Abstract
Endometrial cancers are the most common gynecologic malignancies. The staging of endometrial cancer has evolved from a clinical-based system to a comprehensive surgical-pathologic approach that allows for better risk stratification and treatment planning. Over the past few years, the use of the National Comprehensive Cancer Network sentinel lymph node mapping algorithm for the surgical staging of endometrial cancer has gained significant acceptance and is now commonly applied in many practices. However, the pathologic evaluation of prognostic factors is beset by challenges, including the reproducibility of histologic classification and International Federation of Gynecology and Obstetrics grading, as well as the questionable clinical significance of low-volume tumor in sentinel lymph nodes. With the revelation of major genomic classes of endometrial cancer comes the potential for improved, reproducible, and prognostically relevant classification schemes, which integrate traditional pathologic parameters with genomic findings, to aid in treatment decisions. The pathologic identification of new variants of endometrial cancer, such as undifferentiated carcinoma, continue to advance the phenotypic spectrum of these tumors, spurring genomic and functional studies to further characterize their mechanistic underpinnings and potentially reveal new avenues for treatment. In the era of precision medicine, pathologic assessment of biomarkers (such as mismatch repair proteins) and recognition of phenotypes that are amenable to specific targeted therapies (such as POLE-mutated tumors) have become integral to the management of women with endometrial carcinoma.
Keywords: Endometrial cancer; Genomics; Pathology; Precision oncology; Prognosis; Prognostic factors; Risk stratification; Sentinel lymph node biopsy, Staging; Targeted therapy
1. INTRODUCTION
Endometrial cancers comprise more than half of all gynecologic cancer diagnoses in the United States.1 Pathologic evaluation is an important element of the management of women with this disease. As management approaches continue to evolve, as a result of reported data from clinicopathologic and molecular genetic studies, pathology will continue to play a central role in diagnosis, prognostic assessment, and treatment planning.
2. EVOLUTION OF SURGICAL-PATHOLOGIC STAGING OF ENDOMETRIAL CANCER
Most patients with endometrial cancer present with abnormal bleeding, the investigation of which involves pelvic ultrasonography with endometrial biopsy/curettage. Histopathologic evaluation of the biopsy/curettage specimen confirms the diagnosis.2,3 Early staging schemes were essentially based on clinical findings, but since 1988, a more accurate surgical-pathologic staging approach has been used (Table 1).4
Table 1.
Evolution of FIGO endometrial cancer staging classification over time4
| FIGO staging, 1961–1971 | FIGO staging, 1988 | FIGO staging, 2009 | |
|---|---|---|---|
| Basis | Clinical | Surgical-Pathologic | Surgical-Pathologic |
| Stage 0 | Histological findings suspicious for malignancy, but not proven | ||
| Stage I | Carcinoma confined to uterine corpus *IA: Length of uterine cavity is ≤8 cm *IB: Length of uterine cavity is >8 cm |
IA: Tumor limited to endometrium IB: Invasion limited to less than half of myometrium IC: invasion of half or greater of myometrium |
Tumor confined to corpus uteri IA: No myometrial invasion or invasion to less than half of myometrium; endocervical glandular involvement only IB: Invasion of half or greater of myometrium |
| Stage II | Carcinoma involves uterine corpus and cervix | IIA: Endocervical glandular involvement only IIB: Cervical stromal invasion |
Tumor invades cervical stroma but does not extend beyond uterus |
| Stage III | Carcinoma extends outside uterus but not outside the pelvis | IIIA: Tumor invades serosa and/or adnexa and/or positive peritoneal cytology IIIB: Vaginal metastases IIIC: Metastases to pelvis and/or para-aortic lymph nodes |
Local and/or regional spread of tumor IIIA: Tumor invades serosa of corpus uteri and/or adnexa IIIB: Vaginal and/or parametrial involvement IIIC1: Positive pelvic lymph nodes IIIC2: Positive para-aortic lymph nodes |
| Stage IV | Carcinoma extends outside the true pelvis or obviously invades mucosa of bladder or rectum | IVA: Tumor invasion of bladder and/or bowel mucosa IVB: Distant metastases including intra-abdominal and/or inguinal lymph nodes |
IVA: Tumor invasion of bladder and/or bowel mucosa IVB: Distant metastases including intra-abdominal and/or inguinal lymph nodes |
| Histologic grade |
*Stage I tumors also subgrouped according to histologic type: G1: highly differentiated adenocarcinomas G2: differentiated adenocarcinomas with partly solid areas G3: predominately solid or entirely undifferentiated carcinomas) |
Stage is irrespective of grade G1: ≤5% of non-squamous or non-morular solid growth pattern G2: 6–50% of non-squamous or non-morular solid growth pattern G3: >50% of non-squamous or non-morular solid growth pattern Notable nuclear atypia, inappropriate for architectural grade, raises the grade of a grade 1 or 2 tumor by 1 |
Stage is irrespective of grade G1: ≤5% of non-squamous or non-morular solid growth pattern G2: 6–50% of non-squamous or non-morular solid growth pattern G3: >50% of non-squamous or non-morular solid growth pattern Notable nuclear atypia, inappropriate for architectural grade, raises the grade of a grade 1 or 2 tumor by 1 |
