Abstract
Broadleaf weeds are very costly for crop growers. Additional herbicidal compounds need to be obtained, especially from natural sources. Extracts of Icacina trichantha were evaluated for responses in germinating seeds and seedlings of rice (Oryza sativa) and Arabidopsis (Arabidopsis thaliana). An ethyl acetate fraction of I. trichantha tuber and a diterpenoid constituent, icacinol (1), were found to have impacts on germination and growth of seedlings. The seed germination inhibitory activity on rice was minimal, but significant on Arabidopsis. While rice indicated some growth delay in leaf expansion in the presence of 1, the effects appeared temporary; chlorophyll and anthocyanins were not significantly altered compared to DMSO controls. Rice seedlings attained biomass similar to DMSO controls, and rice grains per panicle were not significantly different than the DMSO-controls. On the other hand, Arabidopsis exhibited damage to leaf expansion, reduced chlorophyll, and increased anthocyanins in aerial portions of the seedlings. Icacinol (1) may be a suitable chemical agent to investigate further for the treatment of eudicot weeds.
Graphical Abstract
Weeds and invasive plant species negatively impact agriculture worldwide. They compete with crop plants for water, nutrients, and light, interfering with crop growth, and can ultimately reduce yields (for reviews, see Refs.1–3). Weeds can be controlled by a variety of means including mechanical, chemical, and biological methods. The chemical methods employed utilize physiologically active compounds, both synthetic and naturally occurring, yet novel chemicals to combat weeds and invasive species require further exploration and validation. Novel chemicals can be identified by screening of chemical libraries, but functional herbicidal studies are inevitably required, as many experiments are done in plant cell cultures.4–6 While seedlings are used in phenotype-based chemical screens to identify small molecules that disrupt processes,7 screening pipelines require improvements in the detection of functional impact, in order to identify new types of molecules that impact plant growth, as well as candidates that do not pose environmental problems. Plants are rich sources of small-molecule organic compounds, with advantages of being renewable and biodegradable in the environment. Useful targeting would include finding compounds that elicit stress responses early in development in weeds, particularly in the seed to seedling transition, with low negative impact on crops of economic importance.
Icacina trichantha Oliv. (Icacinaceae), a drought-resistant shrub native to West and Central Africa, is a food and medicinal plant used by the indigenous tribes in Nigeria and neighboring countries.8 Our group has reported a series of novel pimarane-type diterpene lactones in the tubers of the plant, including (9βH)-pimarane lactones, 17-nor-pimarane lactones, 17-nor-(9βH)-pimarane lactones, rearranged 17-nor-pimarane lactones, and di-nor-pimarane lactones.9–13 Several of these secondary metabolites have displayed cytotoxic activity against human cancer cell lines.11–14 During fractionation and purification of a MeOH extract of the tubers of I. trichantha, it was observed that the EtOAc-soluble fraction induced growth dysregulation in the weed-like dicot plant Arabidopsis thaliana, but not in rice (Oryza sativa), leading to the isolation of icacinol (1) as an active principle. The activities of the EtOAc fraction and 1 are described herein, where the herbicidal characteristics may indicate a useful herbicidal component for monocot crops.
The tubers of I. trichantha were extracted with 80% aqueous MeOH by percolation. The extract was dried, suspended in water, and successively partitioned with petroleum ether, EtOAc and n-BuOH into three fractions denoted S1 – S3, respectively, together with the water-soluble part (S4). All four fractions were tested for seed germination activity using rice (Oryza sativa L. “Nipponbare”) and Arabidopsis (Arabidopsis thaliana Col) in initial screens. The EtOAc-soluble fraction (S2) displayed significant activity. Further plant response assays were conducted with S2 and subsequent chromatographic fractionation afforded an active compound, icacinol (1), which is a (9βH)-pimarane lactone previously found in I. claessensii,15 I. senegalensis,16 and Casimirella sp.14 This compound has also shown cytotoxicity against the A2780 human ovarian cancer,14 MDA-MB-435 human melanoma, MB-231 human breast cancer, and OVCAR3 human ovarian cancer12 cell lines. No other biological activity of this compound has been reported.
