Abstract
Background:
Myasthenic crisis (MC) is a potentially life-threatening complication of myasthenia gravis. Its precipitating factors include surgical procedures, particularly thymectomy. The role of preoperative intravenous immunoglobulin (IVIg) in preventing MC in patients scheduled for thymectomy and other surgery with general anaesthesia is unknown. Our objective was to test the hypothesis that preoperative IVIg is effective in preventing myasthenic crisis in patients with myasthenia gravis scheduled for surgery under general anaesthesia, including thymectomy.
Methods:
A prospective, randomized, double-blind, single-centre study was conducted over a 4-year period. The treatment group received IVIg, 0.4 g/kg/day preoperatively for 5 consecutive days, and the placebo group received saline solution under the same conditions. The two groups were age-matched, with similar functional status, and Myasthenia Gravis Foundation of America class. All patients had well-controlled myasthenia gravis with minimal manifestations before surgery. The primary outcome measured was MC. Intubation times, time in the recovery room, number of postoperative complications, and days of hospitalization were the secondary outcomes measured.
Results:
A total of 47 patients were randomized, 25 to the IVIg group and 22 to placebo. There were 19 men and 28 women, with a mean age of 58.6 years, mean body mass index of 27.8 kg/m2, and mean acetylcholine receptor antibodies of 12.9 nmol/l. The mean forced vital capacity was 84.4%. The mean quantitative myasthenia gravis sum score was 6.3. Ten patients (five in each arm) had a history of MC. Thymectomy was performed in 16 patients. Only one patient in the placebo group presented with MC requiring non-invasive ventilation (but no reintubation) for 6 days. Neither differences between groups in the univariate analysis nor risk factors for MC in the multivariate analysis were found.
Conclusions:
Preoperative IVIg to prevent MC does not appear to be justified in well-controlled myasthenia gravis patients. This study provides class I evidence that preparation with IVIg to prevent MC is not necessary in well-controlled myasthenia gravis patients scheduled for surgery with general anaesthesia.
Keywords: clinical trial, immunoglobulin, myasthenia, myasthenic crisis, thymectomy
Introduction
Myasthenic crisis (MC) is a serious and potentially life-threatening complication of myasthenia gravis (MG) that occurs in approximately 15–20% of patients during their lifetime.1 It is characterized by severe weakness of the bulbar or respiratory muscles, sufficient to cause respiratory failure and to require respiratory support. Patients with MG undergoing a surgical procedure needing intubation and who experienced delayed extubation of >24 h after surgery are also considered to experience MC.2 Common precipitating factors for MC include respiratory infection, aspiration, sepsis, exposure to drugs that may increase myasthenic weakness, pregnancy and surgical operations.3,4 Thymomas are associated with a more fulminant course of MG and are present in one third of patients who experience MC.5 A significant number of MCs also occur in the context of surgical procedures, particularly thymectomy, and often require prolonged postoperative intubation.6–8 Estimates of mortality in MC are as high as 6–10%.9 The respiratory failure that characterizes MC is the most common cause of death.
Thymectomy, alone or combined with immune-suppressive and anticholinesterase therapies, is currently accepted as the standard treatment for MG.10–14 Co-adjuvant therapies with plasma exchange (PLEX) or intravenous immunoglobulin (IVIg) are effective disease-stabilizing regimens to reduce the risk of postoperative respiratory complications and the need for prolonged, assisted ventilation leading to extended hospitalization.15 Small-scale clinical studies have shown significant improvements in respiratory function and muscle strength in thymectomized patients after PLEX.16–19 Complications associated with the use of a large-bore central venous catheter or the occurrence of large volume shifts during the procedure are limiting factors of PLEX.