FIGO – International Federation of Gynecology and Obstetrics
Modifications in 1971
2.1. Comprehensive surgical staging
Preoperatively, staging is performed to estimate recurrence risk, and is based on the imaging evaluation of myometrial invasion, cervical involvement, and lymph node metastasis. Magnetic resonance imaging and transvaginal ultrasonography are effective modalities for the assessment of myometrial and cervical invasion, but imaging is poor at detecting lymph node metastases. Accurate staging, therefore, relies on comprehensive surgical staging to obtain specimens that can be thoroughly examined by pathologists for key prognostic factors, including myometrial invasion; cervical involvement; adnexal, peritoneal, and lymph node metastasis; histologic type and grade; and lymphovascular space involvement. Risk stratification systems incorporating pathologic prognostic factors are crucial in guiding clinical management decisions.5–7
Traditional surgical staging of endometrial cancer involves removing the uterus, cervix, adnexa, pelvic and para-aortic lymph nodes, and obtaining pelvic washings, followed by pathologic examination. This allows accurate diagnosis, identification of disease extent, prognostic assessment, and selection of patients for adjuvant therapy. The advantage of surgical-pathologic staging over clinical staging was reported in Gynecologic Oncology Group (GOG) study 33, which showed that 9% of patients with clinically stage I disease had pelvic nodal involvement, 6% had para-aortic lymphadenopathy, 5% had adnexal involvement, and 6% had other extrauterine metastases.8 Comprehensive surgical staging also identifies patients with advanced-stage disease who require radiation therapy and/or chemotherapy; low-stage patients with high-risk features (high-grade tumors, deep myometrial invasion, lymphovascular space involvement) who should receive adjuvant treatment; and patients without high-risk features who may safely be spared adjuvant chemoradiation and its attendant morbidity.8,9
Despite the benefits of a surgical-pathologic staging system, the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system has its limitations, particularly in the setting of corpus-confined carcinoma. Since the current staging scheme applies uniformly to all cases, irrespective of staging adequacy or tumor type, clinical outcomes are highly variable. For example, using the Memorial Sloan Kettering Cancer Center Endometrial Cancer Nomogram,10 a 65-year-old woman with FIGO grade 1 endometrioid carcinoma, middle-third myometrial invasion, and a benign lymphadenectomy would have an estimated 5-year overall survival rate of 92%, whereas a 65-year-old woman with serous carcinoma, middle-third myometrial invasion, and no lymph node evaluation would have an estimated 5-year overall survival rate of only 64%. This observation prompted a proposal to amend the current FIGO staging scheme.11
Another controversy related to surgical staging in endometrial cancer is the role of para-aortic lymph node dissection. It has been shown that the rate of isolated para-aortic lymph node involvement in the absence of pelvic lymph node involvement is very low (<2%).12 Patients with high-risk disease have a higher frequency of para-aortic lymph node involvement, suggesting that para-aortic lymphadenectomy should be performed as part of surgical staging in these patients.13 However, a classification and regression tree analysis found that overall survival was predicted by FIGO stage and grade (a binary system of low- vs. high-grade) but not by para-aortic lymph node status,14 advocating for an approach with less extensive lymph node dissection.
2.2. Sentinel lymph node mapping for endometrial cancer
Approximately 6–23% of women with endometrial cancer who undergo pelvic lymphadenectomy develop long-term morbidity, such as lymphedema.15,16 This is likely an underestimation, as patient surveys have indicated leg lymphedema rates as high as 20–40%.16 To reduce this morbidity and to improve the detection of lymph node metastases, a sentinel lymph node (SLN) mapping approach to the management of endometrial cancer was introduced and has been incorporated as an option in the National Comprehensive Cancer Network (NCCN) guidelines since 2014.17 The goal of SLN mapping is to initially target and assess the lymph nodes most likely to be involved by metastatic cancer (i.e, the sentinel, or first, nodes in the path of lymphatic flow away from the tumor), thereby limiting the extent of surgery and morbidity associated with extensive lymphadenectomy. This technique identifies SLNs in approximately 85% of patients, of whom 12% have positive SLNs.18 Detailed pathologic examination of SLNs (ultrastaging), which includes the assessment of multiple sections of the SLNs using routine stains as well immunohistochemistry for epithelial markers,19 allows for the detection of low-volume metastases that can be missed with standard techniques.18,19 SLN assessment also can refine surgical-pathologic stage; for example, in one study, SLN biopsy results upstaged 10% of patients with low-risk and 15% of those with intermediate-risk endometrial cancer,20 with implications for adjuvant treatment planning.
