Abstract
Introduction:
We compared the diagnostic accuracy of percentage free prostate-specific antigen (PSA), multiparametric magnetic resonance imaging (mpMRI), and gallium-68 prostate-specific membrane antigen positron emission tomography (Ga-PSMA PET) to detect cancer prostate in men with PSA between 4 and 20 ng/ml in prebiopsy settings.
Materials and Methods:
This prospective study evaluated men with PSA values between 4 and 20 ng/ml, and all patients underwent percentage free PSA estimation, mpMRI, and Ga-PSMA PET scan, followed by cognitive fusion/registration biopsy along with systematic 12-core biopsy to detect cancer prostate. The diagnostic accuracy of percentage free PSA, mpMRI, and Ga-PSMA PET scan was compared with results of cognitive fusion/registration biopsy.
Results:
A total of 15 patients were included, of which 11 had an identifiable lesion on imaging and 9 had malignancy on the final histopathology report. The sensitivity, specificity, positive predictive value, negative predictive value (NPV), and diagnostic accuracy of mpMRI were 62.5%, 71.4%, 71.4%, 62.5%, and 66.6%, respectively, and that of Ga-PSMA PET scan were 88.8%, 66.6%, 80%, 80%, and 80%, respectively. The sensitivity of detection of clinically significant cancers for Ga-PSMA was higher (100%) compared to MRI (33.3%). However, Ga-PSMA also detected a greater number of insignificant lesions as compared to MRI.
Conclusion:
Ga-PSMA PET scan has high NPV and accuracy in predicting presence of cancer and can also be used to direct specific biopsy cores during systematic biopsy.
INTRODUCTION
The traditional method for diagnosing cancer prostate is screening by serum prostate-specific antigen (PSA) or by digital rectal examination (DRE), followed by a 10–12 core transrectal ultrasonography (TRUS)-guided prostate biopsy. However, about 20%–30% of the cancers are missed by this approach, especially in the apical and anterior zones of the prostate.[1,2] On the contrary, among the patients with a serum PSA value between 4 and 10 ng/ml, only about 22%–27% actually harbor prostatic malignancy.[3] Thus, while on the one hand, this approach is an over treatment for a substantial percentage of patients, it still ends up missing a significant number of men with cancer.
The advent of multiparametric magnetic resonance imaging (mpMRI) has improved the sensitivity of detecting cancer prostate.[4] Further, the concept of MRI-targeted biopsy has revolutionized the technique of prostate biopsy from blind sampling to specific targeting and has improved the detection rates of significant prostate cancer.[5] These features have made mpMRI one of the recommended prebiopsy investigations to help decide the need of prostate biopsy, especially in men with a PSA value between 4 and 10 ng/ml or a previous negative biopsy or in patients on active surveillance.[6] However, mpMRI is not disease specific, and many benign conditions such as acute and chronic prostatitis, basal cell hyperplasia, benign prostatic hyperplasia, or postbiopsy changes can give false-positive results and thus may result in an unnecessary biopsy.[7,8] Besides these, the field of evaluation is usually limited to the pelvis, and separate imaging is usually required to image for distant metastasis.
Gallium-68 prostate-specific membrane antigen positron emission tomography (Ga-PSMA PET) has recently been introduced and is gradually establishing its place in the diagnostic algorithm of cancer prostate. Several studies have reported its accuracy in evaluating nodal and bony metastases and for detecting recurrences.[9,10,11,12] A distinct advantage of Ga-PSMA PET scan is that PSMA is overexpressed by 100–1000 folds in cancer prostate as compared to benign tissue which theoretically makes PSMA PET scan relatively specific to malignant transformation as compared to mpMRI, which is not disease specific.[13] However, unlike mpMRI, the current utility of Ga-PSMA PET scan for detection of cancer prostate in prebiopsy settings in patients with equivocal PSA values is still undefined.
We aimed at comparing the diagnostic accuracy of percentage free PSA, mpMRI, and Ga-PSMA PET scan to detect cancer prostate in men with PSA between 4 and 20 ng/ml in prebiopsy settings.
