Abstract
The targets of therapy in inflammatory bowel disease have transformed in the last few years. The standard definition of mucosal healing assessed using white light standard definition endoscopy is being challenged because even when endoscopy suggests mucosal healing, the presence of histological activity can often still be observed. Of note, microscopic signs of inflammation correlate with clinical outcomes such as risk of relapse, hospitalization and colorectal cancer. Therefore, histological healing has increasingly become an important target to achieve. Advanced endoscopic technologies have been developed and many are starting to be adopted in daily clinical practice. They can provide a more detailed view of the mucosal and vascular architecture almost at the histology level, including crypt, vessel architecture and cellular infiltration. So, these can provide a more accurate definition of mucosal and histological healing. In this review we focus on new advanced endoscopic techniques, and how these have the potential to reduce the gap between histological and mucosal healing.
Keywords: Ulcerative colitis, Crohn’s disease, mucosal healing, histological healing, advanced endoscopic techniques, electronic virtual chromoendoscopy
Definitions of endoscopic, transmural and histological healing
The management of inflammatory bowel disease (IBD), a chronic and disabling disease, is still challenging despite the fact that many new drugs have been developed, with more in the pipeline, to achieve tight control of inflammation and improve outcomes of the disease. A symptoms-based treatment is generally suboptimal, as there is wide dissociation between symptoms and inflammation. Hence, we have moved from a purely clinical assessment, to a multi-target approach involving endoscopic mucosal healing (MH), histological healing, cross-sectional imaging and transmural healing (TH), and surrogate markers of inflammation, providing the basis for the concept of ‘treat to target’.1–3
An emerging concept is ‘deep remission’, a profound remission without clinical symptoms and biological activity, with complete MH, usually assessed by colonoscopy in ulcerative colitis (UC) and by ileocolonoscopy in Crohn’s disease (CD), and disappearance of histological inflammatory changes (histological healing). To date there is not a clear and validated definition of deep remission in IBD.4–6 Current studies have also included, in this definition, non-invasive markers of inflammation such as C reactive protein (CRP), faecal calprotectin (FC) and radiological parameters that are being considered as new potential tools for objective assessment of all bowel layers, that is, TH in particular in CD.4,7 Importantly, the achievement of deep remission should alter biological processes of mucosal inflammation and prevent progressive and permanent structural damage typical of IBD patients. Achieving such ‘deep’ remission is an aspirational challenge, feasible in only a minority of IBD patients. Therefore, defining very strict endpoints of intestinal healing will have failed many of the current targeted therapies and challenge future drug development programs.4,8,9
Endoscopic mucosal healing in ulcerative colitis
In the context of IBD, the term MH generally refers to endoscopic assessment of disease activity, though endoscopic healing may be a more specific term.
The International Organization for the Study of Inflammatory Bowel Disease (IOIBD) defined MH in UC as the absence of friability, blood, erosions and ulcers in all visualized segments of the colonic mucosa.3 Therefore this corresponds to Mayo Endoscopic Score (MES) of 0 rather than MES 1, as MES 1 generally has worse disease course than MES 0. It is a topic of intense debate whether MH involves histological healing in UC. Increasing evidence has shown that endoscopic healing does not necessarily mean histological healing and the presence of subtle patchy inflammation, even in an endoscopically quiescent disease, could be associated with adverse outcomes.10 At present, histological remission is not considered a primary endpoint in clinical trials due to a lack of standardization and validation, but in UC it is increasing being used as a secondary outcome measure either independently or in combination with endoscopic healing. MES is commonly used as a major secondary endpoint in clinical trials and in clinical practice and is also integrated into the Mayo Clinical Disease Activity Score, while UC Endoscopic Index of Severity (UCEIS) is used less commonly, as its advantage over MES is not clear.11
Endoscopic mucosal healing and transmural healing in Crohn’s disease
MH in CD generally represents endoscopic MH. Currently, there is no validated score or definition of MH in CD.12 Several studies have defined MH in CD as the resolution of visible mucosal ulcers.3 The EXTEND (Extend the Safety and Efficacy of Adalimumab through Endoscopic Healing) trial was the first placebo-controlled endoscopy trial that evaluated the effects of adalimumab on MH in CD patients (n = 129).13 This study and subsequent accumulating data have shown that the achievement of MH is associated with better outcomes with reduced risk of relapse, long-term corticosteroid-free remission and less surgery and hospitalization.14–19
In a systematic review and meta-analysis of 12 prospective cohorts, including 673 active CD patients, achieving endoscopic remission (or MH) on the first post-treatment endoscopy was associated with a higher likelihood of long-term clinical remission ⩾50 weeks [pooled odds ratio (OR) 2.80, 95% confidence interval (CI), 1.91–4.10], maintenance of MH (14.30, 95% CI, 5.57–36.74) and lower risk of CD-related surgery (2.22, 95% CI, 0.86–5.69).20
However, it is not clear if endoscopic MH and histological remission reflect the healing of the entire bowel wall layers. The resolution of inflammation assessed by cross-sectional imaging has been recognized as a valid target in patients with CD according to the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative by IOIBD.3
Consequently, TH is another dimension that is gradually being appreciated. This means the normalization of bowel wall thickening assessed by cross-sectional imaging: magnetic resonance enterography (MRE), ultrasound (US) and computed tomography enterography (CTE). Many studies have compared, head to head, the accuracy of these techniques, and have concluded that they are similar in assessing inflammation and damage.21
In a multicentre prospective study involving 284 patients, Taylor and colleagues have compared the diagnostic accuracy of MRE and US for the presence, extent and activity of CD. Both reached high accuracy for detecting small bowel CD, but MRE had a higher sensitivity and specificity in terms of presence and extension of small bowel disease.22
Recently, the Image Kids study group has examined in 151 children the association between MH assessed by endoscopy and TH by MRE. In 69% of patients there was concordance in terms of MH and TH. Interestingly, one-third of patients have mucosal inflammation with TH and 6% of patients showed MH with active disease detected by MRE. It is, however, still difficult to identify whether lesions observed by MRE are an index of active disease or of permanent fibrotic damage.23
However, the achievement of TH improves long-term clinical outcome in respect of MH and histologic healing. Indeed, the study by Fernandes and colleagues has shown that patients who have achieved TH have less need for surgery, hospitalization and therapy escalation than patients that have reached only MH.24
Histological healing in ulcerative colitis
European Crohn’s and Colitis Organisation (ECCO) guidelines defined histological healing as resolution of crypt architectural distortion and inflammatory infiltrate.25 This should mean the histological normalization of the bowel mucosa. Many histological scoring systems have been proposed, but none of them have clearly defined the histological healing or ‘deep’ sustained healing, though the newer scores are evolving these definitions.12
The most widely used scores for UC are the Riley Index and the Geboes Index.
The Riley Index involves acute inflammatory infiltrate, crypt abscesses, mucin depletion, epithelial integrity, chronic inflammatory infiltrate and crypt architectural abnormalities, each of which is graded subjectively on a four-point scale and given equal weight.
