Abstract
Background:
Digital pathology has progressed over the last two decades, with many clinical and nonclinical applications. Transplantation pathology is a highly specialized field in which the majority of practicing pathologists do not have sufficient expertise to handle critical needs. In this context, digital pathology has proven to be useful as it allows for timely access to expert second-opinion teleconsultation. The aim of this study was to review the experience of the application of digital pathology to the field of transplantation.
Methods:
Papers on this topic were retrieved using PubMed as a search engine. Inclusion criteria were the presence of transplantation setting and the use of any type of digital image with or without the use of image analysis tools; the search was restricted to English language papers published in the 25 years until December 31, 2018.
Results:
Literature regarding digital transplant pathology is mostly about the digital interpretation of posttransplant biopsies (75 vs. 19), with 15/75 (20%) articles focusing on agreement/reproducibility. Several papers concentrated on the correlation between biopsy features assessed by digital image analysis (DIA) and clinical outcome (45/75, 60%). Whole-slide imaging (WSI) only appeared in recent publications, starting from 2011 (13/75, 17.3%). Papers dealing with preimplantation biopsy are less numerous, the majority (13/19, 68.4%) of which focus on diagnostic agreement between digital microscopy and light microscopy (LM), with WSI technology being used in only a small quota of papers (4/19, 21.1%).
Conclusions:
Overall, published studies show good concordance between digital microscopy and LM modalities for diagnosis. DIA has the potential to increase diagnostic reproducibility and facilitate the identification and quantification of histological parameters. Thus, with advancing technology such as faster scanning times, better image resolution, and novel image algorithms, it is likely that WSI will eventually replace LM.
Keywords: Digital pathology, donor biopsy, graft biopsy, image analysis, transplantation
INTRODUCTION
Digital pathology has progressed over the last two decades and is being used for several clinical and nonclinical applications. Some of these use cases, including primary diagnosis, second-opinion consultation, archiving, education/training, research, and image analysis. Many studies have been performed on the implementation and validation of digital systems. Several reviews have reported on the concordance between whole-slide imaging (WSI) and conventional light microscopy (LM) in surgical pathology[1,2] and highlighted some of the technical challenges related to WSI in cytology.[3] In addition, several digital image analysis (DIA) tools have been developed over the years, and apart from their role in quantitative image analysis of breast biomarkers, these algorithms have been used mainly for research purposes.
Transplantation pathology is a highly specialized field in which the majority of pathologists do not have enough expertise to handle critical practice needs. Digital pathology can be extremely useful in this regard as it allows general pathologists to employ teleconsultation for intraoperative consultation as well as to rapidly gain an expert second opinion. In addition, DIA can be applied to transplant biopsies to facilitate the identification and quantification of several morphological parameters, as well as their spatial relationships.
The aim of this paper was to review the literature on transplantation digital pathology published in the last 25 years and to review the main issues, results, and future directions of the field.
METHODS
Papers on this topic were retrieved using PubMed as a search engine. The search was limited to papers written in the English language and published in the 25 years' time span until December 31, 2018, with the following search strategy: “(“digital” OR “whole slide imaging” OR “WSI” OR “digital pathology” OR “telepathology” OR “telemedicine” OR “image analysis”) AND (“transplant” OR “transplantation” OR “organ” OR “organ procurement” OR “preimplantation biopsy” OR “graft” OR “allograft”) AND (“renal” OR “kidney” OR “liver” OR “heart” OR “lung” OR “pancreas“)”. Inclusion criteria were the presence in the study of the transplantation setting, pre- or post-transplant, and the use of any type of digital pathology image, both with or without the use of image analysis tools. Papers dealing with digital pathology and biopsies but not in transplantation setting, reviews, and commentaries were excluded. Papers retrieved were divided into pre- and post-transplant phase and grouped according to the organ of interest in the study, type of digital pathology, use of image analysis tools, main topic of the study among concordance/reproducibility, assessment of features for organ outcome and rejection, and other morphological or immunohistochemical (IHC) issues.
Distribution of studies
A total of 2207 papers were retrieved with the search strategy, and the main reasons for exclusion on the basis of title and abstract were (i) the absence of the transplantation setting, as the term “transplant” was intended only for tissues in plastic and reconstructive surgery; (ii) the absence of a digitalized image, as the term “digital” was intended for other imaging modalities; and (iii) the use of animal models. The included papers were 93, with the note that a single study[4] comprised both pre- and post-transplant biopsies, so it was counted in both groups. The studies included so represented about 4% of all retrieved items. There were a growing number of publications in the last 15 years as more than 75% of papers have been published after 2004. Subdividing the studies according to the type of digital pathology, it can be seen how the static image modality use has started to decrease after 2008 and how the number of publications using WSI is increasing in the last decade, overcoming the static digitized image in the most recent period 2014–2018. A graphical summary of the distribution of studies over time is shown in Figure 1. Regarding the main issues addressed in the studies, the concordance between modalities was the main topic overall in pretransplant phase papers (14/19, 73.7%), while it was the focus of the study only in 20% (15/75) of posttransplant studies. Indeed, in this group, the correlation of histological features assessed with digital instruments with outcome and the investigation of features related to rejection represented together the most common issues, with total 60% (45/75) of publications. Splitting according to technology type, it can be observed that in studies using WSI, the main topic is the concordance between WSI and conventional LM, both in pretransplant (all 4 studies) and posttransplant (9/13, 69.2%) studies. The assessment of histological features correlated to outcome of organ or with particular attention to rejection was the main topic of the studies using static digitized images (41/70, 58.6%, all posttransplant studies). A diagram of distribution of studies according to transplant phase, type of digital pathology, and main topic is shown in Figure 2.
Figure 1.
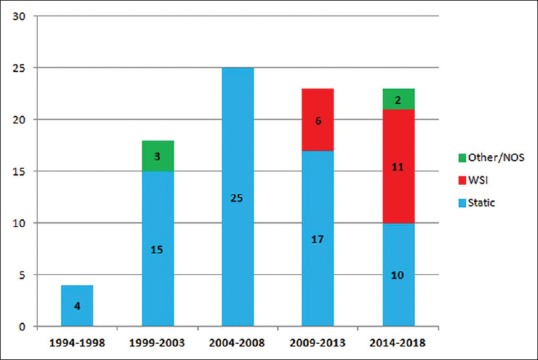
Number of publications over time and according to the type of digital pathology. WSI: Whole-slide imaging, NOS: Not otherwise specified
Figure 2.
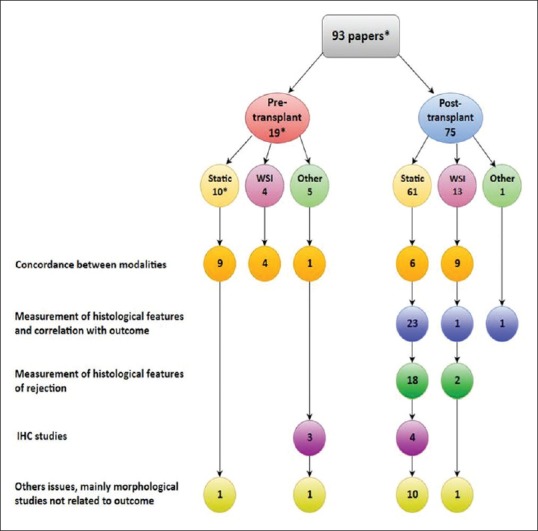
Hierarchy of papers according to transplantation phase, mode of digital pathology, and main topic of study. *A paper is counted in both groups as it comprises both pre- and post-transplant biopsies. IHC: Immunohistochemistry, WSI: Whole-slide imaging
Modes of digital pathology
Static telepathology requires only a microscope with an attached digital camera connected to a monitor or computer, internet access, and secure sharing software. A remote expert pathologist can view these static images but relies on an on-site pathologist who controls the microscope to capture relevant images that are in focus, which makes this inexpensive system restrictive.[5] This can be overcome with robotic or dynamic telepathology, which allows the remote pathologist to control the microscope using software; however, this robotic system is more expensive, is time-consuming, and demands a high network bandwidth.[5] WSI scanners are essentially a microscope and software-driven robotic stage that methodically moves the slide in the x and y axes under the microscopic lens while simultaneously optimizing the Z-plane focus and photographing each microscopic field.[5] WSI scanners can be tile-based (the most common ones), in which a square photosensor is used to capture multiple tiles adjacent to each other, or line scan-based imaging, in which an oblong photosensor is used to continually capture strips of image data as it sweeps through the slide. The quality of focusing is limited by multiple optical and mechanical parameters, notably the numerical aperture (NA) of the objective and movement resolution on the vertical (z) axis. Higher NA allows the distance that can be resolved to become smaller, thus increasing resolution.[6] WSI has proven to be superior in comparison to conventional microscopy in terms of case organization, navigation and annotation of slide, easiness to share for consultation and multiple viewing, and to be reliable for routine surgical pathology diagnosis, after validation of systems.[7] However, scanning time at higher resolutions, storage issues, and costs remain open questions that could have limited widespread adoption of this technology at the beginning; however, nowadays, for academic institutions or community hospitals with a high diagnostic workload, these issues are not to be considered a barrier. Indeed, as reported by a recent international survey, after full implementation of digital pathology, in routine practice, the new step could be the integration of artificial intelligence tools in diagnostic pathology.[8] Finally, hybrid WSI-robotic technology offers pathologists the ability to switch between live robotic viewing and a scanned digital slide.[9] The use of WSI in the transplantation literature only appears after 2011 (13/75, 17.3% of posttransplantation and 4/19, 21.1% of pretransplantation papers).
Telepathology in transplantation
The application of telemedicine to transplantation has lagged significantly compared to other medical fields, despite widespread interest.[10] The clinical benefits of mobile health technologies have been demonstrated in various phases of organ transplantation, including adherence of patients to therapy, clinical monitoring, and increase in life quality of recipients. In addition, in recent years, a number of case series and feasibility studies have highlighted the importance of digital pathology for providing access to expert second opinions. Indeed, this technology can help with real-time allograft selection and assessment of donor/recipient tissue specimens by allowing the teleconsultation of professionals during both pre- and post-transplant phases in medical centers with minimal experience.[10] However, the working scenarios in pre- and post-transplant phases is quite different. The posttransplant phase is best handled by a dedicated subspecialized pathologist, without the need for urgent turnaround times, and if needed availability of ancillary techniques. On the other hand, preimplantation diagnosis can typically be handled by an on-call general pathologist but does need to meet a turnaround time of only a few hours and usually without the luxury of ancillary studies (i.e., diagnoses depend almost entirely on a hematoxylin and eosin stain). In both scenarios, the need for diagnostic teleconsultation may be important.