MATERIALS AND METHODS
This prospective study was carried out at a tertiary care center after obtaining ethical clearance from the Institute Ethical Committee (IEC) (IEC/VMMC/SJH/Project/March/2018/576) from April to November 2018. All male patients >55 years of age presenting to the urology outpatient department with lower urinary tract symptoms (LUTS) were evaluated with a standard protocol consisting of DRE, focused neurological examination, urine routine, uroflowmetry, and ultrasonography of the kidney, bladder, and prostate along with postvoid residual urine estimation. Patients who had a nodule on DRE underwent PSA estimation, whereas those with a probable diagnosis of benign prostatic hyperplasia were also counseled for PSA-based screening of prostate cancer. All patients with a serum PSA between 4 and 20 ng/ml were counseled for inclusion into this study. Those with urinary tract infection or a history of recent instrumentation or urinary retention that could have fallaciously raised the PSA values were excluded. Patients, who gave written informed consent, underwent further evaluation consisting of percentage free PSA estimation, mpMRI of the pelvis, Ga-PSMA PET scan, and TRUS-guided cognitive fusion/registration prostate biopsy. Percentage free PSA was calculated from free: total PSA ratio, and for the study purposes, a value of <10% was considered diagnostic of prostatic malignancy. All patients underwent mpMRI on a 1.5 Tesla clinical MRI system (Philips Healthcare, Eindhoven, the Netherlands) without an endorectal coil. The mpMRI consisted of T1, T2, and dynamic contrast-enhanced (DCE) sequences, and diffusion-weighted imaging was not performed. Each mpMRI was evaluated by a single radiologist who was blinded to the findings of other imaging modalities and Ga-PSMA PET report. The Prostate Imaging Reporting and Data System (PI-RADS) score was calculated as per PIRADS version 2 utilizing T2 and DCE sequences as per the “assessment without adequate DWI” recommendations of PI-RADS steering committee. PI-RADS score (version 2) was assigned to all the suspicious lesions. The lesion with the highest PIRADS score (index lesion) was considered for cognitive fusion/registration biopsy and data analysis, whereas, any other lesions suspicious of malignancy but of lower PIRADS score than the index lesion or any lesion with a PIRADS score <3 was recorded but was not specifically biopsied or used for further evaluation of data. To facilitate specific sampling of index lesion on prostate biopsy (cognitive fusion/registration biopsy), a sector map was made that divided prostate into 24 segments [Figure 1]. First, the gland was divided into right and left lobes and base, mid, and apex regions. Each lobe was then further divided into medial and lateral parts by a sagittal plane running across the center of each lobe and into anterior and posterior parts by a coronal plane running across the center. Thus, there formed 24 segments, 12 in each lobe, for example, the right base mid-posterior sector would be located in the right lobe peripheral zone at the base of the prostate near the midsagittal plane. The radiologist was asked to map the suspicious lesion on the sector map along with its size and PIRADS score to facilitate comparison with Ga-PSMA PET report and adequate sampling on cognitive fusion/registration biopsy.
Figure 1.
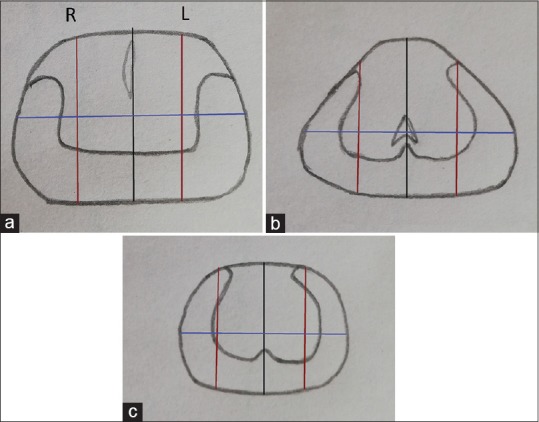
Sector maps. The prostate is first divided into right (r) and left (l) lobes by the midsagittal plane (black line) and then into three regions base (a), mid (b), and apex (c). Each lobe is then further divided into medial and lateral parts by a sagittal plane (red line) running across the center of each lobe and into anterior and posterior parts by a coronal plane (blue line) running across the center
Ga-PSMA PET scan was performed using 68Ga (HBED-CC) (68Ga-PSMA-11) on a Siemens system (Knoxville, Tennessee, USA). Each patient was administered 2–5 mCi of 68Ga-PSMA, and after 60 min, a noncontrast computed tomography (CT) was performed from vertex to toe, followed by an emission scan which was acquired 2 min per bed position for the same anatomic landmarks. Maximum intensity projection, plain PET, plain CT, and fused PET/CT were then evaluated by an experienced nuclear medicine physician who was blinded to the findings of other structural imaging if already performed. As per the Joint European Association of Nuclear Medicine and Society of Nuclear Medicine and Molecular Imaging procedure guidelines, any region of focal/abnormal PSMA ligand accumulation as compared to the background uptake was taken as suspicious of malignancy, and its size and location were marked on the sector map, same as that for mpMRI.[14] Semi-quantitative analysis was performed by drawing the region of interest around the area of focal tracer uptake calculating standard uptake values (maximum standardized uptake value [SUVmax]). However, a suspicious lesion was defined solely on the basis of qualitative assessment rather than a specific SUVmax cutoff value.
