Abstract
Introduction:
Caudal block analgesia is administered to lower the requirements of systemic and inhaled anesthetic drugs during hypospadias surgery. However, recent reports, all clustered in a short time-span have generated controversial and mutually opposing results while evaluating caudal block as an independent risk factor for urethroplasty-related complications after hypospadias repair. There is no consensus statement on the role of caudal block analgesia in formation of urethrocutaneous fistula (UCF) after hypospadias surgery. We performed a systematic review and meta-analysis of the studies evaluating the relative rates of UCF formation after hypospadias surgery in patients who were administered caudal block analgesia versus in those who were not.
Methods:
Electronic searches were performed using PubMed, PubMed Central, Google Scholar, Ovid, and the Cochrane library. Statistical analysis was performed using a fixed-effect model, odds ratios, risk ratios (RR), and heterogeneity (I2) were calculated. Funnel plot was used to assess for publication bias.
Results:
Seven studies with 1706 patients were included. Caudal block analgesia is associated with a significantly higher risk of UCF formation (RR: 1.81; 95% confidence interval [CI]: 1.30–2.53), (P = 0.0004) and other urethroplasty-related complications (RR 2.01; 95% CI: 1.48–2.74), (P < 0.00001) after hypospadias surgery. Funnel plots indicate some publication bias.
Conclusions:
In patients undergoing hypospadias repair, administration of caudal analgesia is associated with a higher risk of UCF formation and other urethroplasty-related complications.
INTRODUCTION
Hypospadias is one of the most common congenital anomalies affecting one in every 125 live male births, and the incidence is rising.[1,2] There are as many techniques of hypospadias surgery and their modifications as there are surgeons performing the repair and the list is still evolving.[2] Formation of urethrocutaneous fistula (UCF) is the most feared complication after hypospadias repair, anticipated though.[3] UCF requires reoperation, the need for another surgery has financial, social, and psychological implications for the patient and their respective families.[4,5]
A caudal block is a safe, effective, and reliable technique to provide intra- and postoperative analgesia in children undergoing hypospadias surgery. Besides, it lowers the requirements of systemic and inhaled anesthetic drugs. However, there is another side to the coin as well: intra-operative penile engorgement because of vasodilatation and pooling of blood in the venous sinuses of the penis is a known complication of caudal epidural block. This vasodilatation results in excessive oozing from the surgical site, the sutures are placed under tension, thereby impeding proper healing and may be responsible for a higher incidence of UCF.
Multiple recent reports, all clustered in a short time-span, have generated controversial and mutually opposing results while evaluating caudal block as an independent risk factor for urethroplasty-related complications after hypospadias repair.[6,7,8,9,10,11,12]
We performed a systematic review and meta-analysis of the studies evaluating the relative rates of urethra-cutaneous fistula formation after hypospadias surgery in patients administered caudal block analgesia versus no caudal block analgesia.
MATERIALS AND METHODS
PICOS statement and hypothesis
This systematic review and meta-analysis (study design) planned to evaluate whether the formation of urethra-cutaneous fistula (outcome) is more common in patients with hypospadias (patient problem or population) undergoing urethroplasty under caudal block analgesia (intervention) as compared to those who are not administered caudal block analgesia (comparison).[13]
Our working hypothesis was that hypospadias surgery without caudal block analgesia is not associated with a lower rate of urethra-cutaneous fistula formation vis-à-vis hypospadias surgery with caudal block analgesia.
Gathering of existing evidence
After an exhaustive literature search, we were unable find a review published on the topic.
Identification of Studies
A thorough literature search on PubMed was performed on March 30, 2017 in agreement with the PRISMA statement[14] by two authors (PG and PK). This was followed by further search on the following databases: PubMed Central, Google Scholar, Ovid, and The Cochrane library. Missing articles were searched by snowballing and reverse snowballing. The search strategy and the PRISMA flow diagram depicting the results of the literature search are outlined in Table 1.
Table 1.
PUBMED search strategy
| Search Term | Results (n) | Included |
|---|---|---|
| Hypospadias | 6226 | - |
| Urethral Fistula | 3244 | - |
| Caudal | 36457 | - |
| 1 AND 2 AND 3 | 12 | 4 |
| Regional Anesthesia | 68374 | - |
| 1 AND 5 | 69 | 4 |
| 2 AND 5 | 13 | 4 |
| Hypospadias repair | 1847 | - |
| Caudal block | 1240 | - |
| 8 AND 9 | 32 | 6 |
| Caudal anesthesia | 2177 | - |
| 11 AND 8 | 35 | 5 |
| Finally relevant=7 |
Inclusion and exclusion criteria
The studies published in the English language, both prospective and retrospective, comparing the rates of UCF development post-hypospadias repair under general anesthesia with or without administration of caudal analgesia were included. Studies reporting adult-based data, duplicate publications, case reports, review articles, editorials, letters to the editor, published replies, viewpoints, and book chapters were excluded from the review. No limitations were imposed on the publication dates in the search strategy.
