Abstract
Photodynamic therapy (PDT) has emerged as a non-invasive modality for treating tumors while a photosensitizer (PS) plays an indispensable role in PDT. Nevertheless, free PSs are limited by their low light stability, rapid blood clearance, and poor water solubility. Constructing a nanocarrier delivering PSs is an appealing and potential way to solve these issues. As a melanin-like biopolymer, polydopamine (PDA) is widely utilized in biomedical applications (drug delivery, tissue engineering, and cancer therapy) for its prominent properties, including favorable biocompatibility, easy preparation, and versatile functionality. PDA-based nanocarriers are thus leveraged to overcome the inherent shortcomings of free PSs. In this Mini-Review, we will firstly present an overview on the recent developments of PDA nanocarriers delivering PSs. Then, we introduce three distinctive strategies developed to combine PSs with PDA nanocarriers. The advantages and disadvantages of each strategy will be discussed. Finally, the current challenges and future opportunities of PDA-based PS nanocarriers will also be addressed.
Keywords: polydopamine, photosensitizers, drug delivery, photodynamic therapy, nanocarriers
Introduction
Currently, cancer therapies commonly used in clinical practice, such as chemotherapy (Hu et al., 2016), surgery (Katz et al., 2005), and radiotherapy (Schaue and McBride, 2015), have reached their bottlenecks. Drug tolerance, non-specificity, and unavoidable side effects hinder their further development (Dean et al., 2005; Xiao et al., 2013). As an alternative emerging treatment, photodynamic therapy (PDT) brings a new dawn (Dolmans et al., 2003; Lismont et al., 2017). Compared with existing treatment modalities, PDT possesses numerous incomparable superiorities, including non-/minimal invasiveness, low side effects, and controllability (Rong et al., 2014). The typical process of PDT can be briefly summarized as follows: when the lesion site enriched with sufficient photosensitizers (PSs) is irradiated with light, the PSs are converted from the ground state to the excited (triplet) state. Subsequently, the excited PSs transfer their energy directly to the oxygen and generate toxic reactive oxygen species (ROS), such as singlet oxygen (1O2) and free radicals, which rapidly kill cancer cells (Calixto et al., 2016). It is not difficult to find that light source, O2, and PSs are three indispensable ingredients for PDT (Agostinis et al., 2011). Among them, PSs are a critical element. In recent years, several types of PSs, such as porphyrin- (Jin et al., 2013), chlorin e6 (Ce6)- (Li et al., 2013), and methylene blue (MB)- (Seo et al., 2014) based therapeutic agents, have progressed largely. However, these PSs still suffer from their low light stability, fast body clearance, and poor water solubility (Teng et al., 2013; Shemesh et al., 2014). To address these issues, suitable nanocarriers are necessary for potent PDT.
Polydopamine (PDA), a melanin-like biopolymer, has a strong binding property, which makes it possible to load chemical drugs or dyes bearing aromatic structure through π-π stacking/hydrophobic interaction (Wang X. et al., 2016). Moreover, many active groups (including catechol, amino, and quinone groups) in PDA are able to react with a variety of drug molecules (Liu et al., 2014). Besides, PDA is an excellent coating material that can easily form a multifunctional core–shell nanostructure through facile dip coating of nanoparticles (NPs) in an aqueous solution of dopamine (DA) (Lee et al., 2007). Importantly, bio-inspired PDA possess excellent biocompatibility and biodegradation, which provide a prerequisite for biological application. These charming features make PDA an ideal nanocarrier for PSs.