2.3. Challenges in the pathologic evaluation of critical prognostic factors
Assignment of histotype is straightforward in the majority of endometrial cancers, but can be exceedingly difficult in some high-grade tumors exhibiting morphologic ambiguity.21,22 There are several risk-group stratification systems based on surgical-pathologic staging of endometrial cancer7,9,13,23–27; however, the poor reproducibility of histotype and grade classification21,22,28–30 presents challenges for accurate prognostic assessment,27 selecting optimal treatment, determining eligibility for clinical trials, and for comparison of treatment interventions between studies. Integrating pathologic parameters with findings of molecular genetic analyses (described below) may provide a more accurate and prognostically relevant classification of these tumors.31–34
Tumor grade and histotype designation in preoperative biopsy and curettage specimens may be incorrect;35 for example, in one study, 1% of preoperative grade 1 endometrioid adenocarcinomas were upgraded to grade 2–3 cancers, and a further 1% harbored a high-risk histotype (serous or clear cell carcinoma) in the hysterectomy specimens.36 Similarly, the undifferentiated component of a dedifferentiated carcinoma, which often lies deep to the well-differentiated component, may not be sampled in a biopsy or curettage specimen.37,38 These sampling errors are more likely to occur with small-volume samples. In these cases, there is a potential for surgical understaging due to the failure to detect high-risk features (high-grade tumor component) in the preoperative specimens.
Although SLN biopsy offers advantages of accurate staging and reduced morbidity from the avoidance of an unnecessary lymphadenectomy, its long-term survival benefits, if any, are unknown.3 Furthermore, the clinical significance of small volumes of tumor (e.g., isolated tumor cells) in SLNs is unknown, and further studies with long-term follow-up are ongoing.
3. EVOLVING DIAGNOSTIC PARADIGMS IN ENDOMETRIAL CANCER AND THEIR CLINICAL IMPLICATIONS
3.1. Molecular genetic findings and integrated pathologic-genetic classification of endometrial cancer
The Cancer Genome Atlas (TCGA) study of endometrioid and serous carcinomas found mutations in several genes (e.g., TP53, PTEN, PIK3CA, PPP2R1A, FBXW7, CTNNB1, KRAS and POLE), but more importantly, identified four major genomically defined classes of tumor (POLE-ultramutated, microsatellite instability-hypermutated [MSI-H], copy-number-low, and copy-number-high). These groups were also clinically significant, as they correlated with progression-free survival; patients with POLE-mutated tumors had an excellent prognosis and those with copy-number-high tumors had poor outcomes, while the MSI-H and copy-number-low groups had intermediate prognoses.34 DNA ploidy was recently shown to differ between TCGA groups, and was highest in the p53-aberrant group. Abnormal DNA ploidy was associated with higher grade, non-endometrioid histotype, and poorer survival (particularly in mismatch repair-deficient tumors).39 A recent study of endometrial clear cell carcinomas identified similar genomic classes, which were also associated with prognosis.40 Uterine carcinosarcomas also frequently harbor mutations in TP53, PTEN, PIK3CA, PPP2R1A, FBXW7, and KRAS, similar to endometrioid and serous carcinomas.41
It is also apparent that genomic classes of endometrial carcinoma are associated with phenotypes. Copy-number-high tumors, which are characterized by TP53 mutations and alterations associated with cell cycle deregulation, comprise some high-grade endometrioid adenocarcinomas and clear cell carcinomas, and all serous cancers.34,40 Copy-number-low tumors are predominantly low-grade endometrioid adenocarcinomas.34 POLE-mutated endometrial carcinomas are typically characterized by: high grade; tumor-infiltrating lymphocytes and/or peritumoral lymphocytes; morphologic heterogeneity/ambiguity; and bizarre/giant tumor cell nuclei.42,43 Endometrioid histotype is most frequent, although POLE mutations have also been reported in clear cell carcinomas,40 undifferentiated carcinomas,44 and carcinosarcomas.45 MSI-H endometrial cancers, which may be associated with germline alterations (Lynch syndrome) or sporadic aberrations, are associated with lower uterine segment location, endometrioid histology, mucinous differentiation, tumor-infiltrating lymphocytes, and peritumoral lymphocytes.46–49
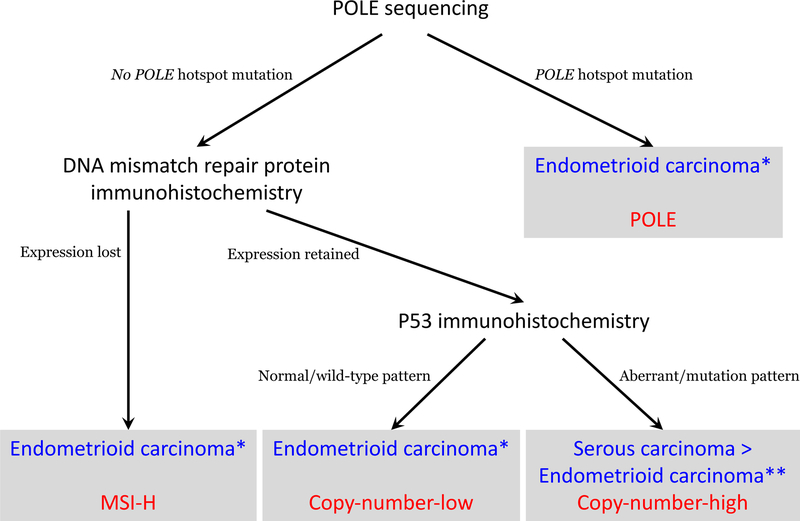
Molecular classification of endometrial cancer has been shown to be reproducible and associated with clinical outcomes.31–34 However, these algorithms do have some limitations. P53 immunohistochemistry does not correlate perfectly with TP53 copy number changes,39,40 and its use in these algorithms may therefore misclassify some copy-number-high tumors. The algorithms do not address how to categorize tumors harboring more than one classifying genomic aberration (POLE mutations, MMR-deficiency or P53-mutations) when the algorithmic components are performed in parallel rather than sequentially. The algorithms do not allow for the exploration of the significant heterogeneity seen within the copy-number-low group.50,51 Finally, in the ProMisE algorithm, DNA MMR immunohistochemistry is performed before POLE sequencing, which may result in failure to detect MMR-deficient tumors with POLE mutations, as well as their incorrect classification as MMR-deficient tumors rather than POLE-mutated tumors; as a result, our approach differs slightly in performing POLE sequencing before DNA MMR immunohistochemistry (Fig. 1). Despite these limitations, an integrated genomic-pathologic classification scheme incorporating genomic-based classifications with traditional clinicopathologic prognostic parameters (Fig. 1) represents the best available method for stratifying patients into prognostically distinct groups that may benefit from tailored treatment approaches.52
Figure 1.