Data extraction
All titles and abstracts were reviewed by two authors (PG and PK) independently. If the abstract information was inadequate to make a decision, the full text was reviewed. Any discrepancies were resolved through consensus. For data extraction, post-operative glandular dehiscence was included with UCF formation.
Quality assessment
Among the studies included, one was a randomized control trial, whereas the others were either prospective or retrospective cohort studies. The risk of bias assessment tool recommended by the GRADE working group was used to assess the quality of each study. The tool was customized to address the following aspects: selection bias, biases in the measurement of exposure and outcome, incomplete outcome data and reporting bias. The risk of bias for each of these domains was categorized into three subcategories: low, high, or unclear.
Statistical analysis
The review manager v5.3 software (The Cochrane Collaboration, Oxford, UK) was used for data analysis as well as the quality of evidence assessment. Meta-analysis was performed taking the rate of UCF development as the outcome. Fixed-effects model was used for statistical analysis. Odds ratio (OR), pooled risk ratios (RR), and 95% confidence intervals (95% CI) were calculated to determine the influence of caudal analgesia on UCF development and forest plots were drawn. I2 was calculated to measure heterogeneity across the studies. Visual assessment of publication bias was performed by drawing the Funnel plot.
RESULTS
Seven studies matched the criteria for inclusion.[6,7,8,9,10,11,12] Characteristics of included studies are summarized in Table 2. Each of these studies were reviewed independently by two authors (PG and VJ) for study designs, patient cohorts, and outcomes. Most of these (n = 5, 71.4%) were retrospective cohort studies, one (14.3%) was a retrospective, nested case-control study and one (14.3%) was a prospective, double-blinded, randomized control trial.
Table 2.
List of studies included in the review and their characteristics
| Author | Country | Study design | Surgeon | Timing of CA | Hypospadias repair with CA (%) | Hypospadias repair without CA (%) | Meatal location | Fistula by meatal location |
|---|---|---|---|---|---|---|---|---|
| Taicher et al., 2017 | Durham, USA | Retro-spective | 1 | Not specified | 230/395 (58) UCF 21 (9.1) |
165/395 (42) UCF 1 (0.61) |
326 distal 69 prox |
n=22 (5.6%) Distal 9 (2.8%) Prox 13 (18.8%) |
| Saavendra et al., 2007 | San Juan, USA | Retro-spective | 1 | Peri-operative | 91/137 (47.4) UCF 9 (9.9) |
101/137 (52.6) UCF 2 (2) |
71 gladular 22 coronal 99 subcoronal |
4 glandular 1 coronal 6 subcoronal |
| Braga et al., 2017 | Toronto, Canada | Retro-spective | 2 | Timing not captured specifically, routine practice to administer CB at beginning of surgery | 367/518 (70.85) UCF 32 (8.72) |
151/518 (29.15) UCF 5 (3.31) |
405 distal 59 mid shaft 54 proximal |
19 distal (14 CB) 18 proximal (18 CB) |
| Kreysing et al., 2016 | Germany | Retrospective | 1 | After surgery | 33/70 (47.14) UCF 3 (9.09) |
37/70 (52.86) UCF 2 (5.41) |
Not analyzed | Not analyzed |
| Kim et al., 2016 | Korea | Retrospective | 1 | Prior to surgery | 216/342 (63.16) 98 distal 87 mid 31 proximal UCF 26 (12.04) Overall complications 53 (24.54)* |
126/342 (36.84) 45 distal 72 mid 9 proximal UCF 15 (11.9) Overall complications 19 (15.08)** |
143 distal 159 mid 40 proximal |
22 distal 37 mid 13 proximal |
| Zaidi et al., 2015 | Ann Arbor, USA | Retrospective, nested case control study | 6 | Not specified | 101/135 (74.82) UCF 32 (31.68) |
34/135 (25.18) UCF 12 (35.3) |
114 distal 8 mid shaft 12 proximal |
25 distal 20 proximal/mid shaft |
| Kundra et al., 2012 | Pondicherry, India | Prospective, RCT, double-blinded | >1 | Prior to surgery | 27/54 (50) UCF 5 (18.5) |
27/54 (50) UCF 0 (0) |
Only distal hypospadias included (meatus distal to mid-shaft) | |
*Includes UCF (n=26), meatal stenosis (n=14), urethral diverticulum (n=8) and wound infection/hematoma (n=5), **Includes UCF (n=15), meatal stenosis (n=4), urethral diverticulum (n=0) and wound infection/hematoma (n=0). UCF=Urethrocutaneous fistula, RCT=Randomized controlled trial, CB=Caudal block, CA=Caudal Analgesia
Most of the studies presented data pertaining to the development of UCF in association with administration of caudal analgesia. In addition, Kim et al. (14.3%) had also commented on the rates of meatal stenosis, urethral diverticulum formation, and post-operative wound infection or hematoma. The principal problem with the nonrandomized studies was that the patient allocation into the caudal analgesia and the noncaudal analgesia group was arbitrary and the decision rested with the anesthetist or the surgeon or both. Sub-group analysis, to find the differential rates of UCF formation for different severities of hypospadias or for the surgical procedure performed, could not be done in view of insufficient data.
