Abstract
Gastrointestinal stromal tumor (GIST) are the most common non epithelial tumor of the gastrointestinal (GI) tract. They arise from interstitial cells of Cajal present in the myenteric plexus. They can also arise outside the GI tract from mesentery, retro peritoneum and omentum. With the advent of new targeted molecular therapy c- tyrosine kinase inhibitor (Imatinib), it has become important to differentiate between response and pseudo-progression of the disease as response evaluation criteria for GIST are different from Response Evaluation Criteria in Solid Tumors (RECIST). Purpose of this pictorial essay is to enumerate the characteristic CT features of GIST, and discuss atypical features and response evaluation criteria.
Keywords: GIST, gastrointestinal, tumor, imatinib
Introduction
Gastrointestinal stromal tumors (GISTs) of Gastrointestinal (GI) tract are the most common non epithelial tumor as published in the western literature as well as in India.[1,2,3,4] The GISTs are thought to arise from the interstitial cells of Cajal located in the myenteric plexus of GI tract and they are clearly different from other mesenchymal tumors like leiomyomas and leiomyosarcomas.[5] GISTs show mutations, most commonly in c-KIT gene which is seen only in GIST and not in other mesenchymal tumors [Figure 1]. c-KIT mutations result in activation of the KIT receptor tyrosine kinase that promotes growth and survival of tumor.[5,6] However, up to 85-90% of GISTs have mutations in KIT and PDGFRA, whereas in the rest 10-15% these mutations are not seen and are referred to as wild type GISTs.[7,8,9] Some of these wild type GISTs are sporadic and others are associated with syndromes like neurofibromatosis, Carney-Stratakis syndrome and Carney triad.[10] In recent studies, it is shown that some of these wild type GISTs show mutations in BRAF V600E and succinate dehydrogenase (SDH) gene.[10,11] Succinate dehydrogenase is the key enzyme in citric acid pathway. SDH deficient GISTs occur in younger patient, commonly gastric in origin and frequently metastasize to lymph nodes in addition to liver and peritoneum.[12] Definitive diagnosis of GIST is required because advanced GISTs are treated with new tyrosine kinase receptor inhibitor Imatinib mesylate, a selective adenosine triphosphate (ATP)–competitive inhibitor of KIT, BCR-ABL, and PDGFR-α and -β. Treatment with Imatinib in advanced GIST is associated with better response and improved survival.[13,14,15] Therefore, immunohistochemical confirmation with c-kit is vital for confirmation of diagnosis. GISTs are friable tumor and hence percutaneous biopsy may theoretically result in tumor rupture and dissemination, upstaging the disease. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) is the alternative to obtain tissue diagnosis and it is safe and accurate procedure. For small resectable lesions, surgery is advised. However, for unresectable or metastatic disease or when treatment needs to be altered (if mass proved to be another neoplastic etiology), percutaneous ultrasound or CT guided biopsy can be performed.[16,17]
Figure 1 (A and B).
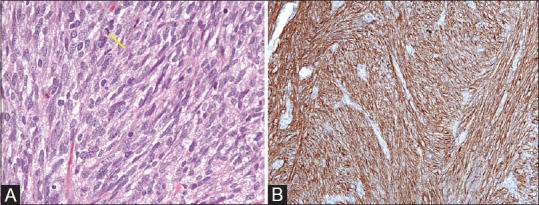
(A) H and E, ×40; Cellular proliferation of spindle cells with pale to eosinophilic fibrillar cytoplasm, arranged in whorls or short intersecting fascicles. Rare mitotic activity (arrow) (B) IHC, ×40; Immunohistochemistry for CD117 (CKIT) shows strong and diffuse cytoplasmic staining
Primary management of small localized primary disease is surgical resection.[8] Locally advanced GIST may benefit from neoadjuvant Imatinib followed by surgery. For metastatic and recurrent GIST, Imatinib remains the mainstay of treatment.[8] For imatinib resistant GIST, sunitinib is a second line agent.[15] Sunitinib targets KIT and PDGFR-α, as well as the vascular endothelial cell growth factor receptor (VEGFR), fms-like tyrosine kinase 3 (Flt3) receptor, and RET receptor. Sorafenib and Regorafenib are the 3rd line of therapy for both Imatinib and Sunitinib resistant GISTs.[18,19,20]
With the advent of these new targeted therapies in GIST, it has now become important to evaluate response to these targeted therapies. After treatment with Imatinib, tumor size usually decreases. However, in early post treatment period, decrease in tumor size is minimal and in some cases it may increase due to development of hemorrhage, necrosis or myxoid degeneration, but there are dramatic changes in the tumor characteristic (like attenuation, nodularity and number of vessels). Because of these factors, the modified response evaluation criteria in solid tumors (mRECIST) cannot be used in response evaluation. Choi Criteria which uses a combination of tumor attenuation (≥15% decrease), and modified tumor size (≥10% decrease) is used to assess partial response.[21,22] PET/CT can also be used in the response assessment of GIST treated with targeted molecular therapies and partial response status is redefined as a decrease in SUV at FDG PET (<70% from baseline or SUVmax <2.5).[21] However, PET/CT is costly and is not widely available. It cannot be used in GISTs which are initially PET negative.
