Abstract
Drugs capable of ameliorating symptoms of depression and anxiety while also improving cognitive function and sociability are highly desirable. Anecdotal reports have suggested that serotonergic psychedelics administered in low doses on a chronic, intermittent schedule, so-called “microdosing”, might produce beneficial effects on mood, anxiety, cognition, and social interaction. Here, we test this hypothesis by subjecting male and female Sprague Dawley rats to behavioral testing following the chronic, intermittent administration of low doses of the psychedelic N,N-dimethyltryptamine (DMT). The behavioral and cellular effects of this dosing regimen were distinct from those induced following a single high dose of the drug. We found that chronic, intermittent, low doses of DMT produced an antidepressant-like phenotype and enhanced fear extinction learning without impacting working memory or social interaction. Additionally, male rats treated with DMT on this schedule gained a significant amount of body weight during the course of the study. Taken together, our results suggest that psychedelic microdosing may alleviate symptoms of mood and anxiety disorders, though the potential hazards of this practice warrant further investigation.
Keywords: Psychedelic, microdosing, DMT, depression, PTSD, anxiety, subhallucinogenic, neural plasticity
Introduction
Mood and anxiety disorders are among the leading causes of disability worldwide,1,2 and antidepressants remain one of the most highly prescribed medications in the United States.3 Current therapeutic strategies for treating these disorders are slow-acting and prove to be ineffective for many patients.4 Thus, there is a critical need to develop new treatment strategies for these disorders.
Serotonergic psychedelics, such as lysergic acid diethylamide (LSD), psilocybin, and N,N-dimethyltryptamine (DMT), have a long history of use as experimental therapeutics in the clinic for treating depression, anxiety, and substance use disorder.5−10 However, it is unclear whether hallucinogenic doses of these drugs are required for them to produce therapeutic effects. Psychedelic microdosing—the practice of administering subhallucinogenic doses of psychedelic compounds on a chronic, intermittent schedule—is rapidly gaining popularity due to its alleged antidepressant and anxiolytic effects.11−13 Despite the prevalence of psychedelic microdosing, there are essentially no peer-reviewed studies that have investigated the potential benefits and risks of this practice.14,15
Psychedelics are potent psychoplastogens,16 and their effects on neural plasticity have been invoked to explain their long-lasting behavioral effects related to mood and anxiety.17 Previously, we observed that even a low dose of DMT caused changes in the frequency and amplitude of spontaneous excitatory postsynaptic currents (EPSCs) in the prefrontal cortex (PFC) of rats that lasted long after the drug had been cleared from the body.16 Therefore, we hypothesized that administration of this low dose on a chronic, intermittent schedule might impact behaviors relevant to mood and anxiety that involve the PFC.18−23
Here, we demonstrate that chronic (∼2 months), intermittent (every third day), low (1 mg/kg) doses of DMT facilitate fear extinction learning and reduce immobility in the forced swim test without producing the anxiogenic-like effects characteristic of a high dose (10 mg/kg).24 However, the former dosing regimen also significantly increases bodyweight in male rats. Taken together, the data presented here suggest that subhallucinogenic doses of psychedelic compounds might possess value for treating and/or preventing mood and anxiety disorders. Despite the therapeutic potential of psychedelic microdosing, this practice is not without risks, and future studies need to better define the potential for negative neurobiological or metabolic repercussions.
Results
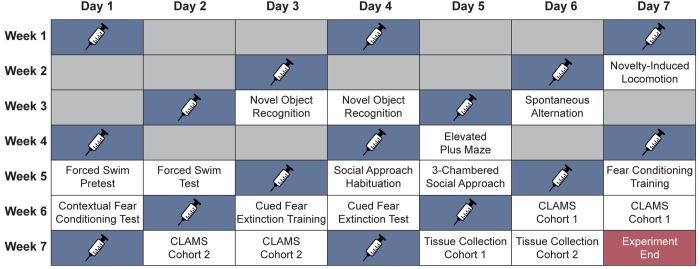
Experimental Design for Assessing the Effects of Chronic, Intermittent, Low Doses of DMT on Rat Behaviors
Anecdotal human reports regarding psychedelic microdosing were informative when designing our rodent studies. First, young adults appear to be the most likely to practice psychedelic microdosing, so we employed similarly aged rats (i.e., postnatal day 56) in our study. Both male and female rats were used. Animals were treated every third day for 2 weeks before beginning behavioral testing (Figure 1), and drug administration continued on this schedule throughout the remainder of the study. Behavioral experiments were generally ordered from least to most stressful, included a variety of tests relevant to mood, anxiety, and cognitive function, and were completed during the 2 day period between administration of doses to ensure that any effects observed were due to persistent neural adaptations as opposed to the acute effects of the drug. Following the completion of behavioral testing, the animals were subjected to metabolic monitoring and sacrificed. Tissue was collected and subjected to additional structural and biochemical analyses.
Figure 1.
Experimental design for testing the effects of chronic, intermittent, low doses of DMT on rats. Blue boxes indicate the days when drug was administered. Behavioral testing was performed on the days between doses. Gray boxes indicate days the animals spent in their home cages with no testing being performed. CLAMS = Comprehensive Lab Animal Monitoring System.
We decided to use the psychedelic N,N-dimethyltryptamine (DMT) in our studies for several reasons. First, DMT constitutes the core structure of all indole-containing psychedelics including LSD and psilocybin.10 Second, DMT is known to impact rodent behaviors relevant to mood, anxiety, cognitive function, and sociability.10,25−31 Third, DMT is the principal psychoactive component in ayahuasca, an Amazonian tisane typically administered on a chronic, intermittent schedule that has been shown to have antidepressant effects.10 Additionally, we have previously demonstrated that an acute hallucinogenic dose of DMT (10 mg/kg) administered to rats produces robust behavioral phenotypes in several paradigms relevant to mood and anxiety disorders, enabling us to compare the effects of a single high dose of the compound to those produced by chronic, intermittent, low doses of the drug. While there is no well-established definition of what constitutes a “microdose”, humans typically employ 1/10 of a hallucinogenic dose,11 so we opted to administer DMT at 1 mg/kg, or 1/10 of the dose used in our acute study.24
Several pieces of evidence suggest that a 1 mg/kg dose of DMT is subhallucinogenic in rats. First, 1 mg/kg DMT only produces ∼20% correct lever responding in Sprague Dawley rats trained to discriminate DMT from saline.32 Additionally, this dose only produces approximately 10–20% drug-appropriate responding in Sprague Dawley rats trained to discriminate other hallucinogens such as (−)-2,5-dimethoxy-4-methylamphetamine (DOM, 0.5 mg/kg) or LSD (0.1 mg/kg) from saline.32 Furthermore, a 1 mg/kg dose of DMT does not produce the drastic change in body posture that is characteristic of a 10 mg/kg dose.24 Finally, the 1 mg/kg dose is predicted to be subhallucinogenic in rats based on allometric scaling33 of known subhallucinogenic doses in humans.34 In these human studies, DMT was administered intravenously, a route that avoids first-pass metabolism and yields a high maximum concentration in the brain. We chose to administer DMT to rats via intraperitoneal injection reasoning that drug administered by this route is slower to enter the bloodstream and more susceptible to hepatic metabolism than intravenous administration,35 thus providing additional assurance that the dose selected for our study would be subhallucinogenic.