The Geboes Index Evaluates architectural change, lamina propria neutrophils and eosinophils, neutrophils in epithelium, crypt destruction and erosion or ulceration. It was not designed to be responsive to change and even if it is routinely used it is only partially and formally validated.12,26
Based on items of the Geboes Index, Mosli and colleagues have developed the Robarts Histopathology Index (RHI), a new validated index comprising chronic inflammatory infiltrate, lamina propria neutrophils, neutrophils in the epithelium and erosion or ulceration.27
Recently, the Nancy Score has been validated for use in clinical practice and clinical trials, showing good intraobserver and interobserver reliability and good responsiveness. It describes three histological items – ulceration, acute inflammatory infiltrate and chronic inflammatory infiltrate – resulting in a five-grade classification.28
Romkens and colleagues reported that there was a high interobserver concordance between Geboes score, Riley score and the Harpaz Gupta Index, a four-point scale including three features: cryptitis, ulcers and erosions.29,30 There was a poor concordance on UC biopsies between general pathologists and gastrointestinal pathologists on histological assessment of remission. Histologic activity was present in 20–80% of patients with MES 0.29
Blanco and colleagues have evaluated the agreement between histological healing and MH. They considered, in 34 moderate-to-severe patients, the histological response to anti-tumour necrosis factor (TNF) therapy with adalimumab at 8 and 52 weeks. At week 52, 31% of patients had histological remission (Geboes score < 3.0) and all of them had MH, while 61.8% achieved MH (MES 0–1) at week 52 but only 42.8% of them had histological healing. Of note, the agreement between histological remission and MH at week 52 was only fair.31
The endoscopic assessment of severe signs of inflammation as erosions and ulcers is generally agreed upon easily, and histology correlate well with MES 2–3, while the main discrepancies concern the evaluation of mild activity, MH and histological healing.32,33 It has been generally accepted that absence of inflammation is MH; however, intestinal healing itself is an active process with its own characteristics, such as regenerative changes visible at endoscopy.
Several authors have reported different outcomes in patients with MES 0 (no mucosal abnormalities) and MES 1 (mild erythema or decreased vascular pattern).
In the subgroup analysis of the ACT1 and 2 trials, patients with MES 0 were significantly more often in corticosteroid-free remission after 1 year of follow up than patients with MES 1 (73% versus 47%; p < 0.001), while no differences were found in the colectomy rate.34
A prospective study by Barreiro-de Acosta and colleagues included patients in clinical remission with either MES 1 or MES 0 and the risk of relapse was significantly higher in patients with MES 1 than those with MES 0 (41.0% versus 19.3%; p < 0.01).35 Similarly, a retrospective study by Carvalho and colleagues including 138 patients has shown that MES 1 was significantly associated with an increased risk of relapse (OR, 2.89; 95% CI, 1.14–7.36; p = 0.026.).36
The importance of achieving histological remission was recently demonstrated by a systematic review and meta-analysis showing that the incidence of clinical relapse was significantly lower among patients with baseline histological remission than those with histological activity [relative risk (RR) = 0.48; 95% CI 0.39–0.60.]37
Similarly, a prospective observational study involved 76 patients affected by UC in clinical remission who were followed for 6 months. Among them, 46 patients were in endoscopic remission (MES ⩽1) and histological remission was observed in 31/46 patients. After 1 year the rate of sustained clinical remission was significantly higher in patients who had been in histological remission than among those with histological activity (87.1% versus 46.6%, p = 0.006). These data support that histological assessment rather than endoscopy better predicts sustained clinical remission.38 However, the endoscopic assessment was with standard-definition white-light endoscopy (WLE).
Bryant and colleagues evaluated the ability of histological remission compared to endoscopic healing by WLE to predict clinical outcome in 91 UC patients followed up over 6 years. The most striking data is that histological remission, and not endoscopic MH, predicted corticosteroid use and acute severe colitis requiring hospitalization over the 6-year follow-up period.39
Recently, Ozaki and colleagues have confirmed the need for histological examination even in patients with endoscopically normal mucosa; 194 UC patients with MH (MES ⩽1) were enrolled with a mean follow-up period of 20 months. Overall, 34.5% relapsed during the follow-up period and mucin depletion was an independent risk factor for clinical relapse in these patients with endoscopically normal mucosa [hazard ratio, 2.18 (1.16–5.82); p = 0.03].40
Many studies have examined different histological microscopic features able to predict clinical outcome. Riley and colleagues, in a pioneering study including 82 patients with UC in asymptomatic and endoscopic remission, have reported a higher relapse within 12 months in the presence of an acute inflammatory cell infiltrate, crypt abscesses and mucin depletion in biopsies.41
Later, Bessissow and colleagues, in a cohort study involving 75 patients with normal endoscopy, demonstrated that the presence of basal plasmacytosis and a Geboes score >3.1 predict clinical relapse in patients with complete MH (OR, 5.13; 95% CI, 1.32–19.99; p = 0.052).42
According to Calafat and colleagues, the presence of neutrophils in the epithelium was considered as a risk factor for clinical relapse in 113 asymptomatic patients affected by UC with both clinically and endoscopic remission.43
The importance of histology as endpoint has been supported by the US Food and Drug Administration (FDA). They stated that ‘endoscopy alone (without histology) only provides an assessment of the visual appearance of the mucosa’. Thereby the definition of MH could not be exhaustive by endoscopic findings alone and requires resolution of histological inflammation. Currently there are no criteria for histological assessment of MH, but the FDA encourages and suggests incorporating histology instruments into the definition of MH.44
Since it has been reported that up to 40% of patients have persistent histological inflammatory activity despite clinical remission and MH, a hot topic of discussion, with a consensus among experts convened by ECCO, has become exit strategies: when is the best time to stop therapies in patients with stable clinical and endoscopic remission, considering the risks and benefits of prolonged treatment?45–47 In future, histological assessment could guide our clinical management, for example by decreasing or stopping therapy, checking for non-adherence or optimizing treatment.48
Several studies have showed histological remission or improvement following therapy.
Magro and colleagues carried out a multicentre, single-cohort, open-label, 52-week trial including 20 moderate–severe bionaive UC patients receiving intravenous infliximab (5 mg/kg). After 8 weeks of treatment the proportion of patients who achieved histological remission (Geboes score ⩽3.0) was 15% compared with 5% at baseline and 35% at week 52.49 Similarly, adalimumab was also able to induce histological remission in a considerable proportion of UC patients at week 52 (26.5%, intention to treat).31 Furthermore, Arijs and colleagues have shown that vedolizumab induced endoscopic MH in 55% of patients who achieved also histological healing with a maximal effect seen at week 52.50
It is important to note that in these studies the rate of histological healing requires more prolonged treatment than clinical or endoscopic remission and may occur differentially within each segment of the colon or bowel. A possible explanation could be the drug mechanisms as well as their pharmacokinetic properties that relate to histological remission.51
Other complications, such as dysplasia, may be influenced by chronicity of inflammation; persistence of histological inflammation can impact on the risk of colorectal carcinoma in IBD patients. Long-term benefit of histological healing on development of colorectal carcinoma was shown by a case–control study of 68 UC patients and 136 controls; a significant correlation between endoscopic scores (OR, 2.5; p = 0.001) and histological scores (OR, 5.1; p < 0.001) was observed. On multivariate analysis, histologic inflammation was the only risk factor for development of colorectal neoplasia in patients with long-standing, extensive UC (p < 0.001).52
A recent meta-analysis by Flores and colleagues has shown that the risk of development of colorectal neoplasia was higher in patients with histological inflammation compared to patients with endoscopic MH (OR, 2.6).53
Histological healing in CD
The relationship between histology and endoscopy in CD may be more complex because of patchy and transmural involvement. It is not yet clear whether only histological remission by endoscopic biopsies could add more practical information to manage CD patients or whether having persistent transmural inflammation is related to development of complications.