The vast majority of papers on digital pathology and transplantation published in the last 25 years dealt with the posttransplant biopsy during graft surveillance (75 posttransplant vs. 19 pretransplant articles, 79.8% vs. 20.2%). Minervini et al. reported their experience with second-opinion teleconsultation using a static telepathology system between the Mediterranean Institute for Transplantation and Advanced Specialized Therapies in collaboration with the University of Pittsburgh Medical Center.[4] In that study, the authors reviewed 18 posttransplant biopsies and five preimplantation frozen section (FS) liver biopsies. They assessed the agreement rates between the referring and consulting pathologist and the reliability and easiness of telepathology for obtaining a rapid second opinion.[4] Low experience with digital pathology in the pretransplantation phase may be attributed to several reasons. Before the development of contemporary WSI scanners, the acquisition of digital images (e.g., static photographs) required a lengthy amount of time that was inconsistent with the rapid turnaround time needed for preimplantation biopsy assessment. Over time, as imaging devices began to allow dynamic and robotic telemicroscopy, so did the use of telepathology to remotely read intraoperative FSs before organ transplantation.[9]
Digital image analysis in transplantation
Although as stated in recent reviews,[11,12] the risk/benefit ratio and relative value of postimplantation biopsy for graft surveillance could appear to be decreasing, compared to less invasive monitoring techniques, given the development of newer noninvasive imaging and fluid techniques. However, advances in digital imaging techniques, robotics, and computing can provide new “toolkits” enabling pathologists to gain more information from tissue samples and to increase the histopathology value.[11] Indeed, starting from the early 90s, image analysis morphometric studies have been performed mainly for the detection of signs of rejection and prediction of organ outcome. The absence of time limitation comparing to pretransplant phase allows the pathologist to use ancillary techniques, to digitalize images, and to ask for consultation and perform image analysis, after slide scanning, and take advantage of DIA techniques for precise quantification of morphological features on biopsies. Among the posttransplant studies, 58/75 (77.3%) were carried out using conventional microscopy plus DIA, 8/75 (10.7%) were performed using WSI plus DIA, while 9/75 (12%) did not use DIA techniques. As clarified by Isse et al., morphometric software programs, which can range from relatively inexpensive basic macro-driven software for color quantification, too expensive and complex, trainable model-based applications for recognizing and quantifying tissue patterns, now consider WSI.[11] Moreover, the development of multiplex staining DIA algorithms and of deep learning algorithms has been rapidly increasing in recent years, with several applications in cancer pathology, that can be also applicable to transplantation biopsy pathology.[12] Therefore, it is reasonable that in the next two decades, the proportion of WSI versus LM in image analysis studies will be reversed as more image analysis studies will use WSI and deep learning algorithms.
Digital pathology in pre-transplantation
Despite the greater number of published studies on posttransplantation biopsies, there is increasing awareness of the potential to use digital pathology in the pretransplantation phase. Pathologists involved in on-call rotations for the transplant service may be asked to classify lesions found during donor assessment and to evaluate the suitability of organs to transplant from small biopsies. For newly discovered lesions, the pathologist performing these duties needs to define their nature and exclude a malignant neoplasm that would preclude safe transplantation.[13] The studies concerning preimplantation biopsies are summarized in Table 1. Among 19 studies concerning the pretransplant phase, none addressed diagnostic issues of newly discovered lesions. However, given that these lesions are typically examined by means of FS, they are probably incorporated in other more general studies about digital pathology for intraoperative consultation. Most studies on organ assessment (14/19, 73.7%) were mainly about liver and kidney biopsy,[4,14,15,16,17,18,19,20,21,22,23,24,25,26] while only a small proportion (5/19, 26.3%)[27,28,29,30,31] dealt with pancreatic islet preparations for transplant. With regard to the type of digital pathology technology used, 12/19 (63.2%) studies discussed DIA applied to LM-acquired images, 4/19 (21.1%) studies used WSI,[15,16,25,26] one study involved only static telepathology without DIA,[4] another referred generally to using a “virtual microscope,”[24] and one did not clarify the type of digital pathology used.[23]
Table 1.
Summary of papers dealing with pre-transplantation phase
| Author, year | Type of digital pathology | Number of patients/biopsies | Type of biopsy | Intervention | Controls or comparisons | Outcomes/Aim of the study | Results |
|---|---|---|---|---|---|---|---|
| Minervini etal., 2001 | Static | 102 | Various case types, among which 5 donor FS liver biopsies | Consultant telepathology review | Referring pathologist original diagnosis | Agreement rates, descriptive | 86% agreement and 14% (only 3% major) disagreement between referring and consultant pathologist |
| Li etal., 2002 | LM plus DIA | 102 | Donor kidney biopsy | DIA software assessment | None | Glomerular volume and sclerosis in different age groups | Glomerular size and global sclerosis increase with age |
| Benkoel etal., 2003 | Confocal laser microscopy plus DIA | 30 | Donor liver biopsy, preimplantation and postreperfusion | DIA assessment of IHC staining for ICAM-1 | None | Difference in ICAM-1 expression between preimplantation and postreperfusion biopsies | Higher expression of ICAM-1 in sinusoidal endothelial cells in postreperfusion biopsies |
| Benkoel etal., 2003 | Confocal laser microscopy plus DIA | 30 | Donor liver biopsy, preimplantation and postreperfusion | DIA assessment of IHC staining for F-actin | None | Difference in F-actin expression between preimplantation and postreperfusion biopsies | Significantly lower expression of F-actin in postreperfusion biopsies |
| Benkoel etal., 2003 | Confocal laser microscopy plus DIA | 30 | Donor liver biopsy, preimplantation and postreperfusion | DIA assessment of IHC staining for NaK-ATPase | None | Difference in NaK-ATPase expression between preimplantation and postreperfusion biopsies | Significantly lower expression of NaK-ATPase in postreperfusion biopsies |
| Marsman etal., 2004 | LM plus DIA | 49 | Donor liver biopsy, FS | DIA software assessment | Pathologist with glass slide | Percentage of total fat, microvesicular and macrovesicular steatosis; correlation with liver function indices, graft and patient survival | Significant correlation between pathologist and software for macrovesicular steatosis and total fat; significant association of macrovesicular steatosis and graft survival both when assessed by pathologist or software |
| Niclauss etal., 2008 | Static, stereo- microscope plus DIA | 12 | Pancreatic islets preparations | Computerized by 2 software and manual counting on digital images | Manual counting at microscope | Number, islet equivalents and purity of islet preparation | Total islet number, equivalents number, and purity were much better correlated between digital manual and computerized analyses than between standard manual and computerized analyses |
| Kissler etal., 2009 | LM plus DIA | 12 | Pancreatic islets preparations | Computerized by software on digital image | Manual counting on digital image | Accuracy, intra- and inter-observer reproducibility for both modalities by means of CV | Digital image analysis is reliable for islet counting, with the advantage of permanent records and quality assurance |
| Biesterfield etal., 2012 | Static LM, point grid counting | 120 | Donor liver biopsy, cut in half for FS and FFPE | Point grid counting | Conventional LM | Interobserver agreement for FS and FFPE, correlation between macro- and micro-vesicular steatosis | Substantial agreement (κ>0.60) and high correlation (r>0.80) between observers and types of steatosis; no advantage for point grid analysis |
| Native etal., 2013 | LM plus DIA | 9 patients, 54 images |
Donor liver biopsy | Model-based segmentation method algorithm | Expert pathologists with LM | Correlation between pathologists’ assessments and automated image analysis-based evaluations of ld-MaS percentages | New algorithm proposed significantly improves separation between large and small macrovesicular lipid droplets (specificity 93.7%, sensibility 99.3%) and correlation with pathologists’ ld-MaS percentage assessments (r=0.97) |
| Gymr etal., 2015 | LM plus DIA | 42 | Pancreatic islets preparations | Automated by software on digital image | Manual counting at LM | Correlation of modalities for total islet number, equivalent number, and purity; intraobserver variability | High correlation between modalities for total islet and equivalent number; high intraobserver reproducibility for the use of software |
| Wang etal., 2015 | LM plus DIA | 25 patients, 84 samples |
Pancreatic islets preparations | Computerized by software on digital image | Manual counting on digital image | Correlation of modalities for total islet number, equivalent number, and purity | Significantly high correlation between modalities; not significant difference for total counts |
| Mammas etal., 2015 | Not clearly defined | 518 images | Donor kidney, liver and pancreas | Diagnosis on digital image on 4 different viewing devices | Diagnosis of reference pathologist, not stated if with LM or digital | Accuracy of diagnosis with different viewing devices | The desktop and the experimental telemedicine platform are more reliable than tablet and mobile phone devices |
| Buchwald etal., 2016 | LM plus DIA | 3 patients, 14 samples |
Pancreatic islets preparations | Computerized by software on digital image | Manual counting at LM | Correlation of modalities for total islet number, equivalent number, and purity; intraobserver variability | Very good overall correlation between modalities; lower intraobserver variability for DIA |
| Eccher etal., 2016 | WSI | 62 patients, 124 biopsies |
Donor kidney wedge biopsy | Pathologist with WSI | Pathologist with glass slide | Intra- and inter-observer reproducibility with weighted Cohen k index | Very high intraobserver agreement (κ=0.961) for WSI and glass slide; slightly lower (κ=0.863) interobserver agreement for WSI than glass slide (κ=0.903) |
| Osband etal., 2016 | Virtual microscope, not otherwise specified | 23 kidneys | Donor kidney wedge biopsy, FS | Experienced pathologist with virtual microscope | On-site pathologist | Time to biopsy read | Shorter time to biopsy read with virtual microscope; improved time to local acceptance but not cold ischemia time or DGF rate |
| Liapis etal., 2017 | WSI | 40 | Donor kidney biopsy | Experienced pathologist with WSI | None | Intraclass correlation coefficient for various parameters of score | Modest agreement among pathologist, only number of glomeruli, sclerosed glomeruli and interstitial fibrosis with ICC >0.5 |
| Cima etal., 2018 | WSI | 28 | 16 donor kidney wedge biopsy, FS 12 donor liver biopsy, FS | Scoring with WSI | Scoring with glass slide | Accuracy rate; intraobserver concordance with weighted Cohen k index; sensibility, specificity, PPV, NPV | 86% accuracy rate, high intraobserver concordance (κ=0.91); 96%, 75%, 96%, 75% sensibility, specificity, PPV, NPV, respectively |
| Marsh etal., 2018 | WSI | 17 patients, 48 biopsy images |
Donor kidney biopsy, FS | Patch-based model and fully convolutional model on WSI | Expert pathologist scoring with WSI | Comparison between the two models and with pathologist’s assessment on WSI in counting total glomeruli and sclerosed glomeruli | Fully convolutional model substantially outperforming the model trained on image patches of isolated glomeruli, in terms of both accuracy and speed |
CV: Coefficient of variation, DIA: Digital image analysis, FFPE: Formalin-fixed, paraffin-embedded, FS: Frozen section, LM: Light microscopy, ld-MaS: Large droplet Macrovesicular steatosis, NPV: Negative predictive value, PPV: Positive predictive value, WSI: Whole slide imaging, ICAM-1: Intercellular adhesion molecule-1, DGF: Delayed graft function, IHC: Immunohistochemistry, ICC: Islet cell counter
The majority of studies (14/19, 73.7%) concerning the pretransplant phase addressed the agreement/concordance of digital pathology with the conventional LM technique. The assessment of agreement was performed with different statistical tests. Minervini et al. reported an agreement rate of 86% between referring pathologist with LM and consultant pathologist with static digital pathology, but they did not specify the agreement rates for the each of the pretransplant cases.[4] Other studies from the same group followed guidelines of the College of American Pathologists for validating WSI systems and compared WSI to LM in the assessment of kidney and liver biopsies. In one of their studies, the intraobserver concordance was excellent (κ = 0.961). The interobserver concordance was excellent for both LM (κ = 0.903) and WSI (κ = 0.863).[26] In another study on the validation of a WSI scanner, the case population included 28 scanned FS slides of the liver and kidney biopsy for organ suitability; the intraobserver concordance was excellent (κ = 0.91) with an accuracy rate of 86%.[15] Biesterfeld et al. analyzed the interobserver concordance in the quantification of macro- and micro-vesicular steatosis in liver biopsies using digital pathology. They found good interobserver agreement (κ >0.70) for all degrees of steatosis (correlation coefficient r > 0.90 and r > 0.60) when the assessment was performed with LM, but the concordance rate was lower when using point grid counting on digitized images. Therefore, they concluded that point grid counting on the digital image does not add value for steatosis quantification.[22] Two other studies analyzed the correlation between macrovesicular steatosis assessed by an experienced pathologist with LM to that assessed by DIA software (r2 = 0.426). One study reported low correlation (r2 = 0.426); however, DIA measurements had stronger correlation with liver function after transplant.[21] In the other study, a high correlation (r2 = 0.97) was found between pathologist's assessment and the DIA method.[18]
Several studies concerned pancreatic islet preparations for islet transplant and compared the assessment of various parameters, including the number of islets, islet equivalents (islets normalized for an average size of 150 μm, IEQ), and purity using different methods. All of them reported high correlation between manual counting on LM[28,29] or on a digitized image[30] and counting using automated/computerized DIA software (determination coefficient r2 = 0.91, r2 = 0.78 and linear coefficient r > 0.819, respectively). Three studies compared manual LM and automated DIA software by means of the coefficient of variation (CV), reporting that the CV is lower for automated software compared to manual counting[27,29] and concluding that DIA is reliable for quantification of IEQ and purity.[30] Finally, one study compared three modalities (i.e., manual assessment on LM, manual assessment of digital images, and counting by DIA using software) and reported a high correlation between assessment of digital images and software analysis (r2 > 0.8) and a lower correlation between standard manual assessment and software analysis (r2 0.62–0.73).[31]
Recently, some authors developed a deep learning model to identify and classify nonsclerosed and sclerosed glomeruli in WSI scans of donor kidney FS biopsies. They reported that their model based on convolutional neural networks yielded results comparable with those achieved by an expert renal pathologist, being robust enough to handle FS artifacts and adding value to the time-sensitive demand of donor biopsy evaluation. Their study is the first to specifically address glomerular recognition and classification in the FS preimplantation biopsy.[16]
The Banff group analyzed reproducibility among pathologists using WSI slides in a population of 40 donor kidney biopsies, with a different proportion of core versus wedge biopsies and FS versus paraffin technique. They reported overall good-to-excellent reproducibility for counting the total number of glomeruli, for assessing the percentage of sclerosed glomeruli and number of sclerosed glomeruli and interstitial fibrosis; however, the interobserver concordance was fair to poor in the assessment of other parameters.[25]
Osband et al. compared the time-to-donor kidney biopsy result between virtual microscopy and standard LM in practice and demonstrated a significant reduction in time-to-biopsy result using digital microscopy.[24] Mammas et al. compared the accuracy rate for the diagnosis of kidney, liver, and pancreas biopsies with a pathologist reading a digital slide on different devices, and they demonstrated that mobile phones and tablets to be less reliable than desktop viewing.[23] Finally, Benkoël et al. examined the expression of different IHC markers in a subset of paired preimplantation and postreperfusion liver biopsies, using DIA of confocal laser scanning microscope images, without comparison to conventional LM IHC.[14,19,20]
Digital pathology in post-transplantation
Among the 75 retrieved studies on posttransplant biopsies, 10 (13.5%) were concerned with liver biopsy, 16 (21.6%) with the heart and lung, and 47 (63.5%) kidney.