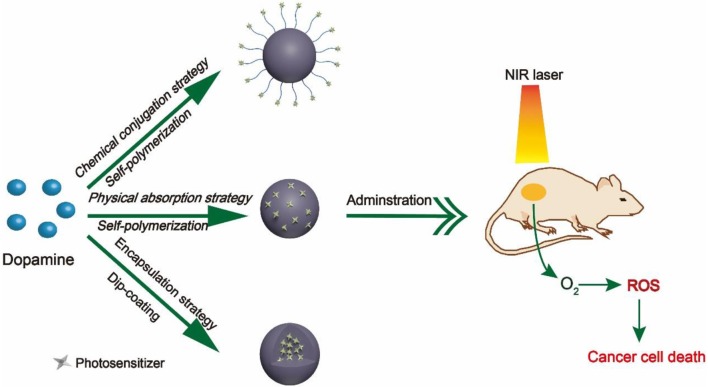
During the past decade, numerous research groups have leveraged PDA as nanocarrier to delivery PSs. According to different interactions between PDA nanocarriers and PSs, we divide these studies into three categories, namely, (1) chemical conjugation strategy, (2) physical absorption strategy, and (3) encapsulation strategy, as summarized in Figure 1. In this Mini-Review, we will highlight the relevant advances regarding PDA-based nanocarriers for PSs and discuss its future prospects and challenges.
Figure 1.
Schematic diagram of three different strategies.
Chemical Conjugation Strategy
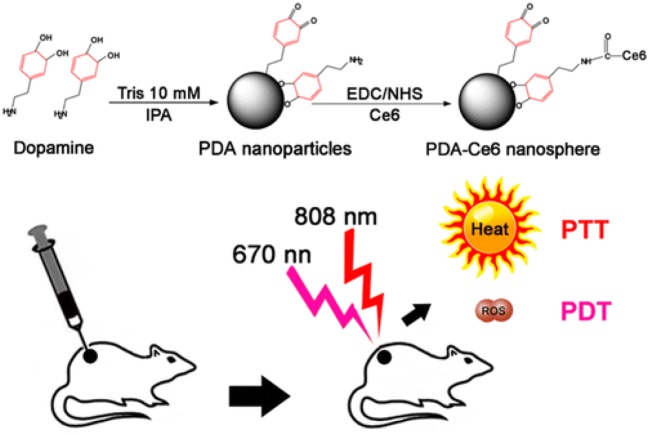
Surface functional groups endow PDA NPs with strong chemical reactivity. The covalent conjugation between PDA and amine/thiol-containing molecules is the most widely used reactions in previous studies (Park et al., 2014). Amine-containing molecules react with PDA via Schiff base reaction while thiol-containing molecules react by facile Michel addition reaction (Lee et al., 2009). Owing to the presence of catechol groups, PDA has redox capacity. Hu et al. (2014) firstly modified graphene with PDA. Subsequently, the PDA–graphene reacted with nucleophilic amine groups of folic acid–C60 by Michael addition/Schiff base reaction. Due to the high quantum yield of 1O2 generation of C60 and photothermal absorption of graphene, the obtained C60-PDA–graphene nanohybrids exhibited synergistic PDT/PTT (photothermal therapy) effect against Hela cancer cells. PDA plays an indispensable role in the whole process, serving as the bridge between graphene and C60. Moreover, the introduction of PDA also increased the biocompatibility and hydrophilicity of C60-PDA–graphene. Besides, the amino groups on the surface of PDA NPs can also react with PSs containing carboxyl groups (Ce6, porphyrin) by a conventional carbodiimide reaction. Under this premise, Zhang et al. (2015) developed a NP to achieve PDT/PTT combination therapy of tumors (Figure 2). In their work, Ce6 was covalently conjugated onto the surface of PDA. Compared to free Ce6, PDA–Ce6 NPs exhibited higher photo-stability and PDT efficacy. Furthermore, the satisfactory NIR absorption enables PDA to be applied in PTT. Under the irradiation of two different lasers (670 and 808 nm), the PDA–Ce6 NPs demonstrated potent antitumor efficacy both in vitro and in vivo. To improve the load efficiency of PSs, Yang et al. (2019) selected black phosphorus nanosheets (BP NSs) as the basic nanocarrier, and BP NSs are then coated with PDA and covalently linked with Ce6. Compared to PDA–Ce6, the larger surface area of BP can significantly increase the load of PSs on PDA.