This pictorial essay describes the CT characteristics of GIST at presentation, its post treatment evaluation and evaluation of disease recurrence during surveillance/follow-up.
GIST on ultrasound
Trans-abdominal ultrasonography is usually the first investigation for the palpable abdominal mass. On ultrasonography, GISTs are seen as heterogeneous masses due to necrosis, hemorrhage or cystic changes and are found in relation to the bowel.[23,24] When large, it becomes difficult to identify the organ of origin. Liver metastases appear as hypoechoic lesions with respect to the liver parenchyma. Contrast enhanced ultrasound helps detect the viable portion of the tumor and hepatic metastasis. Endoscopic ultrasound is useful for small (<2 cm) and incidentally detected submucosal tumors and also facilitates endoscopic biopsy of such lesions.[23]
Computed tomography (CT) characteristics of GIST at presentation
CT plays an important role in the initial evaluation of GIST. Tumors are usually of varying density and show patchy enhancement on contrast enhanced CT scan [Figure 2]. Varying degrees of necrosis may be frequently demonstrated within the mass [Figures 2 and 3].
Figure 2 (A and B).
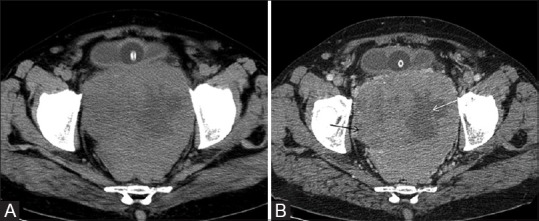
(A), pre contrast CT scan showing rectal GIST with heterogeneous attenuation with hypoattenuating areas within. (B) Post contrast CT scan heterogeneous GIST arising from the rectum with heterogeneous enhancement with non-enhancing cystic/necrotic areas within (arrows). It shows extraserosal extension anteriorly and abuts the urinary bladder without infiltration
Figure 3.
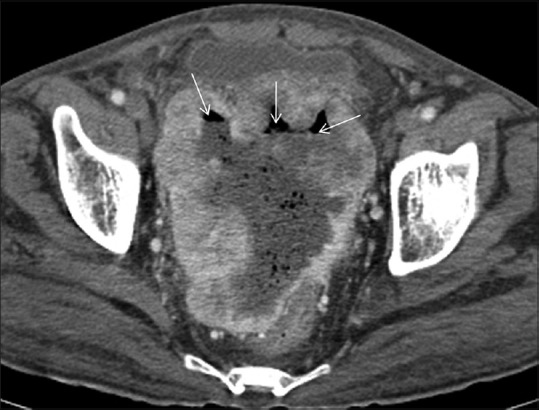
Post contrast axial CT scan showing sigmoid GIST with peripheral post contrast enhancement. Central non enhancing cystic/necrotic area is seen. Air fluid levels (arrows) are also noted within suggestive of communication with gut
GIST can arise from any part of GI tract, from esophagus to anorectum as well as atypical GISTs arising from omentum, mesentery and retroperitoneum termed as Extra-gastrointestinal stromal tumors (EGIST).[25,26,27]
Esophagus
GISTs are uncommon tumors of the esophagus, most common esophageal tumor being leiomyoma. Leiomyomas tend to occur in younger age group with median age of presentation of 35 years and GISTs tend to occur in older age group with median age of 63 years.[28]
Esophageal GISTs more commonly arise in the lower esophagus followed by mid-esophagus. On computed tomography, they are seen as intraluminal (common), intramural or exophytic masses. They are hypodense masses and show mild enhancement. Necrosis and calcifications can be seen within.[29]
The differential diagnoses of esophageal GISTs depend on the predominant growth pattern. Small intraluminal GISTs can be confused with papilloma, adenoma, inflammatory polyp, fibrovascular polyp, and carcinoma which manifest as intraluminal polypoid masses. Large aggressive GISTs with extra serosal extension can mimic advanced stage carcinoma, malignant melanoma, lymphoma and leiomyosarcoma.[25]
Stomach
Stomach is the most common location of GIST and most GISTs in stomach arise in the body followed by fundus and antrum.[25] Gastric GISTs can be endophytic, exophytic or mixed (dumbbell shaped) pattern, most common pattern is exophytic growth.[30] The extragastric extension can be seen in gastrohepatic ligament, gastrosplenic ligament, and lesser sac. Sometimes the large component of gastric GIST is extragastric and it becomes difficult to determine the organ of origin. In these cases, careful evaluation of stomach may show gastric wall thickening which suggests origin of tumor from stomach. Majority of cases (approximately 92%) show peripheral enhancement and this enhancing component represents viable tumor. Non enhancing component could be due to hemorrhage, necrosis or cystic changes. Minority of cases (approximately 8%) show homogeneous enhancement [Figure 4]. The cystic or necrotic areas may form cavities within and these cavities may communicate with the stomach resulting in air/air-fluid level/orally administered contrast in the tumor [Figure 5]. Calcification is unusual in GIST and is seen only in 3% of cases. Calcification can be mottled or extensive diffusely involving the tumor. CT also demonstrates the invasion of adjacent organ, liver, omental and peritoneal metastasis and ascites [Figure 6]. Metastatic lymphadenopathy is not a feature of GIST except for SDH deficient GISTs which can be associated with lymphadenopathy.[12,25]
Figure 4.
