Abstract
Introduction:
In Ayurveda, many natural plant compounds are used to inhibit inflammatory pathways for centuries with less side effects. Different parts of Ficus racemosa L. (Udumber) plant are used in Ayurveda for many diseases. However, few studies have been conducted to evaluate pharmacological activities of F. racemosa.
Objective:
The objective of the study was to in vitro analyze anti-inflammatory property of F. racemosa bark using albumin denaturation activity.
Methodology:
F. racemosa bark extraction was performed using cold water and hot water. The concentration gradient of extracts was prepared using egg albumin and phosphate-buffered saline. The extract was incubated in a water bath at 37°C for 15 min and was heated at 70°C for 5 min. One nonsteroidal anti-inflammatory drug and one steroid were used as reference drugs. The percentage inhibition of protein denaturation was calculated.
Results:
The inhibition rate of egg albumin denaturation for water extraction increased gradually with concentration. Significantly higher inhibition was showed in hot water extracts than cold water extracts at the concentration of 0.01 μg/ml and 0.1 μg/ml. In addition, the inhibition rate of water extraction was significantly higher than the reference drugs (P < 0.05).
Conclusion:
Anti-inflammatory activity increases with the concentration of F. racemosa bark. Furthermore, the action of this plant is significantly higher than the reference drugs.
Keywords: Albumin denaturation, anti-inflammatory activity, Ficus racemosa L
Introduction
Inflammation is a defense mechanism that enables the body to protect itself against infection, burn, toxic chemical allergens, or any other harmful stimuli. Inflammation is a substantial reaction to damage, disease or destruction portrayed by heat, redness, pain, swelling and disturbed physiological functions.
Ficus racemosa L. belongs to the Moraceae family of Ficus L. genus. It is an evergreen, deciduous tree and an average height is 15–18 m where the roots are protruding aerial. The dark green-colored leaves of F. racemosa are ovate, oblong or elliptic – lanceolate. Fruit receptacles have a diameter of 2–5 cm that is present as subglobose or pyriform. The rusty brown-colored bark of F. racemosa appears to be sharp with an effectively removable luminous fragment. The surface of the bark is moderately smooth and soft where the thickness of the bark differs from 0.5 to 2.0 cm.
Protein denaturation is defined as a process where due to external factors such as heat, strong acid or strong base; an organic solvent or a concentrated inorganic salt causes the protein to denature that means the protein’s tertiary structure and secondary structure is disoriented.[1,2] Enzymes lose their activity since the substrates are able to no longer attach to the active site.[3] Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed medications in the world because of their verified effectiveness in reducing pain and inflammation. NSAIDs has accounted for prevention of the protein denaturation, which acts as antigens and prompts autoimmune diseases.[4] These drugs contain several adverse effects, particularly gastric irritation prompting the development of gastric ulcers.[5]
Medicinal plants are accepted to be an essential source of new chemical substances with potential therapeutic effects. In recent years, the use of herbal medicine and natural products has expanded because of minimal cost and lesser side effects.[6] In Ayurvedic medicine, many of natural plant compounds are used to inhibit inflammatory pathways for centuries with low side effects.[7] F. racemosa is an evergreen, deciduous tree in Asia, Africa, America and Australia and exhibits many pharmacological activities such as anti-hyperglycemic activity, antioxidant activity, hepatoprotective activity and antibacterial/antifungal activity.[8] The bark of F. racemosa is used in the medication of menorrhea, hemoptysis, diabetes, dysentery, asthma, piles, burns, swelling and leucorrhea.[8] In addition, it is used as wash for wounds.[8] Few studies evaluated antibacterial and anti-oxidant effects of this plant.[9,10] However, there are no studies under taken to evaluate anti-inflammatory effect of the plant. Therefore, this study was designed to determine anti-inflammatory activity of F. racemosa bark using albumin denaturation method as an indirect measure against inflammation.
Methodology
Collection of the plant material and extraction procedure
F. racemosa bark was collected from Western province, Sri Lanka in March 2017. The plant material was taxonomically identified by Ayurveda doctors. The bark of F. racemosa was air-dried. A sample specimen has been preserved in the laboratory for future studies. After collecting the bark, it was made into powder using the traditional pestle and mortar method and stored in airtight container.
Hot and cold water extraction
The bark specimens of F. racemosa were gently washed under running tap water and then with distilled water for few seconds to reduce the accumulation of impurities. Plant parts were then air-dried at room temperature for 1 week to get constant weight. The dried parts were later ground to powder. About 0.2 g of dried samples of F. racemosa bark powder were added into a conical flask containing 20 ml of distilled water. It was kept at room temperature for 15 min and later, it was kept at 80°C for 20 min. The extract was filtered using a muslin cloth.[9] Thereafter, it was stored in a refrigerator at −4°C until used. Similarly, 0.2 g of the F. racemosa bark powder was kept at 24°C for 60 min for cold water extraction.
