Abstract
Objectives
Eighty-five percent of the 33 million children with epilepsy (CWE) worldwide live in low- and middle-income countries (LMICs). There is limited research into epilepsy-related comorbidities in LMICs, and there are no studies of the long-term progression of behavioral and intellectual difficulties in childhood epilepsy in sub-Saharan Africa. We aimed to assess behavior and cognition at three-year follow-up in CWE in rural Tanzania.
Methods
In 2010, a cross-sectional study identified 112 CWE 6 to 14 years of age and 113 age- and sex-matched controls in the Hai district of northern Tanzania. From March to June 2013, cases and controls (now 10 to 18 years of age) were followed up. At baseline, behavior was assessed using the Rutter A Questionnaire and cognition using the Goodenough–Harris Drawing Test. Details of current seizure frequency and antiepileptic drug (AED) use among CWE were collected.
Results
At follow-up, cases had significantly more behavioral difficulties compared with controls (48% of 108 cases versus 14% of 103 controls (p < 0.001)). Additionally, 69% of the cases and 16% of the controls had cognitive impairment (p < 0.001). In CWE with decreased seizure frequency from baseline to follow-up, behavior had improved significantly. At follow-up, there was no significant difference in behavior between CWE with decreased seizure frequency and those with good seizure control throughout.
Significance
Behavioral difficulties and cognitive impairment are common among CWE in this population. Improved access to AED treatment and subsequent improved seizure control may reduce the frequency of behavioral difficulties seen in this population.
Keywords: Epilepsy, Cognition, Behavior, Pediatric, Sub-Saharan Africa, Tanzania
1. Introduction
There are an estimated 33 million children with epilepsy (CWE) worldwide of whom 85% live in low- and middle-income countries (LMICs), where the global prevalence of epilepsy is 2 to 3 times greater than in high-income countries (HICs) [1,2]. The onset of epilepsy occurs in childhood more commonly in sub-Saharan Africa (SSA) than in HICs [3,4], with 80% of cases with epilepsy having their first seizure between birth and 18 years of age [1].
Neuropsychiatric problems, including disordered behavior and cognitive impairment, are common in CWE and can have a major impact on future life prospects [5,6]. In HICs, the proportion of CWE with cognitive impairment ranges from 31 to 76%, depending on the assessment tool used and characteristics of the population studied [7,8], and there is a higher prevalence of behavioral problems among CWE compared with children with chronic non-neurological disorders such as asthma and diabetes [9]. The etiology of behavioral disorders in epilepsy is not fully understood but is likely due to a combination of biological, psychological, and social factors including type and severity of epilepsy, personal cognitive performance [10], and family support and dynamics [10–12].
We conducted a three-year follow-up case–control study of a cohort of CWE in rural Tanzania [13,14]. There were three aims, namely, to determine the prevalence of behavioral difficulties and cognitive impairment at three-year follow-up; to examine associations between behavioral difficulties and cognitive impairment with other variables in this population, including access to antiepileptic drugs (AEDs) and seizure frequency; and to compare data from 2010 and 2013 to establish any changes in patterns of behavioral difficulties or cognitive impairment over this period. We hypothesized that behavioral difficulties and cognitive impairment would be significantly less common at follow-up than at baseline in children who took AEDs regularly. There is a paucity of research into comorbidities in CWE, and, to our knowledge, this is the first follow-up study from SSA of the long-term impact of epilepsy and epilepsy treatment on behavior and cognition.
2. Methods
The data presented here were collected as part of a three-year follow-up of children with active epilepsy (< 15 years of age) living in the Hai district of Tanzania. Baseline data were collected between January and June 2010 and follow-up data between March and June 2013.
2.1. Setting and study population
Tanzania is one of the world's poorest countries with a gross national income per capita of $630 in 2013 [15]. The rural Hai district is located in the north of Tanzania on the southern side of Mount Kilimanjaro. People live within large family units, and most work as subsistence farmers, although some cash crops are grown. Part of the district was established as a demographic surveillance site (DSS) in the early 1990s, with regular population censuses being conducted [16], most recently in 2009 when the recorded population was 161,119 [17]. Coverage of primary healthcare services is relatively limited within the DSS. The area is served by two district hospitals (Machame and Boman'gombe), with a large tertiary referral hospital located just outside the DSS in the town of Moshi.