Figure 2.
Illustration of the preparation of PDA-Ce6 and their application for PTT and PDT. Reproduced with permission from Zhang et al. (2015). Copyright 2015 American Chemical Society.
To further potentiate therapeutic efficacy and safety, it is advisable to develop delivery systems with controlled drug release property. Zhan et al. (2019) have skillfully designed and fabricated a thermal-triggered PS release system adopting DNA complementary base pairs. In their work, adenine (A) was firstly modified on the surface of PDA NPs via carbodiimide reaction. Then, thymine (T)-ZnPc spontaneously bound to the surface of A-PDA by simple mixing. Upon 808-nm laser irradiation, the photothermal effect of PDA induced the destruction of A–T bonds, leading to the release of ZnPc. When irradiated with a 665-nm laser, ROS was generated from ZnPc. Based on the characteristics of the tumor microenvironment (TME), the introduction of responsive chemical/biological bonds to construct a TME-responsive (pH-, GSH-, enzyme-responsive) release nanoplatform will greatly improve drug delivery efficiency and reduce side effects.
Chemical conjugation strategy is based primarily on the rich functional groups on PDA; any PS with one of these groups, amine, carboxylic, and thiol, is able to be directly combined with PDA. However, there remain some issues to be addressed. For instance, chemical conjugation may affect the surface properties of PDA, such as hydrophilicity/hydrophobicity, roughness, and surface charge, which will hinder cellular endocytosis. Due to the incomplete reaction and restrained reaction sites, the load efficiency of PSs is relatively low, limiting potent antitumor efficacy of PDT.
Physical Absorption Strategy
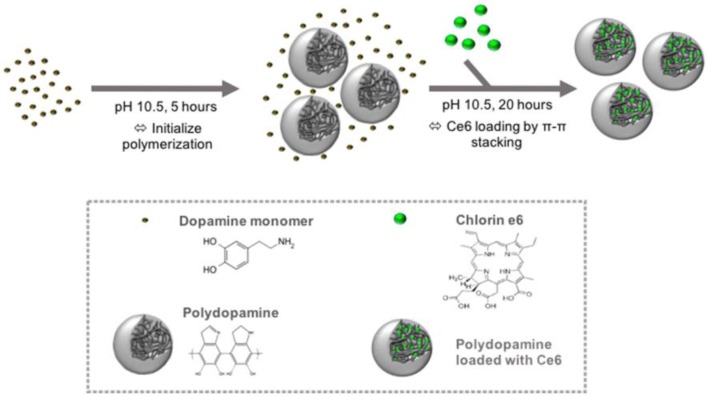
The fabrication of PDA NPs involves physical self-assembly, which occurs due to the non-covalent interactions (Dong et al., 2018), including π-π stacking, electrostatic interaction, and hydrogen bonding (Dreyer et al., 2012). Compared with chemical conjugation, it is easier to load PSs onto PDA NPs by non-covalent bonding, since no activation energy is needed in the binding process and the physical absorption is usually carried out in a mild condition. PDA possesses superior capability to load various drugs (Wang X. et al., 2016) or PSs bearing aromatic groups. For instance, Poinard et al. (2018) reported a two-step method to load Ce6 in the polymer matrix of PDA NPs (Figure 3). As expected, the π-π stacking interaction between PDA and Ce6 enabled a prolonged PSs release and enhanced Ce6 stability. Unlike the chemical conjugation, in this system, after Ce6 was loaded onto PDA, the surface properties of PDA had not changed significantly, which was beneficial for cellular uptake. At present, there are many similar studies on loading PSs onto PDA by π-π stacking interactions, such as PDA@IR820 (Wang et al., 2018), PDA@curcumin/Ce6 (Yu et al., 2017), GNR@PDA@MB (Wang S. et al., 2016), etc. Further, to strengthen the specificity of PSs to tumor sites, Han et al. (2016) used PDA NPs as cores and then shielded PDA core with a PS-conjugated hyaluronic acid (HA) coating (PDA@HA-PSs). PDA NPs are thus used as a fluorescence quencher while HA is used as a targeting moiety. In tumor sites, the HA-PS shell was degraded by hyaluronidase (HAase), thereby separating free PSs and enabling the fluorescence recovery and singlet oxygen generation.