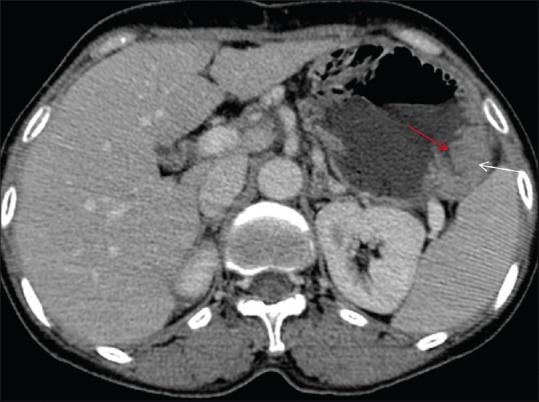
Axial post contrast CT scan showing homogeneously enhancing mass along the greater curvature of stomach (white arrow). Ulceration in the stomach is noted (red arrow)
Figure 5.
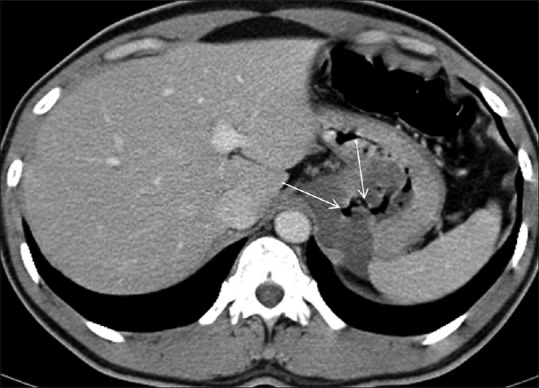
There is hypoattenuating GIST involving the body of the stomach. There is central necrotic component communicating with the lumen of the stomach with resultant air in the tumor cavity (arrows)
Figure 6.
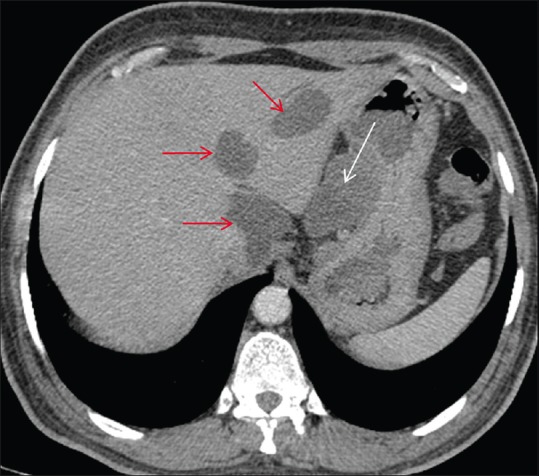
Axial post contrast CT scan showing homogeneously enhancing exophytic gastric mass in the gastrohepatic region (white arrow) with few hypodense metastatic liver lesions (red arrow)
The differential diagnoses of gastric GIST are adenocacinoma and lymphoma. Gastric adenocarcinoma often has perigastric, gastrohepatic and celiac adenopathy, not seen in GIST. Lymphoma has bulky abdominal adenopathy which may extend into the pelvis.
Small intestine
Small intestine is the second most common location of GIST after stomach. Tumor can arise from throughout the small intestine, i.e. duodenum, jejunum and ileum. Tumor extending into the bowel lumen may cause intestinal obstruction while those with extraserosal extension rarely cause intestinal obstruction.
CT depicts the mural, intraluminal and extraserosal component of small intestinal GIST. CT characteristics of small intestinal GISTs are very similar to gastric GIST. Most show heterogeneous post contrast enhancement [Figure 7]. Extraserosal extension into the adjacent small bowel, colon, ureter, urinary bladder or mesentery may occur. Metastasis to liver, omentum and peritoneum may occur and well evaluated by CT scan [Figures 7 and 8].[25]
Figure 7 (A and B).