Liver graft biopsy
The studies concerning posttransplant liver graft biopsies are summarized in Table 2. Two studies[4,32] described the agreement with digital static pathology diagnosis and reported high concordance rates. Two more recent studies explored the reliability of WSI slides when compared to LM or reference diagnosis.[33,34] In the study by Neil et al., pathologists at several centers scored C4d antibody expression in liver biopsy tissue microarrays using WSI and LM. Interobserver agreement was variable with WSI when considering the different compartments of staining in a liver biopsy; in particular, concordance was good for the assessment of portal vein, central vein, and portal capillary compartments (κ = 0.60–0.80) and fair in the evaluation of sinusoidal and hepatic artery endothelium compartments (κ = 0.30–0.40). There was substantial agreement between pathologists with WSI and glass slides although κ indexes were not reported.[33] In the study of Saco et al., where WSI and LM were compared, the authors reported excellent intra- and inter-observer agreement (κ = 0.80–0.90) between modalities. Moreover, the authors highlighted the advantage of using WSI for viewing multiple slides, which is important because, in liver graft pathology, several stains are often used.[34]
Table 2.
Summary of papers dealing with posttransplantation liver graft biopsy
| Author, year | Type of digital pathology | Number of patients/biopsies | Type of biopsy | Intervention | Controls or comparisons | Outcomes/Aim of study | Results |
|---|---|---|---|---|---|---|---|
| Ito etal., 1994 | Static | 22 | Graft liver and kidney biopsy | Telepathology diagnosis | Direct LM diagnosis | Descriptive results | Agreement in 10/12 kidney biopsies and in 9/10 liver biopsies |
| Ben-Hari etal., 1995 | LM plus DIA | 55 (92 biopsies) | Graft liver biopsy | DIA assessment of eosinophil count, cell density and cross-sectional area in portal tract | None | Descriptive correlation of parameters with different degrees of rejection | Positive correlation of all parameters with severity of rejection |
| Minervini etal., 2001 | Static | 102, among which 9 liver graft and 9 kidney graft biopsies | Various case types: Second opinion consultation, transplantation pathology, general surgical pathology | Consultant telepathology review | Referring pathologist original diagnosis | Agreement rates, descriptive | 86% agreement and 14% (only 3% major) disagreement between referring and consultant pathologist |
| El-Refaie etal., 2005 | LM plus DIA | 267 (343 biopsies) | Graft liver biopsy | DIA software quantification of mast cells and IHC staining | None | Correlation of mast cell count and IHC staining with different degrees of rejection | Strong correlation of mast cells with acute rejection and of IHC staining for c-Kit with severity of rejection |
| Calvaruso etal., 2008 | LM plus DIA | 115 (225 biopsies) | Graft liver biopsy | DIA software quantification of collagen proportionate area | None | Descriptive correlation between DIA measurements, Ishak score, and portal hypertension | Collagen proportionate area assessed by DIA correlated with Ishak stage scores and portal hypertension |
| Guzman etal., 2010 | LM plus DIA | 19 (33 biopsies) | Graft liver biopsy | Anisonucleosis and oxidative damage scored by DIA | None | Descriptive correlation of anisonucleosis with different clinical parameters | Higher anisonucleosis in individuals with diabetes and with high expression of oxidative damage marker |
| Manousou etal., 2011 | LM plus DIA | 135 | Graft liver biopsy | Computer-assisted DIA quantification of collagen proportionate area | None | Descriptive correlation between DIA measurements, Ishak score, and decompensation | Collagen proportionate area assessed by DIA correlated with Ishak stage scores and decompensation |
| Calvaruso etal., 2012 | LM plus DIA | 65 | Graft liver biopsy | Computer-assisted DIA quantification of collagen proportionate area | None | Descriptive correlation between DIA measurements, portal hypertension and graft outcome | Collagen proportionate area assessed by DIA correlated with portal hypertension and decompensation |
| Manousou etal., 2013 | LM plus DIA | 155 (587 biopsies) | Graft liver biopsy | Computer-assisted DIA quantification of collagen proportionate area and rate of increase | None | Descriptive correlation of DIA measurements and Ishak score with portal hypertension and graft outcome | Progression rate of fibrosis is a better predictor of clinical outcome than progression by Ishak stage |
| Sclair etal., 2016 | LM plus DIA | 60 | Graft liver biopsy | DIA software assessment of ductular reaction in HCV recurrent recipients with cirrhosis | DIA software assessment of ductular reaction in stable recurrent HCV recipients with no cirrhosis or fibrosing hepatitis | Descriptive difference among the groups | Significantly higher ductular reaction in recipients with cirrhosis |
| Neil etal., 2017 | WSI | 40 | TMAs of graft and native liver, kidney, heart | Pathologists scoring C4d with WSI | Pathologists scoring C4d with LM | Descriptive surveys of pathologists and comparison of staining methods | Strong and diffuse portal vein and capillary C4d staining, determined by both local and central pathologists, distinguished acute antibody-mediated rejection from native livers |
| Saco etal., 2017 | WSI | 64 | Graft liver biopsy | Pathologist with WSI | Pathologist with LM | Intra- and inter-observer agreement | Almost perfect intraobserver concordance between modalities; high interobserver concordance for WSI (κ=0.80) |
DIA: Digital image analysis, HCV: Hepatitis C virus, IHC: Immunohistochemistry, LM: Light microscopy, TMAs: Tissue microarrays, WSI: Whole-slide imaging
Other studies regarding liver biopsy focused on the correlation with clinical parameters and predictive value on organ outcome for several features assessed by DIA software, such as fibrosis determined as collagen proportionate area (CPA) with Sirius red stain,[35,36,37,38] ductular reaction assessed with CK7 staining,[39] nuclear size, and IHC markers of oxidative damage.[40] In particular, CPA assessed as a continuous measure with DIA is reported to be a better predictor of graft outcome than Ishak stage assessed on conventional LM.[35,36,37,38] Ductular reaction area assessed with DIA software is reported to correlate with hepatic progenitor cell number assessed by manual counting and to be associated with hepatitis C virus (HCV) recurrence.[39] Nuclear size and anisonucleosis quantified with DIA software were not associated with any clinical parameters, except diabetes and the presence of a marker of oxidative damage.[40] Two older studies investigated the presence and role of overall inflammatory cells[41] and mast cells[42] for acute and chronic rejection, with quantification of cellular infiltrates or specific subtypes of mast cells with DIA software in digital images; they showed that the number of inflammatory cells assessed by DIA was able to separate mild from severe rejection[41] and that mast cell density both with tryptase and c-Kit staining correlated with the severity of acute and chronic rejection.[42]
Heart and lung graft biopsy
The studies concerning posttransplant heart and lung graft biopsies are summarized in Table 3. Of papers concerning heart and lung graft biopsy, 2/16 (12.5%) dealt with agreement and reproducibility between digital slides and LM. The oldest study by Marchevsky et al. reported concordance rates of 96% and 82.8% with Cohen's κ coefficients of 0.92 and 0.692 for lung and heart biopsy, respectively. Using static digital pathology, images were acquired with a camera attached to a microscope, remotely diagnosed by a pathologist, and then compared to a reference diagnosis.[43] A more recent study by Angelini et al. reported fair interobserver concordance among pathologists (κ = 0.20–0.40) when assessing a set of 20 endomyocardial biopsies (EMBs). The interobserver agreement increased when pathologists were stratified according to their expertise in heart transplant pathology.[44]
Table 3.