Diagnostic algorithm for integrated genomic-pathologic classification of endometrial carcinomas (blue=histotyope; red=TCGA genomic class).
MSI-H: microsatellite instability high; *May also apply to clear cell carcinomas; **This algorithm does not distinguish between histotypes of TP53-mutated copy-number-high tumors, i.e., high-grade endometrioid carcinoma, serous carcinoma, or clear cell carcinoma
3.2. Molecular genetic findings in synchronous endometrial and ovarian carcinomas
The staging of patients with synchronous endometrial and ovarian carcinomas traditionally has been based on pathologic criteria to determine whether the two tumors are independent primaries (each being low-stage disease) or whether one is a metastasis from the other (high-stage disease).53,54 Two recent studies utilizing massively parallel sequencing analyses showed that the majority of synchronous endometrial and ovarian carcinomas are clonally related, and therefore, the latter scenario applies.55,56 Nevertheless, many of these patients have excellent clinical outcomes belying their apparently high stage,57 and further studies are required to determine the mechanisms underlying their indolent behavior.
3.3. Recently recognized types and variants of endometrial carcinoma
There are several recently described phenotypic variants of endometrial carcinoma, which may be associated with specific clinical phenotypes and genotype. A few examples are briefly presented.
3.3.1. Undifferentiated and Dedifferentiated carcinoma
Undifferentiated and dedifferentiated endometrial carcinomas are uncommon, highly aggressive tumors.38,58 Undifferentiated carcinoma is a monomorphic tumor composed of small- to intermediate-sized cells arranged in sheets without any obvious epithelial differentiation, which mimics lymphoma, plasmacytoma, high-grade endometrial stromal sarcoma, or small cell carcinoma.38,58 Approximately 40% of undifferentiated carcinomas are associated with a component of low-grade endometrioid adenocarcinoma; these cases are termed ‘dedifferentiated carcinomas’.37
Most undifferentiated carcinomas display immunohistochemical evidence of epithelial differentiation in the form of intense but focal EMA and cytokeratin 18 expression, along with vimentin and CD138 expression.59,60 Loss of expression of proteins involved in chromatin remodeling through SWI/SNF complexes, such as BRG-1 (the protein product of SMARCA4), INI-1 (the protein product of SMARCB1), or BAF250a (the protein product of ARID1A), may be seen.61,62 DNA mismatch repair deficiency and loss of expression of MLH1 and PMS2, mostly due to hMLH1 promoter methylation, is seen in 50–60% of tumors.63 Genomically, these tumors harbor mutations in POLE, SMARCA4, ARID1B, CTNNB1, PPP2R1A, or TP53.64
3.3.2. Corded and hyalinized endometrioid carcinomas
A subset of endometrioid adenocarcinomas (termed ‘corded and hyalinized endometrioid carcinomas [CHEC]) shows unusual morphologic features including cords of epithelioid cells, spindle cells, and hyalinized stroma that sometimes forms osteoid.65 These tumors present mainly at a low stage and have a good prognosis. Their importance lies in their recognition and distinction from endometrial carcinosarcomas, which are usually seen in older patients and are highly aggressive malignancies.41 Awareness of CHEC allows for the ready morphologic distinction from carcinosarcoma, as the spindle cell component of CHEC lacks conspicuous atypia, in contrast to the high-grade appearance of the sarcomatous component of carcinosarcomas.
3.3.3. Mesonephric-like carcinomas
Mesonephric carcinomas have long been recognized in the uterine cervix. Recent studies have identified tumors involving the uterine corpus that show morphologic and immunohistochemical similarities to the cervical tumors; the uterine tumors are termed ‘mesonephric-like carcinomas’.66,67 The uterine tumors display a uniform appearance, with tubular, solid and papillary architectural patterns, and are composed of cells with atypical, angulated and overlapping, vesicular nuclei. The tubular structures are small and may contain dense luminal eosinophilic material.66,67 Immunohistochemically, the tumors express TTF-1, as well as CD10, calretinin and GATA3, while estrogen and progesterone receptors are negative.67 Mutations in KRAS, NRAS, and chromatin remodeling genes (ARID1A, ARID1B, SMARCA4) have been reported in mesonephric carcinomas.68
3.4. Biomarkers for classification and prognostic assessment
3.4.1. Identification of molecular-prognostic subgroups
MSI-H endometrial carcinomas can be effectively identified by assessing morphologic features (described above) and DNA mismatch repair deficiencies in histologic material with immunohistochemistry using antibodies directed against MLH1, PMS2, MSH2, and MSH6.69,70 There is a high level of concordance between the results of immunohistochemistry and polymerase chain reaction-based microsatellite instability analysis.71 Immunohistochemical expression of p53 (classified as aberrant if absent or diffusely overexpressed) is associated with a poor prognosis in endometrial cancer72,73 and correlates with TP53 mutation status.74 The identification of a POLE mutation in patients with endometrial cancer (based on morphologic features of the tumor, as described above, and POLE sequencing) may help these patients avoid overtreatment given their excellent prognosis.34 POLE-mutated and MSI-H tumors are also amenable to immunotherapy (as discussed below).