2.2. Baseline cohort
Children 6 to 14 years of age (inclusive) with active epilepsy were identified during the 2009 Hai district DSS census using a validated nine-item questionnaire to detect epilepsy [18]. The study pediatrician (KB) assessed each possible case. Active epilepsy was defined according to the International League Against Epilepsy (ILAE) criteria in 1993 as at least two afebrile seizures within the last 5 years, more than 24 h apart and not related to acute infection, metabolic disturbances, neurological disorders, or drugs [19]. Children already taking AEDs were also included as cases with active epilepsy. Exclusion criteria were age of less than 6 years (to exclude febrile seizures) and parental refusal to participate. A control group of age-, sex-, and village-matched children was randomly selected from the census data. Inclusion criteria were residence in Hai during census, age of 6 to 14 years, and parental consent obtained. Exclusion criteria were missing identification data and parental refusal to participate.
A total of 112 CWE and 113 controls were identified at baseline, giving an unadjusted prevalence of epilepsy of 2.9 per 1000 (95% confidence interval = 2.4 to 3.5) [13]. At baseline, 49 (47.5%) of 103 CWE assessed were not attending school regularly, while all 99 controls assessed did attend school regularly [14].
2.3. Follow-up
Cases and controls were followed up by a medical student (KP) in 2013 assisted by four local health professionals employed as supervisors within the local enumeration network.
2.4. Assessments and data collection
At baseline and follow-up, patients were assessed either at home or in a local village hall or their school, as convenient for the patient. All assessment forms were translated into Kiswahili and back-translated for accuracy. Data on current AED use, seizure frequency, behavior, and cognition in cases and on behavior and cognition in controls were collected. Questionnaires were piloted for two weeks and discussed in-depth by the study team before commencement to ensure cultural sensitivity and to reduce interassessor bias.
Behavior was assessed using the Rutter A Questionnaire (RAQ). The original Rutter questionnaire was developed in the UK as a screening tool for behavioral problems in children 7–13 years of age [20], and the RAQ is an adapted version designed for use by parents, rather than teachers, since not all children in the study attended school. The RAQ consists of 29 questions about behavior in the last 12 months, scored 0 (does not apply), 1 (somewhat applicable), or 2 (certainly applies). The RAQ has been shown to have high discriminatory power for distinguishing typical patterns (score < 13) from atypical patterns (score ≥ 13) of behavior [20]. It has been used in LMICs, including in Nigeria [21].
Cognition was assessed using the Goodenough–Harris Drawing Test (GHDT) [22,23]. Children were given specific, identical instructions to draw a picture of themselves, a man and a woman. Drawings of a man and a woman were then marked against an age-standardized index based on inclusion of bodily details. Results have been shown to strongly correlate with general intelligence tests [22]. There are few cognitive screening tools suitable for use in pediatric populations in SSA, and the GHDT was considered the most appropriate. Those with scores of < 70 (equivalent to IQ < 70 [22]) were classified as having cognitive impairment. The GHDT has a high reliability and validity in relation to other available tools and has been used in children up to 18 years of age [23]. All follow-up drawings were coded and marked by one person (KP) who was blinded to the child's identity at the time of assessing the drawings.
Baseline and follow-up GHDTs were scored by different people (KB at baseline and KP at follow-up). To minimize any interrater bias between baseline and follow-up, the two raters discussed the scoring system used prior to scoring the follow-up drawings, and a consensus was reached on the scoring criteria. To assess interrater reliability, both raters marked blindly 16 randomly selected drawings from follow-up.
2.5. Statistical analysis
Statistical analyses were performed using SPSS Statistics version 19 (2010: IBM Corporation, Armonk, NY, USA). When data were missing, it was assumed to be at random and not informative, and no attempt was made to impute missing values.
Age data were normally distributed and so were compared using the Student t-test. All other data were categorized for analysis and compared using the chi-squared test. A child was classified as having seizures if they had had any seizure in the past three months and seizure-free if they had not had any seizures in that period. Rutter A Questionnaire and Goodenough–Harris Drawing Test scores were dichotomized using the cutoffs described above. Interrater reliability for the GHDT follow-up assessments was assessed by calculation of the intraclass correlation coefficient for the raw scores and the kappa statistic for the overall agreement on diagnosis of cognitive impairment.
Univariate logistic regression and multivariable logistic regression were used to identify associations between the variables and the models summarized in terms of odds ratios (ORs) and 95% confidence intervals (CIs). Model validity was checked by assessment of residuals and tolerance. Two-tailed tests and a significance level of 5% were used throughout.
2.6. Ethics
Ethical approval for the study was granted by the National Institute for Medical Research in Tanzania and locally from Kilimanjaro Christian Medical College University, Moshi, Tanzania. Parents and guardians of all the selected children were given written and verbal information in Kiswahili before they gave their consent to participate.