Summary of papers dealing with posttransplantation heart and lung graft biopsy
| Author, year | Type of digital pathology | Number of patients/biopsies | Type of biopsy | Intervention | Controls or comparisons | Outcomes/Aim of the study | Results |
|---|---|---|---|---|---|---|---|
| Armstrong etal., 1998 | LM plus DIA | 101 | EMBs | DIA software assessment of fibrosis and myocyte diameter in recipients | DIA software assessment of fibrosis and myocyte diameter in controls | Descriptive differences between the groups | Larger myocyte diameter in transplanted hearts; fibrosis higher in the first posttransplant EMBs |
| Marchevsky etal., 2002 | Static LM | 108 | Graft lung and heart biopsy | Telepathology diagnosis | Previous LM diagnoses | Agreement rates, descriptive | 96% agreement, κ=0.92, for lung biopsies, 82.8% agreement, κ=0.692, for EMBs |
| Law etal., 2005 | LM plus DIA | 25 | Graft lung biopsy | DIA software quantification of basement membrane thickness | None | Correlation of basement membrane thickness with the development of bronchiolitis obliterans syndrome | Strong negative correlation of basement membrane thickness versus time |
| Ward etal., 2005 | LM plus DIA | 30 (21 biopsies) | Graft lung biopsy | DIA software assessment of basement membrane thickening | Published data on basement membrane thickening in other lung diseases | Descriptive results in lung recipients and correlation with respiratory function parameters | Higher basement membrane thickening compared to published data in other lung diseases; no correlation with lung function |
| Sorrentino etal., 2006 | LM plus DIA | 21 (361 biopsies) | EMBs | DIA of IHC staining | None | Descriptive | Role of IHC assessment in grading rejection |
| Zakliczynski etal., 2006 | LM plus DIA | 43 (129 biopsies) | EMBs | Automated software quantification of nuclei | None | Descriptive | Role of chromatin distribution in nuclei to assess severity of rejection |
| Nozynski etal., 2007 | LM plus DIA | 31 | EMBs | Use of ATG | Standard treatment | Descriptive differences in quantitative assessment of nuclear parameters with automated software in the groups | Nuclear parameters of rejection lower in the ATG group |
| Angelini etal., 2011 | WSI | 20 | EMB | 18 pathologists reading WSI slides | Index diagnosis of referent pathologist | Interobserver reproducibility and agreement with reference | Fair-to-moderate reproducibility (κ=0.39, α=0.55); role of expertise for agreement with reference diagnosis |
| Moreira etal., 2011 | LM plus DIA | Not stated, 658 images | EMBs | Fractal dimension by DIA software | None | Descriptive relation between fractal dimension and degrees of rejection | Fractal dimension can discriminate between degrees of rejection |
| Revelo etal., 2012 | WSI plus DIA | 22 | EMBs | Microvessel density in recipients with AMR | Microvessel density in recipients without AMR | Descriptive | Significantly reduced microvessel density in a subset of patients with pathologic AMR with worse outcome |
| Devitt etal., 2013 | LM plus DIA | 34 | Transplanted hearts in deceased recipients | Measurement on acquired images | None | Descriptive | Consideration of donor-derived accelerated atherosclerosis in heart recipients |
| Pijet etal., 2014 | LM plus DIA | 40 | EMBs | Fractal parameters assessment with DIA software | None | Descriptive differences between grades of rejection | Some digital parameters can aid grading of rejection |
| Tona etal., 2014 | LM plus DIA | 28 | EMBs | Everolimus | Mycophenolate mofetil | Difference in fibrosis, microvascular remodeling, and arteriolar thickening | Capillary density and fibrosis comparable between groups, arteriolar thickening lower in the everolimus group |
| Welsh etal., 2016 | LM plus DIA | 13 | EMBs | DIA software assessment of IHC staining | None | Evaluation of Sirt-1 expression in acute cellular rejection | Increased expression of Sirt-1 in lymphocytes in acute cellular rejection |
| Feingold etal., 2017 | WSI plus DIA | 9 | EMB with LGD | EMBs with WSI | 9 matched control EMBs with WSI | Automated quantification of fibrosis and microvascular changes | Greater fibrosis and microvascular changes in LGD cases |
| Van den Bosch etal., 2017 | WSI plus DIA plus confocal microscopy | 25 (50 EMBs) | EMBs | EMBs at time of rejection | EMBs at no rejection time | Difference in monocyte and macrophage infiltration and degree of fibrosis | CD16+monocyte, M2 macrophage infiltration, and higher fibrosis are associated with rejection |
ATG: Anti-thymocyte globulin, DIA: Digital image analysis, EMBs: Endomyocardial biopsies, IHC: Immunohistochemistry, LGD: Late graft dysfunction, LM: Light microscopy, WSI: Whole-slide imaging, AMR: Antibody-mediated rejection
Most of the studies (9/16, 56.3%) dealt with graft rejection and quantification of parameters that aid in grading the severity of rejection or help elucidate potential pathogenetic mechanisms. Features quantified with DIA software included myocyte diameter,[45] fibrosis with Masson's trichrome stain,[45,46] microvasculature density with CD31[46] or CD34,[47] patterns of inflammatory and immunological cells,[48] monocytes and macrophage profiles,[49] expression of Sirt1, CD8, and FoxP3 on lymphocytes in rejection specimens,[50] and chromatin remodeling expressed as mean gray level.[51] In some publications, digital images were converted in formats adequate for fractal analysis to quantify the inflammatory infiltrate and signs of myocyte damage; it was shown that this kind of DIA can discriminate among different grades of rejection.[52,53] Other parameters assessed on graft biopsy with DIA software on LM images (nuclear parameters of cardiomyocytes[54] or fibrosis with Azan-Mallory stain and microvascular remodeling with IHC staining[55]) were relevant for recipient outcome of different immunosuppressive treatments. Overall, the quantitative assessment of EMBs by means of DIA provided more information than routine, semi-quantitative investigation, even if the application of DIA software required a more reproducible staining quality among slides and a better than routine quality of histological slides.[54] Image analysis was also used to quantify macrophages and T-lymphocytes in autopsy specimens of coronary vessels of transplanted heart recipients to compare several vascular remodeling features.[56] Finally, only two studies concerned lung biopsies and both explored the correlation of basement membrane thickness measured with DIA software with the development of bronchiolitis obliterans in recipients. They found that increased thickness of the basement membrane can be transient and not correlated to respiratory function decline.[57,58] For the majority of the aforementioned studies, DIA was carried out on static digital images acquired with an LM. Only three out of 14 studies where DIA was employed used WSI technology. This is not surprising given that WSI adoption was only adopted more recently.
Kidney graft biopsy
The studies concerning posttransplant kidney graft biopsies are summarized in Table 4. Articles concerning the posttransplantation kidney biopsy were the most numerous (47/75, 62.7%) and dealt with various topics. Apart from the studies by Minervini et al.[4] and Ito et al.[32] that also included kidney biopsies, nine out of 47 studies (19.1%) addressed agreements between LM and digital slide assessment for several parameters.[59,60,61,62,63,64,65,66,67] Ito et al. used a static telepathology system and only evaluated the concordance rate,[59] while more recent studies used WSI and achieved good or substantial (κ > 0.40 and κ > 0.60) intra- and inter-observer agreements, concluding that WSI is as reliable as LM for graft biopsy evaluation.[64,65] Older studies used LM plus DIA software for the quantification of fibrosis, inflammation, and glomerular sclerosis, reporting that DIA assessment had good correlation with manual evaluation, but that it had higher correlation with graft outcome.[60,61,62] More recent studies combining WSI with DIA for the quantification of C4d IHC,[63] fibrosis with PAS staining and collagen IHC,[66] and CD3 for acute rejection[67] showed that digital evaluation had better correlation with organ function and higher reproducibility than LM assessment.[63,66,67]
Table 4.
Summary of papers dealing with posttransplantation kidney graft biopsy
| Author, year | Type of digital pathology | Number of patients/biopsies | Type of biopsy | Intervention | Controls or comparisons | Outcomes/Aim of the study | Results |
|---|---|---|---|---|---|---|---|
| Ito etal., 1994 | Static LM | 22 | Graft liver and kidney biopsy | Telepathology diagnosis | Direct LM diagnosis | Descriptive results | Agreement in 10/12 kidney biopsies and in 9/10 liver biopsies |
| Gandaliano etal., 1997 | LM plus DIA | 20 | Graft kidney biopsy | DIA assessment of IHC staining for CD68 and MCP-1 in acute rejection biopsies | DIA assessment of IHC staining for CD68 and MCP-1 in tubular damage and control biopsies | Descriptive differences in expression between groups and correlation with graft outcome | MCP-1 expression significantly higher in acute rejection biopsies |
| Grimm etal., 1999 | LM plus DIA | 32 | Graft kidney biopsy | DIA assessment of IHC staining of cellular infiltrate in clinical and subclinical rejection biopsies | DIA assessment of IHC staining of cellular infiltrate in normal controls | Descriptive differences in IHC staining among the groups | Significantly higher infiltration of CD8 and CD68 positive cells in clinical rejection |
| Nicholson etal., 1999 | LM plus DIA | 52 | Graft kidney biopsy | Semiautomatic DIA assessment of interstitial fibrosis with IHC | None | Descriptive correlation of interstitial fibrosis with graft outcome | Positive correlation of interstitial fibrosis as stained area with eGFR |
| Bonsib etal., 2000 | LM plus DIA | 14 (42 biopsies) | Graft kidney biopsy | Tubular membrane breaks with methenamine silver assessed on digital images | None | Descriptive correlation with clinical parameters | Correlation of tubular membrane breaks with creatinine level |
| Furukuwa etal., 2001 | LM plus DIA | 21 | Graft kidney biopsy | DIA software assessment of interstitial fibrosis | None | Descriptive correlation of degree of interstitial fibrosis with graft outcome | Usefulness of the computerized imaging diagnosis for quantitative evaluation of interstitial fibrosis in predicting graft failure |
| Ishimura etal., 2001 | LM plus DIA | 21 | Graft kidney biopsy | DIA software assessment of interstitial fibrosis | None | Descriptive correlation between interstitial fibrosis and TGF=beta IHC staining | Strong association between extracellular TGF beta expression and long-term decline in graft function and increased interstitial fibrosis |
| Ito etal., 2001 | Static LM | 31 (37 biopsies) | Graft kidney biopsy | Telepathology diagnosis | Direct LM diagnosis | Descriptive results | Agreement on diagnosis in 30/37cases |
| Minervini etal., 2001 | Static LM | 102 | Various case types, among which 9 kidney graft biopsies | Consultant telepathology review | Referring pathologist original diagnosis | Agreement rates, descriptive | 86% agreement and 14%(only 3% major) disagreement between referring and consultant pathologist |
| Danilewicz etal., 2003 | LM plus DIA | 34 | Graft kidney biopsy | DIA assessment of IHC staining and glomerular area in biopsies with acute rejection | DIA assessment of IHC staining and glomerular area in normal controls | Descriptive differences in IHC staining between the two groups | Significantly higher cellular infiltrate, glomerular area and interstitial area in acute rejection biopsies |
| Encarnacion etal., 2003 | LM plus DIA | 49 | Graft kidney biopsy | Different computerized strategies of DIA | Expert pathologist with LM | Correlation of tubulointerstitial fibrosis with graft function | Different degree of correlation with graft function of tubulointerstitial fibrosis scored with different strategies |
| Grimm etal., 2003 | LM plus DIA | NA | Graft kidney biopsy | Automated DIA software assessment of interstitial fibrosis | None | Correlation of interstitial fibrosis with graft outcome | Cortical fractional interstitial fibrosis volume can be a surrogate for time to graft failure |
| Mui etal., 2003 | LM plus DIA | 30 | Graft kidney biopsy | DIA assessment of IHC staining in ischemic injury | DIA assessment of IHC staining in normal controls | Descriptive | Different pattern of expression of markers in ischemic injury biopsies |
| Pape etal., 2003 | LM plus DIA | 56 | Graft kidney biopsy | DIA assessment of interstitial fibrosis | None | Correlation of interstitial fibrosis with graft outcome | Quantitative measurement of fibrosis by picrosirius red staining is a prognostic indicator for estimating long-term graft function |
| Sugiyama etal., 2003 | LM plus DIA | 25 | Graft kidney biopsy | DIA assessment of mean glomerular area and interstitial area | None | Descriptive differences in recipients with or without focal segmental glomerulosclerosis | No significant difference in mean glomerular area nor interstitial area between the two groups |
| Bains etal., 2004 | LM plus DIA | 112 | Graft kidney biopsy | DIA software assessment of fibrosis in DCD and DBD graft biopsies | None | Difference of fibrosis in the two groups | No significant differences in level of fibrosis |
| Danilewicz etal., 2004 | LM plus DIA | 35 | Graft kidney biopsy | DIA quantification of mast cells and leukocytes with IHC staining in acute rejection biopsies | DIA quantification of mast cells and leukocytes with IHC staining in normal controls | Descriptive differences between the groups | Significantly higher number of mast cells and leukocytes in acute rejection; positive correlation between inflammatory infiltrate and interstitial area |
| Pape etal., 2004 | LM plus DIA | 56 | Graft kidney biopsy | Renal resistance index with Doppler | Interstitial fibrosis assessment with DIA | Correlation between the two measurements and with graft outcome | Positive correlation between the two measures and of the combination of the two with graft outcome |
| Sarioglu etal., 2004 | LM plus DIA | 15 | Graft kidney biopsy | Automated quantification of stained area | None | Descriptive | Strong correlation between stained area and serum creatinine(r=0.64) |
