer term follow-up, highlighting the importance of long-term clinical follow-up for these patients and enrollment in survivorship clinics with regular PSA monitoring.
Although the risk of severe morbidity remains acceptably low in this analysis, radiation toxicity can present long after treatment. Most patients do not manifest new toxicity years after treatment has been completed, although approximately 25% developed grade 2 or higher GU toxicity after 10 years. Radiation toxicity should therefore remain on the differential for patients who present with new-onset urinary symptoms even 10 years after treatment, particularly those with obstructive symptoms and hematuria.
Several factors have been optimized to minimize acute and long-term toxicity among patients receiving 81 Gy or 86.4 Gy, including use of IMRT, strict dose constraints, and daily image guidance along with tighter PTV margins. Although the cohort in the present report received IMRT, they did not receive fiducial markers or daily on-beam imaging with target position correction, all interventions that were later instituted for prostate radiation and have been shown to further decrease late toxicity.10
Increased GI toxicity has been demonstrated in some of the prospective dose escalation studies.18, 23, 24 Patients can be reassured that the large majority of those who developed GI toxicity (83%) also experienced resolution of that toxicity. GU toxicity, however, can be longer-lasting and translate into a more chronic issue, with only about half of these patients experiencing resolution of their symptoms. Nevertheless, some of these patients in this aging population could have concomitant benign hypertrophy symptoms that confound the interpretation of radiation-induced GU toxicity versus benign symptoms not infrequent in older patients.
In studying toxicities, we must also consider that the improved long-term clinical outcomes that have been associated with dose escalation translate into lower rates of salvage therapies, which are associated with their own toxicities.
Our SPM values are similar to those in a larger study in which we previously reported evaluating rates of SPM development in 1310 patients who received external beam radiation therapy (n = 897) or brachytherapy (n = 413).25 With a 7-year mean follow-up, that study reported a 15% rate of SPM among the EBRT cohort, 17% of which were in-field. We now report a 15-year overall SPM rate of 12.6%. Of those, 26% were in-field SPM. Incidence of in-field SPM does seem to increase somewhat with longer-term follow-up, although the in-field SPM were generally early stage, with only 1 diagnosed at greater than stage I; this was the only patient who died secondary to in-field SPM. Certainly, the term in-field SPM does not imply that these were radiation-induced malignancies. In fact, several studies have reported on the increased incidence of bladder and rectal cancers among prostate cancer survivors, regardless of treatment.26, 27 The Surveillance, Epidemiology, and End Results database is therefore not a reliable resource to identify an expected rate of malignancy for these patients; their risk is likely higher than that in the age- and sex-matched general population.
The in-field SPMs reported here included 2 cases of sarcoma diagnosed 14 and 18 years after RT. Although there is certainly no way to ascribe causality, these cases meet previously defined criteria for diagnosing a postirradiation sarcoma (PIS), including a history of radiation, that the lesion had arisen in an irradiated volume and histologically proven, and that there is a latency period between RT and the development of the lesion, with a median of 12.8 years.28, 29 The estimated risk of PIS is 0.03% to 0.8%,30 which is consistent with the 0.7% rate of PIS reported here at a median of 16 years after RT.
Conclusions
This report represents the longest follow-up dataset to our knowledge of patients treated with high-dose IMRT >80 Gy for prostate cancer. Our findings indicate that such treatment is well tolerated, with a 1.0% and 2.3% incidence of long-term grade 3+ GI and GU toxicity, respectively. The 15-year rate of in-field secondary cancer was only 3.0%, and the incidence of PIS was 0.7%. The incidence of toxicity and second malignancy does not seem to increase dramatically after 10 years. Our treated cohort experienced excellent long-term tumor control and prostate cancer–specific survival outcomes.
As prostate cancer radiation treatment paradigms broaden to include hypofractionated approaches, the long-term outcomes reported here can serve as benchmarks for comparison and evaluation of optimal dose and fractionation schemes, taking into account both clinical endpoints and toxicity.