3. Results
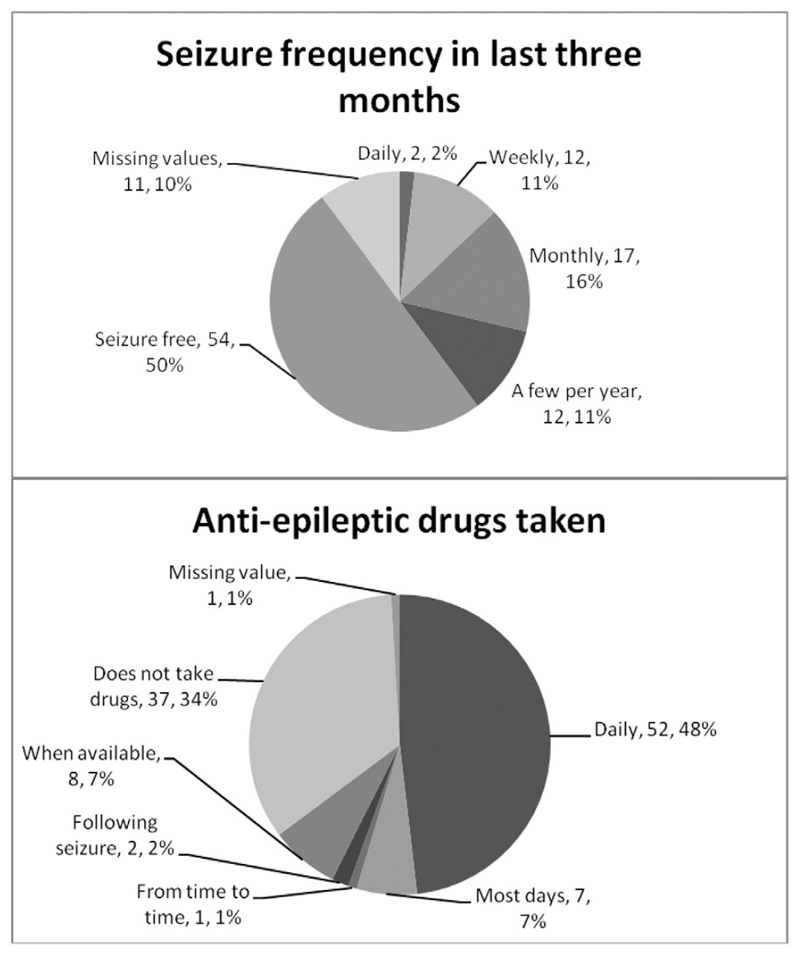
Four cases and ten controls could not be followed up. Of these, three cases and one control had died, and the remaining case and nine controls had either moved away from the district or were at boarding school in a different area of the country. Thus, 108 (96.4%) cases and 103 (91.2%) controls were seen at follow-up in 2013. Demographic and clinical data collected from children seen in the study are summarized in Table 1. Cases and controls were well matched for age and sex. In 107 cases who had data available, 70 (65.4%) were taking AEDs at follow-up. Fig. 1 summarizes data for frequency of seizures and frequency of AED use at follow-up.
Table 1. Demographic, cognitive, and behavioral characteristics of CWE and controls.
| Cases (n = 108) |
Controls (n = 103) |
Significance of difference | |
|---|---|---|---|
| Mean age in years (SD) | 15.1 (2.6) | 14.9 (2.6) | t = 0.504, p = 0.615 |
| Number of females | 55 (50.9%) | 46 (44.7%) | χ2 = 0.829, p = 0.362 |
| Disordered behavior (RAQ ≥ 13) | 50/105 (47.6%) | 14/101 (13.9%) | χ2 = 27.394, p < 0.001 |
| 3 missing values | 2 missing values | ||
| Cognitive impairment (GHDT < 70) | 70/101 (69.3%) | 16/99 (16.2%) | χ2 = 57.612, p < 0.001 |
| 7 missing values | 4 missing values |
SD: standard deviation.
IQR: interquartile range.
RAQ: Rutter A Questionnaire.
GHDT: Goodenough–Harris Drawing Test.
Fig. 1. Antiepileptic drug use and seizure frequency at follow-up.
3.1. Interrater reliability of the GHDT
Sixteen randomly selected subjects (12 cases and 4 controls) were assessed by each rater. The intraclass correlation coefficient for the raw scores was 0.974 (95% CI = 0.927 to 0.991). The raters agreed on the diagnosis of cognitive impairment for 14 of the 16 subjects, giving a kappa value of 0.738. Both measures suggest a high degree of agreement between the two raters.