| Sund etal., 2004 | LM plus DIA | 33 | Graft kidney biopsy | DIA automated quantification | Pathologist with LM | Descriptive | Significant correlation between the two modalities and with graft outcome |
| Nishi etal., 2005 | LM plus DIA | 14 | Graft kidney biopsy | DIA software assessment of the peritubular capillary network in recipients with rejection | DIA software assessment of the peritubular capillary network in recipients without rejection | Descriptive | Significant differences in surface areas of tubulin and glomerular diameter between the groups |
| Sis etal., 2005 | LM plus DIA | 57 (75 biopsies) | Graft kidney biopsy | DIA software assessment of stained area | None | Descriptive correlation among stained areas for fibrosis, Banff scores and rejection | No significant association between serum creatinine at time of biopsy and percentage of stained areas for fibrosis; no predictive value for rejection |
| Danilewicz etal., 2006 | LM plus DIA | 33 | Graft kidney biopsy | DIA of IHC staining in acute rejection recipients | DIA of IHC staining in recipients with no rejection | Differences in IHC staining in the two groups | Higher expression of TGF beta, CD3, CD8 in acute rejection |
| Hoffman etal., 2006 | LM plus DIA | 138 | Graft kidney biopsy | DIA of IHC staining | None | Descriptive expression of CXCR3 | Higher expression of CXCR3 in acute rejection |
| Lauronen etal., 2006 | LM plus DIA | 35 | Graft kidney biopsy | DIA software scoring | Pathologist with LM | Descriptive | No significant difference in scoring between the modalities |
| Roos-van- Groningen etal., 2006 | LM plus DIA | 54 (108 biopsies) | Graft kidney biopsy | Cyclosporine | Tacrolimus | Fibrosis and IHC staining assessed by automated DIA software | No quantitative differences in fibrosis and IHC staining between cyclosporine and tacrolimus |
| Rowshani etal., 2006 | LM plus DIA | 126 | Graft kidney biopsy | Cyclosporine | Tacrolimus | Fibrosis with Sirius red assessed by automated DIA software | No difference in the degree of interstitial stained area between the two treatment groups |
| Sarioglu etal., 2006 | LM plus DIA | 37 (44 biopsies) | Graft kidney biopsy | DIA assessment of periodic acid methenamine silver staining | None | Descriptive relation of stained area to Banff scores and creatinine values | Strong association of stained area with increased interstitial fibrosis and tubular atrophy Banff scores |
| Scholten etal., 2006 | LM plus DIA | 126 | Graft kidney biopsy | Cyclosporine | Tacrolimus | Subacute rejection assessed by pathologist and automated fibrosis quantification | No quantitative differences in fibrosis between cyclosporine and tacrolimus; higher prevalence of subacute rejection in the cyclosporine group but no difference in graft survival |
| Servais etal., 2007 | LM plus DIA | 26 | Graft kidney biopsy | DIA automated quantification of interstitial fibrosis in recipients treated with cyclosporine | None | Descriptive correlation of interstitial fibrosis with graft outcome | Correlation of higher grade of automated interstitial fibrosis with a higher creatinine |
| Servais etal., 2007 | LM plus DIA | 26 | Graft kidney biopsy | DIA automated quantification of interstitial fibrosis in recipients treated with cyclosporine | None | Descriptive correlation of interstitial fibrosis with graft outcome | Association between high grade of automated interstitial fibrosis and worsening of creatinine clearance |
| Birk etal., 2010 | LM plus DIA | 29 (105 biopsies) | Graft kidney biopsy | DIA software quantification of interstitial fibrosis | None | Descriptive correlation of interstitial fibrosis and graft outcome | Significant correlation of interstitial fibrosis assessed by DIA software with graft outcome |
| Yan etal., 2010 | LM plus DIA | 46 | Graft kidney biopsy | DIA quantification of IHC staining | None | Correlation of IHC staining with Banff score for interstitial fibrosis and tubular atrophy | Higher IHC staining expression in higher Banff score classes for interstitial fibrosis and tubular atrophy |
| Brazdziute etal., 2011 | WSI plus DIA | 32 (34 biopsies) | Graft kidney biopsy | Automated software on WSI | Pathologist on LM | Correlation and interobserver variability in C4d scoring | Good-to-high correlation between pathologist and automated software; good manual-automated interobserver agreement |
| Meas-Yedid etal., 2011 | WSI plus DIA | 90 biopsies | Graft kidney biopsy | Automated software on WSI | Expert pathologist on LM | Correlation and interobserver variability in interstitial fibrosis scoring | Good agreement between the two methods(κ=0.75) |
| Miura etal., 2011 | LM plus DIA | 109 | Graft kidney biopsy | DIA software assessment of interstitial fibrosis | None | Correlation of interstitial fibrosis different tacrolimus regimens and cytochrome polymorphism | Higher increase in interstitial fibrosis in absence of cytochrome polymorphism |
| Servais etal., 2011 | LM plus DIA | 140 | Graft kidney biopsy | Automated DIA software assessment of interstitial fibrosis | None | Correlation of interstitial fibrosis with graft outcome | Correlation between interstitial fibrosis at different time points and eGFR |
| Becker etal., 2012 | LM plus DIA | 40 | Graft kidney biopsy | IHC staining in cellular infiltrate of clinical, operational tolerance recipients | IHC staining in cellular infiltrate of rejection recipients | Descriptive expression of IHC staining in inflammatory infiltrate | Different IHC staining in the two groups |
| Ozluk etal., 2012 | WSI | 40 | Graft kidney biopsy | Pathologists with WSI | Pathologists with LM | Intra- and inter-observer reproducibility | Comparable intraobserver reproducibility for both modalities; higher interobserver reproducibility with WSI |
| Yan etal., 2012 | LM plus DIA | 28 | Graft kidney biopsy | DIA software quantification of IHC staining of GSK3 beta at different levels of inflammation | None | Descriptive correlation between GSK3 beta staining and inflammation | Stronger GSK3 beta expression with increasing grade of inflammation or interstitial fibrosis/tubular atrophy |
| Yan etal., 2012 | LM plus DIA | 61 | Graft kidney biopsy | DIA software quantification of IHC staining in recipients with AMR | DIA software quantification of IHC staining in recipients without AMR | Descriptive relationship of IHC staining of extracellular matrix cytokines with interstitial fibrosis and creatinine | Higher expression in grafts with AMR; increasing expression with higher Banff scores of interstitial fibrosis and positive correlation with creatinine |
| Caplin etal., 2013 | LM plus DIA | 246 | Graft kidney biopsy | Serial posttransplant biopsies | No serial biopsies | Descriptive correlation of index of chronic damage with graft function | No significant differences between the two groups; index of chronic damage not predictive of graft function |
| Jen etal., 2013 | WSI | 25 | Graft kidney biopsy | Expert pathologists with WSI | Expert pathologist with LM | Intra- and inter-observer concordance | Substantial intraobserver concordance between modalities (κ=0.60), moderate interobserver concordance (κ=0.41-0.45) |
| Farris etal., 2014 | WSI plus DIA | 30 | Graft kidney biopsies | Pathologists scoring interstitial fibrosis on WSI slides with different stains | Computerized DIA of collagen IHC staining | Interobserver reproducibility and correlation of visual assessment on WSI with DIA assessment and with graft outcome | Poor reproducibility between pathologists; moderate correlation of visual assessment with DIA assessment of collagen-IHC; moderate correlation with graft outcome with no significant differences between the modalities |
| Vuiblet etal., 2015 | LM plus DIA plus spectroscopy (FTIR) | 106 (166 biopsies) | Graft kidney biopsy | Spectroscopy | Pathologist with LM and DIA | Quantification of interstitial fibrosis and inflammation | Poor agreement between scoring LM versus DIA and LM versus FTIR, good agreement in percentages between DIA and FTIR; good correlation between fibrosis with FTIR and graft function |
| Hara etal., 2016 | LM plus DIA | 934 | Graft and native kidney biopsy | 426 graft biopsy | 508 native kidney biopsy | Quantification of GSECs | Prevalence of GSECs slightly increased with posttransplant duration but not statistically significant |
| Yan etal., 2016 | LM plus DIA | 50 | Graft kidney biopsy | DIA software assessment of IHC staining in graft with chronic dysfunction | DIA software assessment of IHC staining in graft with no dysfunction | Difference in markers expression and correlation with Banff scores for interstitial fibrosis/tubular atrophy | Higher expression in grafts with dysfunction; positive correlation between marker expression and Banff scores |
| Bräsens etal., 2017 | WSI plus DIA | 67 | Graft kidney biopsy | Automated software on WSI | None | Correlation of different cellular types digitally quantified with graft function | Predictive value of digitally quantified CD68 cell density for graft function |
| Moon etal., 2017 | WSI plus DIA | 45 | Graft kidney biopsy | DIA automated software assessment of interstitial inflammation with different algorithms | Visual assessment of interstitial inflammation | Descriptive correlation among the modalities | Quantitation algorithms correlated between each other and also with visual assessment |
AMR: Antibody-mediated rejection, DBD: Donor after brain death, DCD: Donor after cardiac death, DIA: Digital image analysis, eGFR: Estimated glomerular filtration rate, FTIR: Fourier-transformed infrared spectroscopy, GSECs: Granular swollen epithelial cells, IHC: Immunohistochemistry, LM: Light microscopy, WSI: Whole-slide imaging, MCP-1: Monocyte chemotactic peptide-1, TGF: Transforming growth factor
Most of the studies on graft kidney biopsy use DIA techniques to explore the role of several biopsy features ranging from fibrosis evaluated with special stains to the expression and quantification of specific IHC markers in determining organ outcome,[68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] as well as signs of acute rejection.[84,85,86,87,88,89,90,91,92,93] In all of these studies, there is no direct comparison of DIA evaluation with manual pathologist results. Moreover, most of these are retrospective or case–control observational studies. The most studied parameter was interstitial fibrosis, with the correlation of DIA quantitative assessment to organ outcome being the main focus of these studies. Interstitial fibrosis was highlighted with special stains or with IHC, and some studies included comparison with other techniques such as spectroscopy[74] or Doppler ultrasound for renal resistance index.[81] Even though organ outcome was assessed slightly differently, most of these studies reinforced the idea that precise and automated quantification of this parameter by DIA technique can add value to biopsy evaluation, providing more reproducible results and permitting comparisons to be made with findings from other researchers. Similarly, studies about rejection mostly compared the IHC expression of several inflammatory markers and immune system cellular infiltration evaluated with DIA software in rejection biopsies and normal control biopsies. The remaining studies on posttransplantation kidney biopsy explored other features that correlated with ischemic injury,[94] levels of glomerular sclerosis,[95] fibrosis in grafts from after-brain-death donor or cardiac-death donor,[96] IHC markers to quantify interstitial fibrosis,[97,98,99] correlation with Banff score parameters[100] and more subtle features such as swollen glomerular epithelial cells.[101] Finally, three studies from the same research group compared fibrosis, assessed with special stains or IHC, and quantified by DIA software, in patients receiving cyclosporine or tacrolimus.[102,103,104]
Two main research themes: concordance and correlation to outcome
As already mentioned, the main issues addressed overall were the concordance between standard LM or manual assessment and WSI or DIA instruments and the correlation of histological features assessed by DIA methods with the outcome. The first topic was the most frequent in pretransplant papers. Intra- and inter-observer concordance with κ index was high when comparing WSI with LM,[15,26] thus reinforcing the point that digital diagnosis could replace conventional glass-slide diagnosis. The group of studies concerning pancreatic islet counting,[27,28,29,30,31] even with slightly different statistical measures, however, pointed toward the same direction, stating that DIA assessment is highly correlated to manual standard assessment and had the advantage of lesser interoperator variability. This remained true also in posttransplant papers addressing the same topic, even if less numerous.[33,34,44,63,64,65,66,67] In particular, more recent studies combining DIA with WSI concluded that DIA assessment of features has not only higher reproducibility than LM but also a better correlation to graft outcome, thus embracing with the second more frequent topic encountered through papers. This applies particularly to liver and kidney graft pathology, where a quota of papers compared DIA to manual assessment of features on LM-digitized images and correlated to outcome. With different grade of strength, they all suggested a better correlation to outcome and the advantage of a higher reproducibility. However, the vast majority of these studies were retrospective, both in the case of only concordance/reproducibility studies and of correlation-to-outcome studies, with the use of archival cases where the reference diagnosis was made previously with LM and sometimes with partly overlapping case populations.[35,36,37,38] Even if a quality assessment of studies was beyond the aims of this work, it is noticeable that only few studies were multicentric with the involvement of pathologists not working together, thus minimizing possible bias.[33,44,66] Moreover, in the majority of studies, digital pathology pertained only to the research field, especially in case of assessment of histological features or particular IHC marker expression, but also for concordance studies, where the value of digital pathology is explored in view of a possible future clinical full implementation.
