Conspectus
Extracellular vesicles are nanoparticles produced by cells. They are composed of cellular membrane with associated membrane proteins that surrounds an aqueous core containing soluble molecules such as proteins and nucleic acids, like miRNA and mRNA. They are important in many physiological and pathological processes as they can transfer biological molecules from producer cells to acceptor cells. Preparation of the niche for cancer metastasis, stimulation of tissue regeneration and orchestration of the immune response are examples of the diverse processes in which extracellular vesicles have been implicated. As a result, these vesicles have formed a source of inspiration for many scientific fields. They could be used, for example, as liquid biopsies in diagnostics, as therapeutics in regenerative medicine, or as drug delivery vehicles for transport of medicines. In this Account, we focus on drug delivery applications.
As we learn more and more about these vesicles, the complexity increases. What originally appeared to be a relatively uniform population of cellular vesicles is increasingly subdivided into different subsets. Cells make various distinct vesicle types whose physicochemical aspects and composition is influenced by parental cell type, cellular activation state, local microenvironment, biogenesis pathway, and intracellular cargo sorting routes. It has proven difficult to assess the effects of changes in production protocol on the characteristics of the cell-derived vesicle population. On top of that, each isolation method for vesicles necessarily enriches certain vesicle classes and subpopulations while depleting others. Also, each method is associated with a varying degree of vesicle purity and concomitant coisolation of nonvesicular material. What emerges is a staggering heterogeneity. This constitutes one of the main challenges of the field as small changes in production and isolation protocols may have large impact on the vesicle characteristics and on subsequent vesicle activity.
We try to meet this challenge by careful experimental design and development of tools that enable robust readouts. By engineering the surface and cargo of extracellular vesicles through chemical and biological techniques, favorable characteristics can be enforced while unfavorable qualities can be overruled or masked. This is coupled to the precise evaluation of the interaction of extracellular vesicles with cells to determine the extracellular vesicle uptake routes and intracellular routing. Sensitive reporter assays enable reproducible analysis of functional delivery.
This systematic evaluation and optimization of extracellular vesicles improves our insight into the critical determinants of extracellular vesicle activity and should improve translation into clinical application of engineered extracellular vesicles as a new class of drug delivery systems.
Extracellular Vesicles
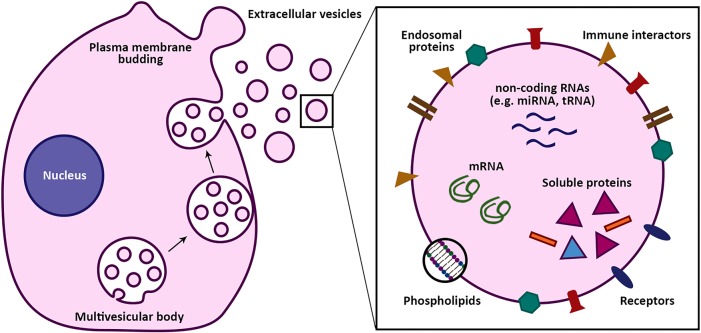
“Extracellular vesicles” is an umbrella term for the small sub-micrometer-sized particles composed of lipid membranes that all cells release. Extracellular vesicles (EVs) are defined by the presence of one or more lipid membranes, but in addition vesicles contain essentially all molecules that are found inside and on cells. These include proteins (such as cell surface proteins, membrane proteins, and cytoplasmic proteins), nucleic acids (such as small noncoding RNAs, mRNAs, and DNA fragments), and soluble small molecules (such as second messengers, carbohydrates, and hormones). A schematic drawing of an EV is shown in Figure 1.
Figure 1.
Origin and cargo of extracellular vesicles (EVs). EVs are cell-derived nanosized vesicles that play an important role in intercellular communication through transfer of biological cargo. Their cargo comprises nucleic acids, lipids and phospholipids, and proteins. Interactions of EVs with the environment are mainly driven by their surface molecules. EV contents are released after uptake by recipient cells, possibly activating cellular pathways and resulting in phenotypical changes.
EVs have captured the imagination of scientists in many different disciplines as the vesicles could transport their bioactive cargo between their parental cell and an acceptor cell. This cell may be in the vicinity but may also be present at a distant site. Because of their lipidic membrane, the signal arrives undiluted and is protected while traveling from, for example, enzymatic degradation. Importantly, EV-mediated intercellular cargo transfer has been shown to play important roles in various physiological as well as pathological processes.1 Various biomedical applications of EVs could be imagined, such as
Liquid biopsies. Being able to isolate these vesicles from bodily fluids would provide a snapshot of the producer cells at the time of vesicle-production enabling near real-time monitoring of disease regression or progression and response to therapy, for example, in cancer.
Therapeutic intervention. Being able to redirect vesicles to tissues of interest could be used to degrade pathological signals or focus their intrinsic therapeutic activity, for example, in regenerative medicine.
Drug delivery. Using the delivery capabilities of EVs for targeting of therapeutics to specific cells and tissues, for example, in the delivery of therapeutic RNAs.
The latter subject, drug delivery with EVs, is the topic of this Account. For the drug delivery field, EVs appear to comprise the holy grail of pharmaceutics, where complex biological cargo could potentially be delivered with cellular and even subcellular specificity.
Imagination versus Reality
Over the past decade, much has been discovered about the biogenesis, release, and function of EVs. The picture that emerges, however, does not lead to straightforward applications.
All cells release vesicles, albeit the number, composition, and physicochemical characteristics vary.
At present, three routes for vesicle formation are recognized: (1) Invaginations of endosomes forms multivesicular bodies from which, upon fusion with the cell membrane, intraluminal vesicles, from that moment onward referred to as exosomes, are released. The size of these vesicles is 30–100 nm. (2) Budding of the cell membrane leads to release of vesicles known as microvesicles. The size of these vesicles is 50–1000 nm. (3) Release of membrane material during cellular apoptosis produces vesicles known as apoptotic bodies. The size of these apoptotic vesicles is ill defined and ranges from 100 nm to several micrometers.
Still, no unique markers for each vesicle type have been determined, and the various classes have partly overlapping physicochemical characteristics. As a result, categorization after release is very difficult. In addition, it appears that each class can be divided into subpopulations of vesicles, further adding to the complexity.2
To add to this, each of these physicochemical aspects is in turn influenced by parental cell type, cellular activation state, local microenvironment, biogenesis pathway, and intracellular cargo sorting routes. On top of that, each isolation method for vesicles necessarily enriches certain vesicle classes and subpopulations while depleting others. Also, each method is associated with a varying degree of vesicle purity and concomitant coisolation of cellular debris, protein aggregates, or other nanosized particles like lipoproteins. The overall result is a staggering heterogeneity. On the one hand, this heterogeneity is in line with the wide variety of functions that have, over the past years, been ascribed to EVs in physiological and pathophysiological processes. However, on the other hand, to be able to reproducibly produce defined EVs for biomedical purposes, a better understanding of the relationship between EV composition and function is required. Over the past years, we have embedded this theme in our research to improve drug delivery, in particular of RNA therapeutics.