Simplified diagnostic algorithms for the molecular classification of endometrial cancers into TCGA classes have been recently proposed.50,75 The ProMisE (Proactive Molecular Risk Classifier for Endometrial Cancer) algorithm involves immunohistochemistry for DNA mismatch repair proteins, sequencing of mismatch-repair-proficient tumors for POLE mutations, and immunohistochemistry for p53 in the POLE-wild-type tumors. This algorithm accurately classifies endometrial cancers as mismatch repair-deficient (MSI-H), POLE-mutated, p53-wild-type (copy-number-low) or p53-aberrant (copy-number-high),75 and has potential as a prognostic and risk stratification assay for clinical use.
3.4.2. High-grade endometrial cancers
The copy-number-high group of endometrial carcinomas identified in TCGA study includes high-grade endometrioid adenocarcinomas and serous carcinomas. The histopathologic and immunohistochemical features of these tumors may overlap considerably, leading to poor interobserver reproducibility in the histotyping of high-grade endometrial carcinomas.22,30 This poor reproducibility doubtless contributes to variability in the reported prognosis of patients with high-grade endometrioid adenocarcinoma compared to those with serous carcinoma.76–80
However, a recent study of copy-number-high endometrial carcinomas showed significant differences between high-grade endometrioid adenocarcinomas and serous carcinomas with respect to their stage distributions and sites of recurrence.81 If these differences also correlate with other differences in clinical behavior, it is important to attempt to distinguish high-grade endometrioid adenocarcinomas from serous carcinomas using available biomarkers to supplement histopathologic interpretation. No single marker is absolutely diagnostic of either histotype, and therefore, a panel of markers, including at least p53 and p16 with either ER or PTEN is recommended. p16-negative/PTEN-negative and/or ARID1A-negative/p16-negative/p53-wild-type tumors are most likely endometrioid, while serous carcinomas are more likely to be p53-aberrant/p16-positive/ER-negative.82 Tumors with discordant findings may be subjected to an expanded immunohistochemical panel that includes DNA mismatch repair proteins (MLH1, PMS2, MSH2, MSH6), loss of expression of at least one of which would support the diagnosis of endometrioid adenocarcinoma.
3.4.3. CTNNB1-mutated endometrial carcinomas
Patients with low-stage endometrial cancer without high-risk features, as described above, generally have excellent outcomes; however, a small proportion of these patients do poorly. A recent study exploring factors associated with poor outcomes in women with low-grade, early-stage endometrial carcinomas found that in patients with endometrioid adenocarcinomas, CTNNB1 mutations were found to be independent predictors of poorer recurrence-free survival.83 In this study, 84% of tumors with CTNNB1 mutations showed nuclear expression of beta catenin (the protein product of CTNNB1) by immunohistochemistry.83
3.5. Pathology and precision medicine in women with endometrial cancer
Pathologists play an important role in the development and implementation of novel therapies targeting molecular/genomic alterations in endometrial cancer. The roles of pathology in the present era of precision oncology include the following: identification of homogenous subsets of tumors, which are critical to obtain meaningful results from exploratory molecular/genomic studies seeking to identify novel targets; evaluation of expression of molecular biomarkers and their localization at the tissue level, which can assist in treatment decisions; phenotype-genotype correlations that assist identification of tumors likely to harbor specific molecular targets or that are likely to be amenable to specific therapy; and selection of suitable patients, based on their phenotypes and biomarker profiles, for entry into clinical trials of novel therapies.
3.5.1. Identification of endometrial cancers that are candidates for immunotherapy
POLE-mutated and mismatch repair-deficient tumors exhibit tumor-infiltrating lymphocytes, high levels of neoantigens, and expression of immune checkpoint regulators, such as programmed death receptor-1 (PD-1)84,85 or its ligand, PDL-1,86 which are thought to promote escape from immune surveillance. Immune checkpoint blockade with the anti-PD1 antibody pembrolizumab has shown responses in patients with POLE-mutated85 and mismatch repair-deficient endometrial cancer,87 and pembrolizumab has been approved by the FDA for metastatic cancers exhibiting mismatch repair deficiency. PDL-1 expression can be directly examined in tissues using immunohistochemistry, but the optimal methods and antibodies are yet to be standardized.88
3.5.2. Identification of endometrial cancers that are candidates for mitogen-activated protein kinase (MAPK) pathway inhibition
KRAS mutations are common in endometrial cancer,34 and are associated with mucinous differentiation.89 ERBB2 amplifications are also identified in endometrial serous carcinomas.90 KRAS is not a direct molecular therapeutic target, but the identification of tumors with MAPK pathway activation might be susceptible to therapy directed against other components of the MAPK/ERK pathway, such as members of the EGFR family.