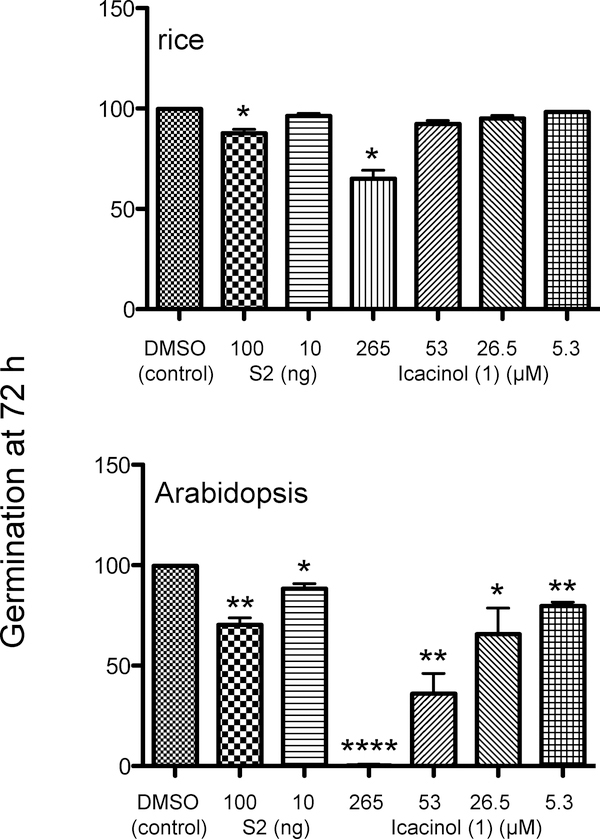
Dilutions of fractions of I. trichantha were screened on 0.5X MS minimal medium, at 10 ng/mL and 100 ng/mL, as an initial test. Most fractions (S1, S3 and S4) did not impede or slow germination of either the monocot rice (Oryza sativa) or dicot Arabidopsis (Arabidopsis thaliana) (Figure S1, Supporting Information). However, the ethyl acetate fraction (S2) and icacinol (1) exhibited significant effects on seed germination (Figure 1). For rice seeds, the effect of S2 was significant at 100 ng/mL (p = 0.0267), with 87% of seeds germinating by 72 h compared to almost 100% of the untreated (DMSO control). Other dilutions were not significant (10 ng/mL shown). The icacinol (1) treatment was significant only at 265 μM. Other dilutions shown did not affect germination. Arabidopsis seed responses, however, indicated much more sensitivity to S2 and 1. When seeds were grown in the presence of S2, the germination rates at 72 h were 62% (100 ng/mL, p = 0.037) and 88% (10 ng/mL, p = 0.0169), compared to the vehicle-only (DMSO) treatment. At 5.3 μM, 1 reduced germination by 72 h, with about 80% of seeds germinating (p = 0.0039). Dose-specific responses were observed at 26.5 μM (66%; p = 0.0459), 53 μM (36%; p = 0.0084), and 265 μM (< 1%; p < 0.0001).
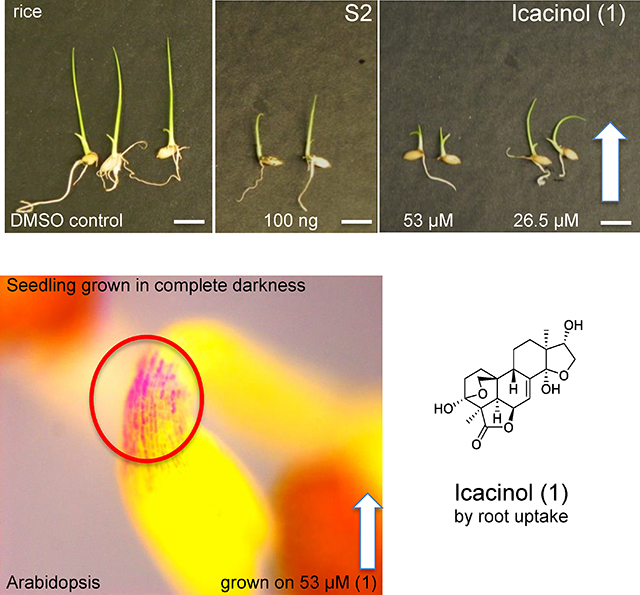
Figure 1.
Icacina tricantha fractions and icacinol (1) effects on seed germination. Extractions were conducted as described in Experimental Section. S2 (EtOAc-soluble fraction) and 1 were mixed in top agarose with seeds at concentrations indicated in the figure; 100 seeds per replicate were sown. Seed germination was scored 72 h postplating at 20°C for Arabidopsis and at 28°C for rice.