Surface of Extracellular Vesicles
The surface of EVs is important as it determines their possible interactions with target tissues and nontarget tissues. Synthetic nanoparticles have a tendency to become rapidly opsonized with proteins, once in contact with biological media.3 The specific profile is dependent on the surface chemistry of the particles. For example, charged surfaces initially tend to be opsonized with proteins bearing opposite charges. Over time the pattern changes in agreement with the Vroman effect, which states that high mobility proteins opsonize first and are subsequently replaced by less mobile proteins with a higher affinity for the surface.4 Usually, the early proteins opsonizing the pristine surface of synthetic nanoparticles cause a rapid clearance by cells of the mononuclear phagocyte system (MPS) followed by degradation in these cells through the endosomal–lysosomal pathway.5 In addition, the immune system can become activated. A variety of nanomedicines and protein conjugates cause unpredictable hypersensitivity reactions upon infusion, especially after intravenous administration. These mild to severe symptoms that resemble allergic reactions are not clearly understood and differ between patients. One factor that appears to be involved in many of these adverse reactions is activation of the complement system. Currently these reactions are therefore classified as complement-activation-related pseudoallergy (CARPA).6
The most popular strategy to avoid rapid clearance is to decorate the nanoparticle surface with a hydrophilic polymer layer such as poly(ethylene glycol) (PEG). By virtue of the resulting decelerated clearance, the nanoparticles can reach other tissues and cell types. Doxil, a PEGylated liposomal formulation of doxorubicin, has been the first marketed nanomedicine based on this principle.7 The PEG is stably anchored in the liposome bilayer through a phospholipid with two saturated C18 acyl chains leading to a prolongation of circulatory half-life to 20–30 h. For RNA delivery, currently the only marketed nanoparticle formulation is Onpattro.8 This is a lipid nanoparticle carrying siRNA that silences mutant transthyretin for the treatment of hereditary transthyretin-based amyloidosis. The lipid nanoparticle encapsulates the siRNA via electrostatic binding to an ionizable fusogenic lipid and is covered with a protective PEG corona. Because of the relatively short C14 lipid anchor of the PEG, this protective layer is gradually lost in the circulation and slowly replaced by blood proteins. One of the dominant proteins that opsonizes the lipid nanoparticles and determines their fate is apolipoprotein E.9 This apolipoprotein is recognized by the low-density lipoprotein receptor, which is present on many cells but especially prominently expressed on hepatocytes. As a result, Onpattro delivers its payload quite efficiently to the liver where the majority of the mutant protein is produced.
Still, even this formulation has substantial drawbacks: it elicits infusion related hypersensitivity reactions and as a result requires pretreatment with a corticosteroid, acetaminophen, and antihistamines (H1 and H2 receptor blockers).10 Furthermore, the apolipoprotein E-based opsonization strategy only favors delivery to hepatocytes. Currently, targeting to alternative tissues and cells with this formulation is not efficient enough to be clinically feasible.
These drawbacks provide opportunities for investigating the application of extracellular vesicles as drug delivery vehicles. As natural materials they might be expected to avoid the hypersensitivity reactions associated with the use of synthetic systems. In addition, their complex surface might enable high specificity and selectivity for specific tissues and cells and a preference for intracellular routing toward functional delivery.
With respect to infusion-related adverse effects, surprisingly little is known about the ability of extracellular vesicles (if any) to avoid these reactions. It is assumed that the combination of natural proteins, carbohydrates and lipids may help to overcome CARPA. However, experimental evidence is still limited. Most studies in which extracellular vesicles have been injected into animals have been performed in rodents. In these species, these infusion-related reactions are not as prominent as in humans, including for synthetic systems. For meaningful studies, sensitive species, such as pigs, should be used. In these animals, however, most studies focus on local rather than intravenous injection as the large-scale isolation of extracellular vesicles is laborious and local injection at the site of action is expected to increase efficacy. One trial reported on extracellular vesicles from virus-infected pigs that were isolated from serum and used as an intramuscular vaccine.11 The studies on applications of extracellular vesicles discussed in this Account are shown in Table 1.
Table 1. Examples of Applications of EVs in Drug Delivery as Mentioned in this Account.
| EV source | engineered content | application | ref |
|---|---|---|---|
| porcine peripheral blood | none; EVs were administered in combination with Montanide adjuvant | vaccination against porcine reproductive and respiratory syndrome virus | (11) |
| human mesenchymal stem cells | none | treatment of chronic myocardial ischemia | (12) |
| autologous dendritic cells | isolated EVs or cells were pulsed with MAGE-derived peptides | vaccination against non-small-cell lung cancer | (13) |
| autologous dendritic cells | isolated EVs or cells were pulsed with MAGE-derived peptides | vaccination against stage III/IV melanoma | (14) |
| mouse neuroblastoma (Neuro2A) cells | surface modified with PEG-nanobodies through postinsertion | improvement of EV circulation time and tumor accumulation | (15) |
| mouse neuroblastoma (Neuro2A) cells | surface modified with GPI-anchored anti-EGFR nanobodies trough cell engineering | improvement of EV binding and internalization by EGFR-positive tumor cell | (17) |
| mouse immature dendritic cells | surface modified with RVG-Lamp2b proteins through cell engineering; loaded with siRNA by electroporation | knockdown of BACE1 expression in the brain | (18) |
| human embryonic kidney (HEK293) cells | surface modified with anti-EGFR peptides (GE11) by cell engineering; loaded with miRNA/siRNA by donor cell transfection with synthetic oligonucleotides | inhibition of breast cancer tumor growth | (19) |
| human cervical cancer (HeLa) cells | surface engineered with cell-penetrating peptides through chemical cross-linking; loaded with saporin through electroporation | improvement of EV uptake and cargo delivery in vitro | (20) |
| human and mouse mesenchymal stromal cells | loaded with paclitaxel through incubation of donor cells with drug | reduction of melanoma tumor growth by coimplantation of paclitaxel-loaded cells | (27) |
| human embryonic kidney (HEK293T) cells | loaded with Cre recombinase protein through cell engineering with reversible light-responsive protein interactors | improved EV-mediated protein delivery to the brain after local injection | (32) |
| various cell lines | loaded with phototoxic porphyrins via EV electroporation, saponin treatment, extrusion, hypotonic dialysis, or passive incubation | EV loading with small molecular weight drugs for improved intracellular delivery | (33) |
| mouse neuroblastoma (Neuro2A) cells | loaded with siRNA via EV incubation with cholesterol-conjugated siRNA | improved in vitro siRNA delivery | (34) |
| human embryonic kidney (HEK293T) and breast cancer (MCF-7) cells | loaded with siRNA, miRNA, and single-stranded DNA via sonication | functional delivery of small nucleic acids in vitro | (36) |
| human umbilical vein endothelial cells (HUVEC) and mouse mesenchymal stem cells | surface engineered and loaded with photosensitizers through EV fusion with liposomes | evasion of macrophage uptake and delivery of small molecular weight compounds in vitro | (37) |
| human breast cancer (MDA-MB-231) cells | loaded with Cre recombinase mRNA (and possibly protein) through donor cell engineering | studying EV-mediated cargo transfer and associated functional effects in vivo | (43) |
| autologous tumor cells | loaded with methotrexate through incubation of the donor cells with the drug | vaccination against advanced lung cancer and malignant pleural effusion | (45) |
In another study, intracardiac administration of mesenchymal stem cell vesicles into an ischemic area of the myocardium was performed.12 In addition, four pigs also received a dose of 50 μg of EVs intravenously via an auricular vein while hemodynamics were continuously monitored. In both studies, CARPA-related adverse effects were not observed, whereas in previous studies with liposomes as little as 5 μL of liposomes could induce shock-like symptoms. These observations may suggest that extracellular vesicles, at least those derived from human mesenchymal stem cells harvested under these particular culture conditions and using these isolation methods, are well tolerated. However, phase I clinical trials revealed that EVs derived from autologous dendritic cells caused mild inflammatory reactions at the site of subcutaneous administration in half of the patients,13,14 suggesting that even autologous EVs may elicit CARPA.
