Abstract
Transthoracic echocardiography is a potent and appealing diagnostic tool by virtue of rapidity, noninvasiveness, and repeatability. Focus-assessed transthoracic echocardiography (FATE) forms quick guidance to interpret the echocardiographic information and relates it to the clinical context. It can be applied in the perioperative period, intensive care units (ICUs), and emergency situations, in trauma and as resuscitation aids. FATE intents to assess cardiac function including contractility, chamber size and hypertrophy, valvular dysfunction, cardiac tamponade, and pericardial and pleural effusions. Thence, FATE has become a quintessential scanning tool perioperatively and in ICUs.
Keywords: Critical care, echocardiography, focus-assessed transthoracic echocardiography, focused transthoracic echocardiography, intensive care, ultrasonography
Introduction
Ultrasound (US) is the only method which enables real-time bedside imaging of the heart.[1] Focused cardiac US provides meritorious diagnostic information to usher changes in the perioperative management.[2] Transthoracic echocardiography (TTE) allows rapid, noninvasive, point-of-care (POC) assessment of ventricular function, valvular integrity, volume status, and fluid responsiveness.[3] Focus-assessed transthoracic echocardiography (FATE), an abbreviated TTE protocol, is an effective supplementary tool.[4] In critically ill patients, it offers a systematic and focused approach to the echocardiographic examination and proposes a skill set that can be readily[5] and quickly learned.[6]
The objectives of FATE protocol are principally the exclusion of the obvious causative pathology, assessing contractility of the left ventricle (LV), estimation of wall thickness and chamber dimensions, exclusion of pleural pathology, and moreover relating the echocardiographic information to the clinical context.[4,7,8]
While the basic FATE is done with the intentions mentioned above, advanced FATE examination aims at comprehensive assessment of hemodynamics, Doppler US for cardiac output and pressure calculations, diastolic dysfunction, severity of valvular heart diseases, and more.[9] This article addresses the basic FATE protocol.
The acumen to image acquisition and analysis ought to be precise and are done by procuring FATE protocol views. In this regard, familiarity with holding and movements of the transducer is essential. The transducer is held in two ways. For most of the images, transducer is held by “under-the-probe” technique like a pencil or “over-the-probe” technique with overhand grip for imaging, especially for subcostal views, which allows application of pressure against the abdominal muscles.
Transducer Movements
To ascertain effective imaging, the transducer is manipulated and the best images are obtained by the movements which include as follows:
Sliding – also called as translation. The transducer is moved on the chest to any position to obtain the best window and in any direction; for instance, sliding the transducer from parasternal to apical area
Tilting – also called as heel-toe movement. The transducer in kept at the same place on the chest and moved side to side to allow other planes in the same axis to come into view
Angulation – The transducer in kept at the same place on the chest and moved cephalad or in caudal direction
Rotation – The transducer is kept at the same place on the chest, and clockwise or counterclockwise movement is done for obtaining different views from the same echocardiographic window. Rotating the transducer approximately from 11 to 2 o’ clock switches from long to short axis.
The basic FATE views are as follows:
Subcostal view
Apical four-chamber view
Parasternal long-axis view
Parasternal short-axis view
Pleura scanning.
Subcostal view [Figure 1]
Figure 1.
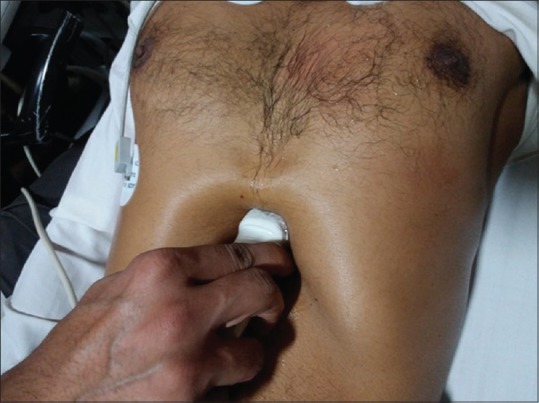
Probe position for subcostal view
The patient is positioned supine with both legs flexed at knees, abdomen relaxed. The transducer is placed in the subxiphoid area with the orientation marker pointing toward the patient's left shoulder. The subcostal four-chamber view is obtained to display left atrium (LA), LV, right atrium (RA), and right ventricle (RV). The interatrial septum (IAS) is best screened in this view as it is perpendicular to the echo signal. The interventricular septum (IVS), mitral valve (MV), and tricuspid valve (TV) are observed while the RV free wall thickness can also be estimated. A deep inspiration avails better imaging of this view. Furthermore, rotating the transducer counterclockwise exhibits short axis of the LV. The annex to the above findings is the inferior vena cava (IVC) diameter and collapsibility which can be determined by directing the probe to the right side to image the IVC entering the RA. In addition, chamber dimensions, contractility, wall thickness, and valvular function can be determined. Pericardial effusion can also be precluded. This view is applicable in particular during the cardiopulmonary resuscitation, patients with poor windows as in the obese, cardiac, or thoracic surgery patients.