Preparation of reference drug (positive control)
NSAID (ibuprofen) and one steroid (prednisolone) were used as reference drugs. Prednisolone was crushed into fine powder. About 0.2 g of prednisolone drug powder was measured using a digital analytical balance (Adam PW 254) and was added to 20.0 ml of distilled water, respectively. The solution was mixed well using a vortex. A similar procedure was done for ibuprofen.[11]
Serial dilutions
Serial dilution from 1000 μg/ml to 0.01 μg/ml was performed for F. racemosa extract and for reference drugs (prednisolone and ibuprofen). All samples contained 5.0 ml of total volume. Reaction mixtures were prepared using 2.8 ml of phosphate-buffered saline (pH 6.4) and 0.2 ml of egg albumin (from fresh hen’s egg). Then 2 ml of F. racemosa bark extract from each different concentration were mixed gently with reaction mixtures. A similar procedure was used for reference drugs (prednisolone and ibuprofen) and they were used as positive controls for this study. In addition, distilled water was used as negative control.
Inhibition of protein denaturation
Reaction mixtures were incubated in a water bath at 37°C ± 2°C for 15–20 min, and later, it was heated at 70°C at which the reaction mixture was maintained for 5 min. Then, the reaction mixture was allowed to cool down at room temperature for 15 min. Absorbance of reaction mixture before and after denaturation was measured for each concentration (1000 μg/ml, 100 μg/ml, 10 μg/ml, 1 μg/ml, 0.1 μg/ml and 0.01 μg/ml) at 680 nm using a colorimeter. Each test was repeated thrice and the mean absorbance was recorded. The percentage of inhibition of protein was determined on a percentage basis with respect to control using the following formula.
Percentage inhibition (%) =
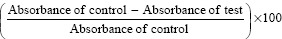
Statistical analysis
All data were analyzed statistically using SPSS version 20 (IBM, Somers, NY, USA). The descriptive data were expressed as mean ± standard error of mean. The percentage of inhibition rate between different groups was analyzed by independent sample t-test. The differences were considered to be statistically significant when P < 0.05.
Results
Anti-inflammatory activity of F. racemosa was evaluated against denaturation of egg albumin method. The highest inhibition rate was observed in both hot and cold water extracts at the concentration of 1000 μg/ml. There was significantly higher inhibition in hot water extractions as compared to cold water extractions at the concentrations of 0.01 μg/ml and 0.1 μg/ml [Table 1].
Table 1.
The percentage of inhibition rate of protein denaturation using cold and hot water
| Concentration (μg/ml) | Rate of inhibition (%) | ||
|---|---|---|---|
| Cold water | Hot water | P | |
| 0.01 | 13.26±0.96 | 19.29±1.34 | 0.021 |
| 0.1 | 16.30±0.93 | 19.58±0.62 | 0.007 |
| 1 | 22.43±1.49 | 20.71±0.66 | 0.351 |
| 10 | 24.74±0.75 | 22.43±1.32 | 0.204 |
| 100 | 25.09±2.27 | 23.73±3.36 | 0.753 |
| 1000 | 27.71±0.72 | 27.65±0.73 | 0.954 |
Results are shown as mean±SEM. SEM: Standard error of the mean
With the comparison of water extracts, the inhibition rates of two reference drugs were low. In addition, the inhibition rate of cold water extract, hot water extract and prednisolone gradually increased with the increase in concentration while a decreasing trend of inhibition rate was observed with ibuprofen [Table 2].
Table 2.
Percentage of inhibition rate of protein denaturation of reference drugs
| Concentration (μg/ml) | Rate of inhibition (%) | |
|---|---|---|
| Ibuprofen | Prednisolone | |
| 0.01 | 15.13±3.56 | 5.43±0.14 |
| 0.1 | 12.09±0.44 | 3.95±1.05 |
| 1 | 11.71±0.51 | 5.03±1.04 |
| 10 | 11.29±4.27 | 5.21±1.04 |
| 100 | 12.16±1.96 | 6.47±1.51 |
| 1000 | 9.77±1.11 | 8.83±1.51 |
Results are shown as mean±SEM. SEM: Standard error of the mean
The inhibition rate of cold and hot water extracts from 0.1 μg/ml was significantly higher than prednisolone and ibuprofen (P < 0.05).
The value of IC50 of cold water and hot water extract was 3691.97 and 4207.1 μg/ml, respectively [Figure 1 and 2]. In addition, the value of IC50 of ibuprofen and prednisolone was 13,377.5 and 11,812.18 μg/ml, respectively [Figure 3 and 4].