Prior to our study, essentially nothing was known about the effects of psychedelic microdosing on animal behaviors. Therefore, our primary goal was to determine which behavioral tests were likely to be impacted by this dosing regimen. We chose to use the highest dose still considered to be subhallucinogenic, reasoning that it should produce the maximal effect with lower doses expected to be less efficacious.
Chronic, Intermittent, Low Doses of DMT Do Not Produce Anxiogenic-like Effects in NIL or EPM Tests
To assess the effects of chronic, intermittent, low doses of DMT on anxiety levels, animals were tested in both novelty-induced locomotion (NIL) and the elevated plus maze (EPM) paradigms. Following exposure to a novel open space, we measured the total distance traveled, degree of thigmotaxis, number of rearings, and time spent rearing (Figure S1a). Using a Z-normalization procedure developed by Guilloux and co-workers,36 we combined all of these related measures into a single NIL Score. No statistical difference between the male and female treatment groups was observed, so these data were combined (Figure 2a). Chronic, intermittent, low doses of DMT did not produce a significant difference between treatment groups in the NIL Score (p = 0.08), though DMT trended toward producing an anxiolytic-like effect.
Figure 2.
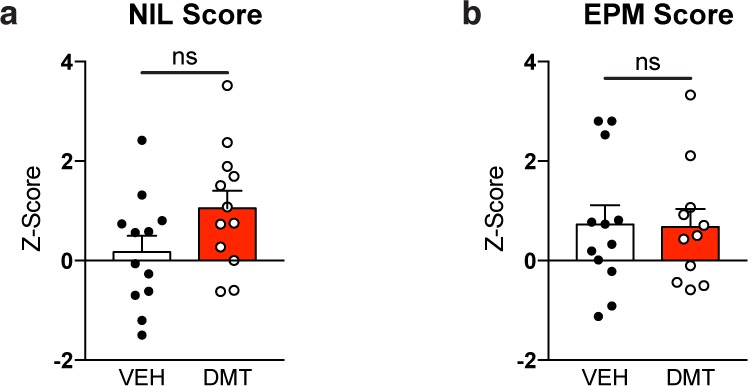
Chronic, intermittent, low doses of DMT do not produce anxiogenic-like effects in rats. (a) DMT-treated and vehicle-treated groups display similar phenotypes in the NIL (a) and EPM (b) paradigms. Error bars represent SEM, ns = not significant. See Supplementary Table 1 for details of all statistical tests.
As in the open field, DMT-treated animals did not display any behavioral signs of anxiety in the EPM as determined by the percentage of time spent in either the open or closed arms of the maze or the number of open or closed arm entries (Figure S1b). Again, these related measures were Z-normalized and combined into a single EPM Score. As no statistical difference between the male and female treatment groups was observed, these data were combined (Figure 2b). Animals treated with DMT were indistinguishable from vehicle controls (p = 0.92). Additionally, the treatment groups displayed similar levels of locomotion in the EPM maze as determined by total distance traveled and average velocity (Figure S1c). This distinct lack of any anxiogenic-like effect in either the NIL or EPM demonstrates a striking difference between the chronic, intermittent, low dose and the single high dose psychedelic treatment paradigms, as a single high dose of DMT is known to produce robust anxiogenic-like effects in both the NIL and EPM behavioral tests.24
Chronic, Intermittent, Low Doses of DMT Facilitate Cued Fear Extinction Learning
Both contextual and cued fear memory were assessed following fear conditioning (Figure 3a). Prior to receiving foot shocks, there was no difference in baseline freezing levels between the vehicle- and DMT-treated groups (Figure 3b). Similarly, the treatment groups were indistinguishable immediately after the last training foot shock (Figure 3c). Again, this latter point highlights the drastic differences between a chronic, intermittent, low dose paradigm and a single high dose of DMT, as the latter significantly increases freezing levels immediately following foot shocks.24
Figure 3.
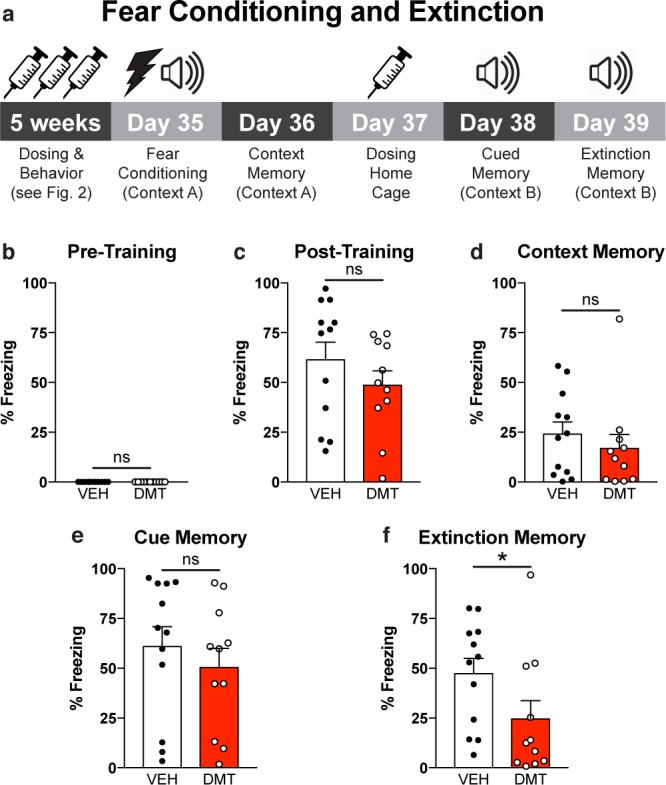
Chronic, intermittent, low doses of DMT enhance fear extinction in rats. (a) Experimental design for the fear conditioning and extinction experiments. (b, c) DMT- and vehicle-treated groups displayed comparable levels of freezing in the 2 min period before (b) and after (c) receiving foot shocks. (d, e) Neither contextual fear memory (d) nor cued fear memory (e) were impaired by chronic, intermittent treatment with low doses of DMT. (f) DMT-treated animals exhibited enhanced cued extinction memory as compared to vehicle-treated controls. n = 11 DMT-treated animals (5 male and 6 female), n = 12 vehicle-treated animals (6 male and 6 female); error bars represent SEM, ns = not significant, *p < 0.05. See Supplementary Table 1 for details of all statistical tests.