Recently, a study by Brennan and colleagues has shown the ability of histology (and not endoscopic activity) to predict clinical flares at 6, 12 and 24 months in 62 CD patients undergoing colonoscopy during clinical remission. Indeed, at month 12 the rate of flares was 25.5% in patients with histologic activity, compared with only 2.4% of patients without histologic activity at baseline.54
Christensen and colleagues, in a retrospective analysis of 101 patients affected by CD, have examined clinical relapse-free survival and reported a fair agreement between mucosal and histologic activity. On multivariate analysis, only histologic remission was associated with a lower risk of clinical relapse (HR 1.85; 95% CI, 1.00–3.44).55
Further studies are required, especially addressing histological scoring systems in CD and patchiness of inflammation.
Surrogate markers to predict histological healing
The relationship between endoscopy and surrogate markers of inflammation is a matter of increasing interest and is well studied, but there are few data available on their role in predicting histological remission.56
Guardiola and colleagues, in a prospective observational study of 59 patients with UC, evaluated the accuracy of FC to identify patients with histologic features of inflammation despite clinical and endoscopic remission. The evidence of active histologic inflammation in at least 1 colonic segment was found in 30.5% of patients in endoscopic remission. A significantly higher median level of FC was found in patients with active histologic inflammation compared with those without active histologic inflammation (p = 0.002). In a multivariable analysis, FC is an independent predictor of histologic inflammation in patients with endoscopic remission.57
Similarly, Magro and colleagues have reported that FC and faecal lactoferrin highly correlate with histological activity.49 However, more prospective studies and larger sample sizes are needed in the future.
Limitation of white-light endoscopy to define endoscopic and histological healing
Conventional WLE, although it remains the most widely used standard procedure in daily practice, is not able to define subtle and mild residual signs of inflammation as well as the ‘depth’ of MH relative to histology, especially when standard-definition settings or scopes are used. WLE does not give us a detailed image of mucosal and especially vascular patterns, and its ability is generally restricted to assessing acute disease activity and extension. Many studies have highlighted that an accurate distinction between different grades of inflammation is crucial to stratify risk of relapse, predict clinical outcomes and guide therapeutic management. WLE healing may not quite define the subtle changes of inflammation that may relate to ‘stableness’ of remission and long-term outcomes.
New advanced high-definition (HD) endoscopic technologies, optical diagnosis narrow-band imaging (NBI; Olympus Japan), i-scan (Pentax, Japan), blue laser image (BLI; Fujifilm, Japan), confocal laser endomicroscopy (CLE; Mauna Kea, France), EndoCytoscopy (EC; Olympus, Japan) and the emerging endoscopic molecular labelling seem to give promising results to improve diagnostic accuracy in IBD.58 Some are already available in routine clinical practice and have revolutionized the classical definition of MH as these can see almost at histology level. A classical description of mucosal friability and loss of vascular pattern are no longer valid, and a new endoscopic language, including crypt architecture, micro-erosions, fine vascular changes around the crypts and distinction between intramucosal and luminal bleeding, has recently been introduced.58,59
In one of the earliest reports, Kudo and colleagues focused on the mucosal vascular pattern (MVP) in patients with asymptomatic or mildly active UC using NBI and HD WLE. Interestingly, they have showed that the determination of MVP was significantly different between conventional colonoscopy and NBI colonoscopy (p = 0.0001). NBI yielded a more precise assessment of inflammation in quiescent UC patients and correlated well with histologic items.60
Significant evidence on the potential of NBI as an electronic chromoendoscopy technique came from a pilot study including 14 IBD patients, conducted by Danese and colleagues. These authors reported that areas that appeared normal on WLE but altered on NBI had an increased leucocyte infiltrate and micro-vessel density as assessed by histology.61
The ability of HD colonoscopy to better assess inflammation was demonstrated by Iacucci and colleagues initially in a retrospective cohort study. A total of 78 patients with UC underwent HD WLE and HD i-scan. A grading system based on mucosal and vascular patterns was designed for i-scan. Overall, there was a statistically significant correlation between i-scan imaging scores (mucosal and vascular patterns) and MES (rs = 0.86; 95% CI, 0.79–0.91; p < 0.0001). Interestingly, 30.4% patients of with MES 0 on WLE had abnormal mucosal pattern and 73.9% had an abnormal vascular pattern. The authors concluded that i-scan colonoscopy can improve the detection of subtle patchy inflammation in patients with MES 0, despite apparently healed mucosa and thus direct targeted biopsies.62 This may be especially relevant for the patchiness of healing after therapy.
Advanced and novel endoscopy technologies to assess mucosal healing
Advanced endoscopic techniques play a potential role in the management of IBD as these reduce the gap between what we can see during conventional endoscopy and patchy microscopic changes of mucosa detected by histology.
Dye chromoendoscopy
Dye chromoendoscopy (DCE) has a stronger correlation to histopathology compared with WLE to assess mucosal inflammation. The principle of DCE is staining mucosa to enhance the mucosal surface pattern. In one of the earliest prospective randomized trials by Kiesslich, 165 patients affected by UC were randomized at a 1:1 ratio to undergo conventional colonoscopy or colonoscopy using 0.1% methylene blue. There was significantly better correlation between the endoscopic assessment of degree of inflammation and the histopathology in the chromoendoscopy group compared with the WLE colonoscopy group (89% versus 52%; p < 0.0001). Methylene blue-aided chromoendoscopy in combination with optical magnification seems to be more accurate in assessing the degree and extent of inflammation compared to standard WLE.63
Ibarra-Palomino and colleagues investigated in UC patients the ability of chromoendoscopy with methylene blue 0.2% or indigo carmine 0.1% to determine extension and severity in relation to histopathology, considered as the gold standard. The assessment of severity of UC, in particular for areas with minimal or silent activity, was improved by chromoendoscopy.64 This reflects the limited bandwidth of MES 0–3, especially at the lower end of the scale.
Nishio and colleagues, in a prospective trial including 113 patients with quiescent UC, analysed the role of magnification endoscopy using methylene blue spray to predict clinical relapse. The authors classified the rectal pit pattern appearance into four grades according to its abnormalities. There was a positive correlation between this grading and the degree of histological inflammation. Concerning clinical outcome, the higher degree of subtle inflammation increased the risk of relapse during 12 months of follow up.65
Dye-less chromoendoscopy
The introduction of dye-less chromoendoscopy techniques has overcome the potential limitations of DCE; indeed, this technique is simple to use by just ‘the push of a button’ and is cost- and time-efficient in comparison to DCE. Newly emerging generation electronic virtual chromoendoscopy (EVC) modalities include: NBI-near focus (Olympus; Tokyo, Japan), the i-scan OE (Pentax; Tokyo, Japan), blue laser image (BLI) and linked colour imaging technology (LCI; Fujinon, Fujifilm, Tokyo, Japan).59 The quality of such image-enhancement technologies is improving by leaps and bounds.