3.2. Behavioral disorders and cognitive impairment at follow-up
Seven CWE were unable to complete the GHDT because of severe motor difficulties. Four control interviews were conducted with parents only, meaning that the GHDT could not be completed. Results of the RAQ and GHDT are summarized in Table 1. There were significant differences in median scores and the frequencies of disordered behavior and cognitive impairment between cases and controls.
3.3. Predictors of disordered behavior and cognitive impairment in CWE at follow-up
On multivariable analysis, cognitive impairment (GHDT < 70) was associated with taking AEDs (OR = 6.02, 95% CI = 2.39 to 15.17) but not with having seizures or disordered behavior. Disordered behavior (RAQ score ≥ 13) was associated with having seizures (OR = 5.37, 95% CI = 2.24 to 12.86) but not with AED use or cognitive impairment.
3.4. Comparison of baseline and follow-up data
Baseline behavioral and cognitive data were compared with those obtained at three-year follow-up (Table 2). Only children for whom both baseline and follow-up data were available were included. There was good evidence of an increase in the number of children with cognitive impairment among controls (5.7% to 16.1%, p = 0.029) and of a decrease in the numbers with disordered behavior among cases (65.6% to 49.5%, p = 0.012).
Table 2. Comparison of cognitive impairment, disordered behavior, and presence of seizures between 2010 and 2013.
| Baseline (2010) | Follow-up (2013) | Significance of difference | |
|---|---|---|---|
| Cases | |||
| Disordered behavior (n = 93) | 61 (65.6%) | 44 (49.5%) | χ2 = 6.320, p = 0.012 |
| Cognitive impairment (n = 88) | 60 (68.2%) | 60 (68.2%) | χ2 = 0.000, p = 1.000 |
| Presence of seizures in the last three months (n = 88) | 54 (61.4%) | 40 (41.5%) | χ2 = 4.475, p = 0.034 |
| Controls | |||
| Disordered behavior (n = 88) | 15 (17.0%) | 12 (13.6%) | χ2 = 0.393, p = 0.530 |
| Cognitive impairment (n = 87) | 5 (5.7%) | 14 (16.1%) | χ2 = 4.786, p = 0.029 |
Nine (10.2%) CWE reported seizures in 2013 who did not have seizures in 2010. Of 54 children with seizures in 2010, however, 23 (45.6%) no longer had seizures in 2013; the remaining 31 had seizures at both time points. Overall, there was good evidence of a decrease in the number of cases having seizures (Table 2). In the 54 CWE with seizures in 2010, there was very strong evidence that disordered behavior was less common in those with improved seizure control in 2013 than in those who still had seizures (6/23 (26.1%) versus 22/31 (71.0%); χ2 = 10.653, p = 0.001).
Finally, there was no evidence of any difference in the prevalence of disordered behavior in CWE with improved seizure control at follow-up compared with those with good seizure control at both baseline and follow-up (6/23 (26.1%) versus 8/25 (32.0%); χ2 = 0.203, p = 0.653). This suggests that improved seizure control in children who previously had seizures may be associated with improved behavior to the level of those with good seizure control.
4. Discussion
4.1. Prevalence of behavioral disorders and cognitive impairment
To date, there have been only two population-based case–control studies of behavioral disorders in CWE in SSA. In Tanzania, Burton et al. [13] found strong evidence of a greater frequency of behavioral problems in CWE compared with age- and sex-matched controls (66% and 19%, respectively, univariable OR = 8.2, 95% CI = 4.3 to 15.6). Using different assessment tools, Kariuki et al. [24] reported a lower prevalence of behavioral disorders in cases (48%) than the Burton et al, but a higher prevalence in controls (26%). Both studies found poorer school attendance in cases than in controls. This was also noted in an observational study in rural Zambia comparing school attendance by CWE with that by age-matched siblings [25]. Poor school attendance and behavioral problems are both associated with reduced quality of life and are likely to result in increased social isolation and reduced life opportunities [26].
This is the first follow-up study of behavior and cognition in CWE in SSA [27]. In this rural Tanzanian population, the prevalence of behavioral difficulties was 47.6% in CWE, which was significantly higher than the 13.9% found in the general population. Based on the same cohort, in 2010, Burton et al. [13] recorded a higher prevalence of 66% in CWE and 19% in the general population, suggesting that there may have been a change in factors that influence behavior (e.g., treatment reducing the seizure frequency) or that behavior, as measured by the tools used in this study, improves with age.
