Abstract
Background:
The objective of the study is to compare stress resistance-promoting effect of triethylene glycol (TEG) and root extract of Ashwagandha (Withania somnifera) i.e. withanolide-free root extract of Withania somnifera (WFWS).
Materials and Methods:
Mice groups treated orally with 10 mg/kg TEG or WFWS (3.3, 10, 33.3, or 100 mg/kg) for 12 consecutive days were subjected to foot shock stress-triggered hyperthermia test on the 1st, 5th, 7th and 10th day and to marble-burying test on the following 2 days. Effects of treatment on stress-triggered alteration in body weight, core temperature, blood glucose, insulin and cortisol level were quantified and statistically analyzed.
Results:
WFWS doses up to 10 mg/kg/day were as effective as TEG in affording protection against stress-triggered alteration in body weight, core temperature and marble-burying behavior. Protection against stress-triggered alteration in blood glucose and insulin level, as well as antidepressants or anxiolytic-like activities in the behavioral test, were observed in the higher two WFWS doses (33.3 and 100 mg/kg) treated groups only.
Conclusion:
Ashwagandha metabolites other than withanolides contribute to its stress resistance increasing effects. The observations suggest that modulation of physiological functions of gut microbiota may be involved in the mode of action of Withania somnifera root extracts.
Keywords: Ashwagandha, body weight regulation, marble-burying test, Rasayana therapy, stress resistance, triethylene glycol, withanolides
Introduction
Dried mature roots of Withania somnifera (L.) Dunal (Ashwagandha), a plant of the Solanaceae family (genus Withania), are used in many Ayurvedic formulations prescribed for a variety of inflammatory conditions (e.g., arthritis and rheumatism) and as a general tonic to increase energy, improve overall health and longevity and prevent diseases.[1] Ashwagandha is classified in Ayurveda as a Rasayana drug useful for promoting physical and mental health, increasing stress resistance against diseases and environmental challenges and prolonging healthy life span.[2-5] The Ayurvedic Pharmacopoeia of India also describes an analytical method for identifying and quantifying the contents of withanolides (a group of structurally analogous steroidal lactones) and alkaloids in Ashwagandha.[6] Withaferin-A was the very first withanolides type constituent isolated from the plant in the 1960s and since then, a number of withanolides encountered in different parts of Withania somnifera and other plants as well as of reports on their antimicrobial and other therapeutically interesting bioactivities have continued to increase.[7-9]
The contents of total withanolides quantified by modern analytical methods are seldom >3%–5%, which vary considerably in different parts and cultivars of the plant. It is now well recognized also that several bioactive substances other than withanolides are also biosynthesized and stored in different amounts in different parts of the plant and that many of them also possess bactericidal, anticancer, brain function modulating and other therapeutically interesting bioactivities.[10,11] Triethylene glycol (TEG) is a relatively new addition to the long list of microbicidal substances with cytostatic or anticancer and sleep-inducing activities.[12,13] However, the presence of TEG has been detected only in the leaves of the plant cultivated under the experimental condition and that too by the same Japanese research group. Although we have not yet been able to identify TEG in crude extracts of Withania somnifera root extracts, observations in laboratories have revealed that its fairly low daily oral doses (10 mg/kg/day) are effective in increasing stress resistance and that its such effectiveness continues to increase with increasing numbers of treatment days.[14] These observations, taken together with our earlier ones made with structurally diverse bactericidal phytochemicals and some plant-derived drugs,[15-21] led us to the working hypothesis that their modulating effects on gut microbiota composition and functions may also contribute to therapeutically interesting bioactivity profiles of Withania somnifera-like adaptogenic herbs often used in Rasayana therapies or as herbal remedies.[22,23] Results of an exploratory experiment added further preclinical evidence in favor of this working hypothesis and suggested that combinations of microbicidal substances other than withanolides are also involved in the traditionally known and more recently, rediscovered medicinal uses of Withania somnifera roots. This experiment was conducted in the realm of our psychopharmacological studies with extracts and bioactive constituents of Ayurvedic Rasayana drugs[24] currently often used for the treatment of obesity and diabetes associated physical and mental health problems.[25,26] Choice of the doses and experimental procedures used in the experiment were based on our earlier observations made with diverse types of Withania somnifera extracts and some of their already known constituents.[25-29] The aim of the experiment was to verify whether or not the presence of withanolides in Withania somnifera root extracts is essential for their obsessive–compulsive behavior suppressing effects observed in rodents and patients.[28,30,31]
Materials and Methods
Animals
Albino male mice of Wistar strain weighing 25 ± 5 g used in this study were procured from Central Animal House of Institute of Medical Sciences, Banaras Hindu University, Varanasi (Registration number: 542/AB/CPCSEA). They were randomly selected and group housed (six animals per cage) in polypropylene cages provided with husk bed at an ambient temperature (25 ± 1°C) and relative humidity (50 ± 10%) with a 12:12 h light/dark cycle. All animals were acclimatized to laboratory conditions for at least 1 week before the start of the experiment. They were always fed with standard rodent diet and water ad libitum. Ethical clearance for animal experimental work was obtained from the Central Animal Ethical Committee of the University (Dean/2014/CAECI/604, dated 30/05/2014) before the commencement of experiments. All experimental groups were always tested in parallel (i.e., on the same day of the experiment) and handled, weighed and observed by a blinded observer.