4. CONCLUSIONS
Over the past two decades, there have been numerous ex vivo, genomic, translational, pathologic, and clinical studies that have significantly expanded our understanding of endometrial cancer. This improved understanding has led to refinements in our approach to the diagnosis and treatment of women with these tumors. As an integral part of any multidisciplinary team, pathology continues to play an important role in diagnosis and prognostic assessment, risk stratification and therapeutic decision-making, and the development and implementation of novel therapeutic agents and strategies for women with these cancers.
Acknowledgments
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Funding: Supported in part by the MSK Cancer Center Support Grant P30 CA008748.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000;89(8):1765–1772. [PubMed] [Google Scholar]
- 3.SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 2014;134(2):385–392. [DOI] [PubMed] [Google Scholar]
- 4.Boronow RC. Surgical staging of endometrial cancer: evolution, evaluation, and responsible challenge--a personal perspective. Gynecol Oncol 1997;66(2):179–189. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Rustum NR, Zhou Q, Gomez JD, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol 2010;116(3):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendifallah S, Canlorbe G, Raimond E, et al. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer 2014;110(11):2640–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong TW, Chang SJ, Paek J, Lee Y, Chun M, Ryu HS. Risk group criteria for tailoring adjuvant treatment in patients with endometrial cancer: a validation study of the Gynecologic Oncology Group criteria. J Gynecol Oncol 2015;26(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987;60(8 Suppl):2035–2041. [DOI] [PubMed] [Google Scholar]
- 9.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92(3):744–751. [DOI] [PubMed] [Google Scholar]
- 10.Memorial Sloan Kettering Cancer Center Endometrial Cancer Nomogram: https://www.mskcc.org/nomograms/endometrial; Accessed 1 October 2017.
- 11.Barlin JN, Soslow RA, Lutz M, et al. Redefining stage I endometrial cancer: incorporating histology, a binary grading system, myometrial invasion, and lymph node assessment. Int J Gynecol Cancer 2013;23(9):1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol 2009;115(2):236–238. [DOI] [PubMed] [Google Scholar]
- 13.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol 2008;109(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlin JN, Zhou Q, St Clair CM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol 2013;130(3):452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 2006;103(2):714–718. [DOI] [PubMed] [Google Scholar]
- 16.Yost KJ, Cheville AL, Al-Hilli MM, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol 2014;124(2 Pt 1):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms (version 1.2018) Vol. 2018; 2017. [DOI] [PubMed] [Google Scholar]
- 18.Khoury-Collado F, St Clair C, Abu-Rustum NR. Sentinel Lymph Node Mapping in Endometrial Cancer: An Update. Oncologist 2016;21(4):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer 2013;23(5):964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballester M, Dubernard G, Lecuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol 2011;12(5):469–476. [DOI] [PubMed] [Google Scholar]
- 21.Soslow RA. Endometrial carcinomas with ambiguous features. Semin Diagn Pathol 2010;27(4):261–273. [DOI] [PubMed] [Google Scholar]
- 22.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013;37(6):874–881. [DOI] [PubMed] [Google Scholar]
- 23.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355(9213):1404–1411. [DOI] [PubMed] [Google Scholar]
- 24.Kwon JS, Qiu F, Saskin R, Carey MS. Are uterine risk factors more important than nodal status in predicting survival in endometrial cancer? Obstet Gynecol 2009;114(4):736–743. [DOI] [PubMed] [Google Scholar]
- 25.Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi33–38. [DOI] [PubMed] [Google Scholar]
- 26.AlHilli MM, Mariani A, Bakkum-Gamez JN, et al. Risk-scoring models for individualized prediction of overall survival in low-grade and high-grade endometrial cancer. Gynecol Oncol 2014;133(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendifallah S, Canlorbe G, Collinet P, et al. Just how accurate are the major risk stratification systems for early-stage endometrial cancer? Br J Cancer 2015;112(5):793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan H, Semaan A, Bandyopadhyay S, et al. Prognosis and reproducibility of new and existing binary grading systems for endometrial carcinoma compared to FIGO grading in hysterectomy specimens. Int J Gynecol Cancer 2011;21(4):654–660. [DOI] [PubMed] [Google Scholar]
- 29.Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013;26(12):1594–1604. [DOI] [PubMed] [Google Scholar]
- 30.Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013;37(9):1421–1432. [DOI] [PubMed] [Google Scholar]