In a recent Nigerian study of 84 CWE in an urban setting, 46.6% were found to have behavioral disorders [28]. In high-income countries, prevalence estimates of behavioral disorders in CWE range from 20 to 60% [29,30]. Our data are towards the upper end of this range, implying that disordered behavior is at least as frequent in CWE in rural Africa as it is in other settings. A large-scale UK study which used the RAQ found a prevalence of behavioral disorders of 48% in children with active epilepsy versus 13% in age-matched controls without epilepsy, remarkably similar to our values [31]. This is surprising, given the high proportion of untreated epilepsy in our cohort.
In societies where education is not universally available, disordered behavior may lead to early withdrawal from school and is likely to limit job opportunities and the life choices open to these children in later years, and disordered behavior has been linked to a decreased quality of life [26]. The high prevalence of behavioral disorders in this population, as well as their potentially detrimental impact on future life choices, highlights the need for increased recognition and improved management of behavioral problems in CWE in SSA.
In this study, 69.3% of the cases had cognitive impairment compared with 16.2% of the controls. The prevalence of cognitive impairment in controls increased significantly between 2010 and 2013 but did not change in CWE. This may be partly due to interrater differences in scoring, although good levels of agreement were found when a random sample of drawings was scored by both the baseline and follow-up assessors, suggesting that this bias was minimal. It may also be that, in an area such as Hai, where coverage of primary health-care services is relatively limited, many people may be living with undiagnosed conditions (such as depression, anxiety, and visual and hearing problems) which may reduce performance on a short cognitive screen. The influence of such comorbidities in CWE may not be apparent because of the high prevalence of apparent cognitive impairment at baseline. Finally, GHDT scores are thought to decrease slightly with age [32], meaning that, at follow-up, some controls who were above the threshold for cognitive impairment may fall below it without having significant changes in cognitive function. The effect of this decrease would be much less obvious in CWE given the much smaller number at risk of developing cognitive impairment at follow-up. The reason for the increase in prevalence of cognitive impairment in controls warrants further investigation.
In high-income countries, estimates of the prevalence of cognitive impairment in CWE are between 31 and 76% [7,8]. Again, the findings of this study are towards the higher end of this range. There are very limited data available on cognitive function in CWE from elsewhere in SSA. In Kenya, a case–control study identified cognitive impairment in 31% of CWE versus 6% of age- and sex-matched controls [24]. The lower prevalence reported could be due to differences in definitions and methodology. The Kenyan study defined cognitive impairment as scoring greater than 2 standard deviations below the group's mean but does not contextualize these findings in terms of a standardized score system such as IQ.
We observed a higher frequency of AED use at follow-up compared with baseline, when there was a treatment gap of at least 69% [27]. At follow-up, 48% of CWE were taking AEDs daily and a further 7% most days (see Fig. 1). Community education had been given to CWE and their carers, and health professionals in the region had received training for diagnosis and management of epilepsy at the time of the baseline study, and this had clearly been effective.
4.2. Variables associated with disordered behavior
On multivariable analysis of baseline and follow-up studies, a significant association was found between ongoing seizures and behavioral disorders [13]. Although cognitive impairment was associated with behavioral disorders in univariate analysis, it was not an independent predictor. Poor seizure control is an indicator of more severe, symptomatic epilepsy, which has been shown to be a risk factor for behavioral disorders [33,34]. More frequent seizures may indicate an underlying structural lesion, poor treatment compliance, or an inappropriate AED regime. Although studies have found behavioral disorders to predate onset of epilepsy [30], there is evidence that repetitive seizures can have a detrimental effect on child development, potentially leading to behavioral problems [10].
4.3. Variables associated with cognitive impairment
In our cohort, taking AEDs at follow-up was a strong independent predictor of greater cognitive impairment. Almost 90% of children taking AEDs were prescribed phenobarbital (PB). Early studies of the use of PB as a treatment for febrile seizures suggested that PB use may cause cognitive impairment [35]. However, a more recent systematic review concluded that there are no significant risks associated with PB use [36]. In our cohort, it is possible that the use of AEDs was a marker for more severe epilepsy at baseline (prior to initiation of AED use in most cases). Despite improved seizure control and improved behavior at follow-up, CWE with high levels of cognitive impairment at baseline may still have cognitive impairment at follow-up. It is also possible that long-term PB use had adversely affected cognitive development. Further studies of long-term cognitive outcomes in CWE in SSA are required.
4.4. Follow-up
Improved seizure control from baseline to follow-up was associated with improved behavior. There were significantly fewer behavioral disorders in the CWE who showed improved seizure control compared with CWE who had continued poor seizure control. Furthermore, there was no significant difference in behavior scores between those whose seizure control improved and those with well-controlled epilepsy throughout. This suggests that in this setting, behavioral problems may be improved with better seizure control to the level expected in those with well-controlled epilepsy. Use of PB as first-line AED treatment has also been postulated as a potential risk factor for behavioral disorders [35,37].