Once both the imaging investigations were available, the respective sector maps were evaluated by a single urologist adequately trained in cognitive as well as software-based fusion prostate biopsy. Because software registration fusion biopsy is not available for Ga-PSMA PET-based data, only cognitive fusion/registration biopsies along with 12-core systematic free hand TRUS-guided biopsies were performed for the patients included in the study. First, the two sector maps, one from mpMRI and the other from Ga-PSMA PET, were assessed for concordance of lesion, in terms of location. Two extra biopsy cores (besides the systematic 12 cores) were planned for each suspicious lesion detected on any of the imaging. If the lesion was discordant, that is, two lesions at two different sites on different imaging, the two extra biopsy cores were planned from each suspicious lesion. If one of the imaging did not detect a lesion whereas others did, two cores were planned from the suspicious lesion whereas the other imaging was considered negative. If the lesion was concordant, then only two extra biopsy cores were planned from that lesion. If no lesion was detected on either of the imaging, only systematic 12-core biopsy was planned. All TRUS-guided prostate biopsies were performed under periprostatic block using a BK Medical Pro Focus ultrasonography system (Herlev, Denmark) with a transrectal probe in the end-firing mode. First, the prostate was imaged, cognitive registration of suspicious lesions as marked on the sector maps was performed, and suspicious lesions were biopsied (2 cores). This was followed by a systematic 12-core prostate biopsy. All biopsy cores were submitted separately for histopathological analysis, and the presence or absence of malignancy along with the Gleason score of each core was separately assessed. Statistical analysis was performed using SPSS 20 (IBM SPSS Statistics for Windows, Version 20.0. IBM Corp., Armonk, NY, USA). Descriptive statistics were used to calculate mean and range for the demographic data. 2 × 2 crosstabs was made comparing the results of percentage free PSA, mpMRI, and Ga-PSMA PET scan with cognitive fusion/registration biopsy results (taken as the gold standard), and sensitivity, specificity, negative predictive value (NPV), positive predictive value, and diagnostic accuracy were calculated.
RESULTS
A total of 15 patients were included, the mean age of the study population was 66.2 years (57–73 years), all the patients presented with bothersome LUTS, and none of them were on indwelling catheter or had culture-positive urinary tract infection at the time of inclusion. The mean prostatic size on ultrasonography was 37.9 g (20–68 g), mean postvoid residual volume was 59 ml (20–78 ml), and none had upper tract changes. The mean total serum PSA was 9.9 ng/ml (5.1–19.5 ng/ml), mean free PSA was 1.3 ng/ml (0.4–2.6 ng/ml), and the mean percentage free PSA was 14.9% (6.1%–34%). Based on a cutoff of <10%, percentage free PSA was suggestive of malignancy in 3 (20%) patients.
All patients underwent mpMRI as described. The scan was unable to identify a lesion in 8 of the 15 patients and was suggestive of malignancy (PIRADS > II) in the rest 7. The most commonly reported PIRADS score was III, and only one patient had PIRADS IV and none had PIRADS V lesion. The mean maximum size of the lesion was 18 mm (12–23 mm), and all of them were located in the peripheral zone, except one lesion which was located in the central zone.
Similarly, all the patients underwent Ga-PSMA PET scan as described, and a focal lesion of increased tracer uptake could be visualized in 10 of the 15 patients (mean SUVmax: 13.8 [range, 4.5–23]). The mean maximum size of the lesion was 19 mm (10–23 mm), and all were located in the posterior part of the prostate except one which was in the central part. In rest of the five patients, the scan was not suggestive of malignancy.
Thus, combining the results of the two imaging techniques, a lesion could be localized in 11 of the 15 patients. In five of these patients, the lesion was seen at the same location both on MRI and Ga-PSMA PET scan (concordant index lesion). In one patient, a lesion was seen only on the MRI scan and the Ga-PSMA PET scan did not show focal tracer uptake, and in four patients, a lesion could be made out only on Ga-PSMA PET scan with the other modality being noncontributory [Figures 2 and 3]. There was one case where two separate lesions could be localized at different places on the two imaging in a single patient (discordant index lesion). Neither of the studies reported seminal vesicle, lymph node involvement, or distant metastasis in any of the patients.