IVIg represents a short-term immunomodulating alternative in patients who are poor candidates for PLEX, and has been demonstrated to be beneficial in controlling MC. A small randomized controlled trial of IVIg 1.2 and 2.0 g/kg over 2–5 days compared with PLEX in MG patients with exacerbations or crisis showed comparable efficacy between the two treatment groups.20 Other studies also failed to show significant differences in the efficacy of PLEX and IVIg in patients during acute exacerbations of MG.21–23 However, experience with the use of IVIg as preparation before surgery to control perioperative and postoperative complications in MG patients is limited to a few studies and noncontrolled randomized trials.24–26 Although these results are encouraging, the design of a prospective, randomized, double-blind, placebo-controlled trial is needed to provide class I evidence of the effectiveness of IVIg in the preoperative setting. A clinical trial with these characteristics was therefore conducted. The main objective was to assess the effectiveness of IVIg as preparation before thymectomy and other surgical procedures under general anaesthesia in MG patients to prevent postoperative MC.
Methods
Ethics
The protocol was designed following the recommendations for clinical research standards of the Myasthenia Gravis Foundation of America (MGFA).27 In accordance with the Declaration of Helsinki, this study was carried out following the protocol approved by the Hospital Universitari Vall d’Hebron Institutional Review Board on 25 May 2012, with registration number MG2012PREP. All participants signed an informed consent agreement following extensive explanation of the aims of the study. The study was registered in the European Clinical Trials Database [EudraCT identifier: 2012-001544].
Design and setting
Our study was a single-centre, prospective, randomized, double-blind, placebo-controlled trial involving parallel treatment with IVIg or placebo preoperatively in consecutive patients diagnosed with MG scheduled for elective surgical procedures, including thymectomy under general anaesthesia between February 2013 and December 2016. The primary objective of the study was to assess the effectiveness of preoperative preparation with IVIg to prevent postoperative MC. Secondary objectives were the effectiveness of IVIg in reducing the length of hospital stays and inducing changes in the quantitative myasthenia gravis (QMG) score for disease severity.
Patients
The patients were males and females aged > 18 years, with generalized MG at the time of screening27 needing elective surgical procedures requiring general anaesthesia, including thymectomy. The eligible individuals belonged to the cohort of MG patients monitored in our unit and new patients who met criteria for thymectomy. Exclusion criteria were as follows: hypersensitivity to homologous immunoglobulins; serum IgA levels < 5% below the lower limit of normal values; presence of severe diseases, such as heart failure, cardiomyopathy, severe coronary heart disease, severe hypertension, and stages IV or V chronic kidney disease; history of thrombotic episodes (pulmonary embolism, acute myocardial infarction, deep venous thrombosis, ischaemic stroke, known hypercoagulable state); history of haemolysis, aseptic meningitis or severe recurrent headache after intravenous infusion of immunoglobulins; pregnancy; and breastfeeding women.
The diagnosis of MG was established by history and clinical signs and symptoms. The following tests were performed to confirm the clinical diagnosis: single-fibre electromyography (SF-EMG) and repetitive nerve stimulation, edrophonium test (Tensilon®, Roche Laboratories, Hoffmann-La Roche Inc., Nutley, NJ), and serum acetylcholine receptor (AChR) antibodies (AChR-Ab; RIA kit, RSR Ltd., Cardiff, UK). For patients negative for AChR-Abs, we tested for antimuscle-specific kinase (anti-MuSK) antibodies (MuSK-Ab; RSR Ltd.). The preoperative diagnosis of thymoma was based on clinical symptoms, conventional radiographs, and chest computed tomographic (CT) scans. Thymectomy was indicated in generalized forms of anti-AChR-positive MG, in patients under 55-years old, and in all patients with thymoma, regardless of age.