The studies collectively included 1706 patients with hypospadias of which 1065 were administered caudal analgesia. Rates of UCF formation ranged from 9% to 32% in patients who were administered caudal analgesia as compared to 0%–35.3% in the noncaudal analgesia group. The cumulative rate of UCF formation was 12% (128 patients out of 1065) in the caudal analgesia group and 5.78% (37 patients of 641) in the noncaudal analgesia group. The cumulative rate of urethroplasty-related complications (including UCF) was 14.6% (155 patients out of 1065) in the caudal analgesia group and 6.4% (41 out of 641) in the noncaudal analgesia group.
Meta-analysis of the data showed an increased risk of UCF formation in patients who were administered caudal analgesia, OR (Mantel-Haenszel, fixed effect, 95% CI), 2.00 (1.36–2.94), P = 0.0005 and RR (Mantel-Haenszel, fixed effect, 95% CI), 1.81 (1.30–2.53), P = 0.0004 [Figure 1a and b]. Another meta-analysis [Figure 1c and d] showed an increased risk of urethroplasty-related complications in patients who were administered caudal analgesia, OR (Mantel-Haenszel, fixed effect, 95% CI), 2.32 (1.60–3.36), P < 0.00001 and RR (Mantel-Haenszel, fixed effect, 95% CI), 2.01 (1.48–2.74) P < 0.00001).
Figure 1.
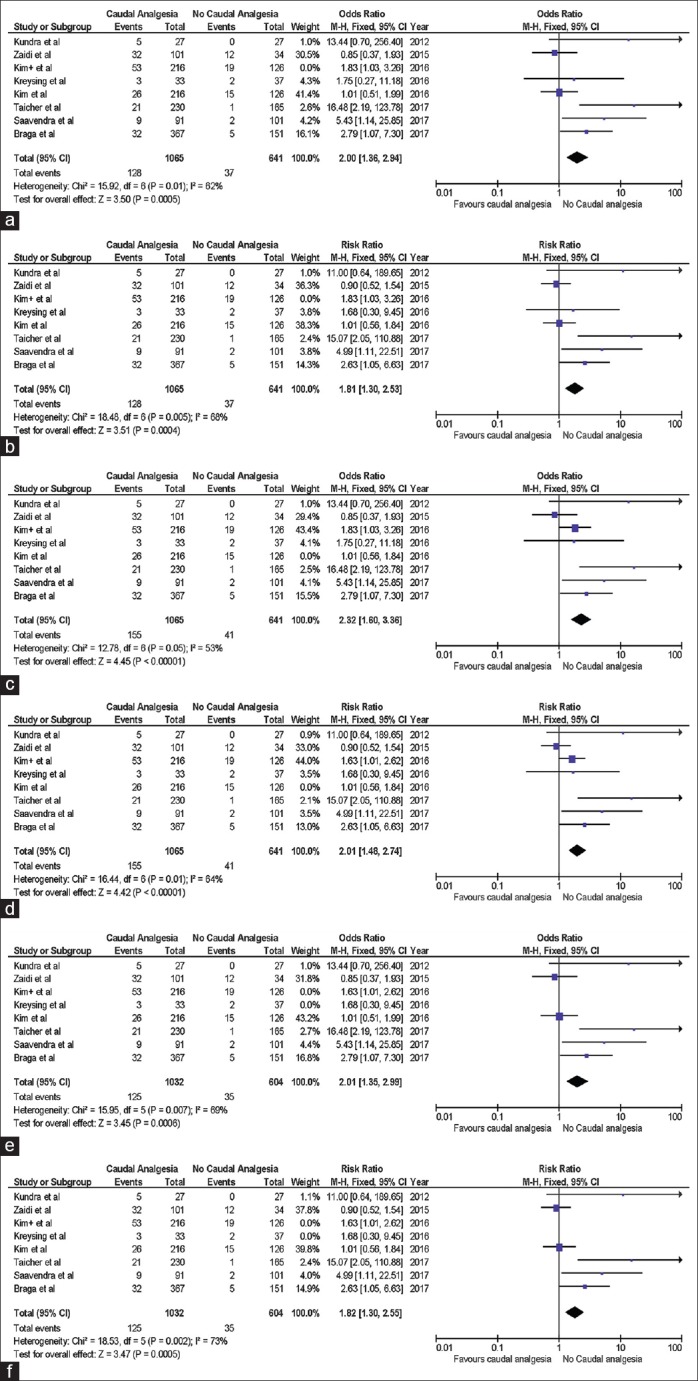
(a and b) Forest plot demonstrating comparison of rates of urethrocutaneous fistula after hypospadias surgery under general anesthesia with caudal analgesia versus general anesthesia without caudal analgesia. (c and d) Forest plot demonstrating comparison of rates of urethroplasty related complications after hypospadias surgery under general anesthesia with caudal analgesia versus general anesthesia without caudal analgesia. (e and f) Forest plot demonstrating comparison of rates of urethrocutaneous fistula after hypospadias surgery under general anesthesia with caudal analgesia administered preoperatively versus general anesthesia without caudal analgesia (Fixed effects analysis model has been used for statistical calculations. Both odds ratio and risk ratio have been used to measure effect)
Kreysing and Höhne have specified that caudal analgesia at their center was usually administered at the end of the hypospadias surgery.[9] This may have a different bearing on caudal analgesia-related complications of urethroplasty, thus a separate meta-analysis was performed after excluding their study from the statistical calculations. Figure 1e and f show an increased risk of urethroplasty-related complications in patients who were administered caudal analgesia, OR (Mantel-Haenszel, fixed effect, 95% CI), 2.01 (1.36–2.99), P = 0.0006 and RR (Mantel–Haenszel, fixed effect, 95% CI), 1.82 (1.30–2.55), P = 0.0005 even after excluding the study by Kreysing et al.