Although safety is important, efficacy still determines the viability of the strategy. We have examined the fate of intravenously administered EVs and found that their tissue distribution profile mimics that of non-PEGylated liposomes.15 Predominant organs of uptake were liver and spleen, typical MPS-rich clearance tissues. Many other studies with EVs from different origins have found similar results. Still, this does not automatically mean that EVs have lost their attractiveness for systemic drug delivery. Again, the heterogeneity of EVs and methods for their production and isolation prevents straightforward interpretation of the data.
Various isolation methods have been applied to isolate the vesicles examined in these studies. It is known that certain procedures may compromise EV integrity and structure. Therefore, it could be argued that these methods may have impacted the in vivo performance and tissue distribution of the EVs. Still, in a direct comparison study between an isolation method known to affect EV structure (ultracentrifugation) and size-exclusion chromatography, the dominance of MPS-mediated clearance was apparent for both methods.16
We have tried to engineer the EV surface by synthetic and biological methods. We have borrowed the PEGylation strategy from the synthetic nanoparticle field.15 By incubation of EVs with micelles of PEG-phospholipids, the surface of the EVs became coated with a PEG corona. The impact of incorporation was, however, limited. Tissue distribution was largely unaffected, and only a slight increase in circulation time was noted. If we compare this to the increase that is observed for synthetic systems following the same strategy, it appears that the increase is far more dramatic. This may suggest that the EVs’ recognition signals are overruling the protective PEG-corona.
We have also used biological engineering of the producing cells in order to achieve surface expression of specific targeting ligands. EV producing cells were transfected with vectors encoding for anti-epidermal growth factor receptor targeted nanobodies fused to glycosylphosphatidylinositol anchor signal peptides derived from decay-accelerating factor.17 It was shown that the nanobodies can be anchored on the surface of extracellular vesicles via the phospholipid, which altered their cell targeting behavior at least in vitro. Similar results have been obtained by other groups, which employed native EV membrane proteins (e.g., Lamp2b and platelet-derived growth factor) as fusion partners for targeting ligands.18,19 Alternatively, the surface of EVs has been manipulated through chemical engineering, such as click chemistry.20,21 However, it remains unclear how such modifications affect EV–cell interactions and delivery capacity.
In the studies that revealed extracellular vesicle tropism for MPS organs, a variety of cell sources have been used. However, it remains conceivable that the release of EVs suitable for systemic administration differs among cell types. One cellular factor that has been identified to possibly benefit EVs’ suitability for systemic administration is the expression of CD47. CD47 is a surface protein that serves as a “do not eat me signal” that appears to limit phagocytic uptake through binding to its receptor signal-regulatory protein (SIRP) α, which is found on cells of the myeloid lineage. It has been shown that EVs without CD47 showed a reduced half-life compared to their CD47-expressing counterparts.22 Similar findings were reported for synthetic nanoparticles decorated with CD47-derived peptides.23
CD47 expression is likely restricted to specific subpopulations of EVs, as the surface composition of EVs is known to be highly heterogeneous.24 EV subpopulation identification and isolation is therefore evolving as an increasingly important element in EV research. It is known that EVs are involved in many different cellular processes, including “waste management”. Vesicles carrying such cellular waste could very well be secreted with MPS tissues as target. One of the important surface molecules in this respect may be the phospholipid phosphatidylserine, which has the opposite effect of CD47. In studies with PEGylated liposomes, we showed that the presence of phosphatidylserine in the liposome bilayer even overrules the dramatic protective PEG-coating effect in liposomes.25 Phosphatidylserine efficiently binds specific opsonins, such as the lactadherin protein, that direct phagocytic uptake by bridging the phospholipid with integrins on the surface of phagocytes. For this reason, phosphatidylserine is carefully withdrawn from the cell surface and only present in the inner leaflet of the cell membrane. Certain subpopulations of EVs, however, display phosphatidylserine on their surface.26 Many phosphatidylserine-binding opsonins, including lactadherin, are described as canonical extracellular vesicle proteins. It is likely that such phosphatidylserine-positive EVs are released with a cellular target in the MPS.
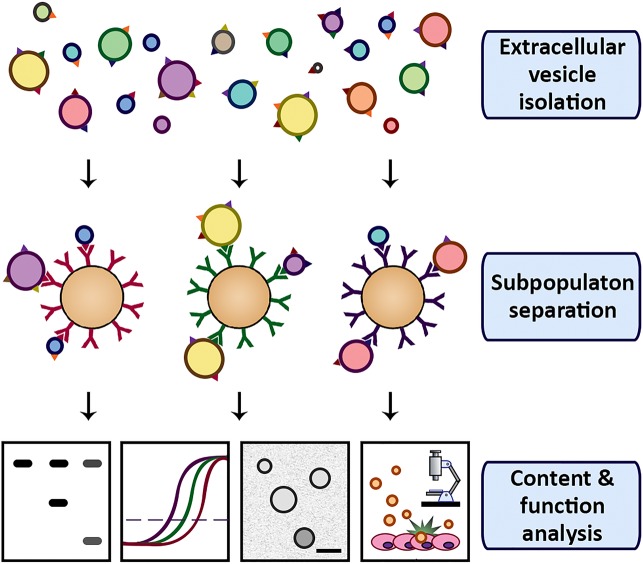
We are currently trying to couple EV surface characteristics to function. In one of our strategies, we isolate various subpopulations and analyze their properties separately. In this setup, EV capture is performed using magnetic microbeads coated with specific antibodies against cognate antigens on the vesicle surface (Figure 2). Subsequent magnetic separation allows separate analysis of EV subpopulations. In theory, the magnetic capture could even be combined with dissociation in order to study the functional behavior of the EV subpopulations. For this, the dissociation should be mild enough to not affect vesicle integrity or protein structure on the surface, while being strong enough to break the antibody–antigen binding.