Apical four-chamber view [Figure 2]
Figure 2.
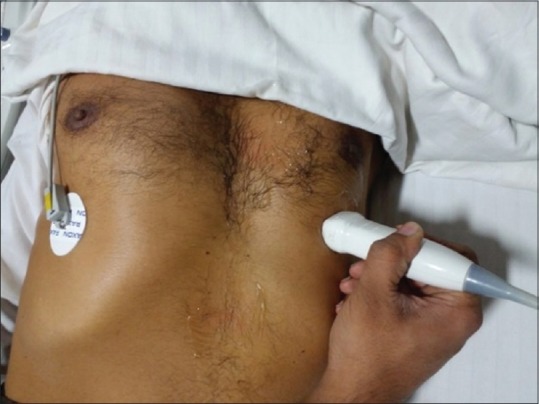
Probe position for apical four-chamber view
Patient is positioned supine with a left lateral tilt. The transducer is placed on the fifth intercostal space in the left midclavicular line or at the point of apex impulse with the orientation marker directing toward the patient's left shoulder. This view displays LA, MV, LV, RA, TV, RV, IAS, and IVS. The IVS should be perpendicular for an ideal view. Analysis of ventricular function, contractility, dilatation and hypertrophy is effected in this view. Pericardial effusion is evident if present.
Parasternal long-axis view [Figure 3]
Figure 3.
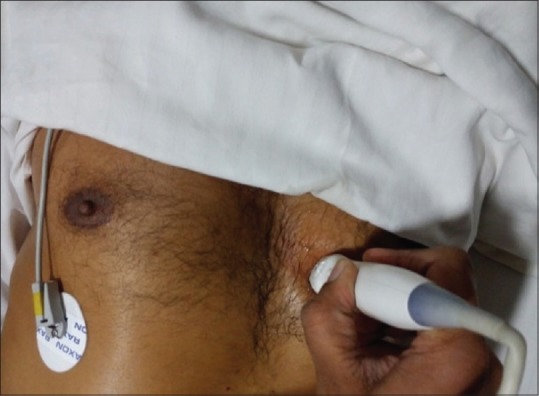
Probe position for parasternal long-axis view
Patient is positioned in the left lateral position; the transducer is placed in the left third to fifth intercostal space near the sternum. The orientation marker points toward patient's right shoulder. This view demonstrates LA, MV, LV, AV, RV, and descending aorta. The LV apex is not imaged in this view. The LV systolic function, dilatation and hypertrophy of chambers, interventricular septal hypertrophy, mitral and aortic valve function, descending aortic dilatation, pericardial and pleural effusion can be assessed. The pericardial effusion can be differentiated from pleural effusion by the fact that pericardial effusion lies anterior to the descending aorta while pleural effusion is seen posterior to descending aorta.
Parasternal short-axis view [Figure 4]
Figure 4.
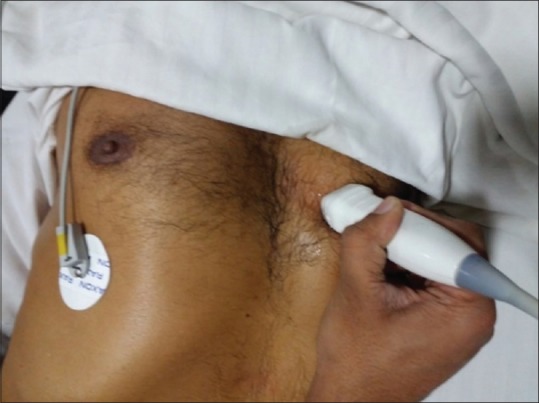
Probe position for parasternal short-axis view
The transducer is rotated 90° clockwise from the parasternal long-axis view to visualize aorta, mitral valve apparatus and LV apex in short axis. The details are discussed in Extended FATE views.