The day after fear conditioning, contextual fear memory was assessed in the original training context. Freezing behavior was comparable between the groups indicating no effect of treatment on contextual fear memory (Figure 3d). The following day, animals were administered DMT or vehicle and allowed to rest in their home cages. The next day, animals were placed in a new context and presented the auditory conditioned stimulus multiple times. Again, freezing levels were indistinguishable between the two groups demonstrating that treatment did not impair the formation of cued fear memories (Figure 3e). This cued memory test also served as the extinction training. After 24 h, the cued memory test was repeated, however, the DMT-treated animals froze significantly less than the vehicle controls (p = 0.03), suggesting that chronic, intermittent, low doses of DMT facilitate fear extinction learning (Figure 3f).
Chronic, Intermittent, Low Doses of DMT Elicit Antidepressant-like Responses in the Forced Swim Test (FST)
To determine if this treatment regimen also produces antidepressant-like effects in rodents, rats were subjected to a 2 day modified forced swim paradigm consisting of a pretest on the first day and a test swim 24 h later. Both male and female rats treated with low doses of DMT on a chronic, intermittent schedule displayed robust antidepressant-like responses when compared to the vehicle-treated controls (Figure 4), with both males and females exhibiting similar behavior. Treatment significantly decreased immobility for females (p = 0.02), and though not significant after controlling for multiple hypothesis testing, a similar trend was observed for males. When individual sex/treatment pairs were analyzed via one-way analysis of variance (ANOVA) with Tukey’s post hoc test, there were no statistical differences in climbing or swimming behavior between sexes or treatment groups, so the data were combined. When males and females were analyzed together, a significant effect of treatment on swimming behavior (p = 0.04) was observed. Similarly, DMT treatment trended toward producing an antidepressant-like effect on climbing behavior (p = 0.05) as well. Because this dosing regimen does not increase locomotion in the open field (Figure S1a), in the EPM (Figure S1c), or in a novel fear conditioning chamber prior to receiving foot shocks (Figure 3b), this response cannot be attributed to a general increase in activity.
Figure 4.
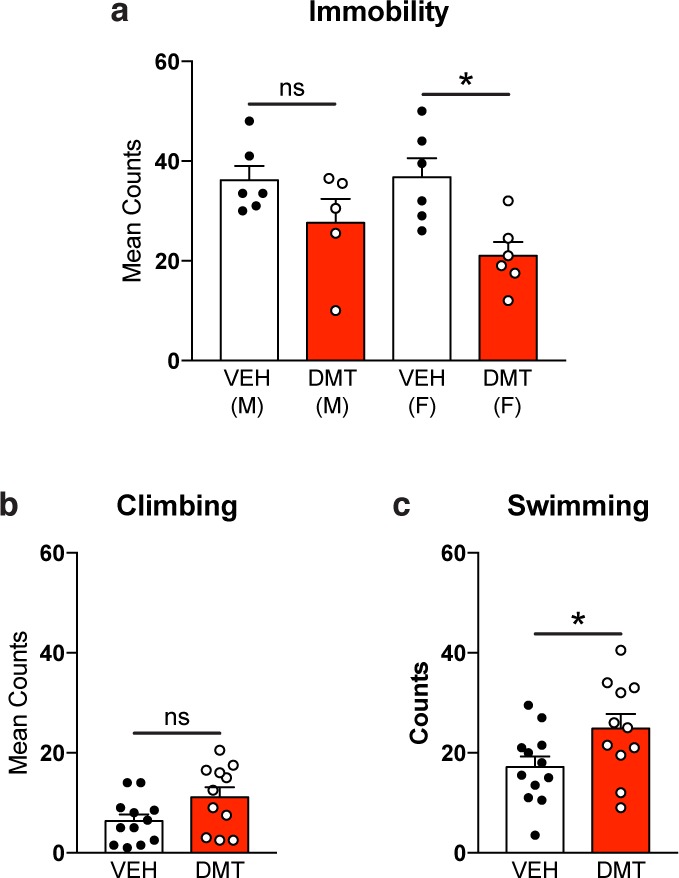
Chronic, intermittent, low doses of DMT produce antidepressant-like effects in rats. Such effects include reduced immobility (a), increased climbing (b), and increased swimming (c) in the FST. Error bars represent SEM, ns = not significant, *p < 0.05. See Supplementary Table 1 for details of all statistical tests.
Unlike the effects observed in the NIL and EPM paradigms, both the enhanced fear extinction learning and antidepressant-like effects in the FST following chronic, intermittent DMT treatment are consistent with the acute effects of a single high dose of DMT. Moreover, these data represent the first experiments in rodents corroborating the antidepressant and anxiolytic effects of psychedelic microdosing reported by humans.
Chronic, Intermittent, Low Doses of DMT Have No Effect on Working/Short-Term Memory or Social Interaction
In addition to positive effects on mood and anxiety, humans also report that psychedelic microdosing can improve various aspects of cognitive function and increase sociability. To assess the effects of chronic, intermittent, low doses of DMT on working memory in rodents, we took advantage of continuous spontaneous alternation behavior (SAB).37 In this paradigm, rodents are allowed to freely explore a T-maze and the number of successive and total arm entries are recorded. In the absence of a reinforcing stimulus (positive or negative), the natural foraging behavior of the animal is to avoid the most recently entered arm after reaching the central node of the maze. Deficits in attention and spatial working memory typically manifest as reductions in percent alternation, however, the animals microdosed with DMT were indistinguishable from vehicle controls (Figure S2a). Moreover, they exhibited similar levels of locomotion as measured by the total number of arm entries (Figure S2b).
Next, we assessed short-term memory in the absence of aversive or appetitive conditioning using novel object recognition (NOR).38 No differences were observed between the treatment groups with respect to the total amount of time spent interacting with the familiar vs the novel object (Figure S2c) or the discrimination index (Figure S2d). Similar results were observed for both male and females in the SAB and NOR paradigms.
Finally, we determined the impact of chronic, intermittent, low doses of DMT on sociability using the three-chambered social approach paradigm.39 Social preference was observed for both the vehicle- and DMT-treated groups, but there was no difference between the groups with respect to the amount of time that they spent interacting with either a novel drug-naïve conspecific or a novel inanimate object (Figure S2e). Taken together, our data demonstrate that chronic, intermittent low doses of DMT do not produce any obvious impairments in working/short-term memory or social interaction in rodents. However, unlike rodent tests of anxiety and depression (Figures 3 and 4), these rodent tests of cognitive function and sociability do not corroborate the beneficial effects of psychedelic microdosing reported by humans.
Chronic, Intermittent, Low Doses of DMT Decrease Dendritic Spine Density in the PFC of Female, but Not Male, Rats
As a single high dose of DMT increases dendritic spine density in the prefrontal cortex (PFC),16 we assessed the effect of chronic, intermittent low doses of DMT on spine density in that brain region. Following 7 weeks of microdosing and behavioral testing (Figure 1), animals were sacrificed and the structure of layer V pyramidal neurons in the PFC were assessed via Golgi–Cox staining. We observed comparable spine density in the PFC of both the vehicle- and DMT-treated male animals (p = 0.98) (Figure 5). However, DMT-treated females showed a significant decrease in the number of spines per 10 μm as compared to vehicle controls (p = 0.03).
Figure 5.