Study procedures
After informed consent, the eligible patients scheduled for elective thymectomy (extended trans-sternal thymectomy) and other surgical procedures under general anaesthesia were randomized to preoperative preparation with IVIg or placebo (see CONSORT flow chart in Figure 1). A computer-generated list of random numbers was used to allocate the patients to the treatment or placebo group. Patients in the treatment group received five doses of IVIg (0.4 g/kg/day; Privigen®, CLS Behring AG, Bern, Switzerland) for 5 consecutive days before surgery, with the final dose administered at least 7 days before the procedure. The first dose was administered at least 12 days beforehand, with the benefit of IVIg peaking around 15 days, just when surgery took place.28–31 Patients assigned to the placebo group received intravenous saline solution for the same time period and under the same conditions. The medications were provided by the pharmacy department in photoprotective bags and opaque tubes to mask the vials of immunoglobulin and placebo. The treatments were administered under the supervision of one of the investigators (JG) in a day hospital setting. Concurrent treatment with rituximab, alemtuzumab, plasmapheresis, tumour necrosis factor alpha (TNF-α) and other immunoglobulin was not allowed.
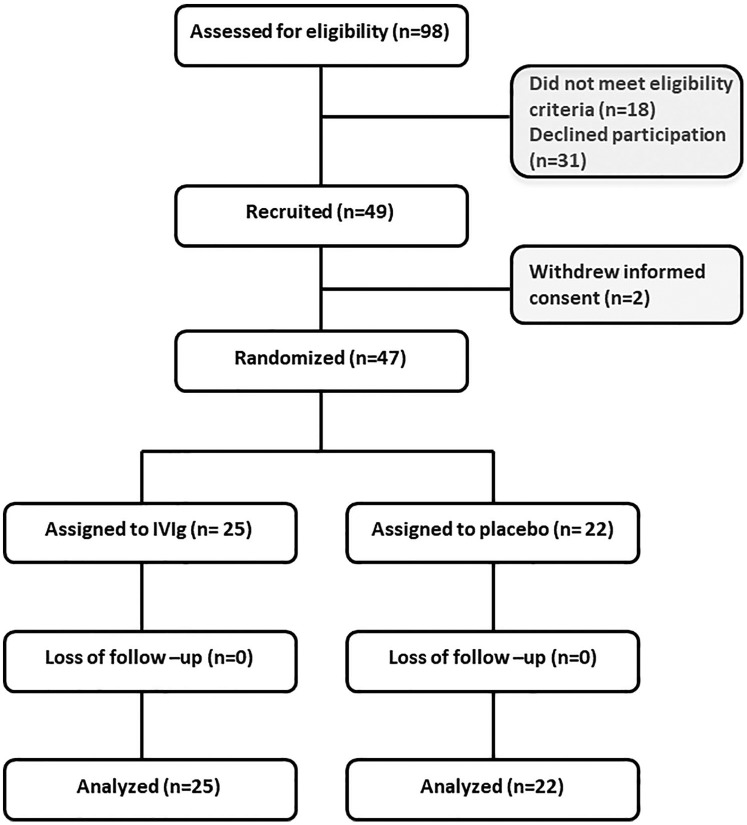
Figure 1.
CONSORT diagram.
Of the 49 patients recruited for the study, 2 withdrew informed consent. A total of 47 patients were subsequently randomized; 25 assigned to the IVIg group and 22 to the placebo group.
IVIg, intravenous immunoglobulin.
Assessments
The patients were evaluated at baseline, that is, at most, 15 days before the surgical procedure, and then every day until the 14th postoperative day. Patients with MG were assessed until discharge from the hospital. Measurements included vital signs, laboratory tests (blood tests, biochemical profile, and serum anti-AChR antibodies), pulmonary function tests (EasyOne™ spirometer, ndd Medizintechnik AG, Zurich, Switzerland), QMG score, MG quality-of-life questionnaire (MGQoL), concomitant treatments/procedures, and adverse events (AEs). The QMG score is a scale ranging from 0 to 39, with higher scores for each of 13 items indicating the most severe weakness. The MGQoL consists of 15 items scored on a 5-point Likert scale (from 0 to 4), with higher scores indicating a lower QoL.