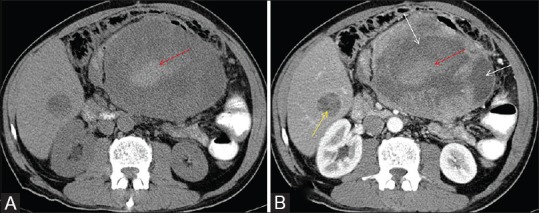
Ileal GIST with hyperdense area within (red arrow in Figure A) and shows no post contrast enhancement (red arrow in B) is hemorrhage within the tumor. Figure B shows heterogeneous enhancement in this tumor with non-enhancing hemorrhagic (red arrow) and necrotic/cystic components within (white arrows). Also there is heterogeneously enhancing liver metastasis (yellow arrow in B)
Figure 8 (A and B).
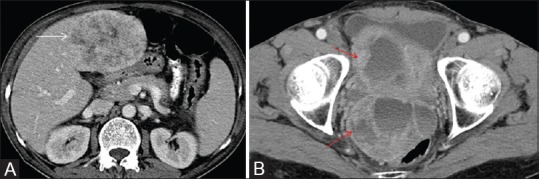
In this case of ileal GIST, axial post contrast CT scan shows (A) heterogeneously enhancing liver metastasis (white arrow) and (B) predominantly peripherally enhancing pelvic peritoneal deposits with central non enhancing cystic/necrotic area (red arrow)
The differentials are adenocarcinoma and lymphoma of small bowel. Neoplasms that originate primarily from the mesentery and secondarily involve the small intestine should also be included in the differential diagnosis. These tumors include mesenteric fibromatosis (desmoid tumor), inflammatory pseudotumor, sclerosing mesenteritis, and metastatic disease.
The adenocarcinoma of small intestine usually manifest as mural thickening with luminal narrowing and may be associated with intestinal obstruction which is uncommon in GIST. Lymphatic metastases can be seen in adenocarcinoma, not a feature of GIST. Small bowel lymphoma associated with luminal dilatation and extraserosal extension can be confused with GIST; however, lymphoma is associated with bulky adenopathy which is uncommon in GIST.[31]
Colon
Primary colonic GISTs are less common. The metastatic GIST to colon can involve the external surface of colon. Primary intramural tumors can involve the intraluminal or external aspect of the colon. Large tumors may have areas of hemorrhage, necrosis, cystic changes and calcification [Figures 3 and 9]. Circumferential growth with dilatation of the affected colon may also be seen.[25]
Figure 9.
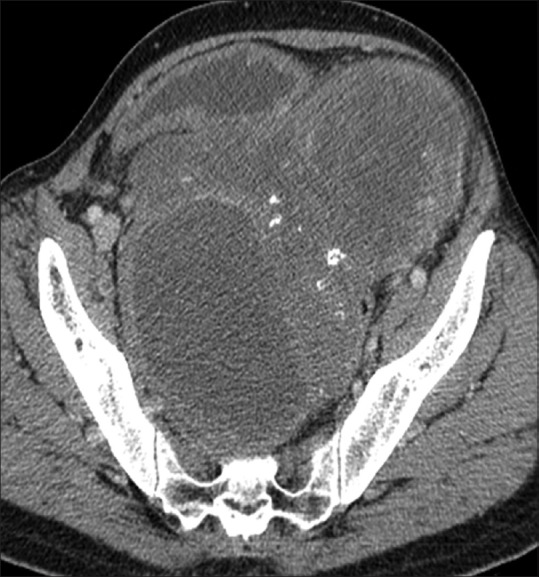
Axial post contrast CT scan showing sigmoid GIST with peripheral enhancement and central non enhancing cystic/necrotic area. Specks of calcifications (hyperdense foci) are also seen within
CT appearances of colonic GISTs are similar to those of gastric or small intestinal GIST. Infiltration to adjacent organs may occur.
The radiologic differential diagnosis for colonic GISTs includes adenocarcinoma, lymphoma, metastatic melanoma, and leiomyosarcoma.
Anorectum
Anorectum is a common site of GISTs. Anorectal GIST can be seen as eccentric mural mass which can have extraserosal extension and may involve ischiorectal fossa, prostate and vagina [Figure 2]. Intraluminal extension is less common in anorectal GISTs [Figure 10]. CT shows heterogeneous post contrast enhancement of Anorectal GIST. Non enhancing areas with low attenuation on CT correspond to the necrotic/cystic areas. Calcification, usually, is not a feature of anorectal GIST. Metastatic adenopathy is uncommon.[32]
Figure 10 (A and B).
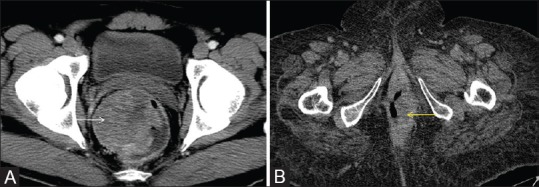
Axial post contrast CT showing (A) rectal GIST with intraluminal extension and showing heterogeneous post contrast enhancement (white arrow) and (B) anal canal GIST with intraluminal extension (yellow arrow)
The differential diagnoses of anorectal GISTs include rectal adenocarcinoma and squamous cell carcinoma, melanoma, lymphoma, carcinoid, leiomyoma and leiomyosarcoma.