Figure 3.
Schematic of the preparation of PDA NPs and loading of hydrophobic PS Ce6 into the polymer matrix of PDA NPs. Reproduced with permission from Poinard et al. (2018). Copyright 2018 American Chemical Society.
The hypoxic environment of tumors represents the biggest obstacle to PDT (Chen et al., 2015). Inspired by the oxygen-carrying properties of red blood cells (RBCs), Zhang et al. (Liu et al., 2018) have skillfully encapsulated hemoglobin (Hb)- and MB (a PS)-loaded PDA NPs into the recombined RBC membranes. Due to the identical origin of RBC membranes, the obtained nanoplatform exhibits outstanding biocompatibility and low immune responses. Importantly, PDA plays a pivotal role, not only as a nanocarrier to load MB but also as an antioxidative factor to protect oxygen-carrying Hb from the oxidation damage during the circulation.
The physical absorption strategy is rather straightforward and does not require any chemical intervention. Moreover, PDA can simultaneously load multiple drugs through non-covalent conjugation to achieve combination therapy. The drug-loaded PDA-based nanocarriers exhibited great drug retaining capability under physiological conditions and could rapidly release the loaded drugs in near-infrared light/low pH environment. However, the reliability of non-covalent bonding and the load efficiency of PSs still need further exploration and improvement.
Encapsulation Strategy
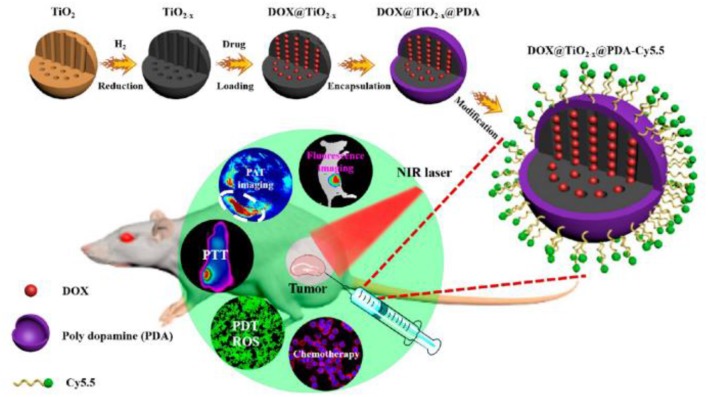
Under weak alkaline condition, DA can undergo simple oxidative self-polymerization reaction, and the generated PDA tends to spontaneously deposit on the surface of various materials (Lee et al., 2007). PDA coating is biocompatible and stable in vivo, rendering it an ideal platform for biomedical applications. Furthermore, the introduction of PDA shell endows the materials with more functions. Owing to these outstanding features, PDA-based encapsulation strategy has been widely adopted in PDT. For example, Guo et al. (2017) reported a TiO2−x based nanoplatform for dual modal imaging and NIR-triggered chem/photodynamic/photothermal combination therapy (Figure 4). The TiO2−x NPs possessed strong NIR absorption and could induce both hyperthermia and ROS under NIR irradiation. PDA coating behaved as a gatekeeper to avoid premature drug release and achieve a controlled drug release under acidic tumor environment and high temperature.
Figure 4.
Schematic illustration of the synthetic method and bioimaging-guided triple therapy. Reproduced with permission from Guo et al. (2017). Copyright 2017 American Chemical Society.