Icacinol (1) was found to be the most active among the tested components on plant germination responses. Importantly, there was a clear difference in seed germination between Arabidopsis (a eudicot) and rice (a monocot), where Arabidopsis germination was severely impaired under the conditions used. The chief difference of monocots and eudicots in germination is related to the fact that monocot seed leaf is thin and the endosperm to feed the seed leaf is outside of the leaf itself, whereas the eudicots like Arabidopsis have two seed leaves with an endosperm to feed the embryonic plant. In addition, Arabidopsis is a very fast-growing weed-like plant, with photosynthesizing cotyledons. It is possible that the metabolic interactions in the cotyledons of Arabidopsis are disrupted in the process of germination and the concomitant greening process, as Arabidopsis cotyledons develop and photosynthesize within 2–3 days with summer (i.e., 16:8 light/dark cycle) conditions. Three pimarane-type diterpenoids, momilactones A-C, have been reported to inhibit germination of Arabidopsis.17–19
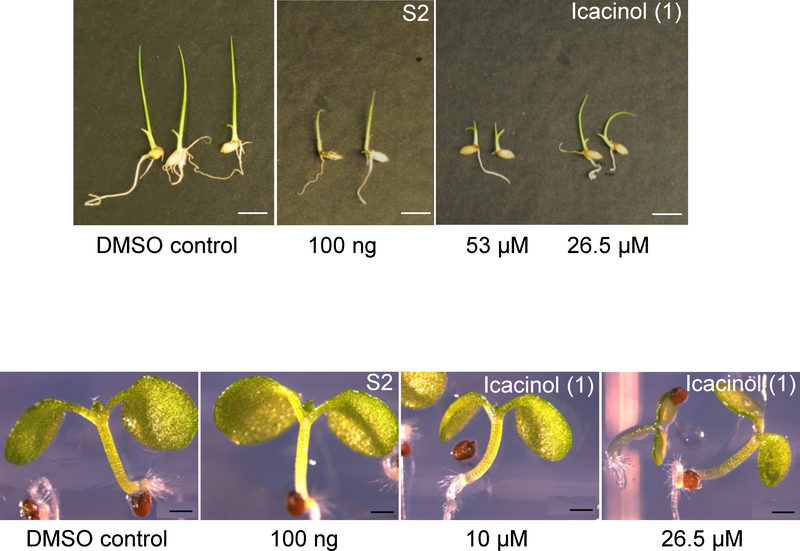
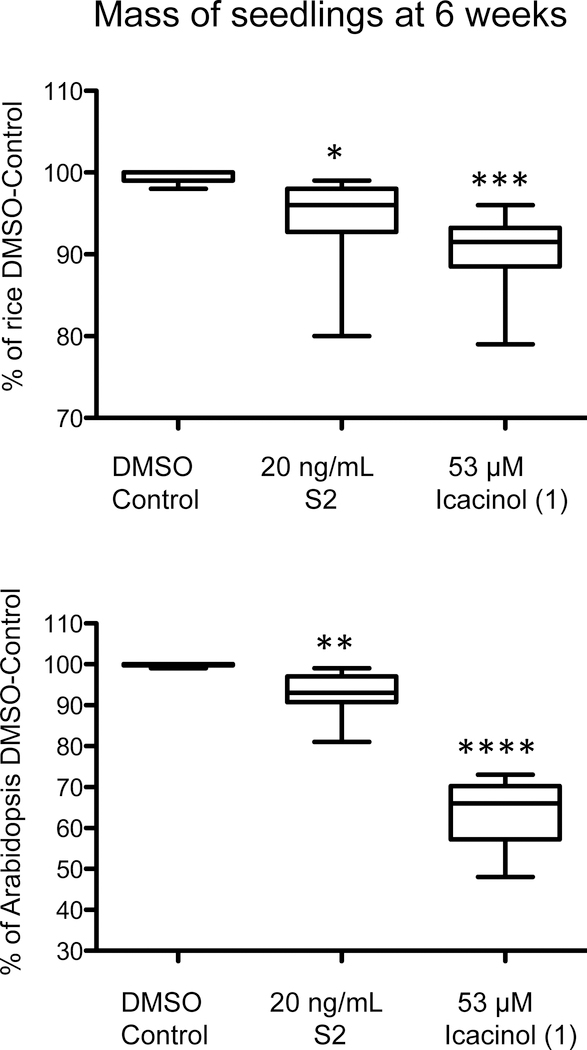
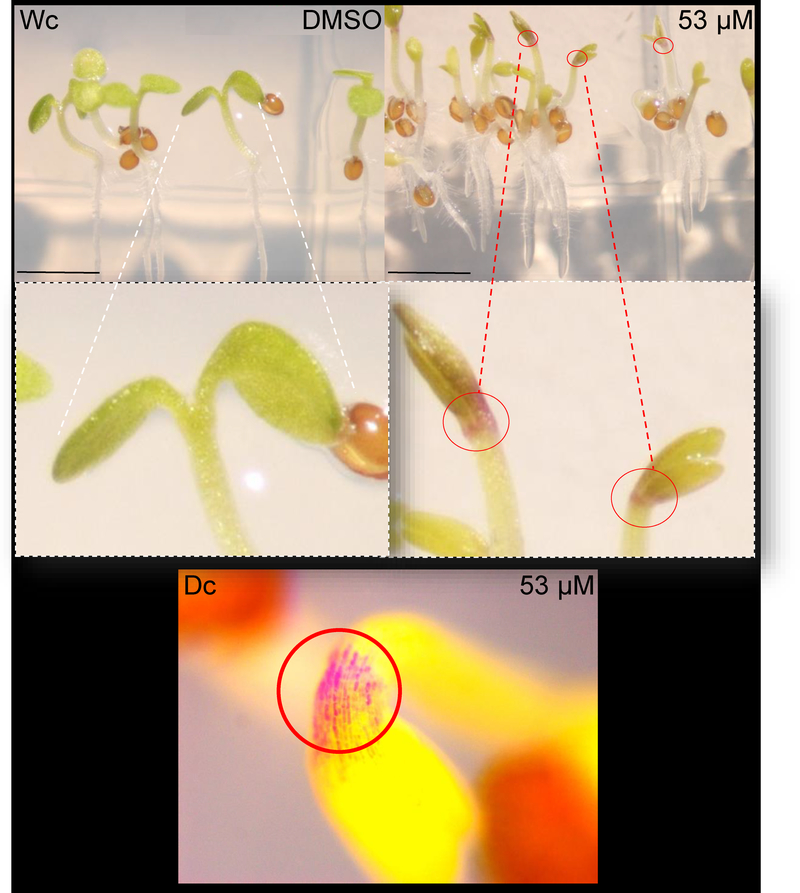
Vegetative development in young seedlings exposed to S2 or 1 was also explored, with seedlings observed daily and specific characteristics evaluated weekly, post-sowing. It was noted over several dilutions of S2 and 1 that rice, while delayed in growth, grew without mortality in vegetative stage, then switched to reproductive mode to seed. Growth (Figure 2) observed at two weeks post-sowing was delayed for rice, where by six weeks the biomass of whole plant presented comparable means (Figure 3; DMSO-control mean 99.6% vs. 94.2% for S2, 90.4% for 1 at 