The following variables were recorded for all patients: demographics (sex, age); body mass index (BMI); age at onset of MG; duration of MG; MGFA class at clinical onset; history of thymoma; previous MC and surgical procedures with general anaesthesia; preoperative bulbar symptoms and bulbar score; anti-AChR antibody levels; forced vital capacity (FVC); forced expiratory volume in 1 s (FEV1); peak expiratory flow (PEF); QMG score; MGQoL score; preoperative dose of pyridostigmine; steroids and other immunosuppressants (azathioprine, tacrolimus, mycophenolate mofetil); duration of surgery; blood transfusion requirement; duration of stay in the post-surgery recovery room; length of hospital stay; and AEs.
Endpoints
The primary endpoint of the study was the occurrence of MC in both arms. The secondary endpoints were the length of hospital stay and changes in QMG score.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, and continuous variables as mean and standard deviation (SD). The distribution of variables in the two study groups was compared with the chi-square test or Fisher’s exact test for categorical data, and the Student’s t test or the Wilcoxon test for quantitative variables when the distribution of data departed from normality. Analysis of variance and analysis of covariance were used to assess differences in QMG scores and other outcome measures. To determine variables associated with the primary endpoint, defined as the presence of MC, variables were fitted in a logistic regression model using the Firth penalized likelihood method,32 with the preoperative QMG score, duration of MG, preoperative FVC, and operative time as independent variables. The Statistical Package for the Social Sciences (SPSS) programme (SPSS Inc., Chicago, IL, USA) version 16 was used for the analysis of data. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
A total of 49 patients were recruited for the study, but 2 of them withdrew informed consent. A total of 47 patients were therefore randomized; 25 were assigned to the IVIg group and 22 to the placebo group. There were 19 men and 28 women, with a mean age of 58.6 years, mean BMI of 27.8 kg/m2. The mean disease interval between clinical onset and randomization was 6.2 years. The distribution of the patients according to MGFA class at clinical onset was 2 patients with class I, 17 with class IIa, 3 with class IIb, 14 with class IIIa, 9 with class IIIb and 2 with class IVb. The two patients with an ocular form at clinical onset had progressed to generalized forms at the time of study inclusion. Of the 47 patients, 45 were anti-AChR-positive and 2 anti-MuSK-positive. None was seronegative for anti-AChR and anti-MuSK (double seronegative). The mean anti-AChR level was 12.9 nmol/l. FVC was 84.4% and FEV1 90.1% of predicted values. None of the patients was in complete clinical remission and all of them belonged to the minimal manifestations category of the MGFA Post-Intervention Status (MGFA-PIS) classification. A total of 5 patients (IVIg 4, placebo 1) were classified as the MM-1 (Minimal manifestations class 1) category, 2 (IVIg 2) as the MM-2 category and 40 (IVIg 19, placebo 21) as the MM-3 category. A total of 45 patients had anti-AChR antibodies (IVIg 21, placebo 24) and 2 patients had anti-MuSK antibodies (IVIg 1, placebo 1), without differences between the study groups. Twenty-six patients had previously undergone surgery with general anaesthesia, half of which were thymectomies. A total of 10 patients (5 in each arm) had previously presented MCs. Of these 10 patients with history of previous MC, 1 patient presented a crisis after thymectomy and 1 after abdominal surgery; in the remaining 8 patients, triggers were respiratory infection in 4, mastoiditis in 1, use of benzodiazepines in 1, poor medication adherence in 1 and spontaneous in 1. As shown in Table 1, statistically significant differences in the distribution of preoperative variables between the study groups were not found, except for a lower level of anti-AChR antibodies in the placebo group as compared with the IVIg group (p = 0.045).
Table 1.