Plant extract, chemicals, and test kits used
Withania somnifera root extract freed from withanolides (WFWS) used in this study was prepared and generously supplied together with its analytical certificate by the Research Department of Natural Remedies Private Limited, Bengaluru, India. In short, Withania somnifera root extract prepared according to the manufacturing procedure used by the company (published elsewhere)[32] for manufacturing and commercializing them was chromatographically subfractionated. Subfractions of the extract devoid of withanolides were combined and evaporated to obtain WFWS (Batch No. RD15052). Traces of any known withanolides encountered in the plant could be detected in WFWS by the chromatographic techniques used by the company for analytically standardizing Ashwagandha extracts commercialized by the company and many others.
Carboxymethyl cellulose (CMC) was procured from Central Drug House (New Delhi, India) and TEG was from Spectrochem (Mumbai, India). Plasma glucose level was estimated by biochemical test kit (ERBA Diagnostics Mannheim GmbH, Germany); plasma insulin level was estimated using enzyme-linked immunosorbent assay (ELISA) test kit (Chemux BioScience Inc., USA) and plasma cortisol was estimated using ELISA test kit (DSI S.r.l., Italy).
Animal grouping and drug treatments
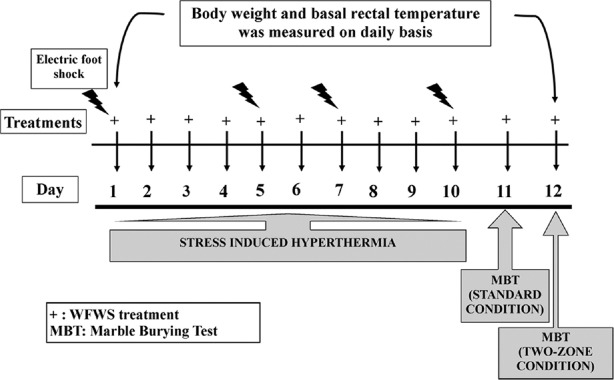
Six randomly selected experimentally naive mice were allotted to each of the seven experimental groups. i.e., group I: stressed control (vehicle treated); group II: nonstressed control (vehicle treated); group III: stressed + TEG (10 mg/kg); group IV: stressed + WFWS (3.3 mg/kg); group V: stressed + WFWS (10 mg/kg); group VI: stressed + WFWS (33.3 mg/kg) and group VII: stressed + WFWS (100 mg/kg). The animals of the stressed and nonstressed control groups were treated once daily with 0.3% CMC (p. o) for 12 consecutive days. For similar oral treatments, TEG and WFWS were suspended in 0.3% CMC and oral application volume for all treatments was 10 ml/kg/day. On each experimental day, body weights and mean rectal temperature of the animals were recorded 1 h before the oral drug administration. All animals were closely observed for apparent behavioral abnormalitie during the entire course of the experiment. Further detail of the experimental procedure used are graphically summarized in Figure 1.
Figure 1.
Summary of experimental procedures used
Foot shock stress-induced hyperthermia test
This test was conducted on the 1st, 5th, 7th and 10th days of the experiment and 60 min after the day's oral treatments. The experimental procedure used for this test was the same as those described in detail in other reports from our research group.[15-21,27-29,33] Temperature was measured using a rectal probe and a calibrated digital thermometer (Easy Care, Mumbai, India).
Marble-burying test
On the 11th day and 60 min after the day's oral treatment, individual mouse from each group was placed in a polypropylene cage (30 cm × 23 cm) provided with husk bed, where 12 glass marbles (color and size of marbles were kept constant) were evenly spaced for standard marble-burying condition test. On the 12th day, 60 min after the oral treatment, they were tested again in the two-zone marble-burying condition, whereupon 8 glass marbles (color and size of marbles were kept constant) were evenly spaced but only on one half of the polypropylene cage. After 15 min (standard condition) or 30 min (two-zone condition) of exposure, the animals were placed back to their home cages and the number of marbles at least two-thirds covered by husk was counted.[34]
Determination of plasma glucose, insulin, cortisol level and organ weight
Immediately after the last observation made in two-zone marble-burying test on the 12th day of the experiment, all animals were sacrificed by decapitation and blood was collected by direct cardiac puncture in EDTA-coated tubes kept in ice. Immediately after blood collection adrenal glands and spleen of the animals were dissected out, washed under slowly running tap water and weighed after removing adhered water by gently drying them on sheets of filter papers.[17,35] Blood glucose, insulin and cortisol levels in the plasma of the blood samples were quantified using commercially available test kits and following the instruction manual of the kits supplied by their manufacturers.
Statistical analysis
Mean ± standard error of mean was calculated for the observed values in each experimental group. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls multiple comparison test and two-way ANOVA followed by Bonferroni post hoc test unless otherwise stated. GraphPad Prism-5 (GraphPad Software Inc., La Jolla, California, USA) was used for statistical analysis. Origin Pro 8 software (Origin Lab Corporation, Massachusetts, USA) was used for graph representation. P < 0.05 was considered as statistically significant.
Results
Body weight
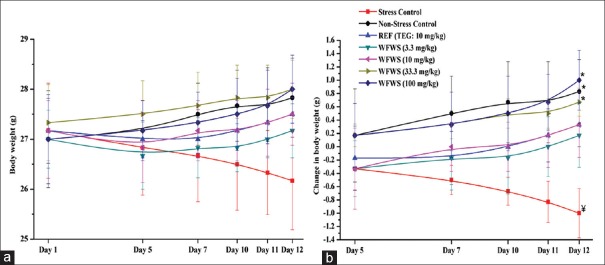
Unlike for the nonstressed control group, mean body weight of the stressed control group decreased during the course of the experiment [Figure 2a and b]. Such body weight losses triggered by occasional exposures to very short duration (50 s) of foot shock stress were not observed in the TEG or any of the WFWS-treated groups. The rate of change in the mean body weights of the 3.3 or 10 mg/kg/day WFWS-treated groups were almost identical to that observed in the 10 mg/kg/day TEG-treated group, whereas those of the 33.3 and 100 mg/kg/day extract-treated groups were like those of the vehicle-treated nonstressed group. These results indicate that 33.3 mg/kg daily oral does of the tested extract is its maximally effective one in affording complete protection against body weight changes caused by repeated exposures to unavoidable stressful stimuli and that its minimally effective ones are 3.3 mg/kg/day or lower.