Extra-gastrointestinal GIST (EGIST)
Extra-gastrointestinal GISTs may arise in the omentum, mesentery or retroperitoneum and are unrelated to the gastrointestinal tract.[27] EGISTs, though reported in literature, are rare. They share histological and immunohistochemical features with gastrointestinal GIST. They show staining with KIT, a marker of interstitial cells of Cajal which are normally present in the GI tract.[33] The reason why these cells, which are normally present in the gut wall, reach the omentum, mesentery and retroperitoneum and then develop into a tumor is not yet clear. Most, if not all, primarily arise from the gut and have extensive extramural component which then lose contact with gut wall.[34,35] Another hypothesis is that this tumor may arise from the multipotent mesenchymal stem cells.[36]
Primary EGIST may be homogeneous hypodense masses showing homogeneous post contrast enhancement or may have cystic, hemorrhagic and necrotic component giving it complex appearance. Solid portion shows contrast enhancement [Figure 11A and B]. The differential diagnoses of primary EGISTs are leiomyosarcoma, fibrosarcoma, liposarcoma and malignant fibrous histiocytoma. Mesenteric fibromatosis are usually homogeneous without any hemorrhage, necrosis or cystic changes within.[25]
Figure 11 (A and B).
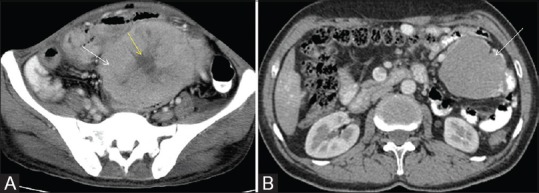
(A) Axial post contrast CT scan showing heterogeneously enhancing mesenteric GIST (white arrow) with central non enhancing cystic/necrotic area (yellow arrow). No communication to GI tract was seen. Histopathology showed features of GIST. (B) Axial post contrast CT scan showing homogeneously enhancing peritoneal GIST in left lumbar region (white arrow). No communication to GI tract was seen. Histopathology showed features of GIST
GIST from the primary gastrointestinal tract origin may metastasize to the peritoneum. In these cases, there are usually multiple masses throughout the peritoneal cavity which is uncommon in primary peritoneal GIST [Figure 8B]. The differential diagnoses are peritoneal carcinomatosis, lymphomatosis and leiomyomatosis peritonealis disseminata.[25]
Metastatic GIST
About 50% of GISTs have metastasis at presentation. GISTs metastasize usually to the liver and peritoneum via hematogenous spread and peritoneal seeding, most common site is liver.[37] Liver metastases are usually multiple, can involve both lobes and can have variegated attenuation. They are hypoattenuating on plain scan and shows heterogeneous post contrast enhancement. Central area is more hypodense than periphery and is due to cystic/necrotic changes. Peripheral enhancing part is solid viable tumor. Sometimes they show early post contrast enhancement in arterial phase and become isodense to rest of the liver parenchyma in delayed phase scan. Hence, triphasic CT scan is essential to look for liver metastases. Sometimes heterogeneous appearance with cystic lesion, fluid-fluid levels or multilocular appearance is seen and is due to intratumoral hemorrhage, necrosis or calcification. Often tumor vessels are seen within the tumors [Figures 6–8].[37,38] Peritoneal metastases can occur when there is tumor extension beyond serosa or can occur after percutaneous biopsy. Peritoneal metastases are in the form of peritoneal or omental nodule or less commonly there can be omental caking. Peritoneal metastasis appear like primary tumor on CT. They can be hyper-enhancing solid masses or can be heterogeneous due to cystic/necrotic changes, bleeding or calcifications [Figure 8B]. Tumor vessels can be seen within the tumors.[38] Ascites is rare and is more attributable to the treatment. Lymph node metastasis is rare though reported in the literature and lymph node metastases are more common in SDH deficient GIST.[12,38] Lymph node metastasis have imaging feature similar to that of primary tumor. Lung, bone, adrenal and cutaneous metastases are rare though reported in literature and commonly seen in association with liver and peritoneal metastases. Bone metastases are usually lytic and are commonly seen in spine and pelvic bones. Lung metastases are seen as soft tissue nodules in association with liver/peritoneal metastases. Adrenal metastases are rare and when present they have attenuation characteristics similar to the primary tumor [Figure 12].[38,39]
Figure 12.