Pleura scanning [Figure 5]
Figure 5.
Probe position for pleural scanning
The FATE examination of the pleural cavity is performed with a cardiac probe. The pleural fluid is easy to differentiate from other tissue as it appears black. The transducer is placed on lateral thoracic wall at 10th rib approximately[4] with the orientation marker directed upward, and scanning is initiated posteriorly in the upper abdomen and is progressed in cranial direction until the diaphragm is visible. Scan as inferior as possible.[9] Pleural effusion, lung collapse, or atelectasis can be identified.
The extended FATE views are as follows:
Subcostal vena cava view
Apical two-chamber view
Apical three-chamber or long-axis view
Apical five-chamber view
Parasternal short-axis mitral plane view
Parasternal aortic short-axis view.
Subcostal vena cava view
The patient is positioned supine with both legs flexed at knees, abdomen relaxed. The transducer is placed in the subxiphoid area with the orientation marker pointing toward the patient's left shoulder. IVC diameter and collapsibility can be determined by directing the probe to right side to image the IVC entering the RA.
Apical two-chamber view
The transducer is rotated counterclockwise from the apical four-chamber position till RA and RV disappear. The LV and the LA are displayed. The anterior and inferior walls of LV are seen.
Apical three-chamber view
This view is also called as apical long-axis view. The transducer is rotated further counterclockwise from the previous position with slight anterior angulation. The LV, the LA, and the ascending aorta are displayed.
Apical five-chamber view
The apical four-chamber is first obtained, and then the transducer is angulated anteriorly towards the patient's head to display LV outflow tract, aortic valve, and ascending aorta in addition to the structures displayed in apical four-chamber view.
Parasternal short-axis mitral plane view
The transducer kept at the same place and tilted toward the left hip obtains the short-axis view of MV. This view displays the anterior mitral leaflet and the posterior mitral leaflet. The walls of LV, IVS, and part of RV are also seen. Further tilting of the transducer gives a view with anterolateral and posteromedial papillary muscles. The walls of LV can be visualized to diagnose RWMAs. Hypovolemia can also be diagnosed in this view. Further tilting of transducer shows the apical segments of LV.
Parasternal aortic short-axis view
The transducer is rotated 90° clockwise from the parasternal long-axis view to visualize aorta in short axis. The indicator should point toward the patient's left shoulder. The three aortic valve leaflets are seen namely the right coronary cusp adjacent to RV, the noncoronary cusp adjacent to the IAS, and the remaining the left coronary cusp. The other structures displayed in this view are the LA, IAS, RA, TV, RV, right ventricular outflow tract, pulmonary valve and the main pulmonary artery. Slight superior angulation displays left main coronary artery at 3–5 o’ clock position and right coronary artery at 11 o’clock position.
Discussion
TTE is an inevitable and indispensable tool for anesthesiologist.[10] Focused TTE performed in the preanesthetic clinic transmutes the management in a substantial proportion of patients, including the asymptomatic ones.[11] It constitutes almost half of all cardiac imaging services.[12] Focused TTE reveals real-time hemodynamics and physiological determinants and holds immediate diagnostic capability.[10] Furthermore, experienced operators can perform FATE examination with patient in sitting position providing flexibility in different clinical scenarios.[13]
FATE can be put to use as a screening and monitoring tool[14] to appraise the effects of therapeutic interventions.[15] The impetus is to screen for significant pathology[5] and evaluate basic hemodynamic determinants such as preload, afterload, contractility, compliance, and relaxation.[6] Studies have shown that clinicians can be trained in a short period of time to determine left ventricular function, determine intravascular fluid status, detect pericardial effusion, and identify valvular pathologies.[14] Its application is distinct as a guide to pericardiocentesis and confirmation of transvenous pacing wire placement.[16]
The characteristics features of various conditions that can be diagnosed by FATE are discussed here:
Chamber dilatation and hypertrophy can be diagnosed by the following indices.[17]
-
Normal ranges for LV internal dimension:
- Diastolic dimension (mm): 50.2 ± 4.1 (males), 45.0 ± 3.6 (females)
- Systolic dimension (mm): 32.4 ± 3.7 (males), 28.2 ± 3.3 (females).