Figure 2.
Effect of stress on (a) mean body weight and (b) change in body weight of male mice treated with withanolide-free Withania somnifera extract. WFWS: Withanolide-free Withania somnifera extract, TEG: Triethylene glycol. Values are mean ± standard error of mean (n = 6). *denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to stress control group (*P < 0.05). ¥denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to nonstress control group (¥P < 0.05)
Mean rectal temperatures
Mean rectal temperature of the stressed control group increased slightly and that of the nonstressed one continued to decrease somewhat during the course of the experiment [Figure 3]. Mean rectal core temperature of TEG- or WFWS-treated and stressed groups also continued to decrease during the course of experiment. On the last 2 observational days, these mean values of the nonstressed group were statistically significantly higher than most of the stressed groups. Therefore, it is apparent that the minimally effective daily oral doses of the tested extract in affording protection against stress-triggered alterations in mean body core temperature of stressed mice are also 3.3 mg/kg or lower. Except for the last 2 observational days, mean rectal temperature of all WFWS- or TEG-treated groups were not statistically significantly different from the nonstressed one. These observations indicate that the observed effects of the test agents in stress-triggered alteration in mean rectal core temperature are due to their stress response suppressing effects evolving slowly during the course of the experiment.
Figure 3.
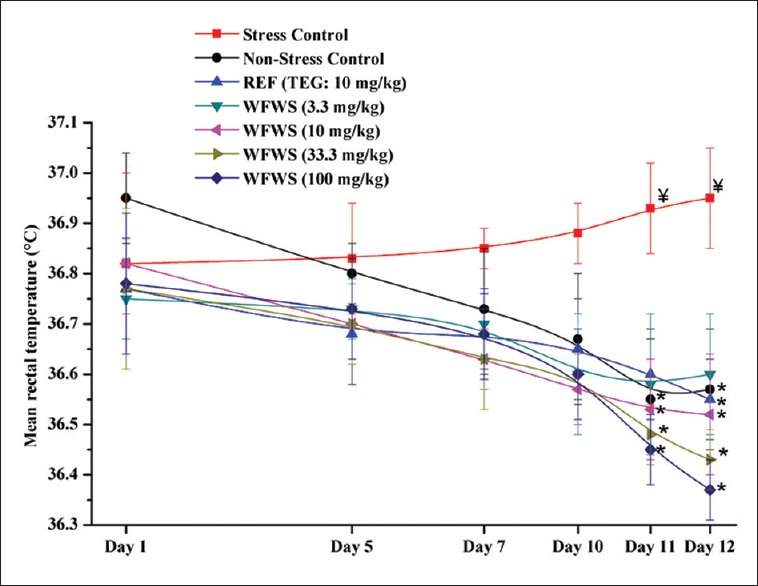
Mean rectal temperature of male mice treated with withanolide-free Withania somnifera extract. WFWS - Withanolide-free Withania somnifera extract, TEG - Triethylene glycol. Values are mean ± standard error of mean (n = 6). *denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to stress control group (*P < 0.05). ¥denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to nonstress control group (¥P < 0.05)
Stress-induced hyperthermia
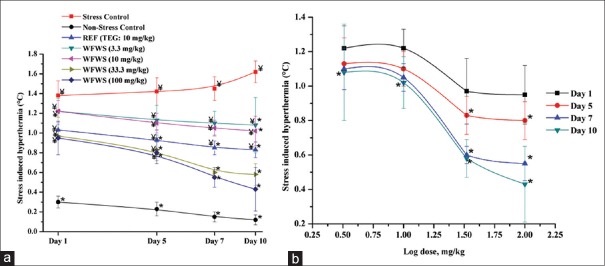
Mean transient hyperthermic response of the stressed control group remained almost constant on all observational days [Figure 4a]. Although numerically these values of the TEG- or WFWS-treated group on the 1st observational day were lower than that of the stressed control group, no statistically significant effect of TEG or WFWS were observed on this day. However, after 12 daily oral 3.3 or 10 mg/kg doses of the extract, this transient hyperthermic response was statistically significantly lower than that of the stressed control group. Observed effectiveness of these two doses of the extract on this day was almost identical and was somewhat lower than that of 10 daily oral doses of 10 mg/kg TEG. Such statistically significant effect of the tested TEG daily dose and of 33.3 or 100 mg/kg WFWS doses were observed even after their 5 daily doses. Effects of 33.3 or 100 mg/kg daily WFWS doses observed from the 5th observational day onward were always almost identical. These observations reveal that the effective daily dose range of the extract in foot shock stress-induced hyperthermia test lies between 3 and 33.3 mg/kg and reaffirm that its efficacy in this test depends also on its daily dose as well as number of days of treatment given [Figure 4b].
Figure 4.