Figure 2.
Schematic representation of EV subpopulation separation and characterization. EVs are captured onto magnetic beads coated with antibodies against EV surface molecules. EV subpopulation content and function is analyzed using a variety of assays.
Cargo Engineering
For synthetic systems, there is usually full flexibility on the timing and method of drug incorporation into the nanoparticles. It may allow the use of organic solvents, chemical coupling to carrier materials, and other strategies that maximize the loading efficiency. For example, for small molecular weight drug remote loading procedures have been demonstrated to result in near complete encapsulation, such as used in the production of the marketed liposomal formulation Doxil. Here, a transmembrane gradient of ammonium sulfate drives intraliposomal precipitation of doxorubicin as the sulfate salt. For Onpattro, the negatively charged siRNA is efficiently encapsulated in the lipid nanoparticle by electrostatic complexation to positively charged ionizable lipids enforced at low pH. Especially for complex and costly pharmaceuticals, like biologicals, drug incorporation can be a critical determinant of the business case for product development.
As EV production and cargo loading are cellular processes, the options are necessarily more limited. Either the methods are applied in the production phase, which requires compatibility with cell culture and viability, or the drug is incorporated after the isolation of vesicles, but then the integrity and composition of the vesicles should be minimally affected.
Several small molecular weight drugs, such as paclitaxel and imatinib, have been loaded into vesicles during production by co-incubation with cells. It seems critical that the producing cell can withstand high concentrations of the drugs in order for this approach to be effective. For example, using mesenchymal stem cells, which displayed high resistance to paclitaxel, 2000 ng/mL could be used in culture conditions to load vesicles during their production, resulting in sufficient loading for functional delivery to block tumor proliferation.27
Proteins and nucleic acids can also be loaded by transfection of the producing cell with the encoding DNA. For RNA, on average, the incorporation is proportional to the intracellular concentrations of the transfected nucleic acid. Generally speaking, the larger the RNA molecule, the lower the absolute amount of RNA that is loaded into the vesicles. To provide a rough estimate of loading efficiency, stoichiometric analyses on EVs have revealed that even for highly expressed small noncoding RNA molecules, only a single RNA copy per several hundred vesicles can be detected.
There are several opportunities to increase loading further. A study has identified sequence motifs at the 3′-end of RNAs that appear to promote loading into vesicles.28 These motifs can bind specific proteins that are enriched in vesicles leading to their sorting into the extracellular vesicle fraction, or they bind to proteins that actively load RNAs into vesicles. At present a variety of these proteins have been identified, including heterogeneous nuclear ribonucleoprotein A2B1,28 Y-box protein 1,29 and synaptotagmin binding cytoplasmic RNA interacting protein,30 among others.
Employing RNA motifs that bind artificially introduced vesicular proteins or domains to facilitate targeted loading of EVs is an additional strategy, which is less reliant on the endogenous machinery operating within a specific cell type and under specific culture conditions. For example, the MS2-domain, derived from the coat protein of the RNA bacteriophage MS2, is responsible for binding the RNA genome of the phage through a specific stem–loop structure. Incorporating this structure into the RNA of interest as well as engineering the MS2 domain onto a vesicular protein has been shown to result in enhanced loading.31 However, as these strategies are currently hampered by limited release kinetics, such approaches may require additional optimization like the use of light-cleavable domains.32 Although these methods can result in significantly enhanced loading, these methods fall short of the complete encapsulation for siRNA in the synthetic lipid nanoparticles based on electrostatic interaction or the encapsulation far above the solubility limit for doxorubicin into liposomes.
An alternative to the encapsulation during vesicle biogenesis is encapsulation after vesicle production and isolation. Postproduction methods include passive loading based on simple co-incubation with or without the presence of detergents, like saponins, to loosen the extracellular vesicle membrane barrier.33 A range of small molecules has been incorporated into or associated with vesicles in this way, such as curcumin, paclitaxel, and doxorubicin. An alternative, also based on passive loading of the active ingredient, is the functionalization with hydrophobic anchors to promote association with the vesicle membrane. Even for hydrophilic biologicals, such as siRNA, this has been shown to result in efficient association and functional delivery,34 albeit the surface exposure of the drug likely limits the level of protection from degradation that would be offered by the encapsulation within the vesicle interior. An advantage of these strategies over active loading, is that the vesicle membrane integrity remains relatively unperturbed, but it may not be suitable for loading of larger molecules.
Active loading strategies generally involve breaking and then rebuilding vesicle bilayer integrity to offer a window of opportunity for drug encapsulation. Especially for biologicals that have a relatively high molecular weight, this is important as spontaneous translocation is negligible. For nucleic acid-based therapeutics, electroporation has emerged as a popular approach.18 Still, this method is controversial as some, including our lab, have reported that aggregation phenomena between the nucleic acid on the one hand and ions liberated during the electroporation pulse on the other hand may artificially overestimate the amount of encapsulated material.35 Still the method remains popular and is even geared up for clinical investigations. Similarly, sonication has been used as a way to temporarily destabilize the membrane and load biologicals.36
An attractive opportunity that, in principle, may avoid the membrane destabilization and concomitant loss of bioactive cargo is based on liposome fusion. In this approach, liposomes composed of fusogenic lipids are co-incubated with extracellular vesicles to merge the cargo of the synthetic vesicles with that of their natural counterparts.37 This approach may facilitate efficient loading of larger molecules, without breaking of the EV membrane.
Intracellular Uptake and Routing
One of the aspects that is of high interest for drug delivery applications of EVs is their uptake pathway and intracellular routing in cells. For synthetic systems, uptake has been shown to be cell type dependent and can occur through both constitutive and inducible pathways. For lipid nanoparticles, clathrin-mediated uptake and macropinocytosis seem dominant uptake pathways, which are followed by endosomal–lysosomal routing. Especially for RNA delivery where spontaneous escape from degradative endosomal/lysosomal organelles is expected to be limited, quantitative examination of uptake routes and functional delivery is important. In a quantitative study on lipid nanoparticles, ∼70% of the cargo was shown to undergo exocytosis through recycling pathways.38 In a similar study, estimated escape of siRNAs from endosomes was a mere 1–2% of the amount that was taken up.39 These numbers indicate that the synthetic systems have limited delivery efficiency in vitro. For Onpattro, these numbers have also been calculated in vivo. The lipid nanoparticles are able to deliver 60% of the injected dose to the liver hepatocytes amounting to 1.6 × 1013 siRNAs/organ or 160 000 siRNAs/cell.40 Of these, 3.1% (∼5000 copies) were associated with the cellular machinery of RNA interference, that is the RNA-induced silencing complex, which is in line with the 1–2% measured in vitro.