NBI involves optical filters to narrow bandwidths of light; a novel system with ‘dual-focus’ function has recently been introduced. These new NBI systems focus more on mucosal tissue and capillary network and the images appear to be brighter and clearer.
The new i-scan OE is a unique combination of optical and digital enhancement chromoendoscopy that improves vessel and mucosal pattern characterization. It involves three types of algorithms: surface enhancement (i-scan 1), tone enhancement (i-scan2) and optical enhancement (i-scan 3).
The BLI and LCI have been developed using LASEREO endoscopic system (Fujifilm, Japan), based on two monochromatic types of diode laser (410 and 450 nm) as the light source, and designed to be able to increase visibility of microvessels and mucosa. BLI images are produced by the combination of spectral and WLE that improve contrast imaging, while LCI expands colour range and enhances slight colour differences in the red region of the mucosa, resulting in more or less visible mucosal redness.66
In a recent study, 52 patients affected by UC underwent LCI endoscopic assessment with corresponding histology. LCI technology significantly improved the diagnosis of subtle inflammation and strongly correlated with Matts’ histopathological score. Interestingly, among areas with MES 0, 41.8% and 4.6% showed images of mucosa redness with visible vessels and redness without visible vessels, respectively. Moreover, non-relapse rates significantly correlated with LCI classification (p = 0.0055), but not with MES (p = 0.0632). So, LCI is a potential tool to better assess MH and inflammation in UC.67
Neumann and colleagues compared HD WLE with computed virtual chromoendoscopy i-scan to detect severity and extent of mucosal inflammation in patients with mild or inactive IBD. They observed that i-scan significantly improved the prediction of extent and severity of disease compared with WLE.68
Recently, Sasanuma and colleagues showed the relationships between magnified NBI findings and histology for 52 patients affected by UC with MES 0–1. There was a statistically significant relationship between NBI findings and pathological findings (p < 0.01). Mucosa, apparently healed, had different vascular patterns with NBI, and several vascular patterns reflected different risks of recurrence.69 Therefore, the vascular pattern in UC may distinguish stable from unstable MH.
A new EVC scoring system, the Paddington International Virtual Chromoendoscopy Score (PICaSSO) was developed using the i-scan platform and validated to better assess and grade inflammation, especially MH in UC. This score involved a more detailed classification of mucosal and vascular changes, reflecting acute and chronic inflammation and healing; it introduced the endoscopic findings of MH and vascular healing using EVC. It correlated with histological scores, including ECAP (extent, chronicity, activity, plus additional findings), a new histological scoring system that includes acute and also chronic inflammation, the Harpaz score and RHI. It can potentially distinguish clearly between absence and presence of mild inflammation.70,71
In addition, Iacucci and colleagues have demonstrated in UC patients the close correlation between i-scan OE magnification and histological scores RHI and ECAP. The overall i-scan OE score correlated with ECAP (r = 0.70; p < 0.001) and RHI (r = 0.61; p < 0.01). The accuracy to detect abnormalities by ECAP was 80% (sensitivity 78%, specificity 100%), while by RHI was 68% (sensitivity 78%, specificity 50%). Significantly, in approximately 31–41% of the patients with MES 0, there was abnormal mucosal or vascular patterns detected by i-scan OE.72 Table 1 presents a summary of the studies exploring the relationship between MH and histological healing.
Table 1.
Relationship between mucosal healing (MH) and histological healing (HH).
| Study | Method | Disease | N patients | Mucosal healing |
Histological healing |
Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Definition | N (%) | Index score | N (%) | |||||
| Fernandez-Blanco et al.31 |
WL | UC | 34 | Mayo score ⩽ 2 | 21 (62%) | Geboes grade ⩽ 3.0 | 9 (26%) | Cohen’s k = 0.293 |
| Bryant et al.39 | WL | UC | 91 | Baron score ⩽1 | 56 (61%) | Truelove and Richards’ Index | 47 (52%) | κ = 0.56 (95% CI 0.36 to 0.77) |
| Iacucci et al.62 | i-scan | UC | 78 | i-scan = 1 | 5 (6.4%) | NYMS = 0 | 18 (78.3%) |
r = 0.65 (95%CI 0.49–0.76) p < 0.0001 |
| Uchiyama et al.67 | LCI | UC | 193 areasa | LCI A | 50 (25.9%) | Matts’ grade = 1 | 47 (24.4%) | p = 0.001b |
| Sasanuma et al.69 | NBI | UC | 52 | BV-BB BV-BH |
41 (78%) | Japanese Ministry of Health, Labour, and Welfare | n/a | p < 0.01 |
| Iacucci et al.72 | OE | UC | 41 UC | i-scan OE mucosal 0–1 vascular 0–1 |
11 (26.8%) 8 (19.5%) |
ECAP RHI |
n/a |
r = 0.70;
p < 0.001 r = 0.61; p < 0.01 |
50 patients, calculations are done on the number of areas.
LCI index at each Matts’ histopathological grade; ANOVA linear contrast test.
ECAP, extent, chronicity, activity, plus additional findings; LCI, linked colour imaging; NBI, narrow-band imaging; NYMS, New York Mount Sinai system; OE, optical enhancement; RHI, Robarts Histological Index; UC, ulcerative colitis; WL, white light.
Confocal laser endomicroscopy
Confocal laser endomicroscopy (CLE) provides in vivo histologic analysis. Due to its resolution and tissue penetration, CLE can describe microscopic imaging such as crypt alterations and microvascular changes. To obtain confocal images, contrast agents have to be administered systemically. The most widely used fluorescent agent is intravenous fluorescein sodium. Different devices are available; one of the most widely used currently is probe CLE (pCLE; Cellvizio, Mauna Kea).73
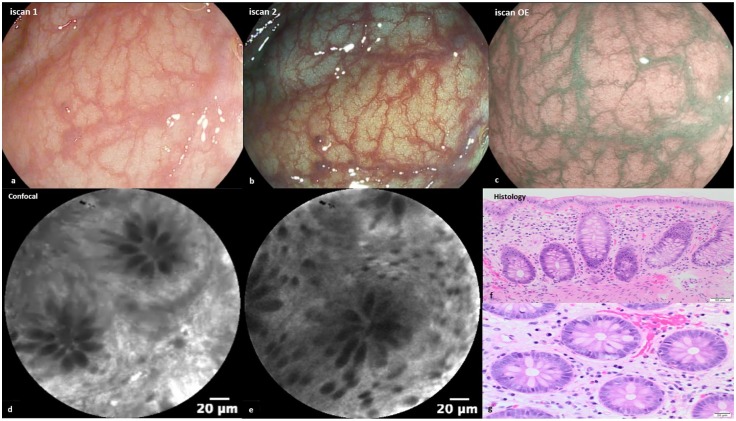
Several studies have assessed the ability of CLE to assess histologic degree and extension of mucosal inflammation in patients affected by IBD (Figure 1).59
Figure 1.