Figure 2.
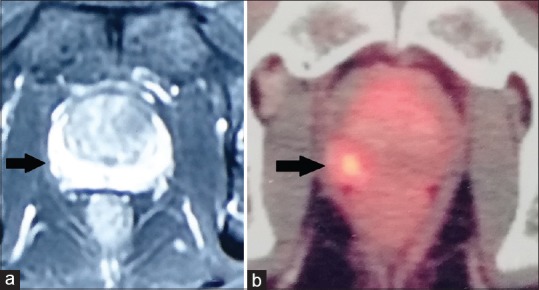
(a) Contrast-enhanced magnetic resonance imaging image showing symmetric enhancement of peripheral zone on both sides. (b) Gallium-68 prostate-specific membrane antigen positron emission tomography scan of the same patient at the same level showing focal tracer accumulation in the right peripheral zone
Figure 3.
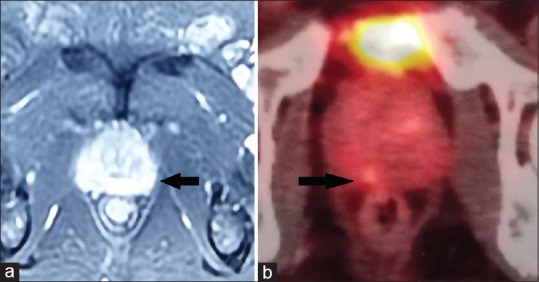
(a) Contrast-enhanced magnetic resonance imaging image showing a suspicious lesion in the left peripheral zone (Prostate Imaging Reporting and Data System III index lesion) and right peripheral zone (Prostate Imaging Reporting and Data System II). (b) Gallium-68 prostate-specific membrane antigen positron emission tomography scan of the same patient at the same level showing focal tracer accumulation in the right peripheral zone (index lesion), with diffuse uptake on both sides
All patients underwent standard 12-core TRUS-guided prostate biopsy which was diagnostic of malignancy in 9 (60%) of the 15 patients. Six of these nine had a Gleason score of 3 + 3, two had 3 + 4, and one had 4 + 4. In addition, as a lesion could be visualized in 11 of the 15 patients on either of the imaging studies (in 1 patient only on MRI, in 4 patients only on Ga-PSMA PET scan, and in 6 patients on both), these 11 patients underwent cognitive fusion/registration biopsy also (one additional site in 10 patients and 2 additional sites in 1 patient – 12 sites total), of which 9 out of 12 sites (75%) had malignancy in the cognitive fusion/registration cores report and 9 out of 11 patients had malignancy on final (12 systematic + cognitive fusion/registration) histopathology report. One patient had an identifiable lesion both on MRI and Ga-PSMA PET scan in the central zone and underwent cognitive fusion/registration biopsy which was benign. Another patient had an identifiable lesion on the MRI in the peripheral zone but not on Ga-PSMA PET scan, the cognitive fusion/registration cores were negative, whereas standard 12-core biopsy revealed malignancy at a different site (adenocarcinoma Gleason score 3 + 3 in 3 cores in the same lobe away from the site of index lesion). One patient had two discordant index lesions, one lesion in each lobe, both of which were malignant on cognitive/registration biopsy (Gleason score 3 + 3 in 7/14 cores – multifocal malignancy). Four patients did not have an identifiable lesion either on the MRI or on Ga-PSMA PET scan, and all had no evidence of malignancy on systematic 12-core TRUS biopsy.
The sensitivity, specificity, NPV, positive predictive value, and diagnostic accuracy of percentage free PSA, mpMRI, and Ga-PSMA PET scan as compared to the cognitive registration/fusion biopsy report are shown in Table 1. On comparing MRI with cognitive fusion/registration histopathology report, the scan was false positive in two and false negative in three patients. In one particular patient, where MRI showed a lesion, but Ga-PSMA PET scan did not, the cognitive fusion/registration biopsy cores were benign, whereas the systematic cores showed malignancy in a different area in the same lobe; this particular case was considered as false positive for MRI. Similarly, on comparing Ga-PSMA PET scan results to cognitive fusion/registration biopsy histopathology results, the scan was false positive in two and false negative in one patient, respectively.
Table 1.