Funnel plot [Figure 2] was drawn to depict publication bias. Figures 3 and 4 summarize the risk of bias (GRADE), presented as percentages across all the included studies.
Figure 2.
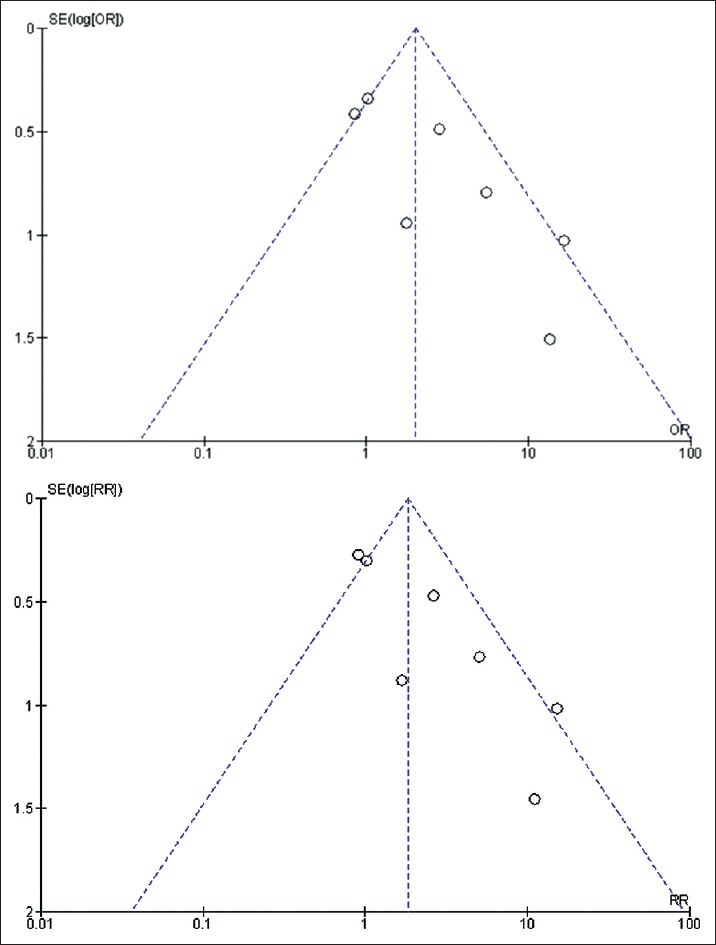
Funnel plot demonstrating comparison of rates of urethrocutaneous fistula after hypospadias surgery under general anesthesia with caudal analgesia versus general anesthesia without caudal analgesia. Fixed effects analysis model has been used for statistical calculations. Both odds ratio and risk ratio have been used to measure effect
Figure 3.
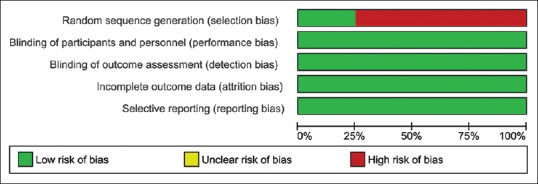
Risk of bias assessment graph (GRADE)
Figure 4.