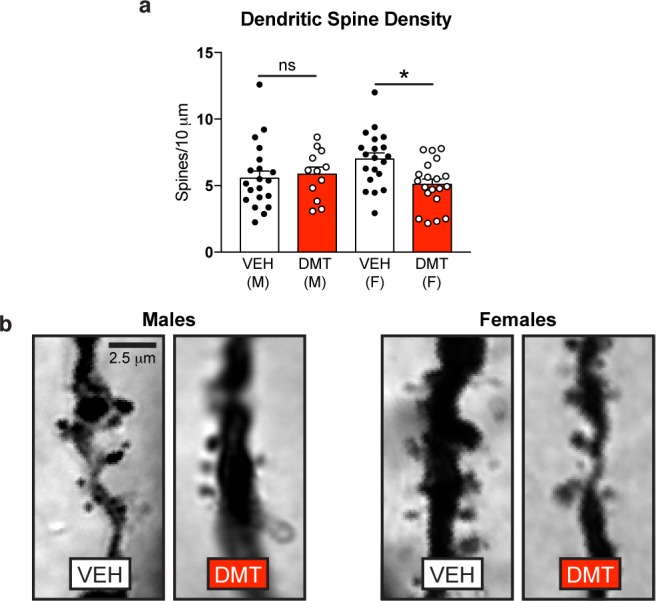
Chronic, intermittent, low doses of DMT decrease dendritic spine density in the PFC of female, but not male, rats. (a) Dendritic spine density on layer V pyramidal neurons is reduced following psychedelic microdosing in females (DMT n = 20 cells from 2 animals; VEH n = 20 cells from 2 animals), but not males (DMT n = 12 cells from 2 animals; VEH n = 21 cells from 2 animals) as measured via Golgi–Cox staining. (b) Representative images of Golgi–Cox stained layer V pyramidal neurons in the PFC of rats. M = males, F = females, Error bars represent SEM, ns = not significant, *p < 0.05. See Supplementary Table 1 for details of all statistical tests.
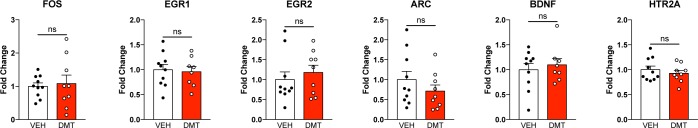
Chronic, Intermittent, Low Doses of DMT Do Not Change Gene Expression in the PFC
In addition to assessing structural changes in the PFC, we also determined if chronic, intermittent, low doses of DMT impacted expression of several key genes (Figure 6). First, we assessed the expression of EGR1, EGR2, ARC, and FOS via droplet digital PCR (ddPCR), as acute doses of psychedelics are known to increase their expression.40 Chronic, intermittent, low doses of DMT did not alter the expression of any of these genes. Furthermore, it did not increase the expression of BDNF, a result that contrasts with increased BDNF expression observed in the cortex of rats following both acute41 and chronic administration42 of high doses of psychedelics. Finally, HTR2A gene expression was unchanged despite chronic stimulation of the 5-HT2A receptor for nearly 2 months.
Figure 6.
Chronic, intermittent, low doses of DMT produce minimal effects on gene expression in the PFC of rats. Gene expression studies (ddPCR) from rat PFC tissue indicate that psychedelic microdosing produces minimal effects on gene expression (n = 5 females and 3–5 males per group). To account for multiple hypothesis testing, a Bonferroni correction was made such that the α = 0.05/6 comparisons = 0.0083 for this family of experiments. Error bars represent SEM, ns = not significant. See Supplementary Table 1 for details of all statistical tests.
Chronic, Intermittent, Low Doses of DMT Impact Metabolism
Over the course of the 7 week study, we noticed that DMT-treated male rats, but not females, gained significantly more bodyweight than their vehicle-treated counterparts (p = 0.003 and 0.99, respectively) (Figure S3a and b). The weights of vehicle-treated male rats increased by 165%, while those of DMT-treated male rats increased by 182% (Figure S3b). Therefore, we subjected the rats to metabolic monitoring for ∼48 h in isolation using a Comprehensive Lab Animal Monitoring System (CLAMS) following completion of behavioral testing (Figure 1). Despite gaining more weight, DMT-treated animals trended toward consuming less food than vehicle controls (Figure S3c). These results could not be easily explained by changes in activity or energy expenditure as horizontal movement, vertical movement, heat dissipated, and respiratory exchange rate (RER) were not statistically different between the treatment groups (Figure S3d–g).
Next, we replicated the DMT microdosing experiment using a larger cohort of male rats without performing any behavioral testing. As observed previously, male rats administered intermittent, low doses of DMT over the course of 1 month gained weight at a faster rate than the vehicle-treated controls (Figure S4a; p = 0.03, two-way repeated measures ANOVA). Divergence between the two groups could be observed as early as 1 week following the initiation of the experiment. After 4 weeks, the animals were sacrificed and their fat pads were dissected and quantified as a percentage of total body weight (Figure S4b). No differences were observed between treatment groups with respect to either white (epidydimal, mesenteric, retroperitoneal, subcutaneous) or brown adipose tissue, suggesting that DMT-induced weight increases do not simply reflect increased adiposity.
In an attempt to shed some light on the metabolic changes induced by chronic, intermittent, low doses of DMT, we measured steroid levels in serum samples from the DMT-treated animals previously subjected to behavioral testing using mass spectrometry-based metabolomics profiling (Supplementary Table 2). As expected, principal components analysis revealed large sex differences in steroid profiles (Figure S5b). However, DMT treatment appeared to have little overall effect (Figure S5c and d), and after controlling for multiple hypothesis testing, there were no statistical differences between the treatment groups.
Discussion
Several clinical studies have demonstrated that acute, hallucinogenic doses of psychedelic compounds can produce rapid, long-lasting changes in mood and behavior in humans.43−45 Recent data also suggests that a single hallucinogenic dose of DMT can change rodent brain structure and behavior long after the drug has been cleared from the body.16,24 Furthermore, we previously demonstrated that a nonhallucinogenic 1 mg/kg dose of DMT leads to increased frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in layer V pyramidal neurons of the PFC 24 h after administration.16 Therefore, we hypothesized that administering DMT on a chronic, intermittent, low dose regimen—similar to psychedelic microdosing—might alter behavior.
To the best of our knowledge, there has only been one peer-reviewed scientific report on the effects of psychedelic microdosing in rodents. This study employed three doses of psilocin spaced over 6 days, with behavioral testing occurring 48 h after the final dose.46 In agreement with our findings, the authors report no statistically significant changes in EPM behavior when two-tailed statistical tests were employed. The distinct lack of robust anxiogenic-like effects following chronic, intermittent, low doses of psychedelics is striking when compared to the effects of acute high doses. A hallucinogenic dose of DMT (10 mg/kg) decreases exploratory behavior in the NIL paradigm (i.e., both locomotion and rearing), decreases the number of open arm entries in the EPM, and increases freezing behavior immediately following the administration of foot shocks.24 None of these effects are observed when a subhallucinogenic dose (1 mg/kg) of DMT is administered on a chronic intermittent schedule (Figures 2 and 3c).