Comparing the diagnostic accuracy of percentage Fee PSA, MRI and Ga-PSMA PET scan
| Investigation | Sensitivity (%) | Specificity (%) | Negative predictive value (%) | Positive predictive value (%) | Diagnostic accuracy (%) |
|---|---|---|---|---|---|
| Percentage free PSA | 33.3 | 100 | 100 | 50 | 60 |
| Magnetic resonance imaging | 62.5 | 71.4 | 71.4 | 62.5 | 66.6 |
| Ga-PSMA PET | 88.8 | 66.6 | 80 | 80 | 80 |
DISCUSSION
We analyzed the accuracy of Ga-PSMA PET scan in detecting cancer prostate in patients with serum PSA between 4 and 20 ng/ml in prebiopsy settings. Ga-PSMA PET scan was able to detect eight out of nine patients with malignancy and had two false positives and one false negative result each, and was superior to mpMRI and percentage free PSA in predicting presence of malignancy. If the results of Ga-PSMA PET were considered decisive for the need of biopsy, 4 (26.6%) unnecessary biopsies could have been avoided, at the cost of missing one patient with cancer prostate. Furthermore, we were able to successfully use Ga-PSMA PET imaging for sector mapping and subsequent cognitive registration/fusion biopsy similar to mpMRI. Of note, the majority of patients (66.6%) in our study had clinically insignificant cancers (Gleason grade group 1). Only three patients had clinically significant cancers (Gleason grade group 2 and above), all of whom could be accurately imaged on Ga-PSMA PET scan as compared to mpMRI, which was able to detect malignancy in only one patient (33.3%). To further distinguish between clinically significant and insignificant cancers on Ga-PSMA PET scan, we compared the mean SUVmax values between patients with significant and insignificant cancers as suggested by Demirci et al. and found the SUVmax to be significantly higher in patients with clinically significant cancers (21.6 vs. 12.4, P = 0.04).[15]
mpMRI has been shown to detect clinically significant cancers and miss insignificant ones, and thus, its use as a screening modality to select patients for biopsy is under evaluation.[5,6,16] However, there is conflicting evidence on the NPV of mpMRI in patients with cancer prostate, with some studies reporting it to be as low as 54%, whereas a recent meta-analysis found the NPV of mpMRI for overall cancer as 82.4%.[17,18] A recent retrospective analysis evaluated patients with normal mpMRI and found that at a follow-up of 38 months, 12.8% of the biopsy-naive patients with normal mpMRI were detected to have cancer, of which 42.3% were clinically significant.[19] The NPV of mpMRI in our study (62.5%) was lower than the recently published meta-analysis and was closer to that reported by Filson et al.[17] We did not perform DWI sequence on our patients, and this could in part explain the poor NPV of mpMRI in our study.
On the other hand, the exact place of Ga-PSMA PET scan in the management of cancer prostate is still under evaluation. PSMA expression in the primary cancer, as seen by immunohistochemical staining, has been shown to correlate with SUVmax of Ga-PSMA PET scan, thus enabling the detection of prostate cancer with high sensitivity.[20] Several authors have compared the accuracy of Ga-PSMA PET scan to mpMRI to detect and locate tumor foci within the prostate and found Ga-PSMA PET to have better accuracy and positive predictive value.[21,22] We also found Ga-PSMA PET scan to be more accurate than mpMRI. None of our patients had a capsular invasion or seminal vesicle invasion on any of the imaging.
MRI-detected lesions in the prostate can be sampled by any of the three ways: cognitive fusion/registration biopsy, software-based MRI-transrectal ultrasonography fusion (MRI-TRUS fusion) biopsy, or in-bore biopsy.[5,23] In a recent analysis, Monda et al. were unable to find a significant difference between cognitive fusion/registration and MRI-TRUS fusion biopsy in the detection of clinically significant cancer.[24] To sample lesions detected on Ga-PSMA PET scan, we performed cognitive fusion/registration biopsies using a sector mapping technique and used the same technique instead of the software fusion biopsy for the mpMRI-detected lesions to avoid sampling bias.
Our study has certain limitations. First, the sample size was small, mainly as patients withdrew consent for biopsy if the imaging turned out negative and also because of financial constraints. Second, majority of the patients included in our study had clinically insignificant cancers, and thus, the ability of Ga-PSMA PET scan in detecting clinically significant cancers as compared to mpMRI cannot be reliably commented upon. Third, we did not rebiopsy patients who had an identifiable lesion on imaging, but the biopsy was negative. Despite these limitations, our data suggest that Ga-PSMA PET scan has higher diagnostic accuracy in detecting localized cancer prostate and predicting the subsequent need of biopsy; however, it should be judiciously utilized in view of higher detection of insignificant lesions.