Indeed, most of the PSs are activated by visible or UV light, which has poor tissue penetration capability, thereby limiting their application in deep-tissue PDT (Wang et al., 2013b). Using NIR-excitable upconversion nanoparticles (UCNPs) as energy donor has been demonstrated to be a valid solution (Wang et al., 2013a). To this end, Cen et al. (2017) designed and prepared a core–shell–shell multifunctional UCNP@SiO2-MB@PDA nanoplatform for mRNA detection and PDT/PTT combination therapy. In this nanoplatform, PSs were entrapped in SiO2 shell, and the outer PDA shell was constructed through an in situ self-polymerization process. Remarkably, the photodynamic performance excited by a 980-nm NIR laser is attributed to the UCNP core. Besides, fluorescently labeled hpDNA was absorbed on PDA shell for sensing tumor-related mRNAs and discriminating cancer cells from normal cells.
To sum up, PDA-based encapsulation strategy is capable of preventing the premature release of PSs in blood circulation effectively, and at the same time, the PDA shell can serve as an active platform for further functionalization, thus assisting cancer PDT. Nevertheless, the PDA shell may hinder the electron transfer of PSs, which will affect the generation of ROS. Furthermore, for organic dye-based PSs, it is usually necessary to introduce a matrix material for assistance, while PDA is only used as a shell. Constructing hollow/capsule PDA NPs to load PSs may be a better method. Besides, the shell thickness of PDA should be tailored well. Due to the broad absorption toward wide range of light, a very thick PDA shell will impede the generation of ROS while a very thin PDA leads to premature leakage of encapsulated PSs.
Summary and Outlook
PDA-based nanocarriers have a broad application in the field of nanomedicine due to its appealing properties, and the simple construction process gives it great potential for commercial-scale production. In this Mini-Review, we summarized the recent advances of PDA as PS nanocarriers, which are summarized in Table 1. Based on different interactions between PDA and PSs, we divided these systems into three aspects. Considering that PDA is full of functional groups, we first reviewed the studies on PS binding to PDA via chemical bond. Moreover, the PSs rich in aromatic ring easily absorb on the surface of PDA through physical absorption. For PDA-based encapsulation strategy, PDA serves both as a biocompatible coating and a versatile nanoplatform. Different strategies have their own merits and limitations. The chemical conjugation strategy binds PSs to PDA firmly, but the surface properties of PDA will change, which may affect the blood circulation and cellular uptake of NPs. Although the method of physical absorption is quite simple, the loading of PSs strongly depends on the surfaces of PDA. While encapsulation strategy can enhance the stability and functionality of PSs, at the same time, strong π-π stacking will lead to fluorescence quenching and hamper the production of ROS. Generally, different strategies are adopted to construct nanocarriers according to different characteristics of PSs.
Table 1.
A summary of PDT based on PDA nanostructures.
| The way PDA combines with PSs | Structure | Size (nm) | Laser parameters | Therapy | Remarks | References |
|---|---|---|---|---|---|---|
| Chemical conjugation | PDA-Ce6 | ~49 | 670 nm, 5 min, 50 mW.cm−2 | PDT/PTT | Enhanced stability and therapeutic effect | Zhang et al., 2015 |
| rGO@PDA-FA-C60 | Sheet | Xe lamp, 15 min, 2 W.cm−2 | PDT/PTT | Excited by a single light | Hu et al., 2014 | |
| Mn3O4@ PDA-GQD | ~100 | 670 nm, 30 min, 4 mW.cm−2 | PDT | Dual mode imaging-guided PDT | Nafiujjaman et al., 2015 | |
| PDA-A = T-ZnPc | ~88 | 665 nm, 15 min, LED: 5 W | PDT/PTT | DNA pairing rules, controlled release | Zhan et al., 2019 | |