- 31.Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A 2009;106(12):4834–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Gallo M, O’Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 2012;44(12):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 2012;228(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchard J, Hirschowitz L. Concordance of FIGO grade of endometrial adenocarcinomas in biopsy and hysterectomy specimens. Histopathology 2003;42(4):372–378. [DOI] [PubMed] [Google Scholar]
- 36.Leitao MM Jr., Kehoe S, Barakat RR, et al. Comparison of D&C and office endometrial biopsy accuracy in patients with FIGO grade 1 endometrial adenocarcinoma. Gynecol Oncol 2009;113(1):105–108. [DOI] [PubMed] [Google Scholar]
- 37.Giordano G, D’Adda T, Bottarelli L, et al. Two cases of low-grade endometriod carcinoma associated with undifferentiated carcinoma of the uterus (dedifferentiated carcinoma): a molecular study. Pathol Oncol Res 2012;18(2):523–528. [DOI] [PubMed] [Google Scholar]
- 38.Tafe LJ, Garg K, Chew I, Tornos C, Soslow RA. Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. Mod Pathol 2010;23(6):781–789. [DOI] [PubMed] [Google Scholar]
- 39.Proctor L, Pradhan M, Leung S, et al. Assessment of DNA Ploidy in the ProMisE molecular subgroups of endometrial cancer. Gynecol Oncol 2017;146(3):596–602. [DOI] [PubMed] [Google Scholar]
- 40.DeLair DF, Burke KA, Selenica P, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol 2017. [DOI] [PMC free article] [PubMed]
- 41.Cherniack AD, Shen H, Walter V, et al. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017;31(3):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol 2015;28(4):505–514. [DOI] [PubMed] [Google Scholar]
- 43.Bakhsh S, Kinloch M, Hoang LN, et al. Histopathological features of endometrial carcinomas associated with POLE mutations: implications for decisions about adjuvant therapy. Histopathology 2016;68(6):916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobel M, Hoang LN, Tessier-Cloutier B, et al. Undifferentiated Endometrial Carcinomas Show Frequent Loss of Core Switch/Sucrose Nonfermentable Complex Proteins. Am J Surg Pathol 2017. [DOI] [PMC free article] [PubMed]
- 45.Hoang LN, Kinloch MA, Leo JM, et al. Interobserver Agreement in Endometrial Carcinoma Histotype Diagnosis Varies Depending on The Cancer Genome Atlas (TCGA)-based Molecular Subgroup. Am J Surg Pathol 2017;41(2):245–252. [DOI] [PubMed] [Google Scholar]
- 46.Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer 2006;106(1):87–94. [DOI] [PubMed] [Google Scholar]
- 47.Shia J, Black D, Hummer AJ, Boyd J, Soslow RA. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol 2008;39(1):116–125. [DOI] [PubMed] [Google Scholar]
- 48.Rabban JT, Calkins SM, Karnezis AN, et al. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. Am J Surg Pathol 2014;38(6):793–800. [DOI] [PubMed] [Google Scholar]
- 49.Sloan EA, Moskaluk CA, Mills AM. Mucinous Differentiation With Tumor Infiltrating Lymphocytes Is a Feature of Sporadically Methylated Endometrial Carcinomas. Int J Gynecol Pathol 2017;36(3):205–216. [DOI] [PubMed] [Google Scholar]
- 50.Stelloo E, Nout RA, Osse EM, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin Cancer Res 2016;22(16):4215–4224. [DOI] [PubMed] [Google Scholar]
- 51.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014;15(7):e268–278. [DOI] [PubMed] [Google Scholar]
- 53.Ulbright TM, Roth LM. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Hum Pathol 1985;16(1):28–34. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura N, Hachisuga T, Yokoyama M, Iwasaka T, Kawarabayashi T. Clinicopathologic analysis of the prognostic factors in women with coexistence of endometrioid adenocarcinoma in the endometrium and ovary. J Obstet Gynaecol Res 2005;31(2):120–126. [DOI] [PubMed] [Google Scholar]
- 55.Schultheis AM, Ng CK, De Filippo MR, et al. Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. J Natl Cancer Inst 2016;108(6):djv427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anglesio MS, Wang YK, Maassen M, et al. Synchronous Endometrial and Ovarian Carcinomas: Evidence of Clonality. J Natl Cancer Inst 2016;108(6):djv428. [DOI] [PubMed] [Google Scholar]
- 57.Williams MG, Bandera EV, Demissie K, Rodriguez-Rodriguez L. Synchronous primary ovarian and endometrial cancers: a population-based assessment of survival. Obstet Gynecol 2009;113(4):783–789. [DOI] [PubMed] [Google Scholar]
- 58.Altrabulsi B, Malpica A, Deavers MT, Bodurka DC, Broaddus R, Silva EG. Undifferentiated carcinoma of the endometrium. Am J Surg Pathol 2005;29(10):1316–1321. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Zhao C. Clinicopathologic and Immunohistochemical Characterization of Dedifferentiated Endometrioid Adenocarcinoma. Appl Immunohistochem Mol Morphol 2016;24(8):562–568. [DOI] [PubMed] [Google Scholar]
- 60.Ramalingam P, Masand RP, Euscher ED, Malpica A. Undifferentiated Carcinoma of the Endometrium: An Expanded Immunohistochemical Analysis Including PAX-8 and Basal-Like Carcinoma Surrogate Markers. Int J Gynecol Pathol 2016;35(5):410–418. [DOI] [PubMed] [Google Scholar]
- 61.Stewart CJ, Crook ML. SWI/SNF complex deficiency and mismatch repair protein expression in undifferentiated and dedifferentiated endometrial carcinoma. Pathology 2015;47(5):439–445. [DOI] [PubMed] [Google Scholar]