CONCLUSION
Ga-PSMA PET scan has a higher negative predictive value and accuracy than mpMRI in detecting tumor foci within the prostate. Thus, Ga-PSMA PET scan can most accurately identify patients suspected of harboring prostate cancer and can be used to direct specific biopsy cores during systematic biopsy to improve the yield.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–9. [PubMed] [Google Scholar]
- 2.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: When should we stop? J Urol. 2001;166:1679–83. [PubMed] [Google Scholar]
- 3.Gretzer MB, Partin AW. PSA levels and the probability of prostate cancer on biopsy. Eur Urol Suppl. 2002;1:21–7. [Google Scholar]
- 4.Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: A diagnostic meta-analysis. Eur Urol. 2015;67:1112–21. doi: 10.1016/j.eururo.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: A systematic review and meta-analysis. Eur Urol. 2015;68:438–50. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Gordetsky JB, Ullman D, Schultz L, Porter KK, Del Carmen Rodriguez Pena M, Calderone CE, et al. Histologic findings associated with false-positive multiparametric magnetic resonance imaging performed for prostate cancer detection. Hum Pathol. 2019;83:159–65. doi: 10.1016/j.humpath.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quon JS, Moosavi B, Khanna M, Flood TA, Lim CS, Schieda N. False positive and false negative diagnoses of prostate cancer at multi-parametric prostate MRI in active surveillance. Insights Imaging. 2015;6:449–63. doi: 10.1007/s13244-015-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68Gallium-PSMAPET compared to conventional imaging in lymph node staging of 130 consecutive patients with intermediate to high-risk prostate cancer. J Urol. 2015;195:1436–43. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty PS, Kumar R, Tripathi M, Das CJ, Bal C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin Nucl Med. 2015;40:328–9. doi: 10.1097/RLU.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 12.Tulsyan S, Das CJ, Tripathi M, Seth A, Kumar R, Bal C. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate cancer68Ga-PSMA PET and MRI in prostate cancer. Nucl Med Commun. 2017;38:1094–102. doi: 10.1097/MNM.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 13.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 14.Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 15.Demirci E, Kabasakal L, Şahin OE, Akgün E, Gültekin MH, Doǧanca T, et al. Can SUVmax values of ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40:86–91. doi: 10.1097/MNM.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–6. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Filson C, Margolis D, Huang J, Natarajan S, Lieu P, Dorey F, et al. MP60-11 should a normal multiparametric MRI preclude prostate biopsy? J Urol. 2017;193:e742. [Google Scholar]
- 18.Moldovan PC, Van den Broeck T, Sylvester R, Marconi L, Bellmunt J, van den Bergh RCN, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72:250–66. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Panebianco V, Barchetti G, Simone G, Del Monte M, Ciardi A, Grompone MD, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: What's next? Eur Urol. 2018;74:48–54. doi: 10.1016/j.eururo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical validation of PSMA expression measured by 68Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2018;59:238–43. doi: 10.2967/jnumed.117.195172. [DOI] [PubMed] [Google Scholar]
- 21.Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F, et al. 68Ga-PSMA PET/CT vs. MpMRI for locoregional prostate cancer staging: Correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21:204–11. doi: 10.1038/s41391-018-0048-7. [DOI] [PubMed] [Google Scholar]
- 22.Rhee H, Thomas P, Shepherd B, Gustafson S, Vela I, Russell PJ, et al. Prostate specific membrane antigen positron emission tomography may improve the diagnostic accuracy of multiparametric magnetic resonance imaging in localized prostate cancer. J Urol. 2016;196:1261–7. doi: 10.1016/j.juro.2016.02.3000. [DOI] [PubMed] [Google Scholar]
- 23.Wegelin O, van Melick HH, Hooft L, Bosch JL, Reitsma HB, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: A Systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71:517–31. doi: 10.1016/j.eururo.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Monda SM, Vetter JM, Andriole GL, Fowler KJ, Shetty AS, Weese JR, et al. Cognitive versus software fusion for MRI-targeted biopsy: Experience before and after implementation of fusion. Urology. 2018;119:115–20. doi: 10.1016/j.urology.2018.06.011. [DOI] [PubMed] [Google Scholar]