Longitudinal studies in high-income countries have reported a relationship between persistent or poorly controlled seizures and behavioral problems [10,38], although the relationship between the two is thought to have a multifactorial etiology [39]. Better control of epilepsy may improve behavior by limiting the psychiatric deterioration associated with seizures [40,41]. Alternatively, it may be that, in some types of epilepsy, a behavioral disorder is a manifestation of underlying brain dysfunction and not reversible [10]. A recent study of behavior in children who were treated for new-onset epilepsy found that behavioral problems may reduce over time [42]. These findings are similar to our own and suggests that seizure treatment and control may improve behavior. Further longer-term studies in different populations of CWE will be necessary to clarify the prognosis and implications of behavioral difficulties in CWE.
4.5. Study strengths
Cases were identified in a large community-based prevalence study which aimed to identify all CWE in the denominator population, there-by eliminating potential bias associated with a sampling frame. Controls were age-, sex-, and village-matched from the same population. In this project, behavior and cognition in CWE and controls were assessed using identical methods, and assessors were blinded wherever possible. All information and questions were independently translated into Kiswahili and back-translated into English to check for consistency.
4.6. Study limitations
We studied a population in a rural district in eastern SSA. There may be unique population characteristics that mean results cannot be generalized to other populations. Although the original study rigorously attempted to identify all cases with epilepsy in the Hai district DSS, it is likely that some cases with epilepsy with nonconvulsive seizures were missed. Follow-up interviews were all conducted by one UK-based medical student, using four locally-trained health professionals as translators. There may have been some interpreter bias because of differences in translation, and, to reduce this as much as possible, questionnaires were discussed with all four translators prior to commencement of interviews. To further minimize bias, the same team conducted all assessments in as similar a manner as possible.
Data on current seizure status and epilepsy management were collected. Epilepsy is known to be a stigmatizing disease in this region, and some interviewees may have been unwilling to reveal the true frequency of their seizures or the full extent of use of traditional healers. Conversely, some parents of CWE may have been more willing to attribute behavioral problems to epilepsy that in a control subject would have been seen as a normal part of a child's behavior by their parent. In an attempt to minimize this bias, question phrasing was discussed with the locally-trained health professionals involved to ensure cultural sensitivity. The GHDT pictures were marked by different people at base-line and follow-up, and this may have resulted in some bias. This is likely to be small, however, as interrater reliability was assessed and found to be acceptable and the GHDT has been shown to have good interrater reliability in previous studies [9]. We recognize that motor and sensory problems in some subjects may have resulted in overreporting of cognitive impairment and that the GDHT does not comprehensively assess cognitive function across all domains.
We acknowledge that data on AED use were based on self-report and so are likely to be subject to some reporting bias.
5. Conclusions
Our study contributes towards the emerging body of data on epilepsy from this region and highlights the need for further research into comorbidities in CWE living in low-income countries. Children with epilepsy in this rural community of Tanzania have a high prevalence of cognitive impairment and behavioral disorders which persisted over time. For those with seizures, better seizure control over time may help improve behavior to the level seen in children with well-controlled epilepsy. Provision of appropriate treatment to achieve optimal epilepsy control will help reduce the detrimental effect of epilepsy on the lives of these children.
6. Key points.
At follow-up, cases had significantly more behavioral difficulties and cognitive impairment compared with controls.
In children with epilepsy (CWE) with decreased seizure frequency from baseline to follow-up, behavior had improved significantly.
At follow-up, there was no significant difference in behavior between CWE with decreased seizure frequency and CWE with good seizure control both at baseline and follow-up.
Improved access to drug treatment may reduce the frequency of behavioral difficulties seen in this population.
Acknowledgments
Northumbria Healthcare NHS Foundation Trust and Kilimanjaro Christian Medical Centre supported this study. We would like to thank Ali Mhina, John Massawe, Addis F. Moshi, John Kissima, and all the health-care workers, officials, carers, and family members who assisted in identification of patients, examination, and assessment.
Role of the funding source
This work received no external funding.
Footnotes
Contributions
Design/conception — Richard Walker, Kathryn Burton, and Ewan Hunter.
Literature search — Richard Walker, Kathryn Powell, and William K. Gray.
Data collection — Kathryn Powell, Jane Rogathi, Kathyrn Burton, and Ewan Hunter.
Data analysis — William K. Gray and Kathyrn Powell.
Results interpretation — all authors.
Paper writing and review — all authors.