(a–c) Endoscopic images of mucosal healing (normal vascular and mucosal pattern) with i-scan OE (optical enhancement). (d,e) Regular crypts and normal vessels with confocal (fluorescein 2.5 ml 10%). (f) Haematoxylin–eosin, original magnification ×200 showed colonic mucosa healing. (g) Haematoxylin–eosin, original magnification ×400.
One of the earliest pilot studies evaluated the morphologic differences of the colonic mucosa between active and inactive UC. The crypts in UC patients with non-active disease were mainly small, round and slightly irregular in arrangement. In contrast, in active UC goblet cells were invisible, colonic crypts appeared large, variously shaped, irregularly arranged with numerous inflammatory cells and capillaries in the lamina propria.74 CLE also seems to be able to differentiate between UC and CD. In a prospective study involving 79 patients with IBD, a CLE scoring system was developed and potentially allowed prediction of the diagnosis of IBD with high overall accuracy.75
Li and colleagues evaluated inflammatory activity in 73 UC patients and created a four-grade CLE classification system. All the parameters considered – crypt architecture, fluorescein leakage, microvasculature – had good correlation with histopathology. Interestingly, more than half of patients with normal mucosa seen on conventional WLE showed acute inflammation on histology, whereas no patients with normal mucosa seen on CLE showed acute inflammation on histology.76
Preliminary results provided by Iacucci and colleagues of a prospective study involving 82 UC patients have shown the ability of PICaSSO and the pCLE grading system to predict histological healing assessed by RHI. A PICaSSO of ⩽4 and pCLE of ⩽10 predicted histological healing at RHI ⩽6, with accuracy of 92.7% (95% CI, 84.8–97.3) and 95.1% (95% CI, 88.0–98.7), respectively. Of note, PICaSSO score and pCLE score can accurately predict histological healing, defined by RH, better than MES, which had an accuracy of 84.2% (95% CI, 74.4–91.3).77
The ability of pCLE to predict disease relapse and clinical outcome was first assessed by Kiesslich and colleagues. They have observed a direct visualization of local epithelial barrier defects at sites of cell shedding in patients with IBD in remission.78 Whether such barrier defects predispose to relapse requires further prospective studies.
Buda and colleagues composed an outcome score by pCLE, combining fluorescence and crypt diameter (p < 0.01), able to predict disease flare during a 12-month follow-up period in patients affected by long-standing UC. ROC analysis revealed that a pericrypt fluorescence >3100 pixels and a crypt diameter >90 μm increased significantly the probability of disease relapse.79
Karstensen and colleagues, in a longitudinal study involving UC patients, showed how endomicroscopic features (fluorescein leakage, micro-erosion, crypt tortuosity, crypt openings, density and vascular alteration, inflammatory infiltrate) could change before and after escalation of therapy and their correlation with endoscopic and histopathologic changes. After intensification of therapy, an improvement of abnormal colonic crypt architecture involving modifications in crypt tortuosity, distorted crypt lumen and decreased crypt density was detectable by CLE. All of these features were significantly correlated with the Geboes Histology Index.80
Tontini and colleagues have evaluated, in a prospective, multicentre, observational study, the ability of CLE to predict long-term clinical course in 49 CD patients. They identified two main hallmarks of active inflammation by CLE – focal cryptitis and crypt architecture abnormality – and evaluated the role of CLE, CRP, Crohn’s Disease Activity Index (CDAI), and Crohn’s Disease Endoscopic Index of Severity (CDEIS) to predict hospitalization, surgery and escalation treatment in a follow-up period of 4 years. At 1-year follow-up there was a significantly high risk of medical treatment escalation (p < 0.001; RR = 2.61; positive likelihood ratio = 3.27), and transmural adverse events (p = 0.025; RR = 4.06; positive likelihood ratio = 1.7) in the presence of endoscopic cryptitis and crypt architecture abnormality by CLE.81
Recently, Hundorfean and colleagues have proposed an endomicroscopic MH score for UC (eMHs) based on CLE imaging. eMHs had a good correlation with the histological score (Gupta Index) and MES. After 3 years, the eMHs responder patients had an overall reduced need for corticosteroids, lower hospitalization rates and a higher surgery-free survival as compared with eMHs non-responders. eMHs was more accurate than endoscopic, histologic and clinical scores for predicting clinical outcomes.82
Molecular imaging with fluorescent antibodies and CLE are a new tool that can be useful to precisely stratify therapy and predict therapeutic responses, enhancing personalized therapy.83 Despite these promising results, standardization of practice, training programmes and more prospective validation studies involving a larger population are essential before their introduction into clinical practice.
Endocytoscopy
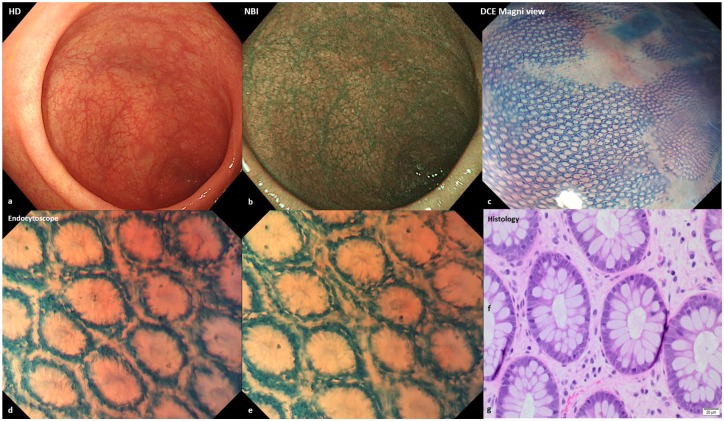
Endocytoscopy (EC) is a high-resolution and high-magnification endoscopic technique that allows real-time observation of cells and nuclei at the mucosal surface during ongoing endoscopy in vivo (Figure 2).
Figure 2.
(a,b) Endoscopic images of mucosal healing (normal vascular and mucosal pattern) with high-definition and narrow-band imaging. (c) Honeycomb appearance of the colonic mucosa with dye chromoendoscopy (methylene blue 0.2%) and Magni view. (d,e) Endocytoscope (methylene blue 0.2%) showed regular crypts and normal spaces between the crypts. (f) Haematoxylin–eosin, original magnification ×200 revealed colonic mucosal healing. (g) Haematoxylin–eosin, original magnification ×400.