-
Normal ranges for LV indices:
- Relative wall thickness (cm): 0.24–0.42 (males), 0.22–0.42 (females)
- Septal thickness (cm): 0.6–1.0 (males), 0.6–0.9 (females)
- Posterior wall thickness (cm): 0.6–1.0 (males), 0.6–0.9 (females).
Contractility and left ventricle function classification
Normal – ejection fraction (EF) >55%
Mild LV dysfunction – EF 45%–54%
Moderate LV dysfunction – EF 30%–44%
Severe LV dysfunction – EF <30%.[18]
Evaluation of myocardial ischemia can also be done by focused TTE.[19]
RWMAs may be present in patients with myocardial ischemia, infarction, or LV dysfunction. RWMAs are classified as:[20]
Normokinesia – Endocardium moves toward the center of LV during systole >30%
Mild hypokinesia – Endocardium moves toward the center of LV during systole <30% but >10%
Severe hypokinesia – Endocardium moves toward the center of LV during systole <10%
Akinesia – Endocardium does not move or thicken
Dyskinesia – Endocardium moves away from the center of LV.
Grading of myocardial contractility
Normally the wall thickening is >30%–50%
Mild Hypokinesia – 30%–50%
Severe Hypokinesia – <30%
Akinesia – <10%
Dyskinesia – None.[20]
Valvular dysfunctions can be diagnosed by inspecting the mitral and aortic valves essentially for stenosis and regurgitations. Restriction or immobility, thickening, calcification, vegetations, coaptation, prolapse, or flail scallops of the leaflets should be looked for.
Pericardial effusion appears as an echofree dark space between the two layers of the pericardium.
Pericardial effusion is classified as:[21,22]
Mild is <10 mm, present posterior to the heart
Moderate is 10–20 mm, present laterally and apically
Large is >20 mm, present circumferentially.
Cardiac tamponade is a life-threatening condition by cause of the accumulation of fluid in the pericardial space compressing the chambers and restricting their normal filling.[21]
Echocardiographic findings of cardiac tamponade:[21,23]
RA or RV diastole collapse
Septal bounce – An inspiratory “bounce” of the IVS toward the LV
Diastolic ventricular size variability with respiration – An exaggerated ventricular interdependence shows increased RV diastolic diameter during inspiration with decreased diameter of the LV with the opposite changes happening on expiration
Respiratory variation of mitral or tricuspid inflow velocities – On inspiration, decrease of mitral E wave >25% and decrease of tricuspid E wave >40%
IVC plethora – IVC dilatation >21 mm with loss of respiratory collapse signifying elevated central venous pressure confirms tamponade
Reversal of hepatic venous flow.
Hypovolemia
The standard criteria for diagnosing hypovolemia[24] are:
LV end-diastolic diameter <25 mm
LV end-diastolic area (LVEDA) <10 cm2 or
LVEDA index (LVEDA/BSA) <5.5 cm2/m2
Systolic obliteration of LV cavity.
In case of true hypovolemia, LV areas in both systole and diastole are small, whereas in case of vasodilatation with hypotension, only the systolic LV area is small.[24]
Acute pulmonary embolism
The diagnosis can be made on the basis of following echocardiographic findings:
-
Qualitatively
- RV hypokinesis
- McConnell's sign – Akinesia of the RV mid-free wall with normal motion at the apex[25]
- Paradoxical septal motion.
-
Quantitatively
- RV to LV end-diastolic ratio >1
- RV to LV end-diastolic area >0.6
- RV end-diastolic diameter >30 mm
- Pulmonary artery systolic pressure >30 mmHg
- Pulmonary artery mean pressure >20 mmHg
- Right pulmonary artery dilatation >30 mm
- Velocity of tricuspid regurgitation >2.8 m/s.
Pleural effusion
It emanates as a dark, anechoic area positioned in between visceral and parietal pleura. Small-to-moderate effusions are present in the dependent parts and the lateral regions, whereas large effusions exist in the anterior area. Large pleural effusions are often associated with compression atelectasis in the form of consolidated lung tissue seen as floating in the effusion[26] [Figure 6].
Figure 6.