53 μM), yet, based on comparison of the means, data were statistically significant. In addition, rice grains per panicle (when plants were permitted to go to seed) were highly similar (DMSO control compared to 1 at 26.5 μM; Figure S2, Supporting Information). However, Arabidopsis treated with 1 showed greater mean biomass differences (Figure 3; DMSO-control mean 99.8% vs. 92.8% for S2, 64.4% for 1 at 53 μM). Arabidopsis did not achieve transition to reproductive stage within typical time when treated with 1 (compared to untreated). Rice treated with 1 also showed no difference in chlorophylls (no reductions) and no increase in anthocyanins by two weeks post-germination (Figure S3). However, Arabidopsis treated with 1 showed indications of stress, where anthocyanins increased and chlorophyll was reduced (Figure S3). The tissue size at this same age (shoot 1.79 vs. 1.59 n.s.; root 5.09 vs. 4.075, p < 0.01) was significant for the roots of Arabidopsis. Arabidopsis signs of damage can be seen in cotyledons (less chlorophyll) and anthocyanin accumulation manifested at the apical hook (Figure 4). Anthocyanin accumulation can occur in response to many chemicals including herbicides or growth regulators.20 Damage to the Arabidopsis leaves by 1 (53 μM) treatment also included chlorosis and lack of cotyledon expansion, with an appearance similar to auxin-related herbicides that in dicots can cause oxidative stress and destruction of the chloroplast (leaving rice and other crops lesser affected) by still poorly understood mechanisms.21 The present experimentation was not on soil for the majority of tests (60 days or less) and this may affect the plant responses. The growth to seed set occurred on soil (transplants, see methods in Experimental Section).