Bessho and colleagues, in a pioneering study, have investigated the potential role of EC system score (ECSS) to assess histological disease activity. These authors developed a UC scoring system based on the shape of crypts, the distance between neighbouring crypts and the visibility of superficial microvessels. The ECSS score had a good correlation with Matts’ histopathological grade and so it could be a good predictor of histopathological activity in UC.84
Similarly, Nakazato and colleagues have shown the diagnostic accuracy of ECSS to determine histological remission in 64 UC patients with endoscopic remission MES 0. The ECSS showed high accuracy to detect histological remission, with a sensitivity of 0.77 (95% CI, 0.59–0.89), a specificity of 0.97 (95% CI, 0.83–0.99) and a diagnostic accuracy of 0.86 (95% CI, 0.75–0.93).85
Neumann and colleagues have determined the role of EC for discriminating inflammatory cells in 40 patients with IBD. The concordance between EC and histopathology for grading intestinal disease activity was 100%. EC was also able to distinguish different histopathological features, which is the strength of this endoscopic technology.86
Recent data have evaluated the role of EC in predicting histology and clinical outcome. Ueda and colleagues reported on the correlation between ECSS and MES for mild-to-moderate UC patients. A simple grading system of four types, depending on the alterations in crypt microstructures (shape of crypts, distance between crypts and small vessels in rectal mucosa of UC patients) has been proposed and was significantly associated with microscopic features. Moreover, there were significant differences in remission rate according to EC classification: deformed pits with distorted crypt lumen, unordered arrangement and disruptive or disappeared pits have higher recurrence rates.87 Table 2 summarizes the studies exploring the correlation between new advanced endoscopic techniques and histology.
Table 2.
Correlation between new advanced endoscopic techniques and histology.
| Study | Disease | N patient | Technique | Histological index | Endomicroscopy findings | Outcome |
|---|---|---|---|---|---|---|
| Li et al.76 | UC | 73 | CLE | Geboes Index | Crypt architecture, fluorescein leakage, microvascular alteration | p < 0.001a |
| Karstensen et al.80 | UC | 22 | CLE | Geboes Index | Fluorescein leakage, micro-erosions, crypt tortuosity, crypt openings, crypt density, inflammatory infiltrates | p < 0.001b |
| Hundorfean et al.82 | UC | 23 | CLE | Gupta score | Crypt number, lumen leakage and perivascular leakage | rs = 0.82,c
p < 0.0001 |
| Bessho et al.84 | UC | 55 | EC | Matt’s score | Shape of crypts, distance between neighbouring crypts and visibility of superficial microvessels | r = 0.713,b p < 0.001 |
| Nakazato et al.85 | UC | 64 | EC | Geboes Index <2 | Shape of the crypts, distance between neighbouring crypts, visibility of superficial microvessels |
κ = 0.72 |
| Neumann et al.86 | IBD | 40 | pEC | Riley | Neutrophils, basophiles, eosinophilic granulocytes and lymphocytes. | κ = 0.81–1.00 |
| Ueda et al.87 | UC | 32 | EC | Severe mucosal inflammation, crypt abscess, goblet cell depletion | Regular arrangement of round to oval pits, irregular
arrangement with/without enlarged spaces between regular pits, deformed pits with distorted crypt lumen, disruptive or disappeared pits. |
p < 0.0005d
p < 0.01 p < 0.001 |
Spearman’s rho correlation and ANOVA, p < 0.01 was considered statistically significant.
Spearman rank test.
Pearson’s correlation.
p < 0.05 was considered statistically significant.
CLE, confocal laser endomicroscopy; EC, endocytoscopy; IBD, inflammatory bowel disease; pEC, probe endocytoscopy; UC, ulcerative colitis.
Finally, there is an emerging attention to the ability of artificial intelligence (AI) system to improve the performance of gastrointestinal endoscopy.88 Exciting data have been reported concerning the feasibility of AI differentiating neoplastic colorectal polyps (adenoma) from non-neoplastic polyps.89–91 A computer-aided diagnosis (CAD) system could play a potential role also in IBD to reduce the need for biopsy and to classify surface and vascular pattern morphology accurately.
Recently, Maeda and colleagues have shown that CAD using EC had a good diagnostic ability to differentiate histologically active versus histological healing in UC. A total of 187 patients were enrolled and retrospectively followed. First, they underwent conventional WLE to determine the area most severely inflamed, according to MES, and then EC was performed. A comparison between CAD, inflammatory activity with MES 0–1 and histologic inflammatory activity per patient was performed. CAD had a sensitivity of 74%, specificity of 97% and accuracy of 91% to predict histological inflammation; its reproducibility was perfect (κ = 1).92
More studies are needed to validate the role of the use of EC combined with AI to grade inflammation and predict clinical outcome in IBD. It is likely that EC can help in characterizing subtle dysplastic lesions better with its high magnification.
Future of assessment of mucosal healing by advanced endoscopy at the horizon
The development and validation of new endoscopic imaging techniques promise to provide new and more precise tools to identify MH and distinguish from subtle mucosal inflammation. These tools have to meaningfully relate to clinical outcome measures in the short and medium term and may radically change the ways we interpret MH, not just as absence of inflammation but as an active process of healing. Regenerative changes may ‘mimic’ inflammation and its distinctive features need to be recognized. Many studies have shown that macroscopic healing determined by WLE may not be sufficient to accurately predict long-term disease outcomes, as it often does not correlate with histological remission. Therefore, histological healing is becoming one of the targets needed to achieve tight control and better clinical outcome for patients with IBD, especially UC. However, clear evidence regarding benefits for optimization and escalation of therapy based on histological activity is still lacking. Growing data have also demonstrated that, in the future, intestinal tissue levels of anti-TNF agents will play a key role in deciding on treatment withdrawal. Indeed, the normalization of mucosal gene expression levels of TNF in CD and recently in UC were a predictor of sustained, long-term remission following discontinuation of infliximab therapy.93–95 It is expected that these new data will have a significant impact on future exit strategies for IBD patients. Colonic mucosal transcriptomic changes in long-duration UC may reflect colitis-associated cancer pathways, but future studies are needed to understand whether such changes identify high-risk patients for dysplasia compared to MH.96
Novel emerging endoscopic techniques can better assess MH, correlate with histology and therefore may better predict clinical outcome. These can help to direct precisely targeted biopsies, especially when there is patchy inflammation and healing. In the future, they can accurately manage therapy, as escalation or exit strategies, and promote personalized therapy. Close interaction is required between endoscopists and pathologists to achieve these ambitious goals.
Footnotes
Author contribution: Study conception and design: MI, SG, ON. Drafting of the article: ON, SG, MI. Figures: ON, RC, DZ, MI. Critical revision of the manuscript: ON, RC, DZ, MI, SG.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: MI and SG are funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Olga Maria Nardone  https://orcid.org/0000-0002-9554-4785
https://orcid.org/0000-0002-9554-4785
Contributor Information
Olga Maria Nardone, Institute of Translational Medicine and Institute of Immunology and Immunotherapy, University of Birmingham, Heritage Building, Mindelsohn Way, Birmingham, B15 2TH, UK.
Rosanna Cannatelli, Institute of Translational Medicine and Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Davide Zardo, University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital Birmingham, Birmingham, UK.
Subrata Ghosh, Institute of Translational Medicine and Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital Birmingham, Birmingham, UK; NIHR Biomedical Research Centre, University of Birmingham and University Hospitals NHS Foundation Trust Birmingham, UK.
Marietta Iacucci, Institute of Translational Medicine and Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital Birmingham, Birmingham, UK; NIHR Biomedical Research Centre, University of Birmingham and University Hospitals NHS Foundation Trust Birmingham, UK; University of Calgary, Calgary, Canada.