| Physical absorption | Ce6-PDA (load) | ~142 | 665 nm,15 min, 250 mW.cm−2 | PDT/PTT | Facile two-step method | Poinard et al., 2018 |
| Ce6@CaCO3-PDA-PEG | ~168 | 660 nm,1 h, 5 mW.cm−2 | PDT | pH sensitivity, biomineralization | Dong et al., 2018 | |
| PDA-PEG@IR820/Fe3+ | ~81 | 808 nm, 10 min, 1.0 W.cm−2 | PDT/PTT | Dual imaging and dual therapy | Wang et al., 2018 | |
| GNR@PDA-MB | ~80/50 | 671 nm, 10 min, 30 mW.cm−2 | PDT/PTT | A promising drug carrier, theranostic | Wang S. et al., 2016 | |
| Encapsulation strategy | UCNP@SiO2-MB@PDA | ~38 | 980 nm,10 min, 1.0 W.cm−2 | PDT/PTT | 980-nm laser for PDT, mRNA target | Cen et al., 2017 |
| DOX@TiO2−X@PDA-Cy5.5 | ~637 | 808 nm, 10 min, 1.0 W.cm−2 | PDT/Chem/PTT | Triple therapy, NIR/pH-triggered drug release | Guo et al., 2017 | |
| MNPs@hy-PDA-lac | ~305 | LED 600 nm, 30 min, 8.6 mW.cm−2 | PDT | Good dispersibility, targeting ability | Shao et al., 2018 | |
| LAP/ICG@PDA-RGD | ~59 | 808 nm, 5 min, 1.2 W.cm−2 | PDT/PTT | High encapsulation efficiency | Xu et al., 2018 |
Although significant efforts have been devoted to develop PDA-based nanocarriers for PDT, there are still many crucial issues that need to be addressed. At the mechanism level, the specific structure and polymerization mechanism of PDA is still elusive, limiting rational design and performance. Because of the limited loading sites of PDA, the load efficiency and stability of PSs on PDA need to be enhanced. Moreover, the strong reactivity of PDA is somewhat favorable, but it may have a negative effect in the organism. The long-term toxicity of PDA-based nanocarriers during the circulation and retention in the organism should be further evaluated. With the joint efforts of scientists, we believe that these issues will be addressed in the near future.
Author Contributions
The manuscript was conceived and prepared by ZL and YX. YX collected papers and contributed to paper writing. ZX and ZL helped to revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by grants from the National Science Foundation of China (Grant Nos. 31700867 and 51573039) and the Scientific Research Foundation of Huazhong University of Science and Technology (Grant No. 3004170130). Program for HUST Academic Frontier Youth Team (Grant No. 2018QYTD01).
References
- Agostinis P., Berg K., Cengel K. A., Foster T. H., Girotti A. W., Gollnick S. O., et al. (2011). Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 61, 250–281. 10.3322/caac.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto G. M., Bernegossi J., de Freitas L. M., Fontana C. R., Chorilli M. (2016). Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: a review. Molecules 21:342. 10.3390/molecules21030342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen Y., Deng W. J., Yang Y., Yu R. Q., Chu X. (2017). Core–shell–shell multifunctional nanoplatform for intracellular tumor-related mRNAs imaging and near-infrared light triggered photodynamic-photothermal synergistic therapy. Anal Chem. 89, 10321–10328. 10.1021/acs.analchem.7b02081 [DOI] [PubMed] [Google Scholar]
- Chen H., Tian J., He W., Guo Z. (2015). H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 137, 1539–1547. 10.1021/ja511420n [DOI] [PubMed] [Google Scholar]
- Dean M., Fojo T., Bates S. (2005). Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284. 10.1038/nrc1590 [DOI] [PubMed] [Google Scholar]
- Dolmans D. E., Fukumura D., Jain R. K. (2003). Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387. 10.1038/nrc1071 [DOI] [PubMed] [Google Scholar]
- Dong Z., Feng L., Hao Y., Chen M., Gao M., Chao Y., et al. (2018). Synthesis of hollow biomineralized CaCO3-polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J. Am. Chem. Soc. 140, 2165–2178. 10.1021/jacs.7b11036 [DOI] [PubMed] [Google Scholar]