Stress-induced hyperthermia of male mice treated with graded doses of (a) WFWS and (b) log dose-response curve of single and daily repeated treatment with WFWS in the foot shock stress induced hyperthermia test. Values are mean ± standard error of mean (n = 6). *denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to stress control group (*P < 0.05). ¥denotes statistically significant difference (two-way ANOVA followed by Bonferroni post hoc test) relative to nonstress control group (¥P < 0.05)
Marble-burying tests
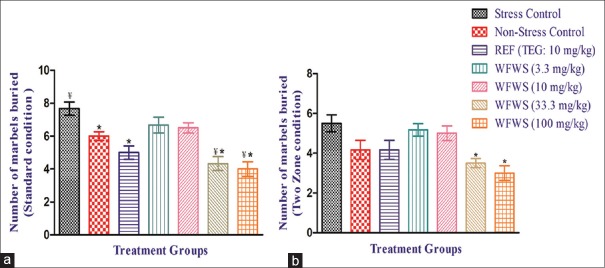
Results of the standard version of the marble-burying test conducted 1 h after 11 daily oral treatment revealed that the mean number of the marbles buried by the animals of the stressed control group was statistically significantly higher than that of the nonstressed control group [Figure 5a]. These values of the 10 mg/kg/day TEG or of the 33.3 or 100 mg/kg/day WFWS-treated groups were statistically significantly lower than the vehicle-treated control group. Effectiveness of these two higher doses of the extract was almost equal and somewhat higher than that of the tested TEG dose. Since no statistically significant effects of 3.3 or 10 mg/kg/day WFWS doses were observed, it seems reasonable to assume that its behaviorally effective daily oral doses are >10 mg/kg. This inference is reaffirmed by the results of the two-zone marble-burying test summarized in Figure 5b. It must be noted, that unlike in the standard version of the test, no statistically significant difference between the mean number of marbles buried by the animals of the stressed and unstressed groups was observed and that unlike in the standard version of the test, no statistically significant effect of the test TEG dose were observed in the two-zone version of the test. These observations indicate that unlike for TEG, beneficial effect of WFWS as an anxiolytic or antidepressant-like bioactive agent can be detected in both versions of the test.
Figure 5.
Effect of withanolide-free Withania somnifera extract on (a) standard condition marble-burying test in male mice on day 11 and (b) two-zone condition marble-burying test in male mice on day 12. Values are mean ± standard error of mean (n = 6). *denotes statistically significant difference (one-way ANOVA followed by Student–Newman–Keuls multiple comparison test) relative to stress control group (*P < 0.05). ¥denotes statistically significant difference (one-way ANOVA followed by Student–Newman–Keuls multiple comparison test) relative to nonstress control group (¥P < 0.05)
Organ weight and plasma glucose, insulin and cortisol levels
The possibility that WFWS is a stress-resistant promoting agent is inferred also from its observed preventive effect against stress-triggered alteration in adrenal gland weight [Table 1] and plasma cortisol level [Table 2]. It was interesting to note that mean plasma glucose level of stressed control group was slightly but significantly higher than the nonstressed group and that no such stress-triggered hyperglycemic response was observed in the 33.3 or 100 mg/kg/day WFWS-treated stressed animals [Table 2]. The observations that plasma insulin level of these two extract-treated groups was not statistically significantly different from that of the stressed control group could indicate that observed anti-stress and/or antihyperglycemic activities of the higher tested doses of WFWS are independent of its effect on insulin homeostasis. However, further more detailed mechanistic studies will be necessary for reaffirming this possibility.
Table 1.
Effect of withanolide-free Withania somnifera extract on the weight of adrenal glands and spleen in male mice
| Treatment groups | Organ weights (mg) | Relative organ weights (mg/g of body weight) | ||
|---|---|---|---|---|
| Spleen | Adrenal glands | Spleen | Adrenal glands | |
| Stress control | 96.78±1.53 | 19.45±0.85¥ | 3.35±0.12 | 0.75±0.05¥ |
| Nonstress control | 92.80±2.07 | 14.03±0.60* | 3.73±0.18 | 0.51±0.03* |
| REF (TEG: 10 mg/kg) | 94.30±1.74 | 17.07±1.24¥ | 3.43±0.05 | 0.62±0.05 |
| WFWS (3.3 mg/kg) | 96.43±1.50 | 18.58±1.05 | 3.57±0.03 | 0.69±0.04 |
| WFWS (10 mg/kg) | 94.32±1.37 | 16.75±0.74 | 3.44±0.12 | 0.61±0.04 |
| WFWS (33.3 mg/kg) | 93.17±1.23 | 15.27±0.69* | 3.33±0.08 | 0.55±0.03* |
| WFWS (100 mg/kg) | 92.78±1.78 | 14.30±1.13* | 3.33±0.11 | 0.52±0.05* |
Values are mean±SEM (n=6). *Statistically significant difference (one-way ANOVA followed by Student-Newman-Keuls multiple comparison test) relative to stress control group (*P<0.05). ¥Statistically significant difference (one-way ANOVA followed by Student-Newman-Keuls multiple comparison test) relative to nonstress control group (¥P<0.05). W. somnifera: Withania somnifera, SEM: Standard error of mean, ANOVA: Analysis of variance, TEG: Triethylene glycol, WFWS: Withanolides free W. somnifera extract, REF: Reference Control Group
Table 2.