Figure 2.
Icacina tricantha extracts and icacinol (1) impacts on vegetative growth. Extractions were conducted as described in the Experimental Section. S2 (EtOAc-soluble fraction) and 1 were mixed in top agarose with seeds at concentration indicated in the figure. Plants were grown on that medium for 2 weeks at 20°C (28°C for rice) in 16:8, then photographed as shown. Images are representative. Top panel rice (size bar 2 cm), lower panel Arabidopsis (size bar 250 μm).
Figure 3.
Icacina tricantha extract and icacinol (1) impacts on longer term vegetative growth. Rice and Arabidopsis seeds were sown; then seedlings were grown for 6 weeks in 16:8 (rice at 28°C; Arabidopsis at 20°C), with S2 or 1 in the medium as described in Figure 2. Ten random representative seedlings were selected from each set. Tissue was harvested, dried, then weighed for comparison of the mass to the untreated control (DMSO control; % of control shown). DMSO controls were compared to completely untreated controls to determine their percent. * P<0.05; ** P<0.01; ***P<0.001; ****P<0.0001
Figure 4.
Icacinol (1) impact on Arabidopsis seedling growth. 1 was mixed in top agarose with seeds at the concentration indicated, sown on vertical plates. Plants were grown for 14 days at 20°C in 16:8, sown on vertical plates (there is some limited contact with gel by stem and leaf edge at emergence), and then photographed on adissecting microscope as shown. Images are representative. The manifestation of anthocyanin response at the apical hook and leaf base is indicated by circled pigmented area and enlargement. Upper 2 panels images grown in continuous white light (Wc); lower image shows seedlings grown in continuous darkness (Dc) on Phytatrays.
In the literature, two (9βH)-pimarane diterpenes, momilactones A and B, found in rice and the moss Hypnum plumaeforme, have shown allelopathic or herbicidal activities.22–25 Momilactone B has exhibited growth inhibition on barnyard grass (Echinochloa crus-galli), a major noxious weed associated with rice and other monocot and dicot plants.26 Further, momilactone B exhibited significant antifungal activity against the rice blast pathogen Magnaporthe oryzae, which is responsible for the devastating disease that has been estimated to cause the loss of 10–30% of the total rice harvest and also infects wheat, barley, and millet crops.27 Momilactones A and B were reported to be allelochemicals in the defense responses of moss (Hypnum plumaeforme and Pseudoleskeella papillosa) as well.17,28 Individual momilactones A and B have already been patented as herbicides and potential anticancer agents.
The discovery of the potential herbicidal activity of icacinol (1) now adds to our knowledge of the (9βH)-pimarane diterpenes. These versatile molecules seem to be the natural choice for plant defense during evolution, and they can serve as prototypes for other lead compounds in the search for crop-friendly herbicides, antifungal agents, and related agricultural substances. Further studies on natural sources to enrich structural diversity of momilactone-like molecules and discovery of more promising lead compounds and adjuvants for treatment are desirable goals for weed management.
EXPERIMENTAL SECTION
General Experimental Procedures
Chemicals for plant experiments, and sterile plastics (square petri dishes, phytatrays™ and phytatray II™) unless otherwise noted, were obtained from Sigma-Aldrich (St. Louis, MO USA). Seeds were sterilized in bleach solution (49% sodium hypochlorite [6.05%], 49% sterile water, 2% 0.1% Triton X-100) with vigorous shaking for 5 min then 4 washes with sterile water. All materials in contact with seeds or plants were sterilized by autoclaving or purchased sterile (gamma irradiated) or sterile (0.22μm) filtered (molecular biology grade water). In order to test specific chemical concentrations per area, 0.5XMS minimal medium trays were made (50mL phytatray™, 50 mL vertical tray) for seed germination and plant growth.31 Rice when transplanted went into autoclaved soil/sand mix.