- Dreyer D. R., Miller D. J., Freeman B. D., Paul D. R., Bielawski C. W. (2012). Elucidating the structure of poly(dopamine). Langmuir 28, 6428–6435. 10.1021/la204831b [DOI] [PubMed] [Google Scholar]
- Guo W., Wang F., Ding D., Song C., Guo C., Liu S. (2017). TiO2–x based nanoplatform for bimodal cancer imaging and NIR-triggered chem/photodynamic/photothermal combination therapy. Chem. Mat. 29, 9262–9274. 10.1021/acs.chemmater.7b03241 [DOI] [Google Scholar]
- Han J., Park W., Park S. J., Na K. (2016). Photosensitizer-conjugated hyaluronic acid-shielded polydopamine nanoparticles for targeted photomediated tumor therapy. ACS Appl. Mater. Interfaces 8, 7739–7747. 10.1021/acsami.6b01664 [DOI] [PubMed] [Google Scholar]
- Hu H., Li Y., Zhou Q., Ao Y., Yu C., Wan Y., et al. (2016). Redox-sensitive hydroxyethyl starch-doxorubicin conjugate for tumor targeted drug delivery. ACS Appl. Mater. Interfaces 8, 30833–30844. 10.1021/acsami.6b11932 [DOI] [PubMed] [Google Scholar]
- Hu Z., Zhao F., Wang Y., Huang Y., Chen L., Li N., et al. (2014). Facile fabrication of a C60-polydopamine-graphene nanohybrid for single light induced photothermal and photodynamic therapy. Chem. Commun. 50, 10815–10818. 10.1039/C4CC04416A [DOI] [PubMed] [Google Scholar]
- Jin C. S., Lovell J. F., Chen J., Zheng G. (2013). Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano 7, 2541–2550. 10.1021/nn3058642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. J., Lantz P. M., Janz N. K., Fagerlin A., Schwartz K., Liu L., et al. (2005). Patient involvement in surgery treatment decisions for breast cancer. J. Clin. Oncol. 23, 5526–5533. 10.1200/JCO.2005.06.217 [DOI] [PubMed] [Google Scholar]
- Lee H., Dellatore S. M., Miller W. M., Messersmith P. B. (2007). Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430. 10.1126/science.1147241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Rho J., Messersmith P. B. (2009). Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 21, 431–434. 10.1002/adma.200801222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang C., Cheng L., Gong H., Yin S., Gong Q., et al. (2013). PEG-functionalized iron oxide nanoclusters loaded with chlorin e6 for targeted, NIR light induced, photodynamic therapy. Biomaterials 34, 9160–9170. 10.1016/j.biomaterials.2013.08.041 [DOI] [PubMed] [Google Scholar]
- Lismont M., Dreesen L., Wuttke S. (2017). Metal–organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv. Funct. Mat. 27 (14). 10.1002/adfm.201606314 [DOI] [Google Scholar]
- Liu W. L., Liu T., Zou M. Z., Yu W. Y., Li C. X., He Z. Y., et al. (2018). Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 30:e1802006. 10.1002/adma.201802006 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ai K., Lu L. (2014). Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 114, 5057–5115. 10.1021/cr400407a [DOI] [PubMed] [Google Scholar]
- Nafiujjaman M., Nurunnabi M., Kang S.-H., Reeck G. R., Khan H. A., Lee Y.-K. (2015). Ternary graphene quantum dot–polydopamine–Mn3O4 nanoparticles for optical imaging guided photodynamic therapy and T1-weighted magnetic resonance imaging. J. Mater. Chem. B 3, 5815–5823. 10.1039/c5tb00479a [DOI] [PubMed] [Google Scholar]
- Park J., Brust T. F., Lee H. J., Lee S. C., Watts V. J., Yeo Y. (2014). Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 8, 3347–3356. 10.1021/nn405809c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinard B., Neo S. Z. Y., Yeo E. L. L., Heng H. P. S., Neoh K. G., Kah J. C. Y. (2018). Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl. Mater. Interfaces 10, 21125–21136. 10.1021/acsami.8b04799 [DOI] [PubMed] [Google Scholar]