CONCLUSION AND FUTURE DIRECTIONS
The aim of this review was to provide a broad overview of accrued international experience in the use of digital pathology in transplantation. Most retrieved studies involved the evaluation of the posttransplantation biopsy. The acquisition, manipulation, and eventual transmission of digital slides, before the advent of WSI, were too slow to be compatible with the time-sensitive needs encountered in the preimplantation setting. DIA was more adequate for outcome studies where time is not necessarily an issue.
It is not surprising that most of the studies using WSI, in particular, those in the pretransplant context, focused on the diagnostic agreement and concordance between LM and WSI. Indeed, it is likely that WSI may soon replace conventional LM diagnosis, especially as newer generation scanners acquire higher resolution images and digital platforms facilitate easier sharing of digital slides among pathologists. Some conventional barriers to implementation of WSI such as costs and storage issues could now be overcome in big centers and academic institutions. Some questions remain open, mainly concerning the regulatory constraints in different countries and economic issues on payer/reimbursement that apply particularly to the transplantation setting, for example, for second-opinion consultations and quality control programs, as transplantation activity is traditionally managed by public national health system.
The number of studies about WSI coupled with DIA is relatively small and restricted to the last 8 years. However, it is foreseeable that in the future, there will be a growing number of studies applying DIA and most likely deep learning algorithms and artificial intelligence to WSI, thereby augmenting the practice and field of transplantation.[8]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2019/10/1/21/261954.
REFERENCES
- 1.Goacher E, Randell R, Williams B, Treanor D. The diagnostic concordance of whole slide imaging and light microscopy: A systematic review. Arch Pathol Lab Med. 2017;141:151–61. doi: 10.5858/arpa.2016-0025-RA. [DOI] [PubMed] [Google Scholar]
- 2.Williams BJ, DaCosta P, Goacher E, Treanor D. A systematic analysis of discordant diagnoses in digital pathology compared with light microscopy. Arch Pathol Lab Med. 2017;141:1712–8. doi: 10.5858/arpa.2016-0494-OA. [DOI] [PubMed] [Google Scholar]
- 3.Capitanio A, Dina RE, Treanor D. Digital cytology: A short review of technical and methodological approaches and applications. Cytopathology. 2018;29:317–25. doi: 10.1111/cyt.12554. [DOI] [PubMed] [Google Scholar]
- 4.Minervini MI, Yagi Y, Marino IR, Lawson A, Nalesnik M, Randhawa P, et al. Development and experience with an integrated system for transplantation telepathology. Hum Pathol. 2001;32:1334–43. doi: 10.1053/hupa.2001.29655. [DOI] [PubMed] [Google Scholar]
- 5.Neil DA, Demetris AJ. Digital pathology services in acute surgical situations. Br J Surg. 2014;101:1185–6. doi: 10.1002/bjs.9576. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Pantanowitz L, Parwani AV. Digital imaging in pathology. Clin Lab Med. 2012;32:557–84. doi: 10.1016/j.cll.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–22. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarwar S, Dent A, Faust K, Richer M, Djuric U, Van Ommeren R, et al. Physician perspectives on integration of artificial intelligence into diagnostic pathology. NPJ Digit Med. 2019;2:28. doi: 10.1038/s41746-019-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantanowitz L, Wiley CA, Demetris A, Lesniak A, Ahmed I, Cable W, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi: 10.4103/2153-3539.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming JN, Taber DJ, McElligott J, McGillicuddy JW, Treiber F. Mobile health in solid organ transplant: The time is now. Am J Transplant. 2017;17:2263–76. doi: 10.1111/ajt.14225. [DOI] [PubMed] [Google Scholar]
- 11.Isse K, Lesniak A, Grama K, Roysam B, Minervini MI, Demetris AJ. Digital transplantation pathology: Combining whole slide imaging, multiplex staining and automated image analysis. Am J Transplant. 2012;12:27–37. doi: 10.1111/j.1600-6143.2011.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood-Trageser MA, Lesniak AJ, Demetris AJ. Enhancing the value of histopathological assessment of allograft biopsy monitoring. Transplantation. 2019 doi: 10.1097/TP.0000000000002656. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neil DA, Roberts IS, Bellamy CO, Wigmore SJ, Neuberger JM. Improved access to histopathology using a digital system could increase the organ donor pool and improve allocation. Transpl Int. 2014;27:759–64. doi: 10.1111/tri.12320. [DOI] [PubMed] [Google Scholar]
- 14.Benkoel L, Dodero F, Hardwigsen J, Benoliel AM, Bongrand P, Botta-Fridlund D, et al. Expression of intercellular adhesion molecule-1 (ICAM- 1) during ischemia-reperfusion in human liver tissue allograft: Image analysis by confocal laser scanning microscopy. Dig Dis Sci. 2003;48:2167–72. doi: 10.1023/b:ddas.0000004521.88660.fd. [DOI] [PubMed] [Google Scholar]
- 15.Cima L, Brunelli M, Parwani A, Girolami I, Ciangherotti A, Riva G, et al. Validation of remote digital frozen sections for cancer and transplant intraoperative services. J Pathol Inform. 2018;9:34. doi: 10.4103/jpi.jpi_52_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh JN, Matlock MK, Kudose S, Liu TC, Stappenbeck TS, Gaut JP, et al. Deep learning global glomerulosclerosis in transplant kidney frozen sections. IEEE Trans Med Imaging. 2018;37:2718–28. doi: 10.1109/TMI.2018.2851150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Nicholls KM, Becker GJ. Glomerular size and global glomerulosclerosis in normal Caucasian donor kidneys: Effects of aging and gender. J Nephrol. 2002;15:614–9. [PubMed] [Google Scholar]
- 18.Nativ NI, Chen AI, Yarmush G, Henry SD, Lefkowitch JH, Klein KM, et al. Automated image analysis method for detecting and quantifying macrovesicular steatosis in hematoxylin and eosin-stained histology images of human livers. Liver Transpl. 2014;20:228–36. doi: 10.1002/lt.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benkoël L, Dodero F, Hardwigsen J, Campan P, Botta-Fridlund D, Lombardo D, et al. Effect of ischemia-reperfusion on bile canalicular F-actin microfilaments in hepatocytes of human liver allograft: Image analysis by confocal laser scanning microscopy. Dig Dis Sci. 2001;46:1663–7. doi: 10.1023/a:1010693218680. [DOI] [PubMed] [Google Scholar]
- 20.Benkoel L, Dodero F, Hardwigsen J, Mas E, Benoliel AM, Botta-Fridlund D, et al. Effect of ischemia-reperfusion on Na+, K+-ATPase expression in human liver tissue allograft: Image analysis by confocal laser scanning microscopy. Dig Dis Sci. 2004;49:1387–93. doi: 10.1023/b:ddas.0000042235.72622.16. [DOI] [PubMed] [Google Scholar]
- 21.Marsman H, Matsushita T, Dierkhising R, Kremers W, Rosen C, Burgart L, et al. Assessment of donor liver steatosis: Pathologist or automated software? Hum Pathol. 2004;35:430–5. doi: 10.1016/j.humpath.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Biesterfeld S, Knapp J, Bittinger F, Götte H, Schramm M, Otto G. Frozen section diagnosis in donor liver biopsies: Observer variation of semiquantitative and quantitative steatosis assessment. Virchows Arch. 2012;461:177–83. doi: 10.1007/s00428-012-1271-6. [DOI] [PubMed] [Google Scholar]
- 23.Mammas CS, Lazaris A, Kostopanagiotou G, Lemonidou C, Patsouris E. The digital microscopy in organ transplantation: Ergonomics of the tele-pathological evaluation of renal and liver grafts. Stud Health Technol Inform. 2015;213:287–90. [PubMed] [Google Scholar]
- 24.Osband AJ, Fyfe B, Laskow DA. Virtual microscopy improves sharing of deceased donor kidneys. Am J Surg. 2016;212:592–5. doi: 10.1016/j.amjsurg.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB, 3rd, et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant. 2017;17:140–50. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eccher A, Neil D, Ciangherotti A, Cima L, Boschiero L, Martignoni G, et al. Digital reporting of whole-slide images is safe and suitable for assessing organ quality in preimplantation renal biopsies. Hum Pathol. 2016;47:115–20. doi: 10.1016/j.humpath.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Kissler HJ, Niland JC, Olack B, Ricordi C, Hering BJ, Naji A, et al. Validation of methodologies for quantifying isolated human islets: An islet cell resources study. Clin Transplant. 2010;24:236–42. doi: 10.1111/j.1399-0012.2009.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gmyr V, Bonner C, Lukowiak B, Pawlowski V, Dellaleau N, Belaich S, et al. Automated digital image analysis of islet cell mass using Nikon's inverted eclipse Ti microscope and software to improve engraftment may help to advance the therapeutic efficacy and accessibility of islet transplantation across centers. Cell Transplant. 2015;24:1–9. doi: 10.3727/096368913X667493. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald P, Bernal A, Echeverri F, Tamayo-Garcia A, Linetsky E, Ricordi C. Fully automated islet cell counter (ICC) for the assessment of islet mass, purity, and size distribution by digital image analysis. Cell Transplant. 2016;25:1747–61. doi: 10.3727/096368916X691655. [DOI] [PubMed] [Google Scholar]
- 30.Wang LJ, Kissler HJ, Wang X, Cochet O, Krzystyniak A, Misawa R, et al. Application of digital image analysis to determine pancreatic islet mass and purity in clinical islet isolation and transplantation. Cell Transplant. 2015;24:1195–204. doi: 10.3727/096368914X681612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niclauss N, Sgroi A, Morel P, Baertschiger R, Armanet M, Wojtusciszyn A, et al. Computer-assisted digital image analysis to quantify the mass and purity of isolated human islets before transplantation. Transplantation. 2008;86:1603–9. doi: 10.1097/TP.0b013e31818f671a. [DOI] [PubMed] [Google Scholar]
- 32.Ito H, Adachi H, Taniyama K, Fukuda Y, Dohi K. Telepathology is available for transplantation-pathology: Experience in Japan using an integrated, low-cost, and high-quality system. Mod Pathol. 1994;7:801–5. [PubMed] [Google Scholar]