Plant Material
Tubers of Icacina trichantha were collected in June 2011 from Orba village, Nsukka, Enugu State, Nigeria, and authenticated by Prof. B. O. Olorede of the Botany Department, University of Abuja, Nigeria, and Mr. A. Ozioko, botanist at BDCP Laboratories, Nsukka, Nigeria. A voucher specimen (UNN/FVM 456) was deposited at the pharmacology laboratory at the University of Nigeria, Nsukka, Nigeria.
Extraction and Isolation
The powdered tubers of I. trichantha (1.5 kg) were extracted with 80% aq. MeOH by percolation to yield 166 g of dry crude extract, which was partitioned into petroleum ether-soluble (S1, 11 g), EtOAc-soluble (S2, 17 g), BuOH-soluble (S3, 15 g), and H2O-soluble (S4, 128 g) fractions. The EtOAc-soluble fraction (S2) was further fractionated. A precipitate from sub-fraction 47–53 was purified by further chromatography using a mixture of CH2Cl2 and MeOH to yield icacinol (1), a crop of 50 mg crystals of which was finally obtained. This compound exhibited physical and spectroscopic parameters consistent with literature data,12 NMR data is shown (Figure S4, Supporting Information).
Plant Growth Response Screening Materials
Stock solutions were made of all final extract fractions at 10 μg/mL, with icacinol (1) resuspended to a 265 μM solution in sterile DMSO, then were diluted for use in 0.5XMS27 for experiments described herein. Arabidopsis thaliana (Arabidopsis) Columbia WT seed was originally obtained from ABRC/TAIR.29 Bulk seed stocks were grown as previously reported.30 Seeds of Oryza sativa (rice) Nipponbare (obtained from KW) were sterilized and sown in the same manner as Arabidopsis seed, as described,31 but incubated at 28 °C for germination.
Assays for Growth Responses
Seeds were surface sterilized in bleach solution, then rinsed in sterile water, and plated on 0.5X MS (pH 5.8) in Phytatrays (Sigma, St. Louis, USA) with no sucrose and no added vitamins as described,31 but instead of a cold treatment, were given a 48 h darkness treatment at 20 °C. At the time of planting, a plant extract of S2 or 1 or DMSO (empty vehicle control) was diluted in 0.5X MS, then was added to 0.8% low melt agarose (0.5X MS; top agarose) when the autoclaved media fell below 50 °C, mixed well, then poured and allowed to solidify with sterilized and washed seeds in the top agarose in a thin layer. Dilutions of chemicals derived from plant were initially made at 1:20 to 1:5000 (depending on the experiment) from extract stock in DMSO (described above), diluted with 0.5X MS right before plating. After sowing seeds on plates, plates were placed into black boxes and moved to 20 °C complete darkness room for 48 h, then subsequently moved to white light at 120 μmol m−2s−1, at 20 °C for Arabidopsis and 28 °C for rice.
Vertical Growth Assays of Arabidopsis
On sterile square petri plates seeds were lined up in a row, 2.5 cm from the designated top edge, and 2.0 cm between each row. A 10-μL aliquot of seed in top agarose spotted onto a 0.5X MS phytatray base plate of different dilutions of S2 or 1. Plated, sterilized seed plates were turned vertical, secured into light-tight black Plexiglas™ boxes, sealed with aluminum foil then were cold placed in a dark room at 20 °C for 48 h. After 48 h the plates were moved to one of several locations: to a 24 h 20 °C dark environmental room (continuous darkness, i.e., Dc), or white light environmental room where lights were on for 24 h continuous (Wc) or lighting regime was 16:8 (16 h light, 8 h darkness),32 for varying numbers of days, ranging from 0 – 60 days, depending on the experiment. Lights sources, including dim green light for handling dark-grown seedlings, have been described.30, 32 The fluence rate received by the vertical plates and phytatrays determined by a LiCor meter was 120 μmol m−2s−1.
Germination and Phenotyping
Successful germination was determined under a dissecting scope by viewing rice and Arabidopsis seeds every 12 h post-sowing, scoring complete emergence of the radical as previously described.30 Phenotypic responses on untreated (control) and experimental extract plates were determined by comparison of chemically treated seeds to untreated plants (DMSO vehicle control), and counted on a Zeiss Stereo Discovery V.8 microscope at 1X using Axiovision (Zeiss). Plates were photographed at different times after stratification using Nikon Coolpix on a light box or black background. At least three sets of 30 seeds were scored for each germination assay on phytatrays at 24 h, 48 h, and 72 h post-stratification.