Conflicts of interest
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
References
- [1].Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- [2].Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- [3].Duggan MB. Epilepsy in rural Ugandan children: seizure pattern, age of onset and associated findings. Afr Health Sci. 2010;10:218–25. [PMC free article] [PubMed] [Google Scholar]
- [4].Mung'ala-Odera V, White S, Meehan R, Otieno GO, Njuguna P, Mturi N, et al. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure. 2008;17:396–404. doi: 10.1016/j.seizure.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hamiwka LD, Wirrell EC. Comorbidities in pediatric epilepsy: beyond “just” treating the seizures. J Child Neurol. 2009;24:734–42. doi: 10.1177/0883073808329527. [DOI] [PubMed] [Google Scholar]
- [6].Chang HJ, Liao CC, Hu CJ, Shen WW, Chen TL. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PLoS One. 2013;8:e59999. doi: 10.1371/journal.pone.0059999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fastenau PS, Jianzhao S, Dunn DW, Austin JK. Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. J Learn Disabil. 2008;41:195–207. doi: 10.1177/0022219408317548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sogawa Y, Masur D, O'Dell C, Moshe SL, Shinnar S. Cognitive outcomes in children who present with a first unprovoked seizure. Epilepsia. 2010;51:2432–9. doi: 10.1111/j.1528-1167.2010.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hysing M, Elgen I, Gillberg C, Lundervold AJ. Emotional and behavioural problems in subgroups of children with chronic illness: results from a large-scale population study. Child Care Health Dev. 2009;35:527–33. doi: 10.1111/j.1365-2214.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- [10].Aldenkamp AP, Bodde N. Behaviour, cognition and epilepsy. Acta Neurol Scand Suppl. 2005;182:19–25. doi: 10.1111/j.1600-0404.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- [11].Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Dekovic M. Psychopathology in children with epilepsy: a meta-analysis. J Pediatr Psychol. 2005;30:453–68. doi: 10.1093/jpepsy/jsi071. [DOI] [PubMed] [Google Scholar]
- [12].Keene DL, Manion I, Whiting S, Belanger E, Brennan R, Jacob P, et al. A survey of behavior problems in children with epilepsy. Epilepsy Behav. 2005;6:581–6. doi: 10.1016/j.yebeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [13].Burton K, Rogathe J, Whittaker RG, Mankad K, Hunter E, Burton MJ, et al. Co-morbidity of epilepsy in Tanzanian children: a community-based case–control study. Seizure. 2012;21:169–74. doi: 10.1016/j.seizure.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burton K, Rogathe J, Hunter E, Burton M, Swai M, Todd J, et al. Behavioural comorbidity in Tanzanian children with epilepsy: a community-based case–control study. Dev Med Child Neurol. 2011;53:1135–42. doi: 10.1111/j.1469-8749.2011.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].The World Bank. Data: Tanzania. 2014. [Google Scholar]
- [16].Adult Morbidity and Mortality Project (AMMP) Policy Implications of Adult Morbidity and Mortality; final report. Dar-es-Salaam: Tanzanian Ministry of Health; 2004. [Google Scholar]
- [17].Hunter E, Rogathi J, Chigudu S, Jusabani A, Jackson M, McNally R, et al. Prevalence of active epilepsy in rural Tanzania: a large community-based survey in an adult population. Seizure. 2012;21:691–8. doi: 10.1016/j.seizure.2012.07.009. [DOI] [PubMed] [Google Scholar]
- [18].Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain. 1992;115:783–98. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- [19].Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34:592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [20].Rutter M. A children's behaviour questionnaire for completion by teachers: preliminary findings. J Child Psychol Psychiatry. 1967;8:1–11. doi: 10.1111/j.1469-7610.1967.tb02175.x. [DOI] [PubMed] [Google Scholar]
- [21].Abiodun OA, Tunde-Ayinmode MF, Adegunloye OA, Ayinmode BA, Sulyman D, Unaogu NN, et al. Psychiatric morbidity in paediatric primary care clinic in Ilorin, Nigeria. J Trop Pediatr. 2011;57:173–8. doi: 10.1093/tropej/fmq072. [DOI] [PubMed] [Google Scholar]
- [22].Harris DB. children's drawings as measures of intellectual maturity. New York, NY, USA: Harcourt, Brace and World, Inc; 1963. [Google Scholar]
- [23].Harris DB, Pinder GD. Goodenough–Harris test estimates of intellectual maturity of youths 12–17 years: demographic and socioeconomic factors. Vital Health Stat. 1977;11 doi: 10.1037/e465402004-001. i–iv [1–43] [DOI] [PubMed] [Google Scholar]