Unlike the anxiogenic-like effects produced by an acute hallucinogenic dose of DMT, the beneficial effects on fear extinction learning and forced swim behavior24 are reproduced by the chronic, intermittent low dose regimen (Figures 3f and 4). These antidepressant-like and anxiolytic-like effects are consistent with the anecdotal human reports regarding psychedelic microdosing providing strong supporting evidence that psychedelic microdosing might actually have therapeutic potential. Compounds capable of enhancing fear extinction learning in rodents, such as 3,4-methylenedioxymethamphetamine (MDMA),47,48 are excellent candidates for treating PTSD symptoms in humans.
We hypothesize that chronic, intermittent low doses of DMT increase the excitability of pyramidal neurons in the PFC that project to the basolateral amygdala and dorsal raphe nucleus to decrease the expression of conditioned fear responses and increase swimming behavior in the forced swim test, respectively. DMT and other psychedelics are potent psychoplastogens—compounds capable of promoting the rapid growth of dendritic branches, spines, and synapses—due to their ability to stimulate the mammalian target of rapamycin (mTOR) through activation of 5-HT2A receptors.16 These receptors are enriched in the prefrontal cortex, an area of the brain that is known to exert top-down control over mood and anxiety. It is likely that the behavioral changes induced by chronic, intermittent low doses of DMT are the result of positive neuroadaptations in circuits involving the PFC that are relevant to fear extinction22 and effortful response to behavioral challenge (i.e., FST)21 as DMT has an extremely short half-life in rats (ca. 15 min)49 and the behaviors were performed on days when DMT was NOT administered. In fact, we have previously shown that a 1 mg/kg dose of DMT increases the frequency and amplitude of spontaneous EPSCs in the PFC long after DMT had been cleared from the body.16
Despite producing similar anxiolytic-like and antidepressant-like behavioral effects, an acute hallucinogenic dose and chronic, intermittent, low doses of DMT produce very different biochemical and structural phenotypes. We have previously shown that a single hallucinogenic dose of DMT (10 mg/kg) increases dendritic spine density on pyramidal neurons of the PFC,16 but a similar effect was not observed following administration of chronic, intermittent low doses (1 mg/kg) of DMT (Figure 5). We hypothesize that chronic, intermittent low doses of DMT promote sufficient plasticity to strengthen key circuits involved in the regulation of mood and fear, thus impacting behavior, but that homeostatic plasticity tightly controls the overall synaptic input to these neurons leading to similar spine densities between treatment groups over time.
While there were no major behavioral differences between male and female rats following DMT treatment, there were distinct changes in neuronal structure. Dendritic spine density on pyramidal neurons of the PFC was unchanged in male rats administered chronic, intermittent, low doses of DMT; however, female rats exhibited a reduction in spine density (Figure 5). As psychedelics are known to increase glutamate release in the cortex,50 the reduced spine density observed in females could reflect greater sensitivity to glutamatergic excitotoxicity. This highlights the fact that the overall psychedelic microdosing load, which includes the amount of drug in each dose, the frequency of administration, and the length of treatment, is likely to be critical for achieving the beneficial effects of psychedelic microdosing without negative repercussions. Long-term intermittent use of ayahuasca is correlated with thinning of the posterior cingulate cortex (PCC) in humans,51 but not increased risk for mental illness.52 However, Nichols and co-workers have shown that chronic (>3 months), intermittent, high doses of LSD administered to rats produce neuroadaptations leading to a persistent behavioral state consisting of hyperactivity, anhedonia, and social deficits.53 These behavioral changes are accompanied by significant gene expression changes in the PFC related to neural plasticity.35 As a similar dose of LSD given over 11 days resulted in antidepressant-like effects,54 it appears that the total length of treatment is critical for determining behavioral outcomes.
A single hallucinogenic dose of several psychedelic compounds has been shown to increase gene expression of several genes related to neural plasticity.55,56 However, we did not observe similar changes following the administration of chronic, intermittent low doses of DMT (Figure 6). Duman and co-workers also observed that repeated dosing of 2,5-dimethoxy-4-iodoamphetamine (DOI), an amphetamine-based psychedelic, caused minimal changes in BDNF expression in the cortex when compared to a single acute dose. They hypothesized that 5-HT2 downregulation might explain the differences observed between the two dosing regimens. However, in the case of chronic, intermittent administration of low dose DMT, we do not observe any changes in HTR2A expression (Figure 6), and it has been previously shown that DMT does not induce receptor downregulation in cellular studies,57 nor does it cause tolerance in humans.58 This distinct lack of increased BDNF expression following chronic, intermittent, low doses of DMT highlights an important mechanistic difference between psychedelic microdosing and chronic treatment with selective serotonin reuptake inhibitors and other slow-acting antidepressants. In the latter case, increased BDNF expression is believed to facilitate the repair of damaged circuits relevant to mood and anxiety.59 Chronic, intermittent low doses of DMT appear to accomplish this without any obvious changes in the expression of BDNF or other genes known to be differentially regulated following administration of a single high dose of a psychedelic.
In addition to neurobiologial effects, psychedelic microdosing might also impact metabolism. After correcting for multiple hypothesis testing, male rats administered chronic, intermittent low doses of DMT exhibited a trend toward eating less food (p = 0.13, Figure S3c). Similar effects have been observed following administration of ayahuasca60 and various serotonergic agonists.61 Surprisingly, despite eating less food than vehicle-treated controls, the DMT-treated animals gained significantly more weight (Figure S3b). This contrasted with a previous report of chronic ayahuasca administration in rats,60 and the reason for the discrepancy is not immediately obvious. Metabolomics experiments revealed that serum levels of most steroids remained unchanged following psychedelic microdosing. However, male rats treated with DMT did experience a nearly 50% reduction in estradiol levels, though the effect was not significant after correcting for multiple hypothesis testing.
In general, more work needs to be done to identify potential risks associated with the increasingly popular practice of psychedelic microdosing. While psychedelic-induced activation of mTOR in young adults might lead to changes in neural plasticity having beneficial effects on mood and anxiety, in some individuals, psychedelic microdosing could cause overstimulation of cortical neurons and actually exacerbate symptoms of neuropsychiatric disorders. Moreover, nothing is known about the effects of psychedelic microdosing on neurodevelopment or the aging brain. As overactivation of mTOR has been proposed to contribute to the development of autism spectrum disorder (ASD)62 and Alzheimer’s disease (AD),63 more research is warranted to fully understand the risks associated with the chronic, intermittent use of psychedelics and related psychoplastogens.
While our study only assessed the impact of a single low dose of DMT administered on a chronic, intermittent schedule, it is important because it provides critical information about which behaviors are sensitive to psychedelic microdosing, and which are not, for both males and females. Moreover, it accomplished this using the minimal number of animals possible—an important ethical consideration. Follow-up studies can now hone in on particular behaviors of interest (e.g., FST or fear extinction) and perform dose–response or time–response studies. We still do not know if (1) lower doses would maintain therapeutic efficacy while minimizing changes in spine density or other potential side effects, or (2) if more complex dose–response relationships (e.g., U-shaped) are operative. Future studies examining dose–response effects and the impact of different dosing schedules are certainly warranted.