References
- 1. Agrawal M, Colombel JF. Treat-to-target in inflammatory bowel diseases: what is the target and how do we treat? Gastrointest Endosc Clin N Am 2019; 29: 421–436. [DOI] [PubMed] [Google Scholar]
- 2. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1042–1050 e2. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 4. Pineton de, Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2016; 10: 915–927. [DOI] [PubMed] [Google Scholar]
- 5. Rogler G, Vavricka S, Schoepfer A, et al. Mucosal healing and deep remission: what does it mean? World J Gastroenterol 2013; 19: 7552–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colombel JF, Louis E, Peyrin-Biroulet L, et al. Deep remission: a new concept? Dig Dis 2012; 30(Suppl. 3): 107–111. [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014; 63: 88–95. [DOI] [PubMed] [Google Scholar]
- 8. Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep 2013; 15: 315. [DOI] [PubMed] [Google Scholar]
- 9. Panaccione R, Colombel JF, Louis E, et al. Evolving definitions of remission in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1645–1653. [DOI] [PubMed] [Google Scholar]
- 10. Travis SP, Higgins PD, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther 2011; 34: 113–124. [DOI] [PubMed] [Google Scholar]
- 11. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015; 148: 37–51 e1. [DOI] [PubMed] [Google Scholar]
- 12. Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 2019; 13: 273–284. [DOI] [PubMed] [Google Scholar]
- 13. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111 e2. [DOI] [PubMed] [Google Scholar]
- 14. Pineton de, Chambrun G, Peyrin-Biroulet L, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 15–29. [DOI] [PubMed] [Google Scholar]
- 15. Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut 2007; 56: 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371: 660–667. [DOI] [PubMed] [Google Scholar]
- 17. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 18. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 19. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468; quiz e10–11. [DOI] [PubMed] [Google Scholar]
- 20. Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016; 43: 317–333. [DOI] [PubMed] [Google Scholar]
- 21. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 22. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol 2018; 3: 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn’s disease. Clin Gastroenterol Hepatol 2018; 16: 1089–1097 e4. [DOI] [PubMed] [Google Scholar]
- 24. Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn’s disease. Inflamm Bowel Dis 2017; 23: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 25. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 26. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut 2017; 66: 50–58. [DOI] [PubMed] [Google Scholar]
- 28. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut 2017; 66: 43–49. [DOI] [PubMed] [Google Scholar]
- 29. Romkens TEH, Kranenburg P, Tilburg AV, et al. Assessment of histological remission in ulcerative colitis: discrepancies between daily practice and expert opinion. J Crohns Colitis 2018; 12: 425–431. [DOI] [PubMed] [Google Scholar]
- 30. Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007; 133: 1099–1105; quiz 340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez-Blanco JI, Fernandez-Diaz G, Cara C, et al. Adalimumab for induction of histological remission in moderately to severely active ulcerative colitis. Dig Dis Sci 2018; 63: 731–737. [DOI] [PubMed] [Google Scholar]
- 32. Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease: is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014; 8: 1582–1597. [DOI] [PubMed] [Google Scholar]
- 33. Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis 2012; 18: 1634–1640. [DOI] [PubMed] [Google Scholar]
- 34. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011; 141: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 35. Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis 2016; 10: 13–19. [DOI] [PubMed] [Google Scholar]
- 36. Boal Carvalho P, Dias de Castro F, Rosa B, et al. Mucosal healing in ulcerative colitis: when zero is better. J Crohns Colitis 2016; 10: 20–25. [DOI] [PubMed] [Google Scholar]
- 37. Park S, Abdi T, Gentry M, et al. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2016; 111: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 38. Narang V, Kaur R, Garg B, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res 2018; 16: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016; 65: 408–414. [DOI] [PubMed] [Google Scholar]
- 40. Ozaki R, Kobayashi T, Okabayashi S, et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J Crohns Colitis 2018; 12: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 41. Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991; 32: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol 2012; 107: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 43. Calafat M, Lobaton T, Hernandez-Gallego A, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig Liver Dis 2017; 49: 1327–1331. [DOI] [PubMed] [Google Scholar]
- 44. Reinisch W, Gottlieb K, Colombel J-F, et al. Comparison of the EMA and FDA guidelines on ulcerative colitis drug development. Clin Gastroenterol Hepatol 2018; 18(S1542–3565): 31195–31199. [DOI] [PubMed] [Google Scholar]
- 45. Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J Crohns Colitis 2018; 12: 17–31. [DOI] [PubMed] [Google Scholar]
- 46. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007; 132: 763–786. [DOI] [PubMed] [Google Scholar]
- 47. Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013; 7: 827–851. [DOI] [PubMed] [Google Scholar]
- 48. Villanacci V, Antonelli E, Geboes K, et al. Histological healing in inflammatory bowel disease: a still unfulfilled promise. World J Gastroenterol 2013; 19: 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magro F, Lopes SI, Lopes J, et al. Histological outcomes and predictive value of faecal markers in moderately to severely active ulcerative colitis patients receiving infliximab. J Crohns Colitis 2016; 10: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 50. Arijs I, De Hertogh G, Lemmens B, et al. Effect of vedolizumab (anti-alpha4beta7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018; 67: 43–52. [DOI] [PubMed] [Google Scholar]
- 51. Jairath V, Peyrin-Biroulet L, Zou G, et al. Responsiveness of histological disease activity indices in ulcerative colitis: a post hoc analysis using data from the TOUCHSTONE randomised controlled trial. Gut 2019; 68: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 52. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004; 126: 451–459. [DOI] [PubMed] [Google Scholar]
- 53. Flores BM, O’Connor A, Moss AC. Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: a systematic review and meta-analysis. Gastrointest Endosc 2017; 86: 1006–1111 e8. [DOI] [PubMed] [Google Scholar]
- 54. Brennan GT, Melton SD, Spechler SJ, et al. Clinical implications of histologic abnormalities in ileocolonic biopsies of patients with Crohn’s disease in remission. J Clin Gastroenterol 2017; 51: 43–48. [DOI] [PubMed] [Google Scholar]
- 55. Christensen B, Erlich J, Gibson P, et al. Histological healing is associated with decreased clinical relapse in patients with ileal Crohn’s disease. Gastroenterology 2018; 154: S128–S129. [Google Scholar]
- 56. Mak WY, Buisson A, Andersen MJ, Jr, et al. Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Dig Dis Sci 2018; 63: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 57. Guardiola J, Lobaton T, Rodriguez-Alonso L, et al. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol 2014; 12: 1865–1870. [DOI] [PubMed] [Google Scholar]