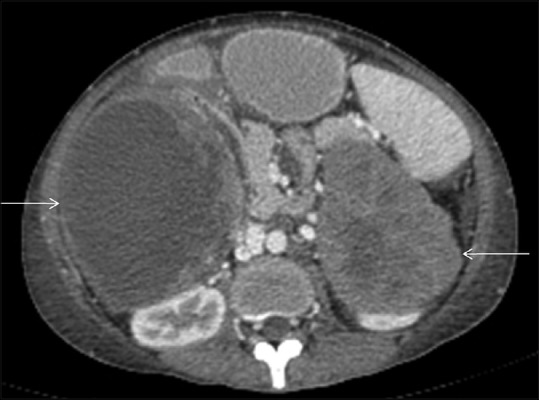
In this case of metastatic gastric GIST (image not shown), axial contrast enhanced CT scan shows heterogeneously enhancing masses in bilateral suprarenal regions involving both adrenal glands with non-enhancing cystic/necrotic areas (arrows), suggestive of bilateral adrenal metastases
Response assessment in GIST
Surgery is mainstay of treatment in the management of GIST. However, targeted therapy with Imatinib and other TKI inhibitors has been widely used in management of GIST. In large tumors, neoadjuvant Imatinib is used to downsize the tumor to avoid morbid surgery and preserving the organs.[40] Post-operative, adjuvant therapy with Imatinib is given for up to 3 years.[41] In metastatic GIST, Imatinib is mainstay of therapy.[42]
Computed Tomography (CT) scan is routinely used to monitor response in primary or metastatic GIST. After treatment with Imatinib, there is decrease in tumor size, attenuation and tumor vessels [Figure 13]. Decrease in tumor size after Imatinib may take several months before satisfying traditional response criteria such as mRECIST. In contrast, on contrast enhanced CT scan, there is dramatically decrease in tumor attenuation value, measured in Hounsfield Units (HU) and this decrease in attenuation is seen within one month. This decrease in tumor attenuation is due to development of myxoid degeneration and occasionally due to hemorrhage and necrosis.[37] Paradoxically, tumor may enlarge after treatment and is due to development of myxoid changes or intratumoral hemorrhage.[37] Accordingly, alternate tumor response criteria incorporating changes in tumor attenuation along with size reduction were proposed by Choi et al. According to the Choi criteria, a 15% decrease in CT attenuation or 10% decrease in unidimensional size indicates response in contrast to 30% decrease in unidimensional size as per RECIST.[22,43]
Figure 13 (A and B).
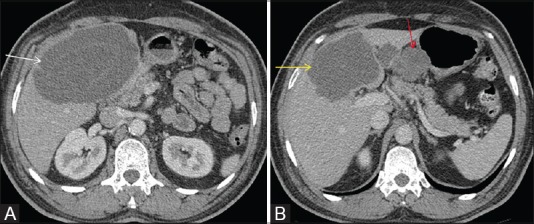
In this case of gastric GIST, axial post contrast CT scan shows a large hypoattenuating metastatic liver lesion (white arrow in A). Post chemotherapy, there is decrease in size as well as attenuation of the liver metastatic lesion (yellow arrow in B). Residual gastric GIST is also seen (red arrow in B; not shown in Figure A)
PET/CT can also be used in the response assessment of GIST treated with targeted molecular therapies. However, PET/CT is costly and is not widely available. It cannot be used in GISTs which are initially PET negative. On PET/CT, partial response status is defined as a decrease in SUV at FDG PET (<70% from baseline or SUVmax < 2.5) [Figure 14].[21]
Figure 14 (A and B).
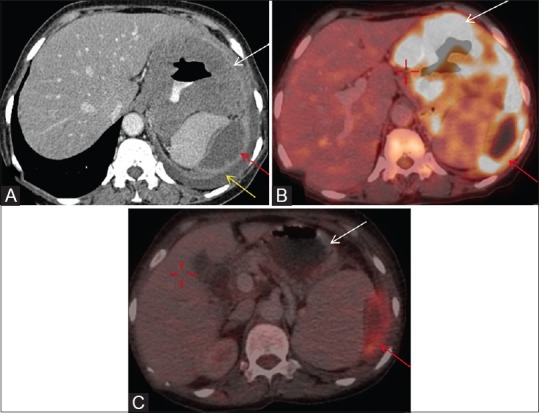
(A) Axial post contrast CT image shows homogeneous wall thickening involving body of stomach (white arrow). Peripherally enhancing cystic/necrotic peritoneal deposit is also seen in left hypochondriac region (red arrow). Left pleural effusion is also seen (yellow arrow). (B) FDG PET/CT fused axial image shows intense FDG uptake in gastric GIST (white arrow). There is also peripheral FDG uptake in peritoneal deposit (red arrow). Central non FDG avid area is cystic/necrotic. (C) Post imatinib, there is no FDG uptake in gastric mass (white arrow) and interval decrease in size and FDG uptake (SUV less than 2.5) in peritoneal deposit (red arrow) suggestive response to imatinib
Imatinib is known to cause fluid retention, diarrhea, nausea, abdominal pain and rash, in patients undergoing treatment for GIST. The most common adverse effect is fluid retention which may manifest as extensive subcutaneous edema, ascites, pleural and pericardial effusion. New onset of ascites in follow up CT scan may be mistakenly interpreted as peritoneal disease and wrongly labeled as progressive disease.[37]
Patients who develop primary or secondary resistance to Imatinib are treated with second line Sunitinib and third line Sorafenib/Regorafenib.[19,20,44] It is not known if dramatic density changes as seen with Imatinib also occur with Sunitinib and Regorafenib. However, in few studies, Choi criteria was not useful for response assessment after treatment with Sunitinib and Regorafenib and RECIST 1.1 was useful in evaluating progression free survival.[45,46,47]
Surveillance
Although surgery is mainstay of treatment, recurrences occur in most patients even after complete resection with negative margins. Once the tumor is resected or has responded to treatment, it is important to detect recurrence or disease progression as early as possible for the benefit of patient.