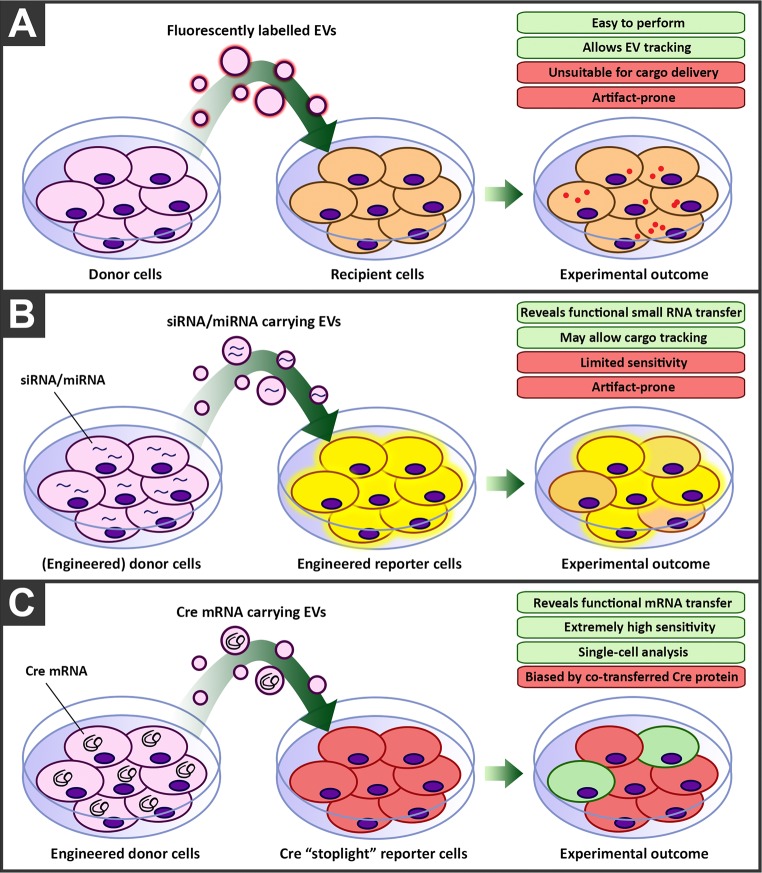
For EVs, we have examined internalization of fluorescently labeled vesicles in HeLa cells, in 2D and 3D cell culture41 (Figure 3A). Using chemical and RNA interference-based inhibition of key proteins of specific endocytic pathways, the effect on uptake could be established. Vesicles appeared to enter cells predominantly via clathrin-independent endocytosis and macropinocytosis but not clathrin-mediated endocytosis, thus seemingly following different internalization pathways than synthetic systems.
Figure 3.
Strategies to evaluate the delivery potential of extracellular vesicles. (A) Uptake and intracellular delivery can be tracked by fluorescently labeled vesicles. (B) Functional analysis can be based on the reduction of specific proteins caused by encapsulated siRNA/miRNAs through RNA interference. (C) Alternatively, in a reporter-based system, delivery of Cre-recombinase mRNA results in translation to the functional enzyme causing DNA recombination visualized as a color change of the reporter cell.
To improve our insight into the uptake and intracellular routing of EVs and evaluate which pathways are important for functional RNA transfer, we are currently setting up genetic screens followed by high-throughput fluorescence microscopy. This will allow us to identify both genes that increase and genes that decrease functional RNA transfer. By evaluating the effect of the identified genes on EV-mediated transfer for other various donor EV–acceptor cell combinations, we aim to demonstrate to what extent the pathways are generic.
Functional Delivery of RNA
One of the key challenges to interpret the functional effects of extracellular vesicles is the abundance of bioactive cargo that could effect phenotypic changes in acceptor cells. Most studies on cellular uptake of EVs employ the use of fluorescently labeled vesicles, by either lipid dyes or fluorescently labeled proteins. Even though these strategies provide invaluable information on EV uptake, intracellular trafficking and processing of these molecules is not necessarily representative of soluble cargo delivery, such as RNA molecules or cytosolic proteins. One work-around for this is the study of EV-mediated miRNA transfer. However, sensitivity of such strategies are generally low, as negative read-outs make smaller effects difficult to observe. Furthermore, measuring miRNA dose-dependent responses is generally more suited for whole cell population analysis rather than single-cell analysis, and as such small effects may be masked by expression levels in the total cell population (Figure 3B). A breakthrough in this area was the use of a highly specific non-natural reporter system that is based on Cre-recombinase.42 Cre-recombinase is an enzyme that catalyzes the site-specific recombination event between two DNA recognition sites known as loxP sites. A reporter cell line was genetically engineered to express a red fluorescent protein gene and stop codon between loxP sites followed by a green fluorescent protein gene. When Cre-mRNA is delivered to this cell and is translated to the enzyme, it cleaves out the red fluorescent protein and stop codon, leading to the irreversible expression of green fluorescent protein. The color change in the cell can then be used as a measure of successful functional delivery (Figure 3C). The only drawback of the system, as is the inherent issue with any reporter system relying on transfer of mRNA, is the potential involvement of the translated enzyme produced in the donor cell. The Cre-recombinase enzyme itself may, similarly as the encoding mRNA, also be transported by the EVs, and this cannot be distinguished from mRNA delivery, as functional delivery of either molecule leads to the same phenotypic outcome. Nevertheless, this system allows the assessment of functional EV cargo delivery at single-cell accuracy and has been an invaluable tool to study EV content delivery. Using this system, it was demonstrated that vesicles functionally deliver Cre protein or mRNA between donor and acceptor tumor cells in vitro and even in vivo, located within the same and within distant tumors and that these EVs are involved in migration and metastasis.43
Still, the inability to separate protein-mediated effects from effects mediated by RNA is a drawback, and it is unknown whether results of a reporter system based on these larger molecules are representative of small RNA transfer, for which evidence for functional importance appears stronger. Thus, within the EV research field there is a need for novel sensitive reporter systems for RNA transfer that would combine the advantages of current miRNA and RNA-based reporter systems: a positive read-out system with single cell accuracy for RNA transfer that does not rely on activity of a translated product but rather on direct activity of the RNA molecule itself. Such a system would allow for the elucidation of the mechanisms, as well as the underlying pathways, that are pivotal for EV-mediated RNA delivery and processing. Ultimately, such insights are vital for designing optimal RNA delivery strategies, in the context of both synthetic and EV-mediated approaches.