- 33.Neil DA, Bellamy CO, Smith M, Haga H, Zen Y, Sebagh M, et al. Global quality assessment of liver allograft C4d staining during acute antibody-mediated rejection in formalin-fixed, paraffin-embedded tissue. Hum Pathol. 2018;73:144–55. doi: 10.1016/j.humpath.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Saco A, Diaz A, Hernandez M, Martinez D, Montironi C, Castillo P, et al. Validation of whole-slide imaging in the primary diagnosis of liver biopsies in a university hospital. Dig Liver Dis. 2017;49:1240–6. doi: 10.1016/j.dld.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Calvaruso V, Dhillon AP, Tsochatzis E, Manousou P, Grillo F, Germani G, et al. Liver collagen proportionate area predicts decompensation in patients with recurrent hepatitis C virus cirrhosis after liver transplantation. J Gastroenterol Hepatol. 2012;27:1227–32. doi: 10.1111/j.1440-1746.2012.07136.x. [DOI] [PubMed] [Google Scholar]
- 36.Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, et al. Computer-assisted image analysis of liver collagen: Relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology. 2009;49:1236–44. doi: 10.1002/hep.22745. [DOI] [PubMed] [Google Scholar]
- 37.Manousou P, Burroughs AK, Tsochatzis E, Isgro G, Hall A, Green A, et al. Digital image analysis of collagen assessment of progression of fibrosis in recurrent HCV after liver transplantation. J Hepatol. 2013;58:962–8. doi: 10.1016/j.jhep.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Manousou P, Dhillon AP, Isgro G, Calvaruso V, Luong TV, Tsochatzis E, et al. Digital image analysis of liver collagen predicts clinical outcome of recurrent hepatitis C virus 1 year after liver transplantation. Liver Transpl. 2011;17:178–88. doi: 10.1002/lt.22209. [DOI] [PubMed] [Google Scholar]
- 39.Sclair SN, Fiel MI, Wu HS, Doucette J, Aloman C, Schiano TD. Increased hepatic progenitor cell response and ductular reaction in patients with severe recurrent HCV post-liver transplantation. Clin Transplant. 2016;30:722–30. doi: 10.1111/ctr.12740. [DOI] [PubMed] [Google Scholar]
- 40.Guzman G, Chennuri R, Voros A, Boumendjel R, Locante A, Patel R, et al. Nucleometric study of anisonucleosis, diabetes and oxidative damage in liver biopsies of orthotopic liver transplant recipients with chronic hepatitis C virus infection. Pathol Oncol Res. 2011;17:191–9. doi: 10.1007/s12253-010-9296-0. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Ari Z, Booth JD, Gupta SD, Rolles K, Dhillon AP, Burroughs AK. Morphometric image analysis and eosinophil counts in human liver allografts. Transpl Int. 1995;8:346–52. doi: 10.1007/BF00337165. [DOI] [PubMed] [Google Scholar]
- 42.El-Refaie AM, Burt AD. Mast cells and c-Kit expression in liver allograft rejection. Histopathology. 2005;47:375–81. doi: 10.1111/j.1365-2559.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 43.Marchevsky AM, Lau SK, Khanafshar E, Lockhart C, Phan A, Michaels PJ, et al. Internet teleconferencing method for telepathology consultations from lung and heart transplant patients. Hum Pathol. 2002;33:410–4. doi: 10.1053/hupa.2002.124722. [DOI] [PubMed] [Google Scholar]
- 44.Angelini A, Andersen CB, Bartoloni G, Black F, Bishop P, Doran H, et al. Aweb-based pilot study of inter-pathologist reproducibility using the ISHLT 2004 working formulation for biopsy diagnosis of cardiac allograft rejection: The European experience. J Heart Lung Transplant. 2011;30:1214–20. doi: 10.1016/j.healun.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong AT, Binkley PF, Baker PB, Myerowitz PD, Leier CV. Quantitative investigation of cardiomyocyte hypertrophy and myocardial fibrosis over 6 years after cardiac transplantation. J Am Coll Cardiol. 1998;32:704–10. doi: 10.1016/s0735-1097(98)00296-4. [DOI] [PubMed] [Google Scholar]
- 46.Feingold B, Picarsic J, Lesniak A, Popp BA, Wood-Trageser MA, Demetris AJ. Late graft dysfunction after pediatric heart transplantation is associated with fibrosis and microvasculopathy by automated, digital whole-slide analysis. J Heart Lung Transplant. 2017;36:1336–43. doi: 10.1016/j.healun.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Revelo MP, Miller DV, Stehlik J, Brunisholz K, Drakos S, Gilbert EM, et al. Longitudinal evaluation of microvessel density in survivors vs. nonsurvivors of cardiac pathologic antibody-mediated rejection. Cardiovasc Pathol. 2012;21:445–54. doi: 10.1016/j.carpath.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Sorrentino C, Scarinci A, D'Antuono T, Piccirilli M, Di Nicola M, Pasquale M, et al. Endomyocardial infiltration by B and NK cells foreshadows the recurrence of cardiac allograft rejection. J Pathol. 2006;209:400–10. doi: 10.1002/path.1980. [DOI] [PubMed] [Google Scholar]
- 49.van den Bosch TP, Caliskan K, Kraaij MD, Constantinescu AA, Manintveld OC, Leenen PJ, et al. CD16+ monocytes and skewed macrophage polarization toward M2 type hallmark heart transplant acute cellular rejection. Front Immunol. 2017;8:346. doi: 10.3389/fimmu.2017.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welsh KJ, Zhao B, Buja LM, Brown RE. Sirt1-positive lymphocytes in acute cellular cardiac allograft rejection: Contributor to pathogenesis and a therapeutic target. ASAIO J. 2016;62:349–53. doi: 10.1097/MAT.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 51.Zakliczynski M, Nozynski J, Lange D, Zembala-Nozynska E, Konecka-Mrówka D, Zembala M. Nuclear mean gray level and chromatin distribution changes in cardiomyocytes of heart transplant recipients suffering from acute cellular rejection. Transplant Proc. 2006;38:325–7. doi: 10.1016/j.transproceed.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 52.Moreira RD, Moriel AR, Murta Junior LO, Neves LA, Godoy MF. Fractal dimension in quantifying the degree of myocardial cellular rejection after cardiac transplantation. Rev Bras Cir Cardiovasc. 2011;26:155–63. doi: 10.1590/s0102-76382011000200004. [DOI] [PubMed] [Google Scholar]
- 53.Pijet M, Nozynski J, Konecka-Mrowka D, Zakliczynski M, Hrapkowicz T, Zembala M. Fractal analysis of heart graft acute rejection microscopic images. Transplant Proc. 2014;46:2864–6. doi: 10.1016/j.transproceed.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 54.Nozynski J, Zakliczynski M, Zembala-Nozynska E, Konecka-Mrowka D, Nikiel B, Lange D, et al. Thymoglobulin administered early after heart transplantation reduces early myocardial hypertrophy assessed by morphometric studies. Transplant Proc. 2007;39:2825–32. doi: 10.1016/j.transproceed.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 55.Tona F, Fedrigo M, Famoso G, Previato M, Tellatin S, Vecchiati A, et al. Everolimus prevents coronary microvasculopathy in heart transplant recipients with normal coronary angiograms: An anatomo-functional study. Transplant Proc. 2014;46:2339–44. doi: 10.1016/j.transproceed.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Devitt JJ, Rice A, McLean D, Murray SK, Hirsch GM, Lee TD, et al. Impact of donor benign intimal thickening on cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32:454–60. doi: 10.1016/j.healun.2013.01.1044. [DOI] [PubMed] [Google Scholar]
- 57.Law L, Zheng L, Orsida B, Levvey B, Oto T, Kotsimbos AT, et al. Early changes in basement membrane thickness in airway walls post-lung transplantation. J Heart Lung Transplant. 2005;24:1571–6. doi: 10.1016/j.healun.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Ward C, De Soyza A, Fisher AJ, Pritchard G, Forrest I, Corris P. A descriptive study of small airway reticular basement membrane thickening in clinically stable lung transplant recipients. J Heart Lung Transplant. 2005;24:533–7. doi: 10.1016/j.healun.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Ito H, Shomori K, Adachi H, Taniyama K. Telepathology for the biopsy specimens from human allografted kidney: Effectiveness and pitfalls. Clin Transplant. 2001;15(Suppl 5):55–8. doi: 10.1034/j.1399-0012.2001.0150s5055.x. [DOI] [PubMed] [Google Scholar]
- 60.Sund S, Grimm P, Reisaeter AV, Hovig T. Computerized image analysis vs. semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant. 2004;19:2838–45. doi: 10.1093/ndt/gfh490. [DOI] [PubMed] [Google Scholar]
- 61.Lauronen J, Häyry P, Paavonen T. An image analysis-based method for quantification of chronic allograft damage index parameters. APMIS. 2006;114:440–8. doi: 10.1111/j.1600-0463.2006.apm_350.x. [DOI] [PubMed] [Google Scholar]
- 62.Meas-Yedid V, Servais A, Noël LH, Panterne C, Landais P, Hervé N, et al. New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation. 2011;92:890–9. doi: 10.1097/TP.0b013e31822d879a. [DOI] [PubMed] [Google Scholar]
- 63.Brazdziute E, Laurinavicius A. Digital pathology evaluation of complement C4d component deposition in the kidney allograft biopsies is a useful tool to improve reproducibility of the scoring. Diagn Pathol. 2011;6(Suppl 1):S5. doi: 10.1186/1746-1596-6-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozluk Y, Blanco PL, Mengel M, Solez K, Halloran PF, Sis B. Superiority of virtual microscopy versus light microscopy in transplantation pathology. Clin Transplant. 2012;26:336–44. doi: 10.1111/j.1399-0012.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 65.Jen KY, Olson JL, Brodsky S, Zhou XJ, Nadasdy T, Laszik ZG. Reliability of whole slide images as a diagnostic modality for renal allograft biopsies. Hum Pathol. 2013;44:888–94. doi: 10.1016/j.humpath.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Farris AB, Chan S, Climenhaga J, Adam B, Bellamy CO, Serón D, et al. Banff fibrosis study: Multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14:897–907. doi: 10.1111/ajt.12641. [DOI] [PubMed] [Google Scholar]
- 67.Moon A, Smith GH, Kong J, Rogers TE, Ellis CL, Farris AB., 3rd Development of CD3 cell quantitation algorithms for renal allograft biopsy rejection assessment utilizing open source image analysis software. Virchows Arch. 2018;472:259–69. doi: 10.1007/s00428-017-2260-6. [DOI] [PubMed] [Google Scholar]
- 68.Nicholson ML, Bailey E, Williams S, Harris KP, Furness PN. Computerized histomorphometric assessment of protocol renal transplant biopsy specimens for surrogate markers of chronic rejection. Transplantation. 1999;68:236–41. doi: 10.1097/00007890-199907270-00013. [DOI] [PubMed] [Google Scholar]