Preoperative characteristics of the study population.
| Variables | All patients (n = 47) |
Study groups |
p
value |
|
|---|---|---|---|---|
| IVIg (n = 25) | Placebo (n = 22) | |||
| Sex, n (%): | 0.718 | |||
| Men | 19 (40.4) | 9 (36) | 10 (45.4) | |
| Women | 28 (59.6) | 16 (64) | 12 (54.5) | |
| Age, years | 58.6 (16.2) | 61.1 (15.3) | 55.7 (17.0) | 0.357 |
| BMI, kg/m2 | 27.8 (4.8) | 27.4 (4.7) | 28.2 (5.0) | 0.898 |
| Age at clinical onset, years | 52.4 (18.6) | 54.3 (17.5) | 50.2 (20.0) | 0.609 |
| Duration of MG, years | 6.2 (8.0) | 6.8 (7.9) | 5.5 (8.3) | 0.639 |
| MGFA class at clinical onset | 0.090 | |||
| I | 2 | 0 | 2 | |
| IIA | 17 | 11 | 6 | |
| IIB | 3 | 3 | 0 | |
| IIIA | 14 | 8 | 6 | |
| IIIB | 9 | 3 | 6 | |
| IVB | 2 | 0 | 2 | |
| MGFA-PIS category | 0.200 | |||
| MM-1 | 5 | 4 | 1 | |
| MM-2 | 2 | 2 | 0 | |
| MM-3 | 40 | 19 | 21 | |
| Patients with prior history of: | ||||
| Thymoma | 13 (27.7) | 6 (24) | 7 (31.8) | 0.786 |
| Surgery with general anaesthesia | 26 (55.3) | 13 (52) | 13 (59.1) | 0.846 |
| Myasthenic crisis | 10 (21.3) | 5 (20) | 5 (22.7) | 1.000 |
| Bulbar symptoms, n (%) | 14 (29.8) | 6 (24) | 8 (36.4) | 0.545 |
| Bulbar score | 0.3 (0.7) | 0.1 (0.3) | 0.4 (1.0) | 0.296 |
| Anti-AChR level, nmol/l | 12.9 (8.3) | 14.4 (8.0) | 11.3 (8.5) | 0.045 |
| Pulmonary function tests: | ||||
| FVC, % | 84.4 (14.9) | 85.1 (15.4) | 83.7 (14.6) | 0.912 |
| FEV1, % predicted | 90.1 (17.9) | 91.0 (18.9) | 89.0 (17.0) | 0.886 |
| Peak flow | 396.4 (142.1) | 359.2 (135.9) | 438.6 (140.2) | 0.796 |
| QMG score | 6.3 (3.7) | 6.1 (3.8) | 6.6 (3.5) | 0.653 |
| MGQoL score | 13.7 (14.7) | 11.6 (13.6) | 16.0 (15.8) | 0.472 |
| Pyridostigmine treatment, n (%) | 42 (89.4) | 21 (84.0) | 21 (95.5) | 0.352 |
| Pyridostigmine, mg | 178.1 (35.4) | 180.0 (30.0) | 175.7 (43.9) | 1.000 |
| Immunosuppressants, mg/day: | ||||
| Prednisone (n = 47) | 11.8 (20.6) | 13.5 (21.7) | 9.9 (19.5) | 0.600 |
| Azathioprine (n = 4) | 62.5 (25.0) | 62.5 (25.0) | 0 | 0.112 |
| Tacrolimus (n = 26) | 3.5 (1.3) | 3.2 (1.2) | 3.9 (1.4) | 0.229 |
| Mycophenolate mofetil (n = 2) | 1250 (1061) | 500 | 2000 | 1.000 |
Data as mean ± SD unless otherwise stated.
AChR, acetylcholine receptor; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; IVIg, intravenous immunoglobulin; MG, myasthenia gravis; MGFA-PIS, Myasthenia Gravis Foundation of America Post-Intervention Status; MGQoL, MG quality-of-life questionnaire; MM, minimal manifestations; MM-1, the patient continues to receive some form of immunosuppression but no cholinesterase inhibitors or other symptomatic therapy; MM-2, the patient has received only low-dose cholinesterase inhibitors (,120 mg pyridostigmine/day) for at least 1 year; MM-3, the patient has received cholinesterase inhibitors or other symptomatic therapy and some form of immunosuppression during the past year; QMG, quantitative myasthenia gravis score; SD, standard deviation.