Toward a Therapeutic Platform
The translation of EVs into a therapeutic platform for drug delivery may already be just beyond the horizon. Following several small clinical trials evaluating EVs as antitumor vaccines,44 the results of a recent phase 2 trial in which autologous tumor cell-derived EVs were used to deliver chemotherapy in lung cancer patients, demonstrated that manufacturing and administering drug-loaded EVs is feasible and safe and suggested a beneficial clinical response.45 Nevertheless, technology for EV production and quality control is far from standardized. While recent efforts have demonstrated the feasibility of GMP production of EVs on a small scale,46,47 challenges in their large scale production remain. Currently, there is no consensus on the best technology for EV production (suggested methods include multilayered culture flasks, bioreactors, hollow fiber cartridges) or EV isolation (ultracentrifugation, precipitation, size-exclusion or affinity chromatography, tangential flow filtration). Also pharmaceutical parameters such as EV storage and stability are ill defined. While storage at 4 °C or even −80 °C seems to have little effect on the physiochemical properties of EVs (i.e., size, charge, number),48,49 how these conditions affect EV bioactivity is unknown.
We are currently evaluating the effects of varying production platform, isolation method, and storage conditions on the yield, purity, and functional properties of EVs. For this, we are mainly focusing on progenitor cell-derived EVs, for which we have previously shown their intrinsic bioactivity in stimulating angiogenesis and cardiac repair in vitro and in vivo.50,51 This systematic optimization of culture, purification, and storage conditions should allow scale-up to clinical scale manufacturing, which will enable a swift translation of the developed therapies to a clinical application.
Biographies
Olivier G. de Jong was born in Arnhem (the Netherlands) in 1984. He received a M.Sc. in Biomedical Sciences from Utrecht University in 2010. He completed Ph.D. studies at UMC Utrecht in 2016. From there, he moved to a postdoctoral research project on extracellular-vesicle mediated RNA trafficking at the University of Oxford (United Kingdom), a topic that he has continued to study since returning to the University Medical Center Utrecht as a postdoctoral researcher in 2019.
Sander A. A. Kooijmans completed his M.Sc. in Drug Innovation at Utrecht University (the Netherlands) in 2011. During his Ph.D. studies in the Schiffelers group at the UMC Utrecht, he focused on extracellular vesicle engineering for drug delivery purposes. After obtaining his Ph.D. in 2016, he moved to the Bioindustry Park Silvano Fumero (Turin, Italy) to study stem cell extracellular vesicle functionality. In 2017, he returned to the Schiffelers lab to continue his work on extracellular vesicles and synthetic drug delivery systems.
Daniel Murphy graduated with a B.Sc. in Biomedical Science from the University of Warwick (U.K.) in 2013. He then undertook a Master’s program in Drug Innovation at Utrecht University during which he became interested in extracellular vesicles. In 2017 he started work on his Ph.D. within the Schiffelers lab at UMC Utrecht. His work currently focuses on extracellular vesicle-mediated RNA delivery.
Linglei Jiang was born in Jianyang, China, in 1988. She received her B.Sc. from Shenyang Pharmaceutical University in 2011 and M.Sc. from Shanghai Institute of Materia Medica in 2015. She started her Ph.D. study at UMC Utrecht in 2015 working on bacterial and mammalian extracellular vesicles.
Martijn J. W. Evers was born in ‘s-Hertogenbosch (The Netherlands) in 1992. He received a M.Sc. in Pharmaceutical Sciences from Utrecht University in 2017. He then joined the group of Raymond Schiffelers as a Ph.D. student working on synthetic drug delivery systems.
Joost Sluijter was born in 1977 in ‘s-Hertogenbosch (the Netherlands). He received his M.Sc. in 2000 from Utrecht University, In 2004, he successfully defended his Ph.D. thesis and went as a postdoctoral researcher to Indiana University–Purdue University (Indianapolis, IN). He has been appointed professor at UMC Utrecht in 2016 leading a research line focusing on improved recovery and diagnosis of cardiac tissue upon injury.
Pieter Vader graduated in Chemistry (B.Sc., 2005) and Drug Innovation (M.Sc., 2007) from the University of Utrecht. He earned his Ph.D. degree in 2012 at University of Utrecht on the subject of targeted delivery of siRNA to inhibit tumor angiogenesis. From 2012 to 2014, he was employed as a postdoctoral fellow at the University of Oxford (U.K.) in the lab of Prof. Matthew Wood. On small RNA-loaded extracellular vesicles for targeted delivery. In 2014, he moved back to the Netherlands to continue his work at the University Medical Center Utrecht. His main research interests are in the field of therapeutic applications of extracellular vesicles.
Raymond M. Schiffelers was born in Den Haag (the Netherlands) in 1971. He received a M.Sc. in Bio-Pharmaceutical Sciences from Leiden University in 1995. He completed Ph.D. studies at Erasmus University Rotterdam in 2001, where he worked on liposomal drug delivery for antimicrobial therapy. From there, he moved to a postdoctoral research project on siRNA delivery within Intradigm Co (Washington, D.C.). Since returning to Utrecht, he worked at the department of Pharmaceutical Sciences on drug delivery of biologicals and conventional therapeutics. In 2014, he has been appointed professor of nanomedicine at the University Medical Center Utrecht.
Author Contributions
⊥ O.G.d.J. and S.A.A.K. contributed equally.
The authors declare the following competing financial interest(s): R.M.S. is CSO of Excytex bv.
Special Issue
Published as part of the Accounts of Chemical Research special issue “Nanomedicine and Beyond”.
