Abstract
Background:
Plantago commonly called as Isabgol (Plantago ovata Forssk.) is a perennial herb that belongs to the family Plantaginaceae. A range of biological activities has been found from plant extracts, including wound healing activity, anti-inflammatory, analgesic, antioxidant, weak antibiotic, immunomodulating and anti-ulcerogenic activity. Periodontal disease is a complex condition as a result of interaction between microorganisms and host inflammatory mediators. Hence, the extract of Isabgol is tested for its antibacterial and anti-inflammatory properties against periodontal disease.
Aim:
The aim of this in vitro study is to evaluate the antibacterial property of Isabgol leaves and seeds against periodontal pathogens, namely Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum and anti-inflammatory property against matrix metalloproteinase-2 (MMP-2) and MMP-9.
Materials and Methods:
In this in vitro study, aqueous extract of Isabgol is tested for its antibacterial property against the stock cultures of specified periodontal pathogens using the tube dilution method and anti-inflammatory property against MMP-2 and MMP-9 using zymogen gel electrography.
Results:
Minimum concentration at which the sensitivity of A. actinomycetemcomitans, P. gingivalis, P. intermedia, and F. nucleatum for the extract observed was 50 μl/ml, 0.8 μl/ml, 0.4 μl/ml and 12.5 μl/ml, respectively, concentrations below these showed no effect on the microorganisms. Zymogen electrographic test for anti-inflammatory activity showed percentage inhibition of 30% and 40% against MMP-2 and MMP-9, respectively.
Conclusion:
Isabgol is effective against the periodontal pathogens and inflammatory mediators which are responsible for periodontal disease.
Keywords: Antibacterial, anti-inflammatory, Isabgol, matrix metalloproteinases, periodontitis, Plantago
Introduction
Plantago commonly called as Isabgol is a perennial herb that belongs to the family Plantaginaceae. The leaves of Plantago ovata[1] have long been used in wound healing and are still being used in traditional medicine.[1] Greek physicians described its usage in wound healing in the first century A.D.[2] In traditional medicine, the juice from the leaves of this plant is used to treat superficial wounds.[3] Norwegian and Swedish people called this plant “groblad” which means “healing leaves.”[4] A range of biological activities has been found from plant extracts, including wound healing activity, anti-inflammatory, analgesic, antioxidant, weak antibiotic, immune-modulating and anti-ulcerogenic activity.[4]
P. ovata is used in many parts of the world in treating skin diseases, infectious diseases and problems concerning the digestive organs, respiratory organs, reproduction, against tumors, for pain relief and for reducing fever.[4]
It is commonly used as a diuretic agent in India and China.[3] In some parts of India, the plant is used as Ayurvedic medicine to treat cut wounds, fever, weakness, respiratory infections and digestive system associated problems. In homeopathic medication, it is used to treat disorders of the epidermis, headache, earache and toothache.[1] Although the plant is known for its antibacterial and anti-inflammatory properties, it has been never evaluated for its efficacy on pathogens and inflammatory mediators resulting in periodontal disease.
Hence, the aim of this study is to evaluate the antibacterial property of Isabgol against Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum and anti-inflammatory property against matrix metalloproteinase-2 (MMP-2) and MMP-9.
Materials and Methods
In this in vitro study, extract of Isabgol leaves and seeds were tested for its antibacterial property against the stock cultures of specified periodontal pathogens using the tube dilution method and anti-inflammatory property against MMP-2 and MMP-9 using the zymogen gel electrography.[4] Extract of Isabgol was purchased from the VHCA herbals through online portal www.ayurvedacart.com. The extract was the aqueous type and was made by the cold maceration process, manufactured according to GMP (goods manufacturing practices) guidelines by the seller VHCA Ayurveda, Gaharaunda, Haryana, India.
Evaluation of antibacterial property
Antibacterial property was evaluated using the minimum inhibitory concentration (MIC) against three periodontal pathogens, i.e., A. actinomycetemcomitans, P. gingivalis, P. intermedia and F. nucleatum using the tube dilution method.