Despite the potential risks associated with psychedelic microdosing, the data presented here suggest several exciting possibilities for the treatment of mood and anxiety disorders. First, a chronic intermittent dosing regimen lends itself to the potential prophylactic treatment of neuropsychiatric diseases. As acute doses of serotonergic psychedelics produce similar effects as an acute dose of the psychoplastogen ketamine, and ketamine has demonstrated promise for preventing stress-induced depression- and anxiety-related phenotypes in animal models,64−66 it will be interesting to see if psychedelic microdosing is also capable of preventing the development of depression and anxiety symptoms. Second, the ability of low doses of DMT to produce positive effects on mood and anxiety in rats suggests that the perceptual effects of psychedelics can be decoupled from their therapeutic properties. This could lead to the development of nonhallucinogenic psychoplastogens with broad therapeutic potential and minimal risk for abuse. Taken together, our results encourage cautious optimism about the potential for psychedelic microdosing to produce beneficial effects on depression and anxiety.
Methods
Drugs
Solid DMT·fumarate (2:1) was prepared as described previously67 and stored in the dark at −20 °C prior to use. For each administration, a solution of DMT·fumarate (2:1) in 0.9% sterile saline was freshly prepared and passed through a 0.2 μm syringe filter. For all experiments, DMT·fumarate (2:1) was administered at 1 mg/kg (calculated based on the weight of the 2:1 DMT:fumarate salt) via intraperitoneal injection using an injection volume of 1 mL/kg. For our vehicle control, 0.9% sterile saline solution was utilized.
Animals
Male and female Sprague Dawley rats were obtained from Charles River Laboratories (Wilmington, MA), were housed two animals of the same sex per cage, and were given ad libitum access to food and water. The experiments began when the rats were 8 weeks of age. Lights in the vivarium were turned on at 07:00 h and turned off at 19:00 h. Behavioral experiments were performed during the light-on phase, with experiments taking place between 08:00 and 18:00 h unless otherwise noted. Treatment groups were randomly assigned, but each cage housed one animal from the DMT- and one from the VEH-treated groups. When appropriate, behavioral tests were counterbalanced to avoid systematic errors. All experimental procedures involving animals were approved by the UC Davis Institutional Animal Care and Use Committee (IACUC) and adhered to principles described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Over the course of one of the microdosing experiments, one animal began to exhibit signs of poor health, and was euthanized according to our IACUC protocol. The University of California, Davis is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Details of behavioral tasks can be found in the Supplementary Methods.
Data Availability
Data that support the findings of this study are available from the corresponding author upon request.
Statistical Analysis
Data analyses were performed by experimenters blinded to treatment conditions. Statistical analyses were performed using GraphPad Prism (version 7.0a). Details of all statistical tests can be found in Supplementary Table 1. Appropriate samples sizes were estimated based on our previous experiences performing similar experiments in conjunction with a power analysis. The behavioral tests selected were based on specific hypotheses regarding the effects of psychedelic microdosing on mood, anxiety, cognitive function, and sociability. All comparisons presented were planned at the outset of the study.
As our study was designed to detect potential sex differences, each experiment was first analyzed using a one-way ANOVA with a Tukey’s post hoc test where each sex/treatment pair served as an independent group. When (1) comparisons between males and females within each treatment group (i.e., vehicle or DMT) were not significant, and (2) there was no difference between treatment groups of the same sex, the data from the two sexes were combined. However, in the case of the metabolism data presented in Figure S3, each sex/treatment pair was treated independently in an attempt to explain the sex differences in weight gain observed for the DMT-treated group.
In cases where several related phenotypes were measured (i.e., NIL and EPM), we performed a Z-normalization according to the method of Guilloux and co-workers36 using vehicle-treated males as the control population. Then, we averaged the Z-scores for each animal across all of the measured phenotypes related to a particular behavioral experiment, to produce a single “behavioral score” (i.e., NIL Score or EPM Score). The Z-normalized values for each behavioral score were then compared using an unpaired Student’s t test.
In the case of fear conditioning and fear extinction experiments (Figure 3), a Mann–Whitney Test was used as a bimodal distribution was observed. Similar results in rat fear extinction experiments have been observed by others68 and suggest that there are responders and nonresponders to extinction training. For the FST (Figure 4), video files were scored by two blinded observers and averaged. For gene expression studies (Figure 6) individual comparisons were made via two-tailed unpaired Student’s t tests, but the alpha for this family of experiments was adjusted using a Bonferroni correction. For the adiposity study, a two-way ANOVA with Sidak’s post hoc test was performed.
For the steroid profiling experiments, samples were split into male and female subgroups prior to statistical analysis. The Shapiro–Wilk normality test revealed normally distributed data in males and females. A heteroscedastic two-tailed Student’s t test and the Benjamini–Hochberg correction69 was used to test for differences in steroid concentrations between treated and control animals. Student’s t test was performed in MS Excel, raw p-values were exported to R-studio where the “p.adjust” function was used to apply the Benjamini–Hochberg multiple comparisons adjustment accounting for all 18 steroid metabolites. For the steroid profiling studies, the ggplots2 package in R studio was used to create boxplots. Principal components analysis was performed using MetaboAnalyst 4.0.
For all statistical analyses, a Grubbs test was used to verify obvious outliers, and the specific data points omitted are listed in Supplementary Table 1. For the social interaction test, a computer error caused us to lose data for one animal.
Acknowledgments
We would like to thank Jennifer Rutkowsky and Jon Ramsey for performing the fat pad dissections, and the Intellectual and Developmental Disabilities Research Center (U54 HD079125) Rat Behavioral Testing Core for equipment use and assistance. We would also like to thank Lee Dunlap for synthesizing DMT, Zefan Hurley for help with the forced swim test, and Whitney Duim for assistance with imaging. The Olympus FV1000 confocal used in this study was purchased using NIH Shared Instrumentation Grant 1S10RR019266-01. We thank the MCB Light Microscopy Imaging Facility, which is a UC-Davis Campus Core Research Facility, for the use of this microscope.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.8b00692.
Author Contributions
D.E.O. conceived the project and was responsible for the overall experimental design. L.P.C. performed the Golgi–Cox staining and gene expression experiments and analyzed all data presented. C.J.B. performed the rat behavioral experiments. B.C.D. and O.F. performed the metabolomics study. L.P.C. and D.E.O. wrote the manuscript with input from all authors.
This work was supported by funds from the UC Davis Department of Chemistry and Department of Biochemistry & Molecular Medicine as well as a UC Davis Genome Center Pilot Grant (D.E.O.), NIH Grant 5T32MH082174-09 (L.P.C.), NIH Grant U24DK097154 (O.F.), NIH Grant R01DK104351 (B.C.D), and National Science Foundation Predoctoral Fellowship #1650042 (C.J.B.).