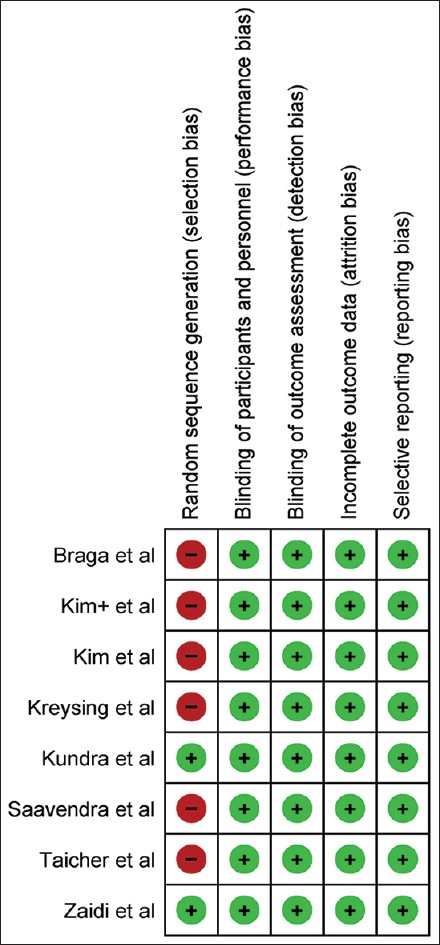
Risk of bias summary
DISCUSSION
Caudal analgesia in pediatric patients undergoing surgery of the groin or the external genitalia is now a well-established, safe, simple, cost-effective, and reliable technique of regional anesthesia.[15] It reduces the requirement of other systemic or inhalational agents administered intraoperatively and a decrease in the need of post-operative pain medications. There is a decrease in stress hormone response and an overall better parent and patient satisfaction.
However, like any other procedure, caudal block has its own set of possible complications. Dural penetration, intravascular injections, drug overdose, and micturition problems have been described.[16,17] Studies have also revealed a decrease in the sympathetic activity, vascular resistance, and cardiac output postepidural anesthesia.[18,19]
Physiology of caudal analgesia
The venous system accounts for almost three-fourth of the blood volume. Caudal neural blockage leads to sympathetic inhibition extending up to T5-L1 vertebral level which leads to a reduction in the vascular tone resulting in pooling of blood in the lower body including the perineum and the external genitalia. Vasodilatation of penile sinuses may lead to penile engorgement and oozing from the surgical site.[20] A messy surgical field is deterrent to quality work. The local tissue edema adversely affects the quality of surgical knots and postoperative wound healing. A modified metabolic response of the tissues in response to caudal neural blockage with decreased coagulability and viscosity of blood predisposing to development of hematoma and bleeding in the postoperative period has been suggested, although in patients undergoing surgery for hip fracture.[21]
Formation of UCF is one of the most common and the most feared complications of hypospadias repair. Reported rates of fistula formation vary from 0% to 23%. Many factors have been incriminated time and again for the development of fistulae. Common factors linked to the development of UCF may be stratified as those related to surgery (technique used, type of suture), surgeon (operating experience, surgical finesse, tissue handling, and postoperative management), disease (severity of hypospadias, size of penis), patient (age, nutritional status), miscellaneous (local infection, postoperative edema, local ischemia, accidental pull-out of stent) or destiny (patient or surgeon).
A systematic review indicates that administration of caudal analgesia is associated with 12% risk of UCF after hypospadias surgery which compares unfavorably with the results (5.8% UCF) of hypospadias surgery without caudal administration. The risk is increased to 14.6% and 6.4% for overall urethroplasty-related complications after hypospadias repair in the caudal analgesia and the noncaudal analgesia groups, respectively.
Kundra et al. conducted a prospective, double-blinded, randomized control trial in a cohort of 54 American Society of Anesthesiologists I and II children within the age-range of 4–12 years with distal hypospadias.[12] In their study, the incidence of urethral fistula formation after primary hypospadias repair was 19.2%. Also, all the children who developed urethral fistula had received caudal epidural (with 0.5 ml/kg of 0.25% bupivacaine). They also observed a significant increase in penile volume following administration of caudal analgesia, calculated by measuring the length of penis from the pubic symphysis to its tip and the circumference in mid-penile region, 10 min after the administration of block as compared prior to the block. The authors' attributed the UCF observed in the caudal analgesia group to the significant increase in penile volume which may have increased penile edema and resulted in inadequate or delayed wound healing. As the measurements were taken 10 min after administration of caudal block (as it was not possible to take measures once the surgery had started), it was likely that the edema continued to worsen further (total duration the engorgement lasted could not be monitored due to application of occlusive dressing at the end of procedure). However, the median age of patients in their study was 6 years for the penile block and 7 years for caudal block group, which is older than the usual age for hypospadias repair. The degree of venous pooling from sympathetic blockade is considerably greater in older children as compared to infants, as evidenced by lack of hypotension and better compensatory mechanisms in infants which may have confounded the results of their study.[22,23]
Also, an independent subgroup analysis within the caudal analgesia group, to assess the difference in percentage increase in penile volume between the patients who developed UCF and those who did not, was not possible with the data available on record. Besides, the primary aim of their study was to compare the efficacy of penile nerve block vis-a-vis caudal anesthesia during hypospadias repair and not to identify a potential association between caudal analgesia and rates of UCF formation. The authors have not specifically assessed for the effects of tissue edema or penile engorgement on wound healing and their assumption is not backed by literature; rather caudal block has been employed as a treatment of penile ischemia following circumcision and to improve cremaster muscle flap tolerance to venous ischemia.[24,25] Various other confounding factors that could have contributed to the development of UCF such as the surgical service or the technique were not controlled.[11] The operating surgeons, besides being multiple belonged to different specialties including urology and plastic surgery.