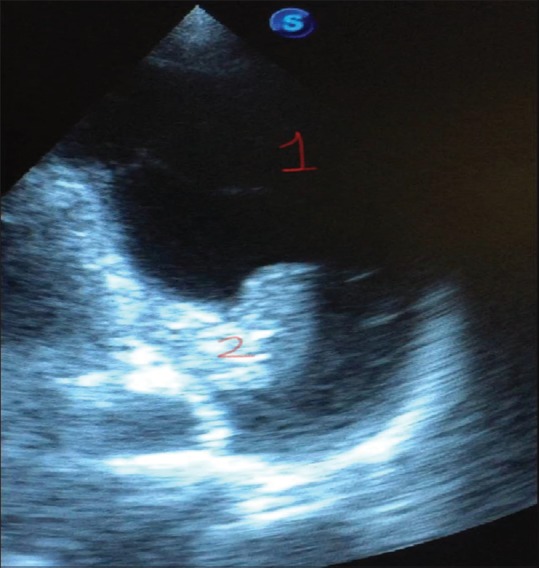
Pleural scan showing left-sided pleural effusion (1) along with the collapsed lung (2)
POC US assessments of the pleural cavity should be incorporated into the primary survey of respiratory and circulatory unstable patients.[13] Early diagnosis of large pleural effusion is crucial as it can culminate into cardiac tamponade-like physiology consequent to the increase in intrapleural pressure transmitting to the pericardium and causing diastolic chamber collapse.[3,13] Regarding pericardial effusions, emergency physicians can reliably evaluate it.[27] Melamed et al. in their study state that minimally trained intensivists using hand-held units have made LV function assessment reasonably accurate despite of challenges such as lung hyperinflation, dressings, drains, anasarca, and suboptimal positioning of the patients in intensive care units (ICUs).[28] Further, the role of TTE in managing high-risk hemodynamically unstable patients for noncardiac surgery has been stressed by Kratz et al., reason being TTE led to variation in the management by discovering the pathologic findings of hypovolemia, right-heart overload, or failure.[29]
POC TTE in addition to diagnosing the causes of hypotension and shock facilitates life-saving interventions.[30] Characterization of perioperative shock in conditions such as cardiac tamponade, hypovolemia, LV dysfunction, acute cor pulmonale, and acute respiratory failures such as pneumothorax, acute pulmonary edema, massive pleural effusion, and major atelectasis can be done.[31]
Like FATE, many abbreviations and protocols have been standardized which define the preponderance of TTE and its efficacious implementation in clinical scenarios such as Focused Assessment with Sonography for Trauma[8,13] protocol to evaluate penetrating chest injuries, cardiac contusions, and tamponade;[30] The Abdominal and Cardiac Evaluation with Sonography in shock;[13,32] and the Focused Echocardiographic Evaluation in Resuscitation.[13,33] Focused Echocardiography in Emergency Life support[34] is a limited echo protocol with limited training for catastrophic conditions.[8] Focused ultrasonography in anesthesia[31] and Rapid Ultrasound in Shock[35] are few more.
Limitations of focused TTE are the challenging acoustic windows in patients with morbid obesity, edema, subcutaneous emphysema,[20] chronic obstructive airway disease, surgical drains, positive pressure ventilation, and positive end-expiratory pressure[36] making application of FATE difficult. Orme et al. stated that TTE could be performed with adequate or good views in mechanically ventilated patients.[36] Further, successful use of focused US in the sitting position has been published.[37] One or more acoustic windows, allowing clinical decision-making, could be obtained in 97% of the ICU patients.[7]
FATE is extremely useful in all aspects of perioperative care.[38] Furthermore, the focused approach can prove beneficial in extracorporeal membrane oxygenation (ECMO) patients as TTE has a role in cardiac screening to select patients, guide cannula insertion and placement, monitoring, detect complications, determine cardiac recovery, and wean off ECMO.[39] Moreover, the concept of focused POC US is promoted by the World Interactive Network Focused on Critical US in critical scenarios in and out of the hospital.[15]
Conclusion
The FATE protocol is a comprehensive, rapid, and noninvasive approach contemplated to find the potential causes of hemodynamic instability. The fundamentals scanned are the chamber dimensions, wall thickness, ventricular function, pleura, and obvious pathology. Moreover, the information incurred from scanning is used analogously to the clinical context for bettering patient management. Hence, FATE protocol can be verily consolidated into routine as well as emergency clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I would like to thank Dr. Hemant Waikar for providing image number 6.