Figure 1.
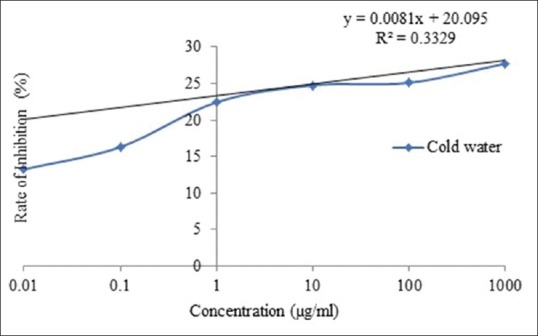
IC50 of Ficus racemosa cold water extract for protein denaturation
Figure 2.
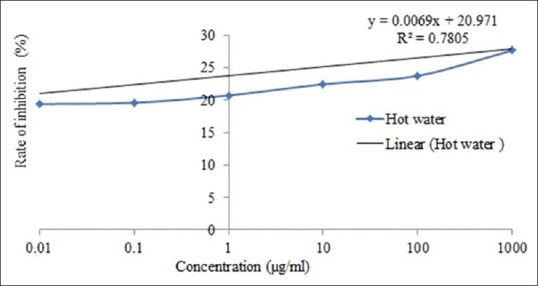
IC50 of Ficus racemosa hot water extract for protein denaturation
Figure 3.
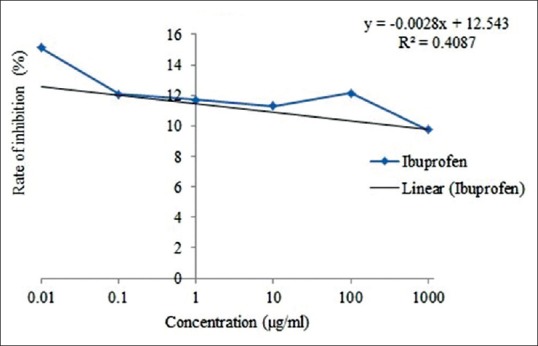
IC50 of protein denaturation in ibuprofen
Figure 4.
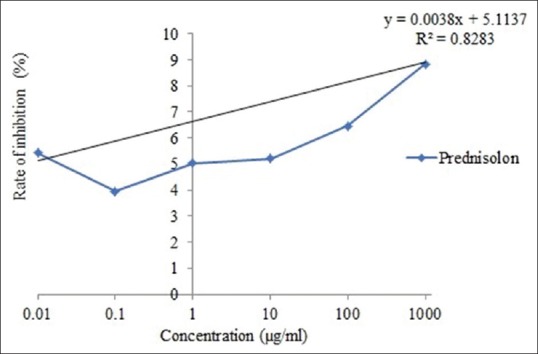
IC50 of protein denaturation in prednisolone
Discussion
Denaturation of protein has an unpredictable mechanism which includes modification in electrostatic hydrogen, hydrophobic and disulfide bonding.[2] Denaturation of protein causes the production of autoantigens in conditions such as rheumatic arthritis, cancer and diabetes which are conditions of inflammation. Hence, by inhibition of protein denaturation, inflammatory activity can be inhibited.[12] In the present study, two types of drugs (NSAIDs and steroids) were used as reference drugs. NSAIDs prevent inflammation by blocking the cyclooxygenase enzyme activity. However, these drugs cause side effects of ulceration, hemorrhage, perforation and obstruction.[13] Anti-inflammatory activity of F. racemosa has been reported in several studies. Anti-inflammatory activity of the leaves was reported using the petroleum ether extract by suppressing histamine and serotonin which are causes for inflammation.[14] The leaf extracts revealed significant ability to reduce the number of fibroblasts and synthesis of collagen and mucopolysaccharide which are needed to form granulation tissues.[15]
Previous studies exhibited analysis and anti-inflammatory properties of leaves and fruit extracts of the same plant. Present investigation is a study of stem bark for the claimed application of the same plant. The results supported the traditional use of this plant in some painful and inflammatory conditions. Because of the presence of biologically active principles, i.e. flavonoids, tannins, phenolic compounds and phytosterols in the same plant from phytochemical investigation, it is suggested that one of the above constituent or in combination together is responsible for producing the analgesic and anti-inflammatory effects. Further studies are in progress to isolate and characterize the active principle from the stem bark of the F. racemosa Linn. The oral LD50 obtained with this plant extract also suggested that it may have a reasonable safety margin with regard to acute toxicity, further justifying its wide application in various communities and lack of any reported side effect with the traditional use of this plant.