Zaidi et al., on the other hand, could not find an association between the use of caudal regional anesthesia and fistula formation.[11] Their study was based on a cohort of 1647 cases from which 45 cases with UCF along with 90 controls were chosen to design a retrospective nested case-control study. A nested case-control study scores superior to a case-control study in its ability to establish a causal relationship and the study design minimizes the selection and the recall bias.[26] However, their study is limited by under-reporting of outcomes and loss to follow-up. Two controls were selected for every case of UCF; the higher the number of controls, stronger the study design. The study included results of six different surgeons which further introduced the possibility of bias. Also, they did not control for factors like the presence of chordee, etc., which may have had an independent bearing on the rate of UCF. Besides UCF, other parameters n were not evaluated. By adjusting for the confounders in a multivariate analysis limited to patients undergoing distal tubularized incised plate repairs, subcutaneous epinephrine injection, and operative time (implicating proximal hypospadias) were the only independent risk factors for UCF formation.
Kim et al. have reported a 2.1 times higher rate (OR) of complications associated with the use of caudal analgesia for hypospadias repair, in addition to the role of surgical duration and location of urinary meatus (severity of hypospadias).[10] By limiting the inclusion criteria to cases operated by a single surgeon, the number of confounding variables were reduced. The study is statistically sound. However, the study is limited by its retrospective design and the possibility of selection bias since the decision for administering caudal analgesia rested with the anesthetist, the basis of which is unclear. It is a general observation of the authors that the anesthetists usually prefer to administer caudal analgesia in surgeries expected to last longer (for better pain relief) which itself correlates directly with the severity of hypospadias and the probable incidence of UCF formation and other complications.
Kreysing and Höhne were unable to find an association between caudal analgesia and the incidence of UCF formation after hypospadias repair.[9] However, in their study, caudal analgesia was administered after completion of the hypospadias surgery. Therefore, the proposed ill effects of caudal analgesia might not come into play, and thus we considered excluding this study from the current meta-analysis and consequently performed a separate meta-analysis. Also, their study was limited by its retrospective design, small case number, and an inconsistent follow-up time range. Furthermore, this study has raised a possibility that postsurgical administration of caudal analgesia may avoid its adverse effects while preserving some of its benefits. Braga et al. were also unable to find an association between the risk of complications following hypospadias repair and caudal block analgesia.[8] The severity of hypospadias (location of urinary meatus and degree of ventral chordee) was the only factor significantly associated with the development of postoperative complications. A post hoc analysis showed that the study was powered at 82% for the primary outcome; the results are unlikely to be due to chance. The mean follow-up of patients in their study was 13.4 ± 17 months, which is significant. The authors' have also questioned the hypothesis that penile engorgement results in impaired postoperative healing and leads to complications. However, the authors' have accepted the possibility of caudal administration after surgery in a small number of patients.
Saavedra-Belaunde et al. reviewed the results of distal hypospadias repair in 192 patients performed by a single surgeon.[7] Fistula formed in 11 patients of which 9 received caudal anesthesia (UCF in 9/91 patients receiving caudal analgesia). The RR for a post-operative complication was 3.70 in the caudal anesthesia group and the probability of complications was calculated at 4.88 (P < 0.027) by the Fischer exact test. The study included patients with distal penile hypospadias only, thereby ruling out disease severity as a confounding factor. All cases were operated by a single surgeon (surgeon's experience was not a confounding factor). However, the retrospective design of the study is a limiting factor and the results of the study may not be extrapolated to all varieties of hypospadias.
Taicher et al.[6] have published their retrospective single surgeon series of 395 patients with distal and proximal hypospadias (homogeneity of treating institution and surgeon). They found that caudal analgesia and the severity of hypospadias were associated with a higher postoperative surgical complication rate after hypospadias repair. Even after adjusting for the disease severity, caudal analgesia was associated with a 13-fold increase in the odds of developing postoperative complications. The OR of “13” with a wide CI of 1.8–101.8 is alarming and implies a huge uncertainty in the results (large effect vs. large error) and inability to draw any meaningful conclusions.[27] Hayder has pointed out the possibility of a lack of internal validity due to unequal administration of caudal anesthesia (selection bias) and referred to Hill's criteria for judging causal association in observational research.[28,29] The OR shrunk and the CI narrowed when the penalization via data augmentation was used to analyze the proposed association.[30]
Inclusion of single surgeon series in a meta-analysis is a double-edged sword. Different surgeons have different skills which act as a confounding factor, which is avoided in a single surgeon series. However, the results of single surgeon study may be unique to the surgeon's practice and may not be applicable to other centers or surgeons. In such situations a meta-analysis has the potential to compare several studies with a common objective conducted at different set-ups in different geographical locations and by different investigators.