References
- 1.Sloth E, Torp P. Focus assessed transthoracic echocardiography (FATE) in aortic valve replacement: P-70. Eur J Anaesthesiol. 2005;22:28. [Google Scholar]
- 2.Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: Feasible and alters patient management. J Cardiothorac Vasc Anesth. 2009;23:450–6. doi: 10.1053/j.jvca.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Cowie BS. Focused transthoracic echocardiography in the perioperative period. Anaesth Intensive Care. 2010;38:823–36. doi: 10.1177/0310057X1003800505. [DOI] [PubMed] [Google Scholar]
- 4.Holm JH, Frederiksen CA, Juhl-Olsen P, Sloth E. Perioperative use of focus assessed transthoracic echocardiography (FATE) Anesth Analg. 2012;115:1029–32. doi: 10.1213/ANE.0b013e31826dd867. [DOI] [PubMed] [Google Scholar]
- 5.Chew MS. Haemodynamic monitoring using echocardiography in the critically ill: A review. Cardiol Res Pract. 2012;2012:139537. doi: 10.1155/2012/139537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macas A, Maciuliene A, Ovsianas J, Juodviroyte G, Bakoyte G. Focus assessed echocardiography performed by inexperienced examiners in a cardiac intensive care unit. Proc Latvian Acad Sci Sect B. 2014;68:242–6. [Google Scholar]
- 7.Jensen MB, Sloth E, Larsen KM, Schmidt MB. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol. 2004;21:700–7. doi: 10.1017/s0265021504009068. [DOI] [PubMed] [Google Scholar]
- 8.Neskovic AN, Edvardsen T, Galderisi M, Garbi M, Gullace G, Jurcut R, et al. Focus cardiac ultrasound: The European Association of Cardiovascular Imaging viewpoint. Eur Heart J Cardiovasc Imaging. 2014;15:956–60. doi: 10.1093/ehjci/jeu081. [DOI] [PubMed] [Google Scholar]
- 9.Ultrasound Airway Breathing Circulation Dolor Organisation; Basic FATE (Focus Assessed Transthoracic Echocardiography) [Last accessed on 2018 Nov 18]. Available from: http://www.usabcd.org .
- 10.Jorgensen MR, Botker MT, Juhl-Olsen P, Frederiksen CA, Sloth E. Point-of-care ultrasonography. OA Crit Care. 2013;1:8. [Google Scholar]
- 11.Canty DJ, Royse CF, Kilpatrick D, Bowman L, Royse AG. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia. 2012;67:618–25. doi: 10.1111/j.1365-2044.2012.07074.x. [DOI] [PubMed] [Google Scholar]
- 12.Matulevicius SA, Rohatgi A, Das SR, Price AL, DeLuna A, Reimold SC, et al. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med. 2013;173:1600–7. doi: 10.1001/jamainternmed.2013.8972. [DOI] [PubMed] [Google Scholar]
- 13.Oveland NP, Bogale N, Waldron B, Bech K, Sloth E. Focus assessed transthoracic echocardiography (FATE) to diagnose pleural effusions causing haemodynamic compromise. Case Rep Clin Med. 2013;2:189–93. [Google Scholar]
- 14.Khasawneh FA, Smalligan RD. Focused transthoracic echocardiography. Postgrad Med. 2010;122:230–7. doi: 10.3810/pgm.2010.05.2162. [DOI] [PubMed] [Google Scholar]
- 15.Price S, Via G, Sloth E, Guarracino F, Breitkreutz R, Catena E, et al. Echocardiography practice, training and accreditation in the intensive care: Document for the world interactive network focused on critical ultrasound (WINFOCUS) Cardiovasc Ultrasound. 2008;6:49. doi: 10.1186/1476-7120-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson BP, Sanghvi A. Point-of-care cardiac ultrasound: Feasibility of performance by noncardiologists. Glob Heart. 2013;8:293–7. doi: 10.1016/j.gheart.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Oommen R, Thomson VS, Jose JV. Assessment of left ventricular systolic function by velocity vector imaging. Indian Heart J. 2012;64:146–9. doi: 10.1016/S0019-4832(12)60050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrus P, Dean A. Focused cardiac ultrasound. Glob Heart. 2013;8:299–303. doi: 10.1016/j.gheart.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Savage RM, Aronson S, Shernan SK. 2nd ed. Philadelphia, United States: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. [Google Scholar]
- 21.Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr. 2017;5:79. doi: 10.3389/fped.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tempe DK, Subramaniam B, Subramaniam K, Ramakrishna H. New Delhi, India: CBS Publishers; 2014. Problem based Transesophageal Echocardiography. [Google Scholar]