D.E.O. is a co-founder of Delix Therapeutics, Inc.
The authors declare no competing financial interest.
Supplementary Material
References
- Whiteford H. A.; et al. (2013) Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Olesen J.; Leonardi M. (2003) The Burden of Brain Diseases in Europe. Eur. J. Neurol. 10, 471–477. 10.1046/j.1468-1331.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- Fuentes A. V.; Pineda M. D.; Venkata K. C. N. (2018) Comprehension of Top 200 Prescribed Drugs in the US as a Resource for Pharmacy Teaching, Training and Practice. Pharmacy (Basel) 6, 43. 10.3390/pharmacy6020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Library of Medicine . Depression: How effective are antidepressants? https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0087089/ (accessed October 2018).
- Nichols D. E.; Johnson M. W.; Nichols C. D. (2017) Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 101, 209–219. 10.1002/cpt.557. [DOI] [PubMed] [Google Scholar]
- Mithoefer M. C.; Grob C. S.; Brewerton T. D. (2016) Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry 3, 481–488. 10.1016/S2215-0366(15)00576-3. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; Goodwin G. M. (2017) The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 42, 2105–2113. 10.1038/npp.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos R. G.; Osório F. L.; Crippa J. A. S.; Riba J.; Zuardi A. W.; Hallak J. E. C. (2016) Antidepressive, Anxiolytic, and Antiaddictive Effects of Ayahuasca, Psilocybin and Lysergic Acid Diethylamide (LSD): A Systematic Review of Clinical Trials Published in the Last 25 Years. Ther. Adv. Psychopharmacol. 6, 193–213. 10.1177/2045125316638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar E. J.; Nichols C. D.; Gainetdinov R. R.; Nichols D. E.; Kalueff A. V. (2017) Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 38, 992–1005. 10.1016/j.tips.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Cameron L. P.; Olson D. E. (2018) Dark Classics in Chemical Neuroscience: N,N-Dimethyltryptamine (DMT). ACS Chem. Neurosci. 9, 2344–2357. 10.1021/acschemneuro.8b00101. [DOI] [PubMed] [Google Scholar]
- Johnstad P. G. (2018) Powerful Substances in Tiny Amounts: An Interview Study of Psychedelic Microdosing. Nord. Stud. Alcohol Drugs 35, 39–51. 10.1177/1455072517753339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T.; Petranker R.; Dinh-Williams L.-A.; Rosenbaum D.; Weissman C.; Hapke E.; Hui K.; Farb N. (2019) Microdosing Psychedelics: Personality, mental health, and creativity differences in microdosers. Psychopharmacology 1–10. 10.1007/s00213-018-5106-2. [DOI] [PubMed] [Google Scholar]
- Polito V.; Stevenson R. J. (2019) A Systematic Study of Microdosing Psychedelics. PLoS One 14, e0211023. 10.1371/journal.pone.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova L.; Lippelt D. P.; Colzato L. S.; Kuchar M.; Sjoerds Z.; Hommel B. (2018) Exploring the effect of microdosing psychedelics on creativity in an open-label natural setting. Psychopharmacology (Berl) 235, 3401–3413. 10.1007/s00213-018-5049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanakieva S.; Polychroni N.; Family N.; Williams L. T. J.; Luke D. P.; Terhune D. B. (2018) The effects of microdose LSD on time perception: a randomised, double-blind, placebo-controlled trial. Psychopharmacology 1–12. 10.1007/s00213-018-5119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; Duim W. C.; Dennis M. Y.; McAllister A. K.; Ori-McKenney K. M.; Gray J. A.; Olson D. E. (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 23, 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D. E. (2018) ″Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics″. J. Exp. Neurosci. 12, 1–4. 10.1177/1179069518800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M. R.; Quirk G. J. (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74. 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Quirk G. J.; Likhtik E.; Pelletier J. G.; Paré D. (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 23, 8800–8807. 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C.; Diwan M.; Macedo C. E.; Brandão M. L.; Shumake J.; Gonzalez-Lima F.; Raymond R.; Lozano A. M.; Fletcher P. J.; Nobrega J. N. (2010) Antidepressant-Like Effects of Medial Prefrontal Cortex Deep Brain Stimulation in Rats. Biol. Psychiatry 67, 117–124. 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Warden M. R.; Selimbeyoglu A.; Mirzabekov J. J.; Lo M.; Thompson K. R.; Kim S.-Y.; Adhikari A.; Tye K. M.; Frank L. M.; Deisseroth K. (2012) A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432. 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A.; et al. (2015) Basomedial Amygdala Mediates Top-down Control of Anxiety and Fear. Nature 527, 179–185. 10.1038/nature15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M.; Thomas A.; Liu R.; Wohleb E. S.; Land B. B.; DiLeone R. J.; Aghajanian G. K.; Duman R. S. (2015) Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. U. S. A. 112, 8106–8111. 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Benson C. J.; Dunlap L. E.; Olson D. E. (2018) Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 9, 1582–1590. 10.1021/acschemneuro.8b00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro T. M.; Gatch M. B. (2016) Neuropharmacology of N,N-dimethyltryptamine. Brain Res. Bull. 126, 74–88. 10.1016/j.brainresbull.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.; Sheard M. H. (1974) Biphasic dose-response effects of N-N-dimethyltryptamine on the rat startle reflex. Pharmacol. Biochem. Behav. 2, 827–829. 10.1016/0091-3057(74)90116-6. [DOI] [PubMed] [Google Scholar]
- Brewster J. M.; Siegel R. K.; Johnson C. A.; Jarvik M. E. (2017) Observational determination of dose-response curves in hallucinogen-treated monkeys. Int. Pharmacopsychiatry 11, 102–108. 10.1159/000468218. [DOI] [PubMed] [Google Scholar]
- File S. E. (1977) Effects of N,N-dimethyltryptamine on behavioural habituation in the rat. Pharmacol. Biochem. Behav. 6, 163–168. 10.1016/0091-3057(77)90067-3. [DOI] [PubMed] [Google Scholar]
- Stoff D. M.; Moja E. A.; Gillin J. C.; Wyatt R. J. (1978) Disruption of conditioned avoidance behavior by n,n-dimethyltryptamine (DMT) and stereotype by beta-phenylethylamine (PEA): animal models of attentional defects in schizophrenia. J. Psychiatr. Res. 14, 225–240. 10.1016/0022-3956(78)90025-0. [DOI] [PubMed] [Google Scholar]
- Walters J. K.; Sheard M. H.; Davis M. (1978) Effects of N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeODMT) on shock elicited fighting in rats. Pharmacol. Biochem. Behav. 9, 87–90. 10.1016/0091-3057(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Sbordone R. J.; Wingard J. A.; Gorelick D. A.; Elliott M. L. (1979) Severe aggression in rats induced by mescaline but not other hallucinogens. Psychopharmacology (Berl) 66, 275–280. 10.1007/BF00428319. [DOI] [PubMed] [Google Scholar]