- Rong P., Yang K., Srivastan A., Kiesewetter D. O., Yue X., Wang F., et al. (2014). Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics 4, 229–239. 10.7150/thno.8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D., McBride W. H. (2015). Opportunities and challenges of radiotherapy for treating cancer. Nat Rev. Clin. Oncol. 12, 527–540. 10.1038/nrclinonc.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S. H., Kim B. M., Joe A., Han H. W., Chen X., Cheng Z., et al. (2014). NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod{{{at}}}SiO2 nanocomposites. Biomaterials 35, 3309–3318. 10.1016/j.biomaterials.2013.12.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C., Shang K., Xu H., Zhang Y., Pei Z., Pei Y. (2018). Facile fabrication of hypericin-entrapped glyconanoparticles for targeted photodynamic therapy. Int J Nanomedicine 13, 4319–4331. 10.2147/IJN.S161262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh C. S., Hardy C. W., Yu D. S., Fernandez B., Zhang H. (2014). Indocyanine green loaded liposome nanocarriers for photodynamic therapy using human triple negative breast cancer cells. Photodiagn. Photodyn Ther. 11, 193–203. 10.1016/j.pdpdt.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Teng I. T., Chang Y. J., Wang L. S., Lu H. Y., Wu L. C., Yang C. M., et al. (2013). Phospholipid-functionalized mesoporous silica nanocarriers for selective photodynamic therapy of cancer. Biomaterials 34, 7462–7470. 10.1016/j.biomaterials.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Wang C., Cheng L., Liu Y., Wang X., Ma X., Deng Z., et al. (2013b). Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light. Adv. Funct. Mat. 23, 3077–3086. 10.1002/adfm.201202992 [DOI] [Google Scholar]
- Wang C., Cheng L., Liu Z. (2013a). Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 3, 317–330. 10.7150/thno.5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Guo Y., Hu J., Li W., Kang Y., Cao Y., et al. (2018). Development of multifunctional polydopamine nanoparticles as a theranostic nanoplatform against cancer cells. Langmuir 34, 9516–9524. 10.1021/acs.langmuir.8b01769 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhao X., Wang S., Qian J., He S. (2016). Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS Appl. Mater. Interfaces 8, 24368–24384. 10.1021/acsami.6b05907 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang J., Wang Y., Wang C., Xiao J., Zhang Q., et al. (2016). Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 81, 114–124. 10.1016/j.biomaterials.2015.11.037 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Zheng X., Bu W., Ge W., Zhang S., Chen F., et al. (2013). A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy. J. Am. Chem. Soc. 135, 13041–13048. 10.1021/ja404985w [DOI] [PubMed] [Google Scholar]
- Xu F., Liu M., Li X., Xiong Z., Cao X., Shi X., et al. (2018). Loading of indocyanine green within polydopamine-coated laponite nanodisks for targeted cancer photothermal and photodynamic therapy. Nanomaterials 8, 347. 10.3390/nano8050347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang D., Zhu J., Xue L., Ou C., Wang W., et al. (2019). Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem. Sci. 10, 3779–3785. 10.1039/C8SC04844D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. J., Tang X., He J. K., Yi X., Xu G. Y., Tian L. L., et al. (2017). Polydopamine nanoparticle as a multifunctional nanocarrier for combined radiophotodynamic therapy of cancer. Part. Part. Syst. Charact. 34:1600296 10.1002/ppsc.201600296 [DOI] [Google Scholar]
- Zhan Q., Shi X., Zhou J., Zhou L., Wei S. (2019). Drug-controlled release based on complementary base pairing rules for photodynamic–photothermal synergistic tumor treatment. Small 15:e1803926. 10.1002/smll.201803926 [DOI] [PubMed] [Google Scholar]
- Zhang D., Wu M., Zeng Y., Wu L., Wang Q., Han X., et al. (2015). Chlorin e6 conjugated poly(dopamine) nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced cancer therapy. ACS Appl. Mater. Interfaces 7, 8176–8187. 10.1021/acsami.5b01027 [DOI] [PubMed] [Google Scholar]