Minimum inhibitory concentration
Stock cultures of the mentioned organisms were obtained from the Department of Microbiology, Bapuji Pharmacy College, Davangere, Karnataka, India. Tube dilution method was carried out to evaluate the antibacterial property.
Tube dilution method
Nine dilutions of each drug was done with thioglycollate broth for MIC.
Initially, 20 μl of the extract was added to 380 μl of thioglycollate broth. For dilutions, 200 μl of broth was added in nine tubes separately. A volume of 200 μl from the initial tube containing extract was added to the first tube, this was 10−1 dilution.
From 10−1 diluted tube, 200 μl was transferred to the second tube to make 10−2 dilution. The serial dilution was repeated up to 10−9 dilution. Thus, the concentrations of the extract obtained after serial dilutions were 0.2, 0.4, 0.8, 1.6, 3.12, 6.25, 12.5, 25, 50, and 100 μl/ml, respectively.
From the maintained stock cultures of required organisms, 5 μl was taken and added into 2 ml of thioglycollate broth. In each serially diluted tube, 200 μl of above culture suspension was added. Tubes were incubated in anaerobic jar at 37°C for 48–72 h and observed for turbidity[5] [Figure 1].
Figure 1.

Tube dilution method
Evaluation of anti-inflammatory property
Anti-inflammatory property against two MMPs, i.e., MMP-2 and MMP-9 were evaluated in vitro through the zymogen electrographic method.
Sample preparation
Excised inflamed tonsil specimen was used as a source of the inflammatory sample. The sample was chopped, and 5 ml of Tris (tris[hydroxymethyl] aminomethane) buffer was added to it and centrifuged at 3000 RPM for 15 min and stored at −20° for further use.
Zymography
Proteolytic activity was examined on 10% polyacrylamide gels-containing 0.05% gelatin. The tissue sample was added to equal volume of nonreducing buffer (2.8 mL distilled water +1 ml 0.5M Tris HCL (pH 6.8) +0.8 ml glycerol +3.2 ml 10% SDS +0.2 ml 0.2% bromophenol blue). 20 μL of the mixture sample was loaded into each well and subjected to electrophoresis.
After electrophoresis, the gel was removed and put into a plastic dish and washed with zymogram renaturing buffer, i.e., 2.5% Triton x-100 for 1 h to remove SDS from the gel and allow proteins to denature. Decanting of the zymogram renaturing buffer and the gel was incubated in zymogram incubation buffer at 37°C overnight. The gel was then stained with Coomassie blue R-250 for 1 h, and then the gels were destained using appropriate destaining solution. After staining, the gels were observed for the white bands which indicate the presence of gelatinases. The anti-inflammatory activity of tested compounds lightens or clears the white bands against the dark background [Figure 2].
Figure 2.
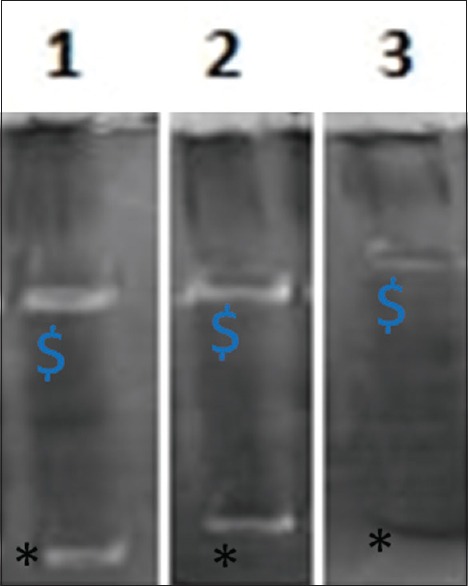
Zymogen gel electrography. $Matrix metalloproteinase-2, *matrix metalloproteinase-9. 1: Isabgol, 2: Negative control, 3: Positive control
Inhibition of metalloproteinase activity by the extract
To examine the effect of Isabgol extract on enzyme activity, conditioned medium containing MMPs was loaded on preparative gelatin-containing polyacrylamide gels. After electrophoresis, the gels were incubated at 37° for 16 h in Tris-CaCl2 buffer containing the extract. The concentration used was 100 μl/ml. After adding the extract to the solution, the pH was adjusted to 7.4, the gels were extensively washed in 2% Triton X-100, and reincubated in Tris-CaCl2 solution at 37°C for 16 h. To quantify the relative inhibition of MMPs by ZnSO4 and CuSO4, electrophoretic bands were scanned, and the transmittance (the transmittance values of the zymogen and active form were added) was analyzed with the SigmaGel software (Sigma – Aldrich Merck, Germany). The percentage inhibition of the extract was determined by comparing the activity of MMPs with control reactions.