- Gatch M. B.; Rutledge M. A.; Carbonaro T.; Forster M. J. (2009) Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacol. (Berl). 204, 715–724. 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A. B.; Jacob S. (2016) A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R. J.; Qualls C. R.; Uhlenhuth E. H.; Kellner R. (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch. Gen. Psychiatry 51, 98–108. 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Turner P.; Brabb T.; Pekow C.; Vasbinder M. A. (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab Anim. Sci. 50, 600–613. [PMC free article] [PubMed] [Google Scholar]
- Guilloux J. P.; Seney M.; Edgar N.; Sibille E. (2011) Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J. Neurosci. Methods 197, 21–31. 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. N. (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 28, 497–505. 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Antunes M.; Biala G. (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O.; Lipina T.; Vukobradovic I.; Roder J.; Woodgett J. R. (2011) Assessment of Social Interaction Behaviors. J. Visualized Exp. 48, e2473 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. A.; Nichols C. D. (2017) The Effects of Hallucinogens on Gene Expression. Curr. Top. Behav. Neurosci. 36, 137–158. 10.1007/7854_2017_479. [DOI] [PubMed] [Google Scholar]
- Vaidya V. A.; Marek G. J.; Aghajanian G. K.; Duman R. S. (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 17, 2785–2795. 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. A.; Marona-Lewicka D.; Nichols D. E.; Nichols C. D. (2014) Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology 83, 1–8. 10.1016/j.neuropharm.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Richards W. A.; McCann U.; Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacol. (Berl) 187, 268–294. 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; McCann U.; Jesse R. (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology 218, 649–665. 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; et al. (2017) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 7, 13817. 10.1038/s41598-017-13282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley R. R.; Pálenícek T.; Kolin J.; Valeš K. (2018) Psilocin and ketamine microdosing: effects of subchronic intermittent microdoses in the elevated plus-maze in male Wistar rats. Behav. Pharmacol. 29, 530–536. 10.1097/FBP.0000000000000394. [DOI] [PubMed] [Google Scholar]
- Young M. B.; Andero R.; Ressler K. J.; Howell L. L. (2015) 3,4-Methylenedioxymethamphetamine facilitates fear extinction learning. Transl. Psychiatry 5, e634 10.1038/tp.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap L. E.; Andrews A. M.; Olson D. E. (2018) Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 9, 2408–2427. 10.1021/acschemneuro.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram B. R.; Lockett L.; Talomsin R.; Blackman G. L.; McLeod W. R. (1987) In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem. Pharmacol. 36, 1509–1512. 10.1016/0006-2952(87)90118-3. [DOI] [PubMed] [Google Scholar]
- Muschamp J. W.; Regina M. J.; Hull E. M.; Winter J. C.; Rabin R. A. (2004) Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 1023, 134–140. 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Bouso J. C.; et al. (2015) Long-Term Use of Psychedelic Drugs Is Associated with Differences in Brain Structure and Personality in Humans. Eur. Neuropsychopharmacol. 25, 483–492. 10.1016/j.euroneuro.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Bouso J. C.; et al. (2012) Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of Ayahuasca: a longitudinal study. PLoS One 7, e42421 10.1371/journal.pone.0042421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D.; Nichols C. D.; Nichols D. E. (2011) An Animal Model of Schizophrenia Based on Chronic LSD Administration: Old Idea, New Results. Neuropharmacology 61, 503–512. 10.1016/j.neuropharm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchborn T.; Schröder H.; Höllt V.; Grecksch G. (2014) Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J. Psychopharmacol. 28, 545–552. 10.1177/0269881114531666. [DOI] [PubMed] [Google Scholar]
- González-Maeso J.; Ebersole B. J.; Wurmbach E.; Lira A.; Zhou M.; Weisstaub N.; Hen R.; Gingrich J. A.; Sealfon S. C. (2003) Transcriptome Fingerprints Distinguish Hallucinogenic and Nonhallucinogenic 5-Hydroxytryptamine 2A Receptor Agonist Effects in Mouse Somatosensory Cortex. J. Neurosci. 23, 8836–8843. 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya V. A.; Marek G. J.; Aghajanian G. K.; Duman R. S. (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 17, 2785–2795. 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L.; Canton H.; Barrett R. J.; Sanders-Bush E. (1998) Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol., Biochem. Behav. 61, 323–30. 10.1016/S0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Strassman R. J.; Qualls C. R.; Berg L. M. (1996) Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol. Psychiatry 39, 784–795. 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Duman R. S.; Monteggia L. M. (2006) A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 59, 1116–1127. 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Santos A. F. A.; Vieira A. L. S.; Pic-Taylor A.; Caldas E. D. (2017) Reproductive effects of the psychoactive beverage ayahuasca in male Wistar rats after chronic exposure. Rev. Bras. Farmacogn. 27, 353–360. 10.1016/j.bjp.2017.01.006. [DOI] [Google Scholar]
- Schreiber R.; Selbach K.; Asmussen M.; Hesse D.; de Vry J. (2000) Effects of serotonin1/2 receptor agonists on dark-phase food and water intake in rats. Pharmacol., Biochem. Behav. 67, 291–305. 10.1016/S0091-3057(00)00357-9. [DOI] [PubMed] [Google Scholar]
- Winden K. D.; Ebrahimi-Fakhari D.; Sahin M. (2018) Abnormal mTOR Activation in Autism. Annu. Rev. Neurosci. 41, 1–23. 10.1146/annurev-neuro-080317-061747. [DOI] [PubMed] [Google Scholar]
- Cai Z.; Zhou Y.; He W.; Xiao M.; Yan L. J. (2015) Activation of mTOR: a culprit of Alzheimer’s disease?. Neuropsychiatr. Dis. Treat. 11, 1015–1030. 10.2147/NDT.S75717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J.; Dolzani S. D.; Tilden S.; Christianson J. P.; Kubala K. H.; Bartholomay K.; Sperr K.; Ciancio N.; Watkins L. R.; Maier S. F. (2016) Previous Ketamine Produces an Enduring Blockade of Neurochemical and Behavioral Effects of Uncontrollable Stress. J. Neurosci. 36, 153–161. 10.1523/JNEUROSCI.3114-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. C.; LaGamma C. T.; Lim S. C.; Tsitsiklis M.; Neria Y.; Brachman R. A.; Denny C. A. (2017) Prophylactic Ketamine Attenuates Learned Fear. Neuropsychopharmacology 42, 1577–1589. 10.1038/npp.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman R. A.; et al. (2016) Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol. Psychiatry 79, 776–786. 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap L. E.; Olson D. E. (2018) Reaction of N,N-Dimethyltryptamine with Dichloromethane Under Common Experimental Conditions. ACS Omega 3, 4968–4973. 10.1021/acsomega.8b00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J.; Furgeson-Moreira S.; Monfils M. H. (2014) Predictability and heritability of individual differences in fear learning. Anim. Cogn. 17, 1207–1221. 10.1007/s10071-014-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y.; Hochberg Y. (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon request.