Effect of withanolide-free Withania somnifera extract on the plasma glucose, insulin and cortisol in male mice
| Treatment groups | Glucose (mg/dl) | Insulin (μIU/ml) | Cortisol (ng/ml) |
|---|---|---|---|
| Stress control | 97.00±1.47¥ | 14.55±0.84¥ | 96.57±1.49¥ |
| Nonstress control | 82.81±1.60* | 19.40±1.27* | 87.50±2.04* |
| REF (TEG: 10 mg/kg) | 91.46±1.74¥ | 15.86±1.14¥ | 89.73±1.62* |
| WFWS (3.3 mg/kg) | 92.74±1.60¥ | 14.75±0.65¥ | 94.16±2.26 |
| WFWS (10 mg/kg) | 91.50±1.45¥ | 14.79±0.76¥ | 93.56±1.11 |
| WFWS (33.3 mg/kg) | 88.87±1.46*,¥ | 14.84±0.43¥ | 89.36±1.40* |
| WFWS (100 mg/kg) | 89.19±2.03*,¥ | 15.08±0.58¥ | 88.13±2.13* |
| Treatment groups | Relative values (per 100 g of body weight) | ||
| Glucose (mg/dl) | Insulin(μIU/ml) | Cortisol (ng/ml) | |
| Stress control | 373.81±17.48¥ | 56.39±4.87 | 370.87±10.12¥ |
| Nonstress control | 299.27±13.02* | 69.89±4.65 | 315.56±11.09* |
| REF (TEG: 10 mg/kg) | 333.06±8.75 | 57.82±4.40 | 326.76±8.18 |
| WFWS (3.3 mg/kg) | 343.93±8.81 | 54.70±2.70 | 349.24±10.88 |
| WFWS (10 mg/kg) | 334.02±12.27 | 54.14±3.81 | 341.22±9.91 |
| WFWS (33.3 mg/kg) | 318.19±9.16* | 53.02±1.34 | 319.99±9.53* |
| WFWS (100 mg/kg) | 319.06±7.47* | 54.11±2.84 | 315.35±8.40* |
Values are mean±SEM (n=6). *Statistically significant difference (one-way ANOVA followed by Student-Newman-Keuls multiple comparison test) relative to stress control group (*P<0.05). ¥Statistically significant difference (one-way ANOVA followed by Student-Newman-Keuls multiple comparison test) relative to nonstress control group (¥P<0.05). W. somnifera: Withania somnifera, SEM: Standard error of mean, ANOVA: Analysis of variance, TEG: Triethylene glycol, WFWS: Withanolides free W. somnifera extract, REF: Reference Control Group
Discussion
Reported observations are the very first one revealing the effect of TEG and Withania somnifera root extract devoid of withanolides on marble-burying behavior of stressed rodents. They suggest that their 10 mg/kg daily oral doses are high enough for preventing stress-triggered alteration in obsessive–compulsive behavior of mice and reveal that even extremely low daily doses (3.3 mg/kg or lower) of WFWS are highly effective in protecting stress-triggered losses in body weight and alterations in thermoregulatory process. However, since we have not yet been able to identify TEG in WFWS or any other extract of the plant, the question whether TEG is one of the bioactive constituents of Withania somnifera root extracts cannot yet be answered with any certainty.
Results of some clinical studies have revealed that Withania somnifera root extract have regulating effect of body weight of stressed and other patients suffering from obsessive–compulsive disorders.[36,37] Chronic exposure to the stressful environment and life events alter brain function regulating eating behavior and addictive or obsessive habits.[38-41] Therefore, our observations strongly suggest that Ayurvedic recommendation of Withania somnifera could as well be due to their ability to counteract compulsive or addictive eating behavior and food choices regulating body weight, temperature and other bodily and mental functions. It is well known that repeated exposures to unavoidable painful stimuli have longer lasting effects on appetite, eating behavior and other habits and physiological responses implicated in pathogenesis and progression of obsessive–compulsive or addictive eating behavior.[38-40] Such behavioral changes lead eventually to diverse malnutrition associated metabolic disorders and diverse spectrums of physical and mental health problems.[42-45] Withania somnifera is also often used by practitioners of Ayurveda for combating addiction, obsession, and diverse so-called “lifestyle disorders”[46] and numerous preclinical and clinical studies conducted with its different types of extract have continued to justify such traditionally known medicinal uses of different parts of the plant.[28,30,31,47-50] However, the therapy relevant questions concerning their doses and treatment regimen necessary for prevention and cure of such disorders still remain unanswered or speculative only.
Results of the earlier dose-finding experiments and other studies with Withania somnifera extracts (characterized or standardized on their withanolide contents) have revealed that like numerous adaptogenic herbal extracts, their activity profiles also depend not only on their daily doses but also on the numbers of days of treatment and that their daily dose necessary for observing their anxiolytic or antidepressant-like activities in marble-burying test and other animal models are somewhat higher than those for protecting rodents against stress-triggered alterations in body weight and core temperature.[27-29] Analogous were also the observations made with several other phytochemicals encountered in Withania somnifera extracts as well.[23,30] Since only 3.3-mg/kg daily oral doses of WFWS afforded almost complete protection against stress triggered in body weight loss and alteration in thermoregulatory process and compulsive behavior, it seems reasonable to assume that TEG-like bioactive substances other than withanolides are also involved in traditionally known medicinal uses of Ashwagandha and diverse types of Withania somnifera root extract currently commercialized or prepared for research purpose.
It was interesting to note also that like withanolides containing extracts, statistically significant anxiolytic or antidepressant like effects of WFWS in the marble-burying test or on adrenal gland weight and blood glucose and cortisol level in stressed animals were also observed after its higher two tested doses (33.3 and 100 mg/kg/day) only. These observations indicate further that higher doses of the combination of substances other than withanolides encountered in Withania somnifera roots are also effective in regulating biological processes and mechanisms involved in unavoidable stress-triggered alterations in mental health accompanying elevated blood glucose and cortisol levels. These observations, taken together with our earlier ones,[10-12,51-55] strongly suggest that all biological processes and mechanisms involved in antidepressant and anxiolytic-like activities of WFWS and other Withania somnifera extract are not necessarily the same as those involved in its protective effect against stress-triggered alterations in body weight and core temperature.