References
- Yanez-Mo M.; Siljander P. R.; Andreu Z.; Zavec A. B.; Borras F. E.; Buzas E. I.; Buzas K.; Casal E.; Cappello F.; Carvalho J.; Colas E.; Cordeiro-da Silva A.; Fais S.; Falcon-Perez J. M.; Ghobrial I. M.; Giebel B.; Gimona M.; Graner M.; Gursel I.; Gursel M.; Heegaard N. H.; Hendrix A.; Kierulf P.; Kokubun K.; Kosanovic M.; Kralj-Iglic V.; Kramer-Albers E. M.; Laitinen S.; Lasser C.; Lener T.; Ligeti E.; Line A.; Lipps G.; Llorente A.; Lotvall J.; Mancek-Keber M.; Marcilla A.; Mittelbrunn M.; Nazarenko I.; Nolte-’t Hoen E. N.; Nyman T. A.; O’Driscoll L.; Olivan M.; Oliveira C.; Pallinger E.; Del Portillo H. A.; Reventos J.; Rigau M.; Rohde E.; Sammar M.; Sanchez-Madrid F.; Santarem N.; Schallmoser K.; Ostenfeld M. S.; Stoorvogel W.; Stukelj R.; Van der Grein S. G.; Vasconcelos M. H.; Wauben M. H.; De Wever O. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E.; Cabanas C.; Mager I.; Wood M. J. A.; Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli M. P.; Aberg C.; Salvati A.; Dawson K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7 (12), 779–86. 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- Vroman L.; Adams A. L.; Fischer G. C.; Munoz P. C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55 (1), 156–9. [PubMed] [Google Scholar]
- Owens D. E. 3rd; Peppas N. A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307 (1), 93–102. 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 2014, 61 (2), 163–73. 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Gabizon A.; Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 1997, 54 (Suppl 4), 15–21. 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- Yang J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expert Rev. Clin. Pharmacol. 2019, 12 (2), 95–99. 10.1080/17512433.2019.1567326. [DOI] [PubMed] [Google Scholar]
- Chen S.; Tam Y. Y. C.; Lin P. J. C.; Sung M. M. H.; Tam Y. K.; Cullis P. R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Controlled Release 2016, 235, 236–244. 10.1016/j.jconrel.2016.05.059. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration.. ONPATTRO Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210922s000lbl.pdf (accessed February 22, 2019).
- Montaner-Tarbes S.; Novell E.; Tarancon V.; Borras F. E.; Montoya M.; Fraile L.; Del Portillo H. A. Targeted-pig trial on safety and immunogenicity of serum-derived extracellular vesicles enriched fractions obtained from Porcine Respiratory and Reproductive virus infections. Sci. Rep. 2018, 8 (1), 17487. 10.1038/s41598-018-36141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potz B. A.; Scrimgeour L. A.; Pavlov V. I.; Sodha N. R.; Abid M. R.; Sellke F. W. Extracellular Vesicle Injection Improves Myocardial Function and Increases Angiogenesis in a Swine Model of Chronic Ischemia. J. Am. Heart Assoc. 2018, 7 (12), 8344. 10.1161/JAHA.117.008344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. A.; Garst J.; Osada T.; Khan S.; Hobeika A.; Clay T. M.; Valente N.; Shreeniwas R.; Sutton M. A.; Delcayre A.; Hsu D. H.; Le Pecq J. B.; Lyerly H. K. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3 (1), 9. 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B.; Dorval T.; Chaput N.; Andre F.; Caby M. P.; Novault S.; Flament C.; Leboulaire C.; Borg C.; Amigorena S.; Boccaccio C.; Bonnerot C.; Dhellin O.; Movassagh M.; Piperno S.; Robert C.; Serra V.; Valente N.; Le Pecq J. B.; Spatz A.; Lantz O.; Tursz T.; Angevin E.; Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3 (1), 10. 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans S. A. A.; Fliervoet L. A. L.; van der Meel R.; Fens M.; Heijnen H. F. G.; van Bergen En Henegouwen P. M. P.; Vader P.; Schiffelers R. M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Controlled Release 2016, 224, 77–85. 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Nordin J. Z.; Lee Y.; Vader P.; Mager I.; Johansson H. J.; Heusermann W.; Wiklander O. P.; Hallbrink M.; Seow Y.; Bultema J. J.; Gilthorpe J.; Davies T.; Fairchild P. J.; Gabrielsson S.; Meisner-Kober N. C.; Lehtio J.; Smith C. I.; Wood M. J.; El Andaloussi S. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015, 11 (4), 879–83. 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Kooijmans S. A.; Aleza C. G.; Roffler S. R.; van Solinge W. W.; Vader P.; Schiffelers R. M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L.; Seow Y.; Yin H.; Betts C.; Lakhal S.; Wood M. J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29 (4), 341–5. 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Ohno S.; Takanashi M.; Sudo K.; Ueda S.; Ishikawa A.; Matsuyama N.; Fujita K.; Mizutani T.; Ohgi T.; Ochiya T.; Gotoh N.; Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21 (1), 185–91. 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I.; Noguchi K.; Aoki A.; Takatani-Nakase T.; Fujii I.; Futaki S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7 (1), 1991. 10.1038/s41598-017-02014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth T.; Petrova K.; Payton N. M.; Persaud I.; Redzic J. S.; Graner M. W.; Smith-Jones P.; Anchordoquy T. J. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 2014, 25 (10), 1777–84. 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar S.; LeBleu V. S.; Sugimoto H.; Yang S.; Ruivo C. F.; Melo S. A.; Lee J. J.; Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546 (7659), 498–503. 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P. L.; Harada T.; Christian D. A.; Pantano D. A.; Tsai R. K.; Discher D. E. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science (Washington, DC, U. S.) 2013, 339 (6122), 971–5. 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J.; Arras G.; Colombo M.; Jouve M.; Morath J. P.; Primdal-Bengtson B.; Dingli F.; Loew D.; Tkach M.; Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (8), E968–77. 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers R. M.; Bakker-Woudenberg I. A.; Storm G. Localization of sterically stabilized liposomes in experimental rat Klebsiella pneumoniae pneumonia: dependence on circulation kinetics and presence of poly(ethylene)glycol coating. Biochim. Biophys. Acta, Biomembr. 2000, 1468 (1–2), 253–61. 10.1016/S0005-2736(00)00265-0. [DOI] [PubMed] [Google Scholar]