- 69.Bonsib SM, Abul-Ezz SR, Ahmad I, Young SM, Ellis EN, Schneider DL, et al. Acute rejection-associated tubular basement membrane defects and chronic allograft nephropathy. Kidney Int. 2000;58:2206–14. doi: 10.1111/j.1523-1755.2000.00395.x. [DOI] [PubMed] [Google Scholar]
- 70.Servais A, Meas-Yedid V, Buchler M, Morelon E, Olivo-Marin JC, Thervet E. Quantification of interstitial fibrosis by image analysis on routine renal biopsy 1 year after transplantation in patients managed by C2 monitoring of cyclosporine microemulsion. Transplant Proc. 2007;39:2560–2. doi: 10.1016/j.transproceed.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 71.Servais A, Meas-Yedid V, Noël LH, Martinez F, Panterne C, Kreis H, et al. Interstitial fibrosis evolution on early sequential screening renal allograft biopsies using quantitative image analysis. Am J Transplant. 2011;11:1456–63. doi: 10.1111/j.1600-6143.2011.03594.x. [DOI] [PubMed] [Google Scholar]
- 72.Birk PE, Gill JS, Blydt-Hansen TD, Gibson IW. Enhanced resolution of interstitial fibrosis in pediatric renal allograft biopsies using image analysis of trichrome stain. Pediatr Transplant. 2010;14:925–30. doi: 10.1111/j.1399-3046.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- 73.Caplin B, Veighey K, Mahenderan A, Manook M, Henry J, Nitsch D, et al. Early changes in scores of chronic damage on transplant kidney protocol biopsies reflect donor characteristics, but not future graft function. Clin Transplant. 2013;27:E669–78. doi: 10.1111/ctr.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vuiblet V, Fere M, Gobinet C, Birembaut P, Piot O, Rieu P. Renal graft fibrosis and inflammation quantification by an automated Fourier-transform infrared imaging technique. J Am Soc Nephrol. 2016;27:2382–91. doi: 10.1681/ASN.2015050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bräsen JH, Khalifa A, Schmitz J, Dai W, Einecke G, Schwarz A, et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017;92:479–89. doi: 10.1016/j.kint.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 76.Furukawa T, Kinukawa T, Sugiyama S, Ono Y, Ohshima S. Prediction of chronic allograft failure using computerized image analysis of postperfusion biopsy specimen: Study of cadaver kidney transplants. Transplant Proc. 2001;33:962–3. doi: 10.1016/s0041-1345(00)02286-7. [DOI] [PubMed] [Google Scholar]
- 77.Ishimura T, Fujisawa M, Isotani S, Higuchi A, Iijima K, Arakawa S, et al. Transforming growth factor-beta1 expression in early biopsy specimen predicts long-term graft function following pediatric renal transplantation. Clin Transplant. 2001;15:185–91. doi: 10.1034/j.1399-0012.2001.150307.x. [DOI] [PubMed] [Google Scholar]
- 78.Diaz Encarnacion MM, Griffin MD, Slezak JM, Bergstralh EJ, Stegall MD, Velosa JA, et al. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant. 2004;4:248–56. doi: 10.1046/j.1600-6143.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 79.Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, et al. Computerized image analysis of Sirius red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14:1662–8. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- 80.Pape L, Henne T, Offner G, Strehlau J, Ehrich JH, Mengel M, et al. Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: A new tool for predicting long-term graft function. Transplantation. 2003;76:955–8. doi: 10.1097/01.TP.0000078899.62040.E5. [DOI] [PubMed] [Google Scholar]
- 81.Pape L, Mengel M, Offner G, Melter M, Ehrich JH, Strehlau J. Renal arterial resistance index and computerized quantification of fibrosis as a combined predictive tool in chronic allograft nephropathy. Pediatr Transplant. 2004;8:565–70. doi: 10.1111/j.1399-3046.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 82.Sarioglu S, Celik A, Sakar M, Sonmez D, Tekis D. Methenamine silver staining quantitative digital histochemistry in chronic allograft nephropathy. Transplant Proc. 2004;36:2991–2. doi: 10.1016/j.transproceed.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 83.Sarioglu S, Sis B, Celik A, Tekis D, Kavukcu S, Bora S, et al. Quantitative digital histochemistry with methenamine silver staining in renal allograft biopsies excluding pure chronic allograft nephropathy cases. Transplant Proc. 2006;38:490–1. doi: 10.1016/j.transproceed.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 84.Grandaliano G, Gesualdo L, Ranieri E, Monno R, Stallone G, Schena FP. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation. 1997;63:414–20. doi: 10.1097/00007890-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 85.Grimm PC, McKenna R, Nickerson P, Russell ME, Gough J, Gospodarek E, et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–9. doi: 10.1681/ASN.V1071582. [DOI] [PubMed] [Google Scholar]
- 86.Danilewicz M, Wagrowska-Danilewicz M. A morphometric insight into glomerular and interstitial lesions in acutely rejected renal allografts. Pol J Pathol. 2003;54:171–7. [PubMed] [Google Scholar]
- 87.Danilewicz M, Wagrowska-Danilewicz M. Immunohistochemical analysis of the interstitial mast cells in acute rejection of human renal allografts. Med Sci Monit. 2004;10:BR151–6. [PubMed] [Google Scholar]
- 88.Nishi S, Imai N, Alchi B, Iguchi S, Ueno M, Fukase S, et al. The morphological compensatory change of peritubular capillary network in chronic allograft rejection. Clin Transplant. 2005;19(Suppl 14):7–11. doi: 10.1111/j.1399-0012.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 89.Sis B, Sarioglu S, Celik A, Kasap B, Yildiz S, Kavukcu S, et al. Renal medullary changes in renal allograft recipients with raised serum creatinine. J Clin Pathol. 2006;59:377–81. doi: 10.1136/jcp.2005.029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danilewicz M, Wagrowska-Danilewicz M. Correlative insights into the immunoexpression of transforming growth factor beta-1 in acutely rejected renal allografts. Pathol Res Pract. 2006;202:9–15. doi: 10.1016/j.prp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Hoffmann U, Segerer S, Rümmele P, Krüger B, Pietrzyk M, Hofstädter F, et al. Expression of the chemokine receptor CXCR3 in human renal allografts – A prospective study. Nephrol Dial Transplant. 2006;21:1373–81. doi: 10.1093/ndt/gfk075. [DOI] [PubMed] [Google Scholar]
- 92.Becker LE, de Oliveira Biazotto F, Conrad H, Schaier M, Kihm LP, Gross-Weissmann ML, et al. Cellular infiltrates and NFκB subunit c-rel signaling in kidney allografts of patients with clinical operational tolerance. Transplantation. 2012;94:729–37. doi: 10.1097/TP.0b013e31826032be. [DOI] [PubMed] [Google Scholar]
- 93.Yan Q, Sui W, Wang B, Zou H, Zou G, Luo H. Expression of MMP-2 and TIMP-1 in renal tissue of patients with chronic active antibody-mediated renal graft rejection. Diagn Pathol. 2012;7:141. doi: 10.1186/1746-1596-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mui KW, van Son WJ, Tiebosch AT, van Goor H, Bakker WW. Clinical relevance of immunohistochemical staining for ecto-AMPase and ecto-ATPase in chronic allograft nephropathy (CAN) Nephrol Dial Transplant. 2003;18:158–63. doi: 10.1093/ndt/18.1.158. [DOI] [PubMed] [Google Scholar]
- 95.Sugiyama S, Asano S, Tomita M, Hasegawa M, Murakami K, Kushimoto H, et al. Focal segmental sclerotic lesions of the glomerulus in transplanted kidneys assessed using computerized image analysis. Clin Transplant. 2003;17(Suppl 10):30–5. doi: 10.1034/j.1399-0012.17.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 96.Bains JC, Sandford RM, Brook NR, Hosgood SA, Lewis GR, Nicholson ML. Comparison of renal allograft fibrosis after transplantation from heart-beating and non-heart-beating donors. Br J Surg. 2005;92:113–8. doi: 10.1002/bjs.4777. [DOI] [PubMed] [Google Scholar]
- 97.Yan Q, Sui W, Xie S, Chen H, Xie S, Zou G, et al. Expression and role of integrin-linked kinase and collagen IV in human renal allografts with interstitial fibrosis and tubular atrophy. Transpl Immunol. 2010;23:1–5. doi: 10.1016/j.trim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Miura Y, Satoh S, Saito M, Numakura K, Inoue T, Obara T, et al. Factors increasing quantitative interstitial fibrosis from 0 Hr to 1 year in living kidney transplant patients receiving tacrolimus. Transplantation. 2011;91:78–85. doi: 10.1097/tp.0b013e3181ff4f7f. [DOI] [PubMed] [Google Scholar]
- 99.Yan Q, Wang B, Sui W, Zou G, Chen H, Xie S, et al. Expression of GSK-3β in renal allograft tissue and its significance in pathogenesis of chronic allograft dysfunction. Diagn Pathol. 2012;7:5. doi: 10.1186/1746-1596-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan Q, Jiang H, Wang B, Sui W, Zhou H, Zou G, et al. Expression and significance of RANTES and MCP-1 in renal tissue with chronic renal allograft dysfunction. Transplant Proc. 2016;48:2034–9. doi: 10.1016/j.transproceed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Hara S, Ishimura T, Fujisawa M, Nishi S, Itoh T. Granular swollen epithelial cells in the kidney allograft: A clinicopathological study with special emphasis on possible marker for kidney allograft aging. Nephrology (Carlton) 2016;21(Suppl 1):14–9. doi: 10.1111/nep.12764. [DOI] [PubMed] [Google Scholar]
- 102.Roos-van Groningen MC, Scholten EM, Lelieveld PM, Rowshani AT, Baelde HJ, Bajema IM, et al. Molecular comparison of calcineurin inhibitor-induced fibrogenic responses in protocol renal transplant biopsies. J Am Soc Nephrol. 2006;17:881–8. doi: 10.1681/ASN.2005080891. [DOI] [PubMed] [Google Scholar]
- 103.Rowshani AT, Scholten EM, Bemelman F, Eikmans M, Idu M, Roos-van Groningen MC, et al. No difference in degree of interstitial Sirius red-stained area in serial biopsies from area under concentration-over-time curves-guided cyclosporine versus tacrolimus-treated renal transplant recipients at one year. J Am Soc Nephrol. 2006;17:305–12. doi: 10.1681/ASN.2005030249. [DOI] [PubMed] [Google Scholar]
- 104.Scholten EM, Rowshani AT, Cremers S, Bemelman FJ, Eikmans M, van Kan E, et al. Untreated rejection in 6-month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of graft function. J Am Soc Nephrol. 2006;17:2622–32. doi: 10.1681/ASN.2006030227. [DOI] [PubMed] [Google Scholar]