Vegetative Assessment
Rice and Arabidopsis seeds were sown on phytatray II to assess how they would grow over 7–60 days, depending on experiments. At four weeks, rice seedlings were transferred to soil/sand mix submerged in water in white catering 1-gallon buckets, where they were moved to greenhouse of similar fluence levels, and where daytime temperature was above 25 °C, in order to grow long-term, including for seed set (rice grain per panicle). Sets of plants were washed at dried to determine root and shoot dried weight at six weeks. Images and samples (100 seedlings × 3 replicates) for chlorophylls extraction33 and anthocyanins extraction34 were taken at two weeks. Seedlings were imaged on dissecting scope at various times to capture phenotypes as indicated in Figures.
Statistical Analysis
Phenotypes and plant responses were quantitated and the values entered into GraphPad/Prism V.5 for Mac, similar in the manner described.31 Equal variances were not assumed in plant materials (seeds and seedlings) and differences of means compared (typically to the DMSO-control data) were assessed by unpaired t-test (two-tailed) with Welch’s correction.
Supplementary Material
ACKNOWLEDGMENTS
B. Guo acknowledges the NIH Office of the Director and the National Center for Complementary and Integrative Health for a trainee fellowship (T32AT007533). M. M. Onakpa acknowledges a Fulbright Junior Scholar award (No. 15120356) to conduct research at the University of Illinois at Chicago (UIC). K. Warpeha acknowledges start-up funds from UIC, and thanks to Shamaila Zaheer for technical assistance.
REFERENCES
- (1).Shaik RS; Burrows GE; Urwin NAR; Gopurenko D; Lepschi BJ; Weston LA Crop Prot. 2017, 92, 29–40. [Google Scholar]
- (2).Tshewang S; Sindel BM; Ghimiray M; Chauhan BS Crop Prot. 2016, 90, 117–124. [Google Scholar]
- (3).Korres NE; Norwsorthy JK; Tehranchian P; Gitsopoulos TK; Loka DA; Oosterhuis DM; Gealy DR; Moss SR; Burgos NR; Miller MR; Palhano M Agron. Sustain. Dev 2016, 36, 12. [Google Scholar]
- (4).Macias FA; Castellano D; Molinillo JMG J. Agric. Food Chem 2000, 48, 2512–2521. [DOI] [PubMed] [Google Scholar]
- (5).Duke SO; Dayan FE; Rimando AM; Schrader KK; Aliotta G; Oliva A; Romagni JG Weed Sci. 2002, 50, 138–151. [Google Scholar]
- (6).Chung IM; Ahn JK; Yun SJ Can. J. Plant. Sci 2001, 81, 815–819. [Google Scholar]
- (7).Reigosa MJ; Pazos-Malvido EJ Chem. Ecol 2007, 33, 1456–1466. [DOI] [PubMed] [Google Scholar]
- (8).Burkill HM, The Useful Plants of West Tropical Africa, 2nd edn.; Royal Botanical Gardens: Kew, U.K., 1994; Vol. 2, pp 418–419. [Google Scholar]
- (9).Guo B; Onakpa MM; Huang X-J; Santarsiero BD; Chen W-L; Zhao M; Zhang X-Q; Swanson SM; Burdette JE; Che C-TJ Nat. Prod 2016, 79, 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zhao M; Onakpa MM; Zhang X-Q; Cheng J-J; Che C-T; Santarsiero BD; Huang X-J; Chen J; Longnecker R Org. Lett 2015, 17, 3834–3837. [DOI] [PubMed] [Google Scholar]
- (11).Zhao M; Onakpa MM; Chen W-L; Santarsiero BD; Swanson SM; Burdette JE; Asuzu IU; Che C-TJ Nat. Prod 2015, 78, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Onakpa MM; Zhao M; Goedecke T; Chen W-L; Che C-T; Santarsiero BD; Swanson SM; Uzoma Asuzu I Chem. Biodivers 2014, 11, 1914–1922. [DOI] [PubMed] [Google Scholar]