Footnotes
These data were presented at the American Society for Radiation Oncology Annual Meeting in September 2017 in San Diego, CA.
Sources of support: This work had no specific funding.
Disclosures: Dr McBride received research funding from Janssen and honoraria from Bristol-Myers Squibb, unrelated to this project. Dr Zelefsky is a consultant and has an advisory role at Augmenix.
References
- 1.Zietman A.L., DeSilvio M.L., Slater J.D. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky M.J., Fuks Z., Hunt M. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky M.J., Chan H., Hunt M. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176(4 Pt 1):1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Pollack A., Zagars G.K., Starkschall G. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 5.Pollack A., Hanlon A.L., Horwitz E.M. Prostate cancer radiotherapy dose response: An update of the fox chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 6.Peeters S.T., Heemsbergen W.D., Koper P.C. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 7.Dearnaley D.P., Jovic G., Syndikus I. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 8.Cahlon O., Zelefsky M.J., Shippy A. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: Toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky M.J., Pei X., Chou J.F. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelefsky M.J., Kollmeier M., Cox B. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Spratt D.E., Pei X., Yamada J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alicikus Z.A., Yamada Y., Zhang Z. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky M.J., Fuks Z., Hunt M. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 14.Zelefsky M.J., Fuks Z., Happersett L. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55:241–249. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 15.Burman C., Chui C.S., Kutcher G. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: A strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;39:863–873. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 16.Chui C.S., Spirou S.V. Inverse planning algorithms for external beam radiation therapy. Med Dosim. 2001;26:189–197. doi: 10.1016/s0958-3947(01)00069-3. [DOI] [PubMed] [Google Scholar]
- 17.Mohan R., Wang X., Jackson A. The potential and limitations of the inverse radiotherapy technique. Radiother Oncol. 1994;32:232–248. doi: 10.1016/0167-8140(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuban D.A., Tucker S.L., Dong L. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Zietman A.L., Bae K., Slater J.D. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heemsbergen W.D., Al-Mamgani A., Slot A. Long-term results of the Dutch randomized prostate cancer trial: Impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110:104–109. doi: 10.1016/j.radonc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Beckendorf V., Guerif S., Le Prise E. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Michalski J.M., Moughan J., Purdy J. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4:e180039. doi: 10.1001/jamaoncol.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Mamgani A., van Putten W.L., Heemsbergen W.D. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–988. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 24.Syndikus I., Morgan R.C., Sydes M.R. Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: Results from the UK Medical Research Council RT01 trial (ISRCTN47772397) Int J Radiat Oncol Biol Phys. 2010;77:773–783. doi: 10.1016/j.ijrobp.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelefsky M.J., Housman D.M., Pei X. Incidence of secondary cancer development after high-dose intensity-modulated radiotherapy and image-guided brachytherapy for the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:953–959. doi: 10.1016/j.ijrobp.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Singh A., Kinoshita Y., Rovito P.M. Higher than expected association of clinical prostate and bladder cancers. J Urol. 2005;173:1526–1529. doi: 10.1097/01.ju.0000154700.80042.c6. [DOI] [PubMed] [Google Scholar]
- 27.Pickles T., Phillips N. The risk of second malignancy in men with prostate cancer treated with or without radiation in British Columbia, 1984–2000. Radiother Oncol. 2002;65:145–151. doi: 10.1016/s0167-8140(02)00307-9. [DOI] [PubMed] [Google Scholar]
- 28.Huvos A.G., Woodard H.Q., Cahan W.G. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer. 1985;55:1244–1255. doi: 10.1002/1097-0142(19850315)55:6<1244::aid-cncr2820550617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Cahan W.G., Woodard H.Q., Higinbotham N.L. Sarcoma in irradiated bone. Report of eleven cases. Cancer. 1948;1:3–29. doi: 10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Mark R.J., Poen J., Tran L.M. Postirradiation sarcomas. A single-institution study and review of the literature. Cancer. 1994;73:2653–2662. doi: 10.1002/1097-0142(19940515)73:10<2653::aid-cncr2820731030>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]