Although statistically significant effect of TEG in the standard version of marble-burying test was observed, its 10-mg/kg/day oral dose had no effects on the stress-triggered alteration in adrenal gland weight or on circulating blood glucose and cortisol level. These observations could indicate that its therapeutically interesting bioactivity profile is mainly due to its broad spectrum of bactericidal, antiviral and antifungal activities and that it is a fairly potent and specific regulator of the endocrinological function of gut microbiota and gastrointestinal tract involved in digestive and metabolic processes regulating body weight and temperature. Efforts to reconfirm these observations and to define its pharmacological activity profile after its repeated daily higher oral doses will be necessary for judging whether or not it could as well be a substitute or a cheaper therapeutic option of Withania somnifera root extract. It can be suggested that TEG is certainly a useful and safe pharmacological tool for better understanding the role of the gut microbiota and gut endocrine functions and in regulating stress-triggered alteration in body weight, temperature and eating behavior.
The very first report on therapeutically interesting bioactivities of TEG in cellular and animal bioassays had revealed that it is a cytostatic agent with cancer growth-inhibiting properties.[12] Although structurally and functionally diverse cancer chemotherapeutic and antibiotics are effective in suppressing cancerous growth and proliferation, therapeutic responses of currently available treatment against cancer cachexia are often fairly poor or unsatisfactory.[56,57] Safety margins of TEG and many other microbicidal substances encountered in Withania somnifera extracts and several phytochemicals encountered in them are much larger than most anticancer and other drugs currently recommended or prescribed for combating cancer and cancer-associated cachexia. Since many of them are readily available, cheap, pharmaceutically very stable, they could also be considered as the potential therapeutic lead against cancer and associated cachexia and other physical and mental health problems accompanying this and other life-threatening diseases. Appropriate uses of mice models of cancer and other diseases and the bioassay procedure used in this study seem to be a more reliable and time-saving starting point for achieving such goals.
Observations made during such effort will certainly be useful not only for more rational medicinal and commercial uses of Withania somnifera but also for better understanding of biological and pharmacological principles behind traditionally known medicinal uses of Ashwagandha and other Rasayana (rejuvenating) drugs. Although many questions concerning their bioactive constituents and modes of action still remain open, it can now be said that substances other than withanolides with TEG-like microbicidal and other bioactivities extractable from the roots of Withania somnifera are also involved in their therapeutically interesting activity profiles. These observations, taken together with current knowledge on the role of the composition and physiological functions of gut microbiota in dictating healthy life span of individuals and their ability to resist against diseases and environmental challenges,[58,59] strongly suggest that the bioassay procedure used in this study is particularly well suited for pharmacological standardization of Ayurvedic Rasayana drugs necessary for obtaining more reliable and reproducible health benefits from them even in the 21st century.
Among all plants used in Ayurveda and other traditionally known systems of medicine and health care, Withania somnifera is a unique stress resistance increasing or adaptogenic plant with sedative and sleep-regulating activities,[60] and it is now well recognized that it is a rich source of naturally occurring bactericidal agent and their combinations.[61] Therefore, it seems to be a particularly well suited not only for better understanding of biological and pharmacological aspects behind Rasayana therapies but also for better understanding of the role of gut microbiota in systems pharmacology of many plants and plant-derived drugs, paradoxical effects of many of which in regulating brain functions have often been reported.[62] Therefore, an effort to better define Ayurvedic system pharmacology of Ashwagandha using the more holistic experimental strategy used in this and our earlier studies[23] can be warranted.
Conclusion
TEG is a useful pharmacological tool for Ayurvedic translation research as well as for better understanding of system pharmacology of Ashwagandha and other Rasayana drugs. However, the question whether this bactericidal molecule is a bioactive constituent of Ashwagandha or not still remains open. Ashwagandha metabolites other than withanolides contribute to its stress resistance increasing effects. The observations suggest that modulation of physiological functions of gut microbiota are involved in the mode of action of Withania somnifera root extracts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 2.Kulkarni SK, Dhir A. Withania somnifera: An Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093–105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Dey A, Hadimani MB, Marcović T, Emerald M. Chemistry and pharmacology of Withania somnifera: An update. TANG [Hum Med] 2015;5:e1. [Google Scholar]
- 4.Puri HS. Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation: Traditional Herbal Medicines for Modern Times. London: Taylor &Francis; 2003. pp. 46–58. [Google Scholar]
- 5.Narinderpal K, Junaid N, Raman B. A review on pharmacological profile of Withania somnifera (Ashwagandha) Res Rev J Bot Sci. 2013;2:6–14. [Google Scholar]