Limitation of the review
Although the review encompasses the best available evidence, some limitations should be considered. First, all except one study included in this analysis are retrospective and most of them are statistically sub-optimal. However, there are few trials underway, results of which may be available over time. Second, there is an element of heterogeneity across the studies. This heterogeneity is introduced by inclusion of more than one surgeon, more than one type of hypospadias, use of different drugs for caudal analgesia, more than one surgical technique, lack of anthropometric data of the patient database as in indirect indicator of nutritional status, different climatic conditions, etc., Moreover, the patient demographics have not been consistently reported in all the included studies. Third, it is a well-known statistical fact that in any study, more the number of confounding factors; more is the required sample strength to generate meaningful results. However, most of the studies available in the literature are based on relatively small number of patients. Fourth, the use of caudal analgesia or dorsal penile block was not randomized; it was the anesthetist's decision in nearly all the retrospective studies The penis is mainly supplied by the dorsal nerve of the penis; dorsal penile block is more effective as a pain relief procedure for patients with distal hypospadias. A single lateral injection of a local anesthetic under the pubis is insufficient for proximal varieties of hypospadias since the proximal (penile and perineal) parts are supplied by the posterior branches of the nerve of the penis which branch behind the pubis and receive twigs from the branches of the genitofemoral and ilioinguinal nerve. The relative utility of caudal analgesia vis-à-vis dorsal penile block may bias the anesthetists' decision to preferentially use caudal block for proximal hypospadias. Fifth, the asymmetrical funnel plot [Figure 2] subtly hints towards some degree of publication bias in studies that did not report an increased rate of UCF in patients receiving caudal anesthesia.
CONCLUSIONS
From this meta-analysis, it can be concluded that administration of caudal analgesia in patients undergoing hypospadias repair may be associated with a higher risk of UCF and other urethroplasty related complications. However, a randomized control trial is required to draw definitive conclusions.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–4. doi: 10.1542/peds.100.5.831. Paulozzi LJ. [DOI] [PubMed] [Google Scholar]
- 2.Subramaniam R, Spinoit AF, Hoebeke P. Hypospadias repair: An overview of the actual techniques. Semin Plast Surg. 2011;25:206–12. doi: 10.1055/s-0031-1281490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal K, Misra A. Unfavourable results in hypospadias. Indian J Plast Surg. 2013;46:419–27. doi: 10.4103/0970-0358.118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodhouse CR, Christie D. Nonsurgical factors in the success of hypospadias repair. BJU Int. 2005;96:22–7. doi: 10.1111/j.1464-410X.2005.05560.x. [DOI] [PubMed] [Google Scholar]
- 5.Mondaini N, Ponchietti R, Bonafè M, Biscioni S, Di Loro F, Agostini P, et al. Hypospadias: Incidence and effects on psychosexual development as evaluated with the Minnesota Multiphasic Personality Inventory test in a sample of 11,649 young Italian men. Urol Int. 2002;68:81–5. doi: 10.1159/000048423. [DOI] [PubMed] [Google Scholar]
- 6.Taicher BM, Routh JC, Eck JB, Ross SS, Wiener JS, Ross AK, et al. The association between caudal anesthesia and increased risk of postoperative surgical complications in boys undergoing hypospadias repair. Paediatr Anaesth. 2017;27:688–94. doi: 10.1111/pan.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra-Belaunde JA, Soto-Aviles O, Jorge J, Escudero K, Vazquez-Cruz M, Perez-Brayfield M, et al. Can regional anesthesia have an effect on surgical outcomes in patients undergoing distal hypospadia surgery? J Pediatr Urol. 2017;13:45.e1–45.e4. doi: 10.1016/j.jpurol.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Braga LH, Jegatheeswaran K, McGrath M, Easterbrook B, Rickard M, DeMaria J, et al. Cause and effect versus confounding-is there a true association between caudal blocks and tubularized incised plate repair complications? J Urol. 2017;197:845–51. doi: 10.1016/j.juro.2016.08.110. [DOI] [PubMed] [Google Scholar]
- 9.Kreysing L, Höhne C. A retrospective evaluation of fistula formation in children undergoing hypospadias repair and caudal anesthesia. Paediatr Anaesth. 2016;26:329–30. doi: 10.1111/pan.12845. [DOI] [PubMed] [Google Scholar]