- [24].Kariuki SM, Abubakar A, Holding PA, Mung'ala-Odera V, Chengo E, Kihara M, et al. Behavioral problems in children with epilepsy in rural Kenya. Epilepsy Behav. 2012;23:41–6. doi: 10.1016/j.yebeh.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Birbeck GL, Kim S, Hays RD, Vickrey BG. Quality of life measures in epilepsy: how well can they detect change over time? Neurology. 2000;54:1822–7. doi: 10.1212/wnl.54.9.1822. [DOI] [PubMed] [Google Scholar]
- [26].Osungbade KO, Siyanbade SL. Myths, misconceptions, and misunderstandings about epilepsy in a Nigerian rural community: implications for community health interventions. Epilepsy Behav. 2011;21:425–9. doi: 10.1016/j.yebeh.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [27].Mushi D, Burton K, Mtuya C, Gona JK, Walker R, Newton CR. Perceptions, social life, treatment and education gap of Tanzanian children with epilepsy: a community-based study. Epilepsy Behav. 2012;23:224–9. doi: 10.1016/j.yebeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lagunju IA, Bella-Awusah TT, Takon I, Omigbodun OO. Mental health problems in Nigerian children with epilepsy: associations and risk factors. Epilepsy Behav. 2012;25:214–8. doi: 10.1016/j.yebeh.2012.08.006. [DOI] [PubMed] [Google Scholar]
- [29].Ott D, Caplan R, Guthrie D, Siddarth P, Komo S, Shields WD, et al. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–14. doi: 10.1097/00004583-200108000-00012. [DOI] [PubMed] [Google Scholar]
- [30].Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–22. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- [31].Hoare P. The development of psychiatric disorder among schoolchildren with epilepsy. Dev Med Child Neurol. 1984;26:3–13. doi: 10.1111/j.1469-8749.1984.tb04399.x. [DOI] [PubMed] [Google Scholar]
- [32].Lotz L, Loxton H, Naidoo AV. Visual–motor integration functioning in a South African middle childhood sample. J Child Adolesc Ment Health. 2005;17:63–7. doi: 10.2989/17280580509486602. [DOI] [PubMed] [Google Scholar]
- [33].Austin JK, Dunn DW, Caffrey HM, Perkins SM, Harezlak J, Rose DF. Recurrent seizures and behavior problems in children with first recognized seizures: a prospective study. Epilepsia. 2002;43:1564–73. doi: 10.1046/j.1528-1157.2002.26002.x. [DOI] [PubMed] [Google Scholar]
- [34].Sabaz M, Lawson JA, Cairns DR, Duchowny MS, Resnick TJ, Dean PM, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav. 2003;4:680–91. doi: 10.1016/j.yebeh.2003.08.012. [DOI] [PubMed] [Google Scholar]
- [35].de Silva M, MacArdle B, McGowan M, Hughes E, Stewart J, Neville BG, et al. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carba-mazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet. 1996;347:709–13. doi: 10.1016/s0140-6736(96)90074-4. [DOI] [PubMed] [Google Scholar]
- [36].Pal DK. Phenobarbital for childhood epilepsy: systematic review. Paediatr Perinat Drug Ther. 2006;7:31–42. doi: 10.1185/146300905X75361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vining EP, Mellitis ED, Dorsen MM, Cataldo MF, Quaskey SA, Spielberg SP, et al. Psychologic and behavioral effects of antiepileptic drugs in children: a double-blind comparison between phenobarbital and valproic acid. Pediatrics. 1987;80:165–74. [PubMed] [Google Scholar]
- [38].Mitchell WG, Scheier LM, Baker SA. Psychosocial, behavioral, and medical outcomes in children with epilepsy: a developmental risk factor model using longitudinal data. Pediatrics. 1994;94:471–7. [PubMed] [Google Scholar]
- [39].Schmitz EB, Robertson MM, Trimble MR. Depression and schizophrenia in epilepsy: social and biological risk factors. Epilepsy Res. 1999;35:59–68. doi: 10.1016/s0920-1211(98)00129-6. [DOI] [PubMed] [Google Scholar]
- [40].Amruth G, Praveen-Kumar S, Nataraju B, Kasturi P. Study of psychiatric comorbidities in epilepsy by using the Mini International Neuropsychiatric Interview. Epilepsy Behav. 2014;33:94–100. doi: 10.1016/j.yebeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [41].Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav. 2011;20:550–5. doi: 10.1016/j.yebeh.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao Q, Rathouz PJ, Jones JE, Jackson DC, Hsu DA, Stafstrom CE, et al. Longitudinal trajectories of behavior problems and social competence in children with new onset epilepsy. Dev Med Child Neurol. 2015;57:37–44. doi: 10.1111/dmcn.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]