MMPs sample without any compound was used for the negative control. MMPs sample with tetracycline stored for 1 h was used as the positive control.
Results
Of all the concentrations tested, the concentration at which the organisms have started showing sensitivity is recorded as MIC [Table 1]. The results varied among the organisms. A. actinomycetemcomitans showed more resistance when compared to other three organisms as the MIC required for A. actinomycetemcomitans was 50 μl/mL, whereas P. intermedia showed sensitivity at the lowest concentration when compared to others that was at 0.4 μl/mL. P. gingivalis and F. nucleatum showed sensitivity at a concentration of 0.8 μl/mL and 12.5 μl/mL, respectively.
Table 1.
Minimum inhibitory concentration
| Isabgol (Plantago ovata Forssk.) | 100 (μl/ml) | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Aa | S | S* | R | R | R | R | R | R | R | R |
| Pg | S | S | S | S | S | S | S | S* | R | R |
| Pi | S | S | S | S | S | S | S | S | S* | R |
| Fn | S | S | S | S* | R | R | R | R | R | R |
*MIC of the extract found using the tube dilution method. MIC for Aa is 50 μl/ml, Pg is 0.8 μl/ml, Pi is 0.4 μl/ml, Fn is 12.5 μl/ml, respectively. S: Sensitive, R: Resistant, Aa: Aggregatibacter actinomycetemcomitans, Pg: Porphyromonas gingivalis, Pi: Prevotella intermedia, Fn: Fusobacterium nucleatum, MIC: Minimum inhibitory concentration
Anti-inflammatory results were interpreted in percentage (%) inhibition of MMP-2 and MMP-9 when compared to the positive control (tetracycline) and negative control (no compound).
Isabgol showed 30% inhibitory activity against MMP-2, which was three times lesser than that of positive control but three times more than that of the negative control. Against MMP-9 Isabgol showed 40% inhibitory concentration which was 2.5 times lesser than that of positive control and two times more than that of the negative control [Table 2].
Table 2.
Anti-inflammatory activity
| Sample name | Anti-inflammatory activity against MMP-2 (%) | Anti-inflammatory activity against MMP-9 (%) |
|---|---|---|
| Isabgol (Plantago ovata Forssk. )-1st | 30%* | 40%* |
| PC | 90%# | 100%# |
| NC | 10%$ | 20%$ |
*Percentage inhibition of MMP-2 and MMP-9 by isabgol is 30% and 40%, respectively, #Percentage inhibition of MMP-2 and MMP-9 by positive control (tetracycle) is 90% and 100% respectively, $Percentage inhibition of MMP-2 and MMP-9 by negative control (no compound) is 10% and 20% respectively. PC: Positive control, NC: Negative control, MMP: Matrix metalloproteinase
Discussion
Recent ethnopharmacological studies reported that Plantago Ovata is used in many parts of the world, in the treatment of a number of diseases.[6] Plantago leaves and seeds contain carbhohydrates,[7-10] lipids,[11] alkaloids,[12] caffeic acid derivatives,[13,14] flavonoids,[15] iridoid glycosides,[16] vitamins and other organic substances owing to its diverse medicinal properties. Each of the constituents has unique medicinal property. The polysaccharide called plantaglucid extracted from Isabgol has shown to reduce the ulcer index in the rat stomach.[9] Clinical and histological studies showed that saturated C26–C30 primary alcohols with even numbers of carbon atoms from the n-hexane extract and the nonhydrolysable fractions of the n-hexane extract made from isabgol leaves had powerful curative effects on superficial injuries in rabbits.[17] Aucubin which is one of the iridoid glycosides present in leaves is known to have anti-inflammatory property through the inhibitory effect of TPA (12-o-tetradecanoylphorbol-13-acetate).[18] Isabgol has also been tested for wound healing properties by assessing the proliferation and migration of oral epithelial cells in vitro and the results showed that the extracts of Isabgol have beneficial effects on proliferation of epithelial cells suggesting its wound healing properties.