- 6.Anonymous. The Ayurvedic Pharmacopoeia of India. Part I. 1st ed. Vol. 1. New Delhi: Government of India, Ministry of Health and Family Welfare, Department of Ayush; 2001. pp. 19–20. [Google Scholar]
- 7.Chen LX, He H, Qiu F. Natural withanolides: An overview. Nat Prod Rep. 2011;28:705–40. doi: 10.1039/c0np00045k. [DOI] [PubMed] [Google Scholar]
- 8.Rai M, Jogee PS, Agarkar G, dos Santos CA. Anticancer activities of Withania somnifera: Current research, formulations and future perspectives. Pharm Biol. 2016;54:189–97. doi: 10.3109/13880209.2015.1027778. [DOI] [PubMed] [Google Scholar]
- 9.Maurya R. Withanolides: A prospective drug for infectious and tropical diseases. In: Kaul S, Wadhwa R, editors. Science of Ashwagandha: Preventive and Therapeutic Potentials. Cham: Springer; 2017. pp. 105–20. [Google Scholar]
- 10.Wadhwa R, Konar A, Kaul SC. Nootropic potential of ashwagandha leaves: Beyond traditional root extracts. Neurochem Int. 2016;95:109–18. doi: 10.1016/j.neuint.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS, Sidhu OP, et al. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry. 2010;71:1085–94. doi: 10.1016/j.phytochem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa R, Singh R, Gao R, Shah N, Widodo N, Nakamoto T, et al. Water extract of Ashwagandha leaves has anticancer activity: Identification of an active component and its mechanism of action. PLoS One. 2013;8:e77189. doi: 10.1371/journal.pone.0077189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik MK, Kaul SC, Wadhwa R, Yanagisawa M, Urade Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS One. 2017;12:e0172508. doi: 10.1371/journal.pone.0172508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson OH, Puck TT, Lemon HF, Loosli CG. The lethal effect of triethylene glycol vapor on air-borne bacteria and influenza virus. Science. 1943;97:142–4. doi: 10.1126/science.97.2510.142. [DOI] [PubMed] [Google Scholar]
- 15.Khan SA, Chatterjee SS, Kumar V. Potential anti-stress, anxiolytic and antidepressant like activities of mono-hydroxybenzoic acids and aspirin in rodents: A comparative study. Aust J Pharmacol Ther. 2015;3:1073. [Google Scholar]
- 16.Shakya A, Chatterjee SS, Kumar V. Role of fumarates in adaptogenics like efficacies of traditionally used Fumaria indica extracts. TANG [Hum Med] 2015;5:28–37. [Google Scholar]
- 17.Khan SA, Chatterjee SS, Kumar V. Low dose aspirin like analgesic and anti-inflammatory activities of mono-hydroxybenzoic acids in stressed rodents. Life Sci. 2016;148:53–62. doi: 10.1016/j.lfs.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Shivavedi N, Chatterjee SS, Kumar V. Stress response modulating effects of lactic acid in mice. Ther Targets Neurol Dis. 2014;1:e418. [Google Scholar]
- 19.Langstieh AJ, Verma P, Thakur AK, Chatterjee SS, Kumar V. Desensitization of mild stress triggered responses in mice by a Brassica juncea leaf extract and some ubiquitous secondary plant metabolites. Pharmacologia. 2014;5:326–38. [Google Scholar]
- 20.Yadav V, Chatterjee SS, Majeed M, Kumar V. Long lasting preventive effects of piperlongumine and a Piper longum extract against stress triggered pathologies in mice. J Intercult Ethnopharmacol. 2015;4:277–83. doi: 10.5455/jice.20150921010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav V, Chatterjee SS, Majeed M, Kumar V. Preventive potentials of piperlongumine and a Piper longum extract against stress responses and pain. J Tradit Complement Med. 2016;6:413–23. doi: 10.1016/j.jtcme.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakur AK, Shakya A, Husain GM, Emerald M, Kumar V. Gut-microbiota and mental health: current and future perspectives. J Pharmacol Clin Toxicol. 2014;2:1016–31. [Google Scholar]
- 23.Chatterjee SS, Kumar V. Quantitative systems pharmacology: Lessons from fumaric acid and herbal remedies. Drug Des. 2017;6:152. [Google Scholar]
- 24.Chatterjee SS, Kumar V. Holistic psychopharmacology and promiscuous plants and principles of Ayurveda. Am J Plant Sci. 2012;3:1015–21. [Google Scholar]
- 25.Kumar V, Thakur AK, Verma S, Yadav V, Chatterjee SS. Potential of some traditionally used edible plants for prevention and cure of disability associated comorbidities. TANG [Hum Med] 2015;5:8.1–8.22. [Google Scholar]
- 26.Kumar V, Dey A, Chatterjee SS. Phytopharmacology of Ashwagandha as an anti-diabetic herb. In: Kaul S, Wadhwa R, editors. Science of Ashwagandha: Preventive and Therapeutic Potentials. Cham: Springer; 2017. pp. 37–68. [Google Scholar]
- 27.Thakur AK, Dey A, Chatterjee SS, Kumar V. Reverse ayurvedic pharmacology of Ashwagandha as an adaptogenic anti-diabetic plant: A pilot study. Curr Tradit Med. 2015;1:51–61. [Google Scholar]
- 28.Dey A, Chatterjee SS, Kumar V. Low dose effects of a Withania somnifera extract on altered marble burying behavior in stressed mice. J Intercult Ethnopharmacol. 2016;5:274–7. doi: 10.5455/jice.20160414104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey A, Chatterjee SS, Kumar V. Analgesic activity of a Withania somnifera extract in stressed mice. Orient Pharm Exp Med. 2016;16:295–302. [Google Scholar]
- 30.Kaurav BP, Wanjari MM, Chandekar A, Chauhan NS, Upmanyu N. Influence of Withania somnifera on obsessive compulsive disorder in mice. Asian Pac J Trop Med. 2012;5:380–4. doi: 10.1016/S1995-7645(12)60063-7. [DOI] [PubMed] [Google Scholar]
- 31.Jahanbakhsh SP, Manteghi AA, Emami SA, Mahyari S, Gholampour B, Mohammadpour AH, et al. Evaluation of the efficacy of Withania somnifera (Ashwagandha) root extract in patients with obsessive-compulsive disorder: A randomized double-blind placebo controlled trial. Complement Ther Med. 2016;27:25–9. doi: 10.1016/j.ctim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Orrù A, Casu MA, Tambaro S, Marchese G, Casu G, Ruiu S, et al. Withania somnifera (L.) Dunal root extract alleviates formalin-induced nociception in mice: Involvement of the opioidergic system. Behav Pharmacol. 2016;27:57–68. doi: 10.1097/FBP.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 33.Shrivastava N, Dey A, Chatterjee SS, Kumar V. Adaptogenic potential of triethylene glycol and quercetin in stressed mice. Pharm Pharmacol Int J. 2015;2(6):197–206. [Google Scholar]