- 62.Strehl JD, Wachter DL, Fiedler J, et al. Pattern of SMARCB1 (INI1) and SMARCA4 (BRG1) in poorly differentiated endometrioid adenocarcinoma of the uterus: analysis of a series with emphasis on a novel SMARCA4-deficient dedifferentiated rhabdoid variant. Ann Diagn Pathol 2015;19(4):198–202. [DOI] [PubMed] [Google Scholar]
- 63.Garg K, Leitao MM Jr., Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol 2009;33(6):925–933. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn E, Ayhan A, Bahadirli-Talbott A, Zhao C, Shih Ie M. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am J Surg Pathol 2014;38(5):660–665. [DOI] [PubMed] [Google Scholar]
- 65.Murray SK, Clement PB, Young RH. Endometrioid carcinomas of the uterine corpus with sex cord-like formations, hyalinization, and other unusual morphologic features: a report of 31 cases of a neoplasm that may be confused with carcinosarcoma and other uterine neoplasms. Am J Surg Pathol 2005;29(2):157–166. [DOI] [PubMed] [Google Scholar]
- 66.Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol 2012;36(6):799–807. [DOI] [PubMed] [Google Scholar]
- 67.McFarland M, Quick CM, McCluggage WG. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology 2016;68(7):1013–1020. [DOI] [PubMed] [Google Scholar]
- 68.Mirkovic J, Sholl LM, Garcia E, et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol 2015;28(11):1504–1514. [DOI] [PubMed] [Google Scholar]
- 69.de Leeuw WJ, Dierssen J, Vasen HF, et al. Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients. J Pathol 2000;192(3):328–335. [DOI] [PubMed] [Google Scholar]
- 70.Peterson LM, Kipp BR, Halling KC, et al. Molecular characterization of endometrial cancer: a correlative study assessing microsatellite instability, MLH1 hypermethylation, DNA mismatch repair protein expression, and PTEN, PIK3CA, KRAS, and BRAF mutation analysis. Int J Gynecol Pathol 2012;31(3):195–205. [DOI] [PubMed] [Google Scholar]
- 71.McConechy MK, Talhouk A, Li-Chang HH, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol 2015;137(2):306–310. [DOI] [PubMed] [Google Scholar]
- 72.Alkushi A, Lim P, Coldman A, Huntsman D, Miller D, Gilks CB. Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a clinically relevant cut-off level. Int J Gynecol Pathol 2004;23(2):129–137. [DOI] [PubMed] [Google Scholar]
- 73.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015;113(2):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 2000;88(4):814–824. [PubMed] [Google Scholar]
- 75.Talhouk A, Hoang LN, McConechy MK, et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: Earlier prognostic information to guide treatment. Gynecol Oncol 2016;143(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altman AD, Ferguson SE, Atenafu EG, et al. Canadian high risk endometrial cancer (CHREC) consortium: analyzing the clinical behavior of high risk endometrial cancers. Gynecol Oncol 2015;139(2):268–274. [DOI] [PubMed] [Google Scholar]
- 77.Alkushi A, Kobel M, Kalloger SE, Gilks CB. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol 2010;29(4):343–350. [DOI] [PubMed] [Google Scholar]
- 78.Voss MA, Ganesan R, Ludeman L, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol 2012;124(1):15–20. [DOI] [PubMed] [Google Scholar]
- 79.Soslow RA, Bissonnette JP, Wilton A, et al. Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol 2007;31(7):979–987. [DOI] [PubMed] [Google Scholar]
- 80.Ayeni TA, Bakkum-Gamez JN, Mariani A, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol 2013;129(3):478–485. [DOI] [PubMed] [Google Scholar]
- 81.Bosse T, Nout RA, McAlpine JN, et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol 2017. [DOI] [PMC free article] [PubMed]
- 82.Chen W, Husain A, Nelson GS, et al. Immunohistochemical Profiling of Endometrial Serous Carcinoma. Int J Gynecol Pathol 2017;36(2):128–139. [DOI] [PubMed] [Google Scholar]
- 83.Kurnit KC, Kim GN, Fellman BM, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol 2017;30(7):1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howitt BE, Shukla SA, Sholl LM, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol 2015;1(9):1319–1323. [DOI] [PubMed] [Google Scholar]
- 85.Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest 2016;126(6):2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23(12):2965–2970. [DOI] [PubMed] [Google Scholar]
- 87.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sholl LM, Aisner DL, Allen TC, et al. Programmed Death Ligand-1 Immunohistochemistry--A New Challenge for Pathologists: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016;140(4):341–344. [DOI] [PubMed] [Google Scholar]
- 89.Xiong J, He M, Jackson C, et al. Endometrial carcinomas with significant mucinous differentiation associated with higher frequency of k-ras mutations: a morphologic and molecular correlation study. Int J Gynecol Cancer 2013;23(7):1231–1236. [DOI] [PubMed] [Google Scholar]
- 90.Buza N, Hui P. Marked heterogeneity of HER2/NEU gene amplification in endometrial serous carcinoma. Genes Chromosomes Cancer 2013;52(12):1178–1186. [DOI] [PubMed] [Google Scholar]