Studies showed that the extracts made from Isabgol have shown antibacterial activity against many bacteria such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis and methicillin-resistant S. aureus. The exact mechanism is not understood, but this antibacterial activity can be majorly attributed to a caffeic acid derivative called plantamajoside.[19] Plantamajoside is also known to have anti-inflammatory activity through its inhibitory effect on arachidonic acid metabolism,[20] and is also known to have anti-oxidant effect[21] and radical scavenging property.[22] The present study was undertaken to assess the efficacy of Isabgol on periodontal pathogens, and the results show that Isabgol is effective against P. gingivalis, A. Actinomycetemcomitans and F. nucleatum. Reason for selecting specific microorganisms is that the flora present in the dental plaque is predominated by anaerobic bacteria such as P. gingivalis, F. nucleatum, A. actinomycetemcomitans and has shown to be associated with onset and progression of periodontal disease.[23] However, it is now recognized that during active periodontitis, degradation of gingival tissue (mainly collagen) is due in part to MMPs expressed in situ by inflammatory cells and resident cells.[24,25] The proteolytic activity of MMP’s is under the control of endogenous tissue-specific inhibitors, the tissue inhibitors of metalloproteinases, as well as a2-macroglobulin.[26] Imbalance between these results in pathological processes. Hence, it has been tested for its anti-inflammatory properties against MMP-2 and MMP-9. The results showed its effect on these MMPs is weak when compared with the positive control. MMP-2 and MMP-9, also known as gelatinases A and B, respectively, are active in the degradation of denatured fibrillar collagens, elastase, and several other components of the extracellular matrix[27-29] MMP-2 and MMP-9 were selected because there are several evidence indicating that MMP-2 and MMP-9 play an important role in tissue destruction during periodontal disease.[28] Periodontitis patients have significantly higher levels of MMP-2 and MMP-9 than healthy participants, and the amount of gelatinases decreases after periodontal treatment.
As it is tested only against MMP-2 and MMP-9, the results cannot be extrapolated to other MMP’s.
Conclusion
Within the limitations of the study, Isabgol extract has shown to be an effective antibacterial and a weak anti-inflammatory agent. This is the first time, it is tested for its efficacy against periodontal pathogens and MMPs. Further confirmatory studies need to be conducted to prove it as an effective alternative for regularly used antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sharma PK, Chauhan NS, Lal B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in Western Himalaya, India. J Ethnopharmacol. 2004;92:167–76. doi: 10.1016/j.jep.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Roca-Garcia H. Weeds: A link with the past. Arnoldia. 1972;30:23–4. [Google Scholar]
- 3.Brondegaard V.J. Folk Flora. Kobenhavn: Rosenkilde Bagger; 1987. pp. 68–77. [Google Scholar]
- 4.de Souza AP, Gerlach RF, Line SR. Inhibition of human gingival gelatinases (MMP-2 and MMP-9) by metal salts. Dent Mater. 2000;16:103–8. doi: 10.1016/s0109-5641(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 5.Praveen NC, Rajesh A, Madan M, Chaurasia VR, Hiremath NV, Sharma AM, et al. In vitro evaluation of antibacterial efficacy of pineapple extract (Bromelain) on periodontal pathogens. J Int Oral Health. 2014;6:96–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorin AG. Polysaccharides from Plantago major leaves. I. Analysis of monosaccharide composition of polysaccharide complex. Chem Abstracts. 1966a;64:8277. [Google Scholar]
- 8.Gorin AG. Polysaccharides from Plantago major leaves. II. Pectic acid. Chem Abstracts. 1966b;64:11552. [Google Scholar]