Ibuprofen is a nonselective NSAID which is a derivative of propionic acid and it is prescribed as an analgesic, anti-inflammatory and antipyretic agent. Prednisolone is considered as oral steroid utilized as an alternative because of its mitigating impact, especially in asthma and allergic condition and infections.
In the present study, it was found that the inhibition rate of cold water extract, hot water extract and prednisolone gradually increases with the increase in the concentration. In addition, a decreasing trend of inhibition rate was observed with ibuprofen. It was found that 1000 μg/ml of the F. racemosa bark extract showed the highest anti-inflammatory activity against 1;32ZEX denaturation of the protein. Similarly, Mandal et al. (2000) reported that F. racemosa Linn. leaf extract (400 mg/kg) exhibited around 32% of anti-inflammatory activity.[16] Furthermore, the effect against albumin denaturation in high concentration of F. racemosa extracts was significantly more than reference drugs. Denaturation of protein causes production of auto-antigens in conditions such as rheumatic arthritis, cancer and diabetes which are conditions of inflammation as mentioned above. Hence, by inhibition of protein denaturation, inflammatory activity can also be inhibited.[13]
In vivo studies can be undertaken where in vivo techniques such as carrageenan-induced rat paw edema test and egg albumin-induced rat paw edema test can be carried out using different concentrations of the specimens in the future. Other in vitro studies such as bovine serum albumin and membrane stabilization test can be further performed for an effective result.
Egg albumin method provides a cheap alternative method of testing the anti-inflammatory activity of herbal medicine using denaturation technique and this method should be validated by conducting further studies. Finally, can be concluded that F. racemosa has a proven anti-inflammatory effect against the egg albumin denaturation method. This study showed that cold water and hot water extract of F. racemosa bark possess in vitro anti-inflammatory effect.
Conclusion
F. racemosa bark contains anti-inflammatory property against egg albumin denaturation method and has significantly more potent activity than reference drugs (ibuprofen and prednisolone). Further in vivo and in vitro studies should be performed to establish anti-inflammatory activity of F. racemosa bark.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The author would like to thank the staff of the Institute of Ayurveda and alternative medicine for providing the knowledge of uses of F. racemosa bark in Sri Lankan traditional medicine and for infrastructure support.
References
- 1.Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. J Drug Dev Res. 2011;3:189–96. [Google Scholar]
- 2.SSen S, Chakraborty R, Maramsa N, Basak M, Deka S, et al. In vitro anti-inflammatory activity of Amaranthus caudatus L leaves. Indian J Nat Prod Resour. 2015;6:326–9. [Google Scholar]
- 3.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Can Soc Allergy Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel PA. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman A, editors. The Pharmacological Basics of Therapeutics. 9th ed. New York: McGraw Hill; 1996. pp. 617–57. [Google Scholar]
- 5.Marliyah M, Ananthi T. In vitro anti-inflammatory activity of extract of Zea mays (L.) J Glob Biosci. 2015;4:2168–73. [Google Scholar]
- 6.Nostro A, Germanò MP, D'angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379–84. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 7.Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed F, Urooj A. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: A review. Pharm Biol. 2010;48:672–81. doi: 10.3109/13880200903241861. [DOI] [PubMed] [Google Scholar]
- 9.Sharma SK, Gupta VK. In vitro antioxidant studies of Ficus racemosa Linn. Root. Pharmacogn Mag. 2008;4:70–3. [Google Scholar]
- 10.Vasudevan K, Sophia D, Balakrishanan S, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Ficus racemosa (Linn.) bark extracts in alloxan induced diabetic rats. J Med Sci. 2007;7:330–8. [Google Scholar]
- 11.Handa SS, Khanuja SP, Longo G, Rakesh DD. Technologies for Medicinal and Aromatic Plants. No. 66. 1st ed. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology; 2008. [Google Scholar]
- 12.Sangeetha G, Vidhya R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.) Int J Herb Med. 2016;4:31–6. [Google Scholar]
- 13.Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:121–32. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh MN, Banerjie RM, Mukherji SK. Capillary permeability-increasing property of hyaluronidase in rat. Indian J Physiol Pharmacol. 1963;7:17–21. [PubMed] [Google Scholar]
- 15.Arrigoni Martellie E. Inflammation and Anti-Inflammatories. New York: Spectrum Publications; 1977. p. 1190. [Google Scholar]
- 16.Mandal SC, Maity TK, Das J, Saba BP, Pal M. Anti-inflammatory evaluation of Ficus racemosa linn. Leaf extract. J Ethnopharmacol. 2000;72:87–92. doi: 10.1016/s0378-8741(00)00210-5. [DOI] [PubMed] [Google Scholar]