Recurrences typically occur first in liver or peritoneum, so these sites along with site of primary tumor should be carefully evaluated to look for recurrence. Traditional criteria for disease progression are increase in tumor size, development of new lesion and appearance of metastasis and these are also of value in monitoring patients with GIST. In previously treated GIST with hypoattenuating cystic lesion, increase in size or density and development of new enhancing intratumoral nodule referred to as nodule within mass is also consistent with disease recurrence or progression, regardless of change in size of tumor.[37,48]
Conclusion
Gastrointestinal stromal tumor (GIST) is the most common non epithelial tumor of the gastrointestinal (GI) tract. With advancement in treatment modalities in GIST, it is important to diagnose and accurately stage GIST for optimal benefit of patient. With new targeted molecular therapy, response assessment is important in which case modified response evaluation criteria in solid tumors (mRECIST) may not be helpful after Imatinib treatment but is useful for response evaluation after second and third line Sunitinib and Sorafenib/Regorafenib treatment respectively. We also have discussed about the surveillance in treated GIST.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lakshmi VA, Chacko RT, Kurian S. Gastrointestinal stromal tumors: A 7-year experience from a tertiary care hospital. Indian J Pathol Microbiol. 2010;53:628–33. doi: 10.4103/0377-4929.72005. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64. doi: 10.1016/s1470-2045(02)00899-9. [DOI] [PubMed] [Google Scholar]
- 3.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;19(369):1731–41. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 4.Steigen SE, Eide TJ. Gastrointestinal stromal tumors (GISTs): A review. APMIS. 2009;12(117):73–86. doi: 10.1111/j.1600-0463.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 5.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 6.DeMatteo RP. The GIST of targeted cancer therapy: A tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571) Ann Surg Oncol. 2002;9:831–9. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 7.Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012;136:483–9. doi: 10.5858/arpa.2011-0082-RA. [DOI] [PubMed] [Google Scholar]
- 8.Poveda A, García Del Muro X, Antonio López-Guerrero J, Cubedo R, Martínez V, Romero I, et al. Tumour review GEIS guidelines for gastrointestinal sarcomas (GIST) Cancer Treat Rev. 2017;55:107–19. doi: 10.1016/j.ctrv.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH. Imatinib and beyond in gastrointestinal stromal tumors: A radiologist's perspective. Am J Roentgenol. 2013;201:801–10. doi: 10.2214/AJR.12.10003. [DOI] [PubMed] [Google Scholar]
- 10.Tirumani SH, Baheti AD, Tirumani H, O’Neill A, Jagannathan JP. Update on Gastrointestinal stromal tumors for radiologists. Korean J Radiol. 2017;18:84–93. doi: 10.3348/kjr.2017.18.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Regan KN, Shinagare AB, Saboo SS, Ramaiya NH, Jagannathan JP, Tirumani SH. Gastrointestinal stromal tumors (GIST): Lesser known facts. Clin Imaging. 2013;37:821–9. doi: 10.1016/j.clinimag.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Tirumani SH, Tirumani H, Jagannathan JP, Shinagare AB, Hornick JL, George S, et al. MDCT features of succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumours. Br J Radiol. 2014;87:20140476. doi: 10.1259/bjr.20140476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–6. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results. JAMA Oncol. 2017;3:944. doi: 10.1001/jamaoncol.2016.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamboat ZM, Dematteo RP. Updates on the management of gastrointestinal stromal tumors. Surg Oncol Clin N Am. 2012;21:301–16. doi: 10.1016/j.soc.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN task force report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;Suppl 2:S1–41. doi: 10.6004/jnccn.2010.0116. quiz S42-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano C, George S. Recent advances in the treatment of gastrointestinal stromal tumors. Ther Adv Med Oncol. 2014;6:115–27. doi: 10.1177/1758834014522491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montemurro M, Gelderblom H, Bitz U, Schütte J, Blay JY, Joensuu H, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: A retrospective analysis. Eur J Cancer. 2013;49:1027–31. doi: 10.1016/j.ejca.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Demetri GD, Reichardt P, Kang Y-K, Blay J-Y, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: Review of traditional and new criteria. RadioGraphics. 2013;33:1323–41. doi: 10.1148/rg.335125214. [DOI] [PubMed] [Google Scholar]
- 22.Choi H, Charnsangavej C, Faria S de C, Tamm EP, Benjamin RS, Johnson MM, et al. CT Evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. Am J Roentgenol. 2004;183:1619–28. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 23.Vernuccio F, Taibbi A, Picone D, LA Grutta L, Midiri M, Lagalla R, et al. Imaging of gastrointestinal stromal tumors: From diagnosis to evaluation of therapeutic response. Anticancer Res. 2016;36:2639–48. [PubMed] [Google Scholar]