- 10.Kim MH, Im YJ, Kil HK, Han SW, Joe YE, Lee JH, et al. Impact of caudal block on postoperative complications in children undergoing tubularised incised plate urethroplasty for hypospadias repair: A retrospective cohort study. Anaesthesia. 2016;71:773–8. doi: 10.1111/anae.13463. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi RH, Casanova NF, Haydar B, Voepel-Lewis T, Wan JH. Urethrocutaneous fistula following hypospadias repair: Regional anesthesia and other factors. Paediatr Anaesth. 2015;25:1144–50. doi: 10.1111/pan.12719. [DOI] [PubMed] [Google Scholar]
- 12.Kundra P, Yuvaraj K, Agrawal K, Krishnappa S, Kumar LT. Surgical outcome in children undergoing hypospadias repair under caudal epidural vs. penile block. Paediatr Anaesth. 2012;22:707–12. doi: 10.1111/j.1460-9592.2011.03702.x. [DOI] [PubMed] [Google Scholar]
- 13.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. JAMA. 2015;313:1657–65. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 15.Uguralp S, Mutus M, Koroglu A, Gurbuz N, Koltuksuz U, Demircan M. Regional anesthesia is a good alternative to general anesthesia in pediatric surgery: Experience in 1,554 children. J Pediatr Surg. 2002;37:610–3. doi: 10.1053/jpsu.2002.31619. [DOI] [PubMed] [Google Scholar]
- 16.Ecoffey C, Lacroix F, Giaufré E, Orliaguet G, Courrèges P; Association Des Anesthésistes Réanimateurs Pédiatriques d'Expression Française (ADARPEF), et al. Epidemiology and morbidity of regional anesthesia in children: A follow-up one-year prospective survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF) Paediatr Anaesth. 2010;20:1061–9. doi: 10.1111/j.1460-9592.2010.03448.x. [DOI] [PubMed] [Google Scholar]
- 17.Metzelder ML, Kuebler JF, Glueer S, Suempelmann R, Ure BM, Petersen C, et al. Penile block is associated with less urinary retention than caudal anesthesia in distal hypospadias repair in children. World J Urol. 2010;28:87–91. doi: 10.1007/s00345-009-0420-2. [DOI] [PubMed] [Google Scholar]
- 18.Shimosato S, Etsten BE. The role of the venous system in cardiocirculatory dynamics during spinal and epidural anesthesia in man. Anesthesiology. 1969;30:619–28. doi: 10.1097/00000542-196906000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Malmqvist LA, Bengtsson M, Björnsson G, Jorfeldt L, Löfström JB. Sympathetic activity and hemodynamic variables during spinal analgesia in man. Acta Anaesthesiol Scand. 1987;31:467–73. doi: 10.1111/j.1399-6576.1987.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 20.Kakiuchi M. Reduction of blood loss during spinal surgery by epidural blockade under normotensive general anesthesia. Spine (Phila Pa 1976) 1997;22:889–94. doi: 10.1097/00007632-199704150-00012. [DOI] [PubMed] [Google Scholar]
- 21.Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: A meta-analysis of randomized trials. Br J Anaesth. 2000;84:450–5. doi: 10.1093/oxfordjournals.bja.a013468. [DOI] [PubMed] [Google Scholar]
- 22.Raux O, Rochette A, Morau E, Dadure C, Vergnes C, Capdevila X, et al. The effects of spread of block and adrenaline on cardiac output after epidural anesthesia in young children: A randomized, double-blind, prospective study. Anesth Analg. 2004;98:948–55. doi: 10.1213/01.ANE.0000108133.63310.AF. [DOI] [PubMed] [Google Scholar]
- 23.McCann ME, Withington DE, Arnup SJ, Davidson AJ, Disma N, Frawley G, et al. Differences in blood pressure in infants after general anesthesia compared to awake regional anesthesia (GAS study-A prospective randomized trial) Anesth Analg. 2017;125:837–45. doi: 10.1213/ANE.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplanian S, Chambers NA, Forsyth I. Caudal anaesthesia as a treatment for penile ischaemia following circumcision. Anaesthesia. 2007;62:741–3. doi: 10.1111/j.1365-2044.2007.05060.x. [DOI] [PubMed] [Google Scholar]
- 25.Cayci C, Cinar C, Yucel OA, Tekinay T, Ascherman JA. The effect of epidural anesthesia on muscle flap tolerance to venous ischemia. Plast Reconstr Surg. 2010;125:89–98. doi: 10.1097/PRS.0b013e3181c49544. [DOI] [PubMed] [Google Scholar]
- 26.Sedgwick P. Nested case-control studies: Advantages and disadvantages. BMJ. 2014;348:1532. doi: 10.1136/bmj.f7707. [DOI] [PubMed] [Google Scholar]
- 27.Polaner DM, Almenrader N, Vemulakonda V. Caudal analgesia, hypospadias, and urethrocutaneous fistula: Does association mean causality? Paediatr Anaesth. 2017;27:676–7. doi: 10.1111/pan.13167. [DOI] [PubMed] [Google Scholar]
- 28.Haydar B. Judging causal associations in observational research on caudal anesthesia and hypospadias repair. Paediatr Anaesth. 2017;27:1279. doi: 10.1111/pan.13260. [DOI] [PubMed] [Google Scholar]
- 29.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 30.Ayubi E, Safiri S. The association between caudal anesthesia and increased risk of postoperative surgical complications in boys undergoing hypospadias repair: Comment on data sparsity. Paediatr Anaesth. 2017;27:974. doi: 10.1111/pan.13207. [DOI] [PubMed] [Google Scholar]