- 23.Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5:72–5. doi: 10.4103/0974-2700.93118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarti A, Luca Lorini F. Springer Science & Business Media; 2012. Echocardiography for Intensivists. [Google Scholar]
- 25.Sosland RP, Gupta K. Images in cardiovascular medicine: McConnell's sign. Circulation. 2008;118:e517–8. doi: 10.1161/CIRCULATIONAHA.107.746602. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Gomez J, Ripoll JG, Ratzlaff RA, Tavazzi G, Via G, Mookadam F, et al. Perioperative lung ultrasound for the cardiothoracic anesthesiologist: Emerging importance and clinical applications. J Cardiothorac Vasc Anesth. 2017;31:610–25. [Google Scholar]
- 27.Mandavia DP, Hoffner RJ, Mahaney K, Henderson SO. Bedside echocardiography by emergency physicians. Ann Emerg Med. 2001;38:377–82. doi: 10.1067/mem.2001.118224. [DOI] [PubMed] [Google Scholar]
- 28.Melamed R, Sprenkle MD, Ulstad VK, Herzog CA, Leatherman JW. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest. 2009;135:1416–20. doi: 10.1378/chest.08-2440. [DOI] [PubMed] [Google Scholar]
- 29.Kratz T, Steinfeldt T, Exner M, Dell Orto MC, Timmesfeld N, Kratz C, et al. Impact of focused intraoperative transthoracic echocardiography by anesthesiologists on management in hemodynamically unstable high-risk noncardiac surgery patients. J Cardiothorac Vasc Anesth. 2017;31:602–9. doi: 10.1053/j.jvca.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Margale S, Marudhachalam K, Natani S. Clinical application of point of care transthoracic echocardiography in perioperative period. Indian J Anaesth. 2017;61:7–16. doi: 10.4103/0019-5049.198407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Díaz-Gómez JL, Via G, Ramakrishna H. Focused cardiac and lung ultrasonography: Implications and applicability in the perioperative period. Rom J Anaesth Intensive Care. 2016;23:41–54. doi: 10.21454/rjaic.7518.231.lus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson PR, McAuley DJ, Kendall RJ, Abeyakoon O, Reid CG, Connolly J, et al. Abdominal and cardiac evaluation with sonography in shock (ACES): An approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg Med J. 2009;26:87–91. doi: 10.1136/emj.2007.056242. [DOI] [PubMed] [Google Scholar]
- 33.Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resuscitation management: Concept of an advanced life support-conformed algorithm. Crit Care Med. 2007;35:S150–61. doi: 10.1097/01.CCM.0000260626.23848.FC. [DOI] [PubMed] [Google Scholar]
- 34.Breitkreutz R, Price S, Steiger HV, Seeger FH, Ilper H, Ackermann H, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: A prospective trial. Resuscitation. 2010;81:1527–33. doi: 10.1016/j.resuscitation.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Ghane MR, Gharib M, Ebrahimi A, Saeedi M, Akbari-Kamrani M, Rezaee M, et al. Accuracy of early rapid ultrasound in shock (RUSH) examination performed by emergency physician for diagnosis of shock etiology in critically ill patients. J Emerg Trauma Shock. 2015;8:5–10. doi: 10.4103/0974-2700.145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orme RM, Oram MP, McKinstry CE. Impact of echocardiography on patient management in the Intensive Care Unit: An audit of district general hospital practice. Br J Anaesth. 2009;102:340–4. doi: 10.1093/bja/aen378. [DOI] [PubMed] [Google Scholar]
- 37.Frederiksen CA, Knudsen L, Juhl-Olsen P, Sloth E. Focus-assessed transthoracic echocardiography in the sitting position: Two life-saving cases. Acta Anaesthesiol Scand. 2011;55:126–9. doi: 10.1111/j.1399-6576.2010.02330.x. [DOI] [PubMed] [Google Scholar]
- 38.Faris JG, Veltman MG, Royse C. Focused transthoracic echocardiography in the perioperative period. Anaesth Intensive Care. 2011;39:306–7. [PubMed] [Google Scholar]
- 39.Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012;25:131–41. doi: 10.1016/j.echo.2011.11.009. [DOI] [PubMed] [Google Scholar]