- (13).Zhao M; Onakpa MM; Santarsiero BD; Chen W-L; Szymulanska-Ramamurthy KM; Swanson SM; Burdette JE; Che C-TJ Nat. Prod 2015, 78, 2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Adou E; Williams RB; Schilling JK; Malone S; Meyer J; Wisse JH; Frederik D; Koese D; Werkhoven MCM; Snipes CE; Werk TL; Kingston DG I. Bioorg. Med. Chem 2005, 13, 6009–6014. [DOI] [PubMed] [Google Scholar]
- (15).On’okoko P; Vanhaelen M; Vanhaelen-Fastre R; Declercq JP; Van Meerssche M Tetrahedron 1985, 41, 745–748. [Google Scholar]
- (16).Vanhaelen M; Planchon C; Vanhaelen-Fastre R; On’Okoko PJ Nat. Prod 1987, 50, 312. [Google Scholar]
- (17).Liu N; Wang S; Lou H Acta Pharm. Sin. B 2012, 2, 256–259. [Google Scholar]
- (18).Kato-Noguchi H; Ota K; Kujime H; Ogawa M Weed Biol. Manage 2013, 13, 19–23. [Google Scholar]
- (19).Kato-Noguchi H; Kitajima S Nat. Prod. Commun 2015, 10, 729–732. [PubMed] [Google Scholar]
- (20).Chalker-Scott L Phytochem. Phytobiol 1999, 70, 1–9. [Google Scholar]
- (21).Kelley KB; Riechers DE Pest. Chem. Physiol 2007, 89, 1–11. [Google Scholar]
- (22).Kato-Noguchi H; Hasegawa M; Ino T; Ota K; Kujime HJ Plant Physiol. 2010, 167, 787–791. [DOI] [PubMed] [Google Scholar]
- (23).Chung IM; Kim JT; Kim S-HJ Agric. Food Chem 2006, 54, 2527–2536. [DOI] [PubMed] [Google Scholar]
- (24).Kato-Noguchi HJ Plant Physiol. 2011, 168, 1511–1516. [DOI] [PubMed] [Google Scholar]
- (25).Kato-Noguchi H; Peters RJ J. Chem. Ecol 2013, 39, 175–185. [DOI] [PubMed] [Google Scholar]
- (26).Kato-Noguchi H In Rice Allelopathy and Momilactones A and B; Nova Science Publishers, Inc.: 2010; pp 193–216. [Google Scholar]
- (27).Hasegawa M; Mitsuhara I; Seo S; Imai T; Koga J; Okada K; Yamane H; Ohashi Y Mol. Plant-Microbe Interact 2010, 23, 1000–1011. [DOI] [PubMed] [Google Scholar]
- (28).Kato-Noguchi H Plant Signaling Behav. 2009, 4, 737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Alonso JM; Stepanova AN; Leisse TJ; Kim CJ; Chen H; Shinn P; Stevenson DK; Zimmerman J; Barajas P; Chheuk R; Gadrinab C; Heller C; Jeske A; Koesema E; Meyers CC; Parker H; Prednis L; Ansari Y; Deen H; Geralt M; Hazari N; Hom E; Karnes M; Mulholland C; Ndibali R; Schmidt I; guzman P; Aguilar-Henonin L; Schmid M; Weigel D; Carter DE; Marchand T; Risseeuw E; Brogden D; Zeko A; Crosby WL; Berry CC; Ecker JR Science 2003, 301, 653–657. [DOI] [PubMed] [Google Scholar]
- (30).Orozco-Nunnelly DA; Muhammad D; Mezzich R; Lee BS; Jayathilaka L; Kaufman LS; Warpeha KM PLoS One 2014, 9, e93371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Para A; Muhammad D; Orozco-Nunnelly DA; Memishi R; Alvarez S; Naldrett M; Warpeha KM Plant Physiol. 2016, 172, 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Warpeha KM; Gibbons J; Carol A; Slusser J; Tree R; Durham W; Kaufman LS Plant Cell Environ. 2008, 31, 1756–1770. [DOI] [PubMed] [Google Scholar]
- (33).Pruzinska A; Tanner G; Aubry S; Anders I; Moser S; Muller T; Ongania KH; Krautler B; Youn JY; Liljegren SJ; Hortensteiner S Plant Physiol. 2005, 139, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Montgomery BL; Yeh AC; Crepeau MW; Lagarias JC Plant Physiol. 1999, 121, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.