- 34.Nicolas LB, Kolb Y, Prinssen EP. A combined marble burying-locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547:106–15. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Salman TM, Alagbonsi IA, Biliaminu SA. Blood glucose-lowering effect of Telfairia occidentalis: A preliminary study on the underlying mechanism and responses. Biokemistri. 2013;25:133–9. [Google Scholar]
- 36.Choudhary D, Bhattacharyya S, Joshi K. Body weight management in adults under chronic stress through treatment with Ashwagandha root extract: A double-blind, randomized, placebo-controlled trial. J Evid Based Complementary Altern Med. 2017;22:96–106. doi: 10.1177/2156587216641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295. [PMC free article] [PubMed] [Google Scholar]
- 38.Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41:765–82. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- 39.Ball K, Lee C. Relationships between psychological stress, coping and disordered eating: A review. Psychol Health. 2000;14:1007–35. doi: 10.1080/08870440008407364. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim M, Thearle MS, Krakoff J, Gluck ME. Perceived stress and anhedonia predict short-and long-term weight change, respectively, in healthy adults. Eat Behav. 2016;21:214–9. doi: 10.1016/j.eatbeh.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebebrand J, Albayrak Ö, Adan R, Antel J, Dieguez C, de Jong J, et al. Eating addiction, rather than food addiction, better captures addictive-like eating behavior. Neurosci Biobehav Rev. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 43.Sato AF, Fahrenkamp AJ. From bench to bedside: Understanding stress-obesity research within the context of translation to improve pediatric behavioral weight management. Pediatr Clin North Am. 2016;63:401–23. doi: 10.1016/j.pcl.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36–45. doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes Rev. 2016;17:159–77. doi: 10.1111/obr.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandola HM. Lifestyle disorders: Ayurveda with lots of potential for prevention. Ayu. 2012;33:327. doi: 10.4103/0974-8520.108814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pingali U, Pilli R, Fatima N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacognosy Res. 2014;6:12–8. doi: 10.4103/0974-8490.122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni SK, Ninan I. Inhibition of morphine tolerance and dependence by Withania somnifera in mice. J Ethnopharmacol. 1997;57:213–7. doi: 10.1016/s0378-8741(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 49.Peana AT, Muggironi G, Spina L, Rosas M, Kasture SB, Cotti E, et al. Effects of Withania somnifera on oral ethanol self-administration in rats. Behav Pharmacol. 2014;25:618–28. doi: 10.1097/FBP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 50.Bansal P, Banerjee S. Effect of Withinia somnifera and shilajit on alcohol addiction in mice. Pharmacogn Mag. 2016;12:S121–8. doi: 10.4103/0973-1296.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatia A, Bharti SK, Tewari SK, Sidhu OP, Roy R. Metabolic profiling for studying chemotype variations in Withinia somnifera (L.) Dunal fruits using GC-MS and NMR spectroscopy. Phytochemistry. 2013;93:105–15. doi: 10.1016/j.phytochem.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Bharti SK, Bhatia A, Tewari SK, Sidhu OP, Roy R. Application of HR-MAS NMR spectroscopy for studying chemotype variations of Withinia somnifera (L.) Dunal. Magn Reson Chem. 2011;49:659–67. doi: 10.1002/mrc.2817. [DOI] [PubMed] [Google Scholar]
- 53.Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withinia somnifera the Indian ginseng. Cell Mol Life Sci. 2015;72:4445–60. doi: 10.1007/s00018-015-2012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh B, Saxena AK, Chandan BK, Gupta DK, Bhutani KK, Anand KK, et al. Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withinia somnifera dun. Phytother Res. 2001;15:311–8. doi: 10.1002/ptr.858. [DOI] [PubMed] [Google Scholar]
- 55.Singh B, Chandan BK, Gupta DK. Adaptogenic activity of a novel withanolide-free aqueous fraction from the roots of Withinia somnifera Dun. (Part II) Phytother Res. 2003;17:531–6. doi: 10.1002/ptr.1189. [DOI] [PubMed] [Google Scholar]
- 56.Lainscak M, Podbregar M, Anker SD. How does cachexia influence survival in cancer, heart failure and other chronic diseases? Curr Opin Support Palliat Care. 2007;1:299–305. doi: 10.1097/SPC.0b013e3282f31667. [DOI] [PubMed] [Google Scholar]
- 57.Bossola M, Pacelli F, Doglietto GB. Novel treatments for cancer cachexia. Expert Opin Investig Drugs. 2007;16:1241–53. doi: 10.1517/13543784.16.8.1241. [DOI] [PubMed] [Google Scholar]
- 58.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–5. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 59.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 60.David W, Maimes SA. Adaptogens: Herbs for Strength, Stamina and Stress Relief. Rochester Vermont: Healing Arts Press. 2007:138–41. [Google Scholar]
- 61.Lai W, Chen J, Cock IE, Cheesman MJ. The interactive antimicrobial activity of Withania somnifera (L.) Dunal root extracts and conventional antibiotics against some bacterial triggers of autoimmune inflammatory diseases. Pharmacogn Commun. 2018;8:86–92. [Google Scholar]
- 62.Kennedy DO. Plants and the Human Brain. London: Oxford University Press; 2014. pp. 237–52. [Google Scholar]