- 58. Iacucci M, Panaccione R, Ghosh S. Advances in novel diagnostic endoscopic imaging techniques in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 873–880. [DOI] [PubMed] [Google Scholar]
- 59. Sivanathan V, Tontini GE, Mohler M, et al. Advanced endoscopic imaging for diagnosis of inflammatory bowel diseases: present and future perspectives. Dig Endosc 2018; 30: 441–448. [DOI] [PubMed] [Google Scholar]
- 60. Kudo T, Matsumoto T, Esaki M, et al. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis 2009; 24: 495–501. [DOI] [PubMed] [Google Scholar]
- 61. Danese S, Fiorino G, Angelucci E, et al. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: a pilot study. World J Gastroenterol 2010; 16: 2396–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iacucci M, Fort Gasia M, Hassan C, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy 2015; 47: 726–734. [DOI] [PubMed] [Google Scholar]
- 63. Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003; 124: 880–888. [DOI] [PubMed] [Google Scholar]
- 64. Ibarra-Palomino J, Barreto-Zuniga R, Elizondo-Rivera J, et al. [Application of chromoendoscopy to evaluate the severity and interobserver variation in chronic non-specific ulcerative colitis]. Rev Gastroenterol Mex 2002; 67: 236–240. [PubMed] [Google Scholar]
- 65. Nishio Y, Ando T, Maeda O, et al. Pit patterns in rectal mucosa assessed by magnifying colonoscope are predictive of relapse in patients with quiescent ulcerative colitis. Gut 2006; 55: 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iacucci M, Furfaro F, Matsumoto T, et al. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: new technology, new era. Gut 2018; 68: gutjnl-2017-315235. [DOI] [PubMed] [Google Scholar]
- 67. Uchiyama K, Takagi T, Kashiwagi S, et al. Assessment of endoscopic mucosal healing of ulcerative colitis using linked colour imaging, a novel endoscopic enhancement system. J Crohns Colitis 2017; 11: 963–969. [DOI] [PubMed] [Google Scholar]
- 68. Neumann H, Vieth M, Gunther C, et al. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis 2013; 19: 1935–1942. [DOI] [PubMed] [Google Scholar]
- 69. Sasanuma S, Ohtsuka K, Kudo SE, et al. Narrow band imaging efficiency in evaluation of mucosal healing/relapse of ulcerative colitis. Endosc Int Open 2018; 6: E518–E523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Iacucci M, Daperno M, Lazarev M, et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: the PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest Endosc 2017; 86: 1118–1127 e5. [DOI] [PubMed] [Google Scholar]
- 71. Trivedi PJ, Kiesslich R, Hodson J, et al. The Paddington International Virtual Chromoendoscopy Score in ulcerative colitis exhibits very good inter-rater agreement after computerized module training: a multicenter study across academic and community practice (with video). Gastrointest Endosc 2018; 88: 95–106 e2. [DOI] [PubMed] [Google Scholar]
- 72. Iacucci M, Kiesslich R, Gui X, et al. Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy 2017; 49: 553–559. [DOI] [PubMed] [Google Scholar]
- 73. Neumann H, Kiesslich R, Wallace MB, et al. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology 2010; 139: 388–392. [DOI] [PubMed] [Google Scholar]
- 74. Watanabe O, Ando T, Maeda O, et al. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol 2008; 23(Suppl. 2): S286–S290. [DOI] [PubMed] [Google Scholar]
- 75. Tontini GE, Mudter J, Vieth M, et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: a pilot study. Endoscopy 2015; 47: 437–443. [DOI] [PubMed] [Google Scholar]
- 76. Li CQ, Xie XJ, Yu T, et al. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol 2010; 105: 1391–1396. [DOI] [PubMed] [Google Scholar]
- 77. Iacucci M, Cannatelli R, Gui SX, et al. P254 re-defining the concept of endoscopic and histological healing by using electronic virtual chromoendoscopy and probe confocal endomicroscopy in ulcerative colitis. J Crohns Colitis 2019; 13(S1): S224. [Google Scholar]
- 78. Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012; 61: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Buda A, Hatem G, Neumann H, et al. Confocal laser endomicroscopy for prediction of disease relapse in ulcerative colitis: a pilot study. J Crohns Colitis 2014; 8: 304–311. [DOI] [PubMed] [Google Scholar]
- 80. Karstensen JG, Saftoiu A, Brynskov J, et al. Confocal laser endomicroscopy in ulcerative colitis: a longitudinal study of endomicroscopic changes and response to medical therapy (with videos). Gastrointest Endosc 2016; 84: 279–286 e1. [DOI] [PubMed] [Google Scholar]
- 81. Tontini GE, Mudter J, Vieth M, et al. Prediction of clinical outcomes in Crohn’s disease by using confocal laser endomicroscopy: results from a prospective multicenter study. Gastrointest Endosc 2018; 87: 1505–1514 e3. [DOI] [PubMed] [Google Scholar]
- 82. Hundorfean G, Chiriac MT, Mihai S, et al. Development and validation of a confocal laser endomicroscopy-based score for in vivo assessment of mucosal healing in ulcerative colitis patients. Inflamm Bowel Dis 2017; 24: 35–44. [DOI] [PubMed] [Google Scholar]
- 83. Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med 2014; 20: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bessho R, Kanai T, Hosoe N, et al. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol 2011; 46: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 85. Nakazato Y, Naganuma M, Sugimoto S, et al. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy 2017; 49: 560–563. [DOI] [PubMed] [Google Scholar]
- 86. Neumann H, Vieth M, Neurath MF, et al. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis 2013; 19: 356–362. [DOI] [PubMed] [Google Scholar]
- 87. Ueda N, Isomoto H, Ikebuchi Y, et al. Endocytoscopic classification can be predictive for relapse in ulcerative colitis. Medicine (Baltimore) 2018; 97: e0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Leggett CL, Wang KK. Computer-aided diagnosis in GI endoscopy: looking into the future. Gastrointest Endosc 2016; 84: 842–844. [DOI] [PubMed] [Google Scholar]
- 89. Mori Y, Kudo SE, Misawa M, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: a prospective study. Ann Intern Med 2018; 169: 357–366. [DOI] [PubMed] [Google Scholar]
- 90. Takeda K, Kudo SE, Mori Y, et al. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy 2017; 49: 798–802. [DOI] [PubMed] [Google Scholar]
- 91. Misawa M, Kudo SE, Mori Y, et al. Artificial intelligence-assisted polyp detection for colonoscopy: initial experience. Gastroenterology 2018; 154: 2027–2029 e3. [DOI] [PubMed] [Google Scholar]
- 92. Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc 2019; 89: 408–415. [DOI] [PubMed] [Google Scholar]
- 93. Olsen T, Rismo R, Gundersen MD, et al. Normalization of mucosal tumor necrosis factor-alpha: a new criterion for discontinuing infliximab therapy in ulcerative colitis. Cytokine 2016; 79: 90–95. [DOI] [PubMed] [Google Scholar]
- 94. Johnsen KM, Goll R, Hansen V, et al. Repeated intensified infliximab induction: results from an 11-year prospective study of ulcerative colitis using a novel treatment algorithm. Eur J Gastroenterol Hepatol 2017; 29: 98–104. [DOI] [PubMed] [Google Scholar]
- 95. Rismo R, Olsen T, Cui G, et al. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn’s disease. Scand J Gastroenterol 2013; 48: 311–319. [DOI] [PubMed] [Google Scholar]
- 96. Low END, Mokhtar NM, Wong Z, et al. Colonic mucosal transcriptomic changes in patients with long-duration ulcerative colitis revealed colitis-associated cancer pathways. J Crohns Colitis 2019; 13: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]