- Arraud N.; Linares R.; Tan S.; Gounou C.; Pasquet J. M.; Mornet S.; Brisson A. R. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemostasis 2014, 12 (5), 614–27. 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- Pessina A.; Bonomi A.; Cocce V.; Invernici G.; Navone S.; Cavicchini L.; Sisto F.; Ferrari M.; Vigano L.; Locatelli A.; Ciusani E.; Cappelletti G.; Cartelli D.; Arnaldo C.; Parati E.; Marfia G.; Pallini R.; Falchetti M. L.; Alessandri G. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS One 2011, 6 (12), e28321 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C.; Gutierrez-Vazquez C.; Sanchez-Cabo F.; Perez-Hernandez D.; Vazquez J.; Martin-Cofreces N.; Martinez-Herrera D. J.; Pascual-Montano A.; Mittelbrunn M.; Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff M. J.; Temoche-Diaz M. M.; Karfilis K. V.; Ri S.; Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 2016, 5, e19276. 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo L.; Giurato G.; Cicchini C.; Montaldo C.; Mancone C.; Tarallo R.; Battistelli C.; Alonzi T.; Weisz A.; Tripodi M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17 (3), 799–808. 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Hung M. E.; Leonard J. N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles 2016, 5, 31027. 10.3402/jev.v5.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim N.; Ryu S. W.; Choi K.; Lee K. R.; Lee S.; Choi H.; Kim J.; Shaker M. R.; Sun W.; Park J. H.; Kim D.; Heo W. D.; Choi C. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016, 7, 12277. 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G.; Serio A.; Mazo M.; Nair R.; Stevens M. M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Controlled Release 2015, 205, 35–44. 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- O’Loughlin A. J.; Mager I.; de Jong O. G.; Varela M. A.; Schiffelers R. M.; El Andaloussi S.; Wood M. J. A.; Vader P. Functional Delivery of Lipid-Conjugated siRNA by Extracellular Vesicles. Mol. Ther. 2017, 25 (7), 1580–1587. 10.1016/j.ymthe.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijmans S. A. A.; Stremersch S.; Braeckmans K.; de Smedt S. C.; Hendrix A.; Wood M. J. A.; Schiffelers R. M.; Raemdonck K.; Vader P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Controlled Release 2013, 172 (1), 229–238. 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Lamichhane T. N.; Jeyaram A.; Patel D. B.; Parajuli B.; Livingston N. K.; Arumugasaamy N.; Schardt J. S.; Jay S. M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9 (3), 315–324. 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffoux M.; Silva A. K. A.; Wilhelm C.; Gazeau F.; Tareste D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12 (7), 6830–6842. 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- Sahay G.; Querbes W.; Alabi C.; Eltoukhy A.; Sarkar S.; Zurenko C.; Karagiannis E.; Love K.; Chen D.; Zoncu R.; Buganim Y.; Schroeder A.; Langer R.; Anderson D. G. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013, 31 (7), 653–8. 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J.; Querbes W.; Zeigerer A.; Borodovsky A.; Marsico G.; Schubert U.; Manygoats K.; Seifert S.; Andree C.; Stoter M.; Epstein-Barash H.; Zhang L.; Koteliansky V.; Fitzgerald K.; Fava E.; Bickle M.; Kalaidzidis Y.; Akinc A.; Maier M.; Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31 (7), 638–46. 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- Maier M.Recent Advances in Lipid Nanoparticle-Mediated Delivery of RNAi Therapeutics, Presented at the 8th Annual Meeting of the Oligonucleotide Therapeutics Society, 2012http://www.alnylam.com/web/assets/ALNY-OTS-LNP-Oct2012.pdf (accessed May 20, 2019).
- Costa Verdera H.; Gitz-Francois J. J.; Schiffelers R. M.; Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Controlled Release 2017, 266, 100–108. 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Zomer A.; Steenbeek S. C.; Maynard C.; van Rheenen J. Studying extracellular vesicle transfer by a Cre-loxP method. Nat. Protoc. 2016, 11 (1), 87–101. 10.1038/nprot.2015.138. [DOI] [PubMed] [Google Scholar]
- Zomer A.; Maynard C.; Verweij F. J.; Kamermans A.; Schafer R.; Beerling E.; Schiffelers R. M.; de Wit E.; Berenguer J.; Ellenbroek S. I. J.; Wurdinger T.; Pegtel D. M.; van Rheenen J. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015, 161 (5), 1046–1057. 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener T.; Gimona M.; Aigner L.; Borger V.; Buzas E.; Camussi G.; Chaput N.; Chatterjee D.; Court F. A.; Del Portillo H. A.; O’Driscoll L.; Fais S.; Falcon-Perez J. M.; Felderhoff-Mueser U.; Fraile L.; Gho Y. S.; Gorgens A.; Gupta R. C.; Hendrix A.; Hermann D. M.; Hill A. F.; Hochberg F.; Horn P. A.; de Kleijn D.; Kordelas L.; Kramer B. W.; Kramer-Albers E. M.; Laner-Plamberger S.; Laitinen S.; Leonardi T.; Lorenowicz M. J.; Lim S. K.; Lotvall J.; Maguire C. A.; Marcilla A.; Nazarenko I.; Ochiya T.; Patel T.; Pedersen S.; Pocsfalvi G.; Pluchino S.; Quesenberry P.; Reischl I. G.; Rivera F. J.; Sanzenbacher R.; Schallmoser K.; Slaper-Cortenbach I.; Strunk D.; Tonn T.; Vader P.; van Balkom B. W.; Wauben M.; Andaloussi S. E.; Thery C.; Rohde E.; Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.; Wu F.; Hu G.; Chen L.; Xu J.; Xu P.; Wang X.; Li Y.; Liu S.; Zhang S.; Huang Q.; Fan J.; Lv Z.; Zhou M.; Duan L.; Liao T.; Yang G.; Tang K.; Liu B.; Liao X.; Tao X.; Jin Y. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci. Transl Med. 2019, 11 (474), eaat5690. 10.1126/scitranslmed.aat5690. [DOI] [PubMed] [Google Scholar]
- Andriolo G.; Provasi E.; Lo Cicero V.; Brambilla A.; Soncin S.; Torre T.; Milano G.; Biemmi V.; Vassalli G.; Turchetto L.; Barile L.; Radrizzani M. Exosomes From Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method. Front. Physiol. 2018, 9, 1169. 10.3389/fphys.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt M.; Kamerkar S.; Sugimoto H.; McAndrews K. M.; Wu C. C.; Gagea M.; Yang S.; Blanko E. V. R.; Peng Q.; Ma X.; Marszalek J. R.; Maitra A.; Yee C.; Rezvani K.; Shpall E.; LeBleu V. S.; Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3 (8), e99263. 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R.; Zhao Y.; Jamaluddin M.; Popov V. L.; Wang H.; Kalubowilage M.; Zhang Y.; Luisi J.; Sun H.; Culbertson C. T.; Bossmann S. H.; Motamedi M.; Brasier A. R. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6 (1), 1359478. 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Zeng Q.; Han Q.; Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295. 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maring J. A.; Lodder K.; Mol E.; Verhage V.; Wiesmeijer K. C.; Dingenouts C. K. E.; Moerkamp A. T.; Deddens J. C.; Vader P.; Smits A. M.; Sluijter J. P. G.; Goumans M. J. Cardiac Progenitor Cell-Derived Extracellular Vesicles Reduce Infarct Size and Associate with Increased Cardiovascular Cell Proliferation. J. Cardiovasc Transl Res. 2019, 12 (1), 5–17. 10.1007/s12265-018-9842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen K. R.; Maring J. A.; Chamuleau S. A.; Verhage V.; Mol E. A.; Deddens J. C.; Metz C. H.; Lodder K.; van Eeuwijk E. C.; van Dommelen S. M.; Doevendans P. A.; Smits A. M.; Goumans M. J.; Sluijter J. P. Exosomes from Cardiomyocyte Progenitor Cells and Mesenchymal Stem Cells Stimulate Angiogenesis Via EMMPRIN. Adv. Healthcare Mater. 2016, 5 (19), 2555–2565. 10.1002/adhm.201600308. [DOI] [PubMed] [Google Scholar]