- 9.Gorin AG, Maksyutina NP, Kolesnikov DG. New ulcer remedy from the leaves of Plantago major. Chem Abstracts. 1969;65:175–81. [Google Scholar]
- 10.Samuelsen AB, Paulsen BS, Wold JK, Otsuka H, Yamada H, Espevik T. Isolation and partial characterization of biologically active polysaccharides from Plantago major L. Phytother Res. 1995;9:211–8. [Google Scholar]
- 11.Ahmed ZF, Hammouda FM, Rizk AM, Wassel GM. Phyochemical studies of Egyptian Plantago species. Planta Med. 1968;4:404–10. doi: 10.1055/s-0028-1099927. [DOI] [PubMed] [Google Scholar]
- 12.Schneider G. Arzneidrogen, Ein Kompendium für Pharmazeuten, Biologien und Chemiker. Germany: Wissenschaftsverlag, Mannheim; 1990. p. 131. [Google Scholar]
- 13.Pailer VM, Haschke-Hofmeister E. Inhaltstoffe aus Plantago major. Planta Med. 1969;17:139–45. doi: 10.1055/s-0028-1099839. [DOI] [PubMed] [Google Scholar]
- 14.Maksyutina NP. Hydroxycinnamic acids of Plantago major and P. lanceolata. Chem Natl Compounds. 1971b;7:795. [Google Scholar]
- 15.Kawashty SA, Gamal el Din E, Abdalla MF, Saleh NA. Flavonoids of Plantago species in Egypt. Biochem Syst Ecol. 1994;22:729–33. [Google Scholar]
- 16.Handjieva N, Spassov S, Bodurova G. Majoroside, an iridoid glucoside from Plantago major. Phytochemistry. 1991;30:1317–8. [Google Scholar]
- 17.Mironov VA, Vasil'ev GS, Matrosov VS, Filipova TM, Zamureenko VA, Mishchenko VV, et al. Physiologically active alcohols from great plantain. Khimiko Farmatsevticheskii Zhurnal. 1983;17:1321–5. [Google Scholar]
- 18.Recio MC, Giner RM, Máñez S, Ríos JL. Structural considerations on the iridoids as anti-inflammatory agents. Planta Med. 1994;60:232–4. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- 19.Ravn H, Brimer L. Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major subsp. major. Phytochemistry. 1988;27:3433–7. [Google Scholar]
- 20.Murai M, Tamayama Y, Nishibe S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Med. 1995;61:479–80. doi: 10.1055/s-2006-958143. [DOI] [PubMed] [Google Scholar]
- 21.Miyase T, Ishino M, Akahori C, Ueno A, Ohkawa Y, Tanizawa H. Phenylethanoid glycosides from Plantago asiatica. Phytochemistry. 1991;30:2015–8. [Google Scholar]
- 22.Skari KP, Malterud KE, Haugli T. Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major a medicinal plant. In: Kumpulainen JT, Salone JT, editors. Proceedings of the 2nd International Conference on Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease. Cambridge: The Royal Society of Chemistry; 1999a. pp. 200–2. [Google Scholar]
- 23.Perinetti G, Paolantonio M, Cordella C, D’Ercole S, Serra E, Piccolomini R. Clinical and microbiological effects of subgingival administration of two active gels on persistent pockets of chronic periodontitis patients. J Clin Periodontol. 2004;31:273–81. doi: 10.1111/j.1600-051x.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994;8:312–9. doi: 10.1177/08959374940080022701. [DOI] [PubMed] [Google Scholar]
- 25.van der Zee E, Everts V, Beertsen W. Cytokines modulate routes of collagen breakdown. Review with special emphasis on mechanisms of collagen degradation in the periodontium and the burst hypothesis of periodontal disease progression. J Clin Periodontol. 1997;24:297–305. doi: 10.1111/j.1600-051x.1997.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 26.Starkey PM, Barrett AJ. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973;131:823–31. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkedal Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 28.Mäkelä M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity:Cellular origin and relationship to periodontal status. J Dent Res. 1994;73:1397–406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 29.Creemers LB, Jansen ID, Docherty AJ, Reynolds JJ, Beertsen W, Everts V. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 1998;17:35–46. doi: 10.1016/s0945-053x(98)90123-8. [DOI] [PubMed] [Google Scholar]