- 24.Chan KP. What's the Mass? The gist of point-of-care ultrasound in gastrointestinal stromal tumors. Clin Pract Cases Emerg Med. 2018;2:82–5. doi: 10.5811/cpcem.2017.12.36375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: Radiologic features with pathologic correlation1. RadioGraphics. 2003;23:283–304. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa I, Morishita H, Matsuki S, Komeyama T, Emura I, Hara N. Primary gastrointestinal stromal tumor in the retroperitoneum. Int J Urol. 2006;13:1245–8. doi: 10.1111/j.1442-2042.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 27.Casella C, Villanacci V, D’Adda F, Codazzi M, Salerni B. Primary extra-gastrointestinal stromal tumor of retroperitoneum. Clin Med Insights Oncol. 2012;6:189. doi: 10.4137/CMO.S9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211–22. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Shinagare AB, Zukotynski KA, Krajewski KM, Jagannathan JP, Butrynski J, Hornicke JL, et al. Esophageal gastrointestinal stromal tumor: Report of 7 patients. Cancer Imaging. 2012;12:100–8. doi: 10.1102/1470-7330.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J, Kang W, Zhu J, Xu J. CT and MR imaging of gastrointestinal stromal tumor of stomach: A pictorial review. Quant Imaging Med Surg. 2012;2:274–9. doi: 10.3978/j.issn.2223-4292.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley J, Fishman EK. CT evaluation of small bowel neoplasms: Spectrum of disease1. Radiographics. 1998;18:379–92. doi: 10.1148/radiographics.18.2.9536485. [DOI] [PubMed] [Google Scholar]
- 32.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Anorectal gastrointestinal stromal tumors: CT and MR imaging features with clinical and pathologic correlation. Am J Roentgenol. 2003;180:1607–12. doi: 10.2214/ajr.180.6.1801607. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto H, Oda Y, Kawaguchi K, Nakamura N, Takahira T, Tamiya S, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–88. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Goh BK, Chow PK, Kesavan SM, Yap WM, Chung YF, Wong WK. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: Critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg. 2009;13:1094–8. doi: 10.1007/s11605-009-0828-4. [DOI] [PubMed] [Google Scholar]
- 35.Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: A regular origin in the muscularis propria, but an extremely diverse gross presentation. Langenbeck's Arch Surg. 2006;391:322–9. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Huan X, Liang X, Li Z, Tan A. Clinicopathological and immunohistochemical study of extra-gastrointestinal stromal tumors arising from the omentum and mesentery. Chinese J Pathol. 2005;34:11–4. [PubMed] [Google Scholar]
- 37.Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: Role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. RadioGraphics. 2006;26:481–95. doi: 10.1148/rg.262055097. [DOI] [PubMed] [Google Scholar]
- 38.Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci. 2012;2:43. doi: 10.4103/2156-7514.99177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripathi S, Rajagopal K, Srivastava RK, Ayachit A. CT features, mimics and atypical presentations of gastrointestinal stromal tumor (GIST) Indian J Radiol Imaging. 2011;21:176–81. doi: 10.4103/0971-3026.85364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH, Raut CP. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol. 2014;40:420–8. doi: 10.1016/j.ejso.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs Three years of adjuvant imatinib for operable gastrointestinal stromal tumor. JAMA. 2012;307:1265. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 42.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 43.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 44.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 45.Schramm N, Englhart E, Schlemmer M, Hittinger M, Übleis C, Becker CR, et al. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: Comparison of RECIST, Choi and volumetric criteria. Eur J Radiol. 2013;82:951–8. doi: 10.1016/j.ejrad.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 46.Shinagare AB, Barysauskas CM, Braschi-Amirfarzan M, O’Neill AC, Catalano PJ, George S, et al. Comparison of performance of various tumor response criteria in assessment of sunitinib activity in advanced gastrointestinal stromal tumors. Clin Imaging. 2016;40:880–4. doi: 10.1016/j.clinimag.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Shinagare AB, Jagannathan JP, Kurra V, Urban T, Manola J, Choy E, et al. Comparison of performance of various tumour response criteria in assessment of regorafenib activity in advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib. Eur J Cancer. 2014;50:981–6. doi: 10.1016/j.ejca.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shankar S, vanSonnenberg E, Desai J, DiPiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: New nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892–8. doi: 10.1148/radiol.2353040332. [DOI] [PubMed] [Google Scholar]