In relation to preoperative treatment, 45 patients received anticholinesterase drugs as symptomatic treatment. Treatment with immunosuppressants included prednisone in 31 cases, tacrolimus in 26, azathioprine in 4, and mycophenolate mofetil in 2. Six patients received pyridostigmine only and two patients tacrolimus only. The remaining patients were treated with double or triple combinations of pyridostigmine and immunosuppressant drugs.
Perioperative data
Thymectomy was the most common surgical procedure, and was performed in 16 (34.0%) patients. Differences in preoperative variables between patients treated with IVIg (n = 9) or placebo (n = 7) in the subset of 16 thymectomized patients were not observed (Table 2). Details of the procedures are shown in Table 3. The mean duration of surgery was 120.7 min in the overall study population and 159.1 min in patients undergoing thymectomy (Table 4). Only one thymectomized patient in the IVIg group required perioperative blood transfusion. Differences in postoperative AEs per preparation regimen were not observed. Surgical complications were recorded in two patients in the IVIg group and in three patients treated with placebo.
Table 2.
Preoperative characteristics of patients undergoing thymectomy.
| Variables | All patients (n = 16) |
Study groups |
p
value |
|
|---|---|---|---|---|
| IVIg (n = 9) | Placebo (n = 7) | |||
| Sex, n (%) | 0.358 | |||
| Men | 7 (43.7) | 5 (55.6) | 2 (28.6) | |
| Women | 9 (56.2) | 4 (44.4) | 5 (71.4) | |
| Age, years | 53.9 (13.4) | 52.7 (10.4) | 55.5 (17.3) | 0.681 |
| BMI, kg/m2 | 27.1 (6.0) | 25.4 (4.6) | 29.4 (7.1) | 0.596 |
| Age at clinical onset, years | 53.1 (13.6) | 51.9 (10.7) | 54.5 (17.5) | 0.681 |
| Duration of MG, years | 0.8 (0.6) | 0.8 (0.6) | 0.8 (0.5) | 0.918 |
| MGFA class at clinical onset, no.: | 0.090 | |||
| I | 0 | 0 | 0 | |
| IIA | 6 | 4 | 2 | |
| IIB | 1 | 1 | 0 | |
| IIIA | 5 | 3 | 2 | |
| IIIB | 3 | 2 | 1 | |
| IVB | 1 | 0 | 1 | |
| Patients with prior history of: | ||||
| Thymoma | 9 (56.2) | 5 (55.5) | 4 (57.1) | 1.000 |
| Surgery with general anaesthesia | 5 (31.2) | 4 (44.4) | 1 (14.3) | 0.308 |
| Myasthenic crisis | 4 (25) | 1 (11.1) | 3 (42.9) | 0.261 |
| Bulbar symptoms, n (%) | 5 (31.2) | 2 (22.2) | 3 (42.9) | 0.596 |
| Bulbar score | 0.4 (1.0) | 0.2 (0.4) | 0.7 (1.5) | 0.727 |
| Anti-AChR level, nmol/l | 15.5 (8.1) | 16.3 (7.4) | 14.4 (8.5) | 0.440 |
| Pulmonary function tests: | ||||
| FVC, % | 86.4 (14.8) | 84.3 (17.1) | 89.1 (11.8) | 0.408 |
| FEV1, % predicted | 92.7 (16.1) | 90.1 (17.4) | 96.1 (14.8) | 0.681 |
| Peak flow | 433.7 (110.4) | 438.6 (127.2) | 430.0 (103.3) | 0.832 |
| QMG score | 6.1 (3.0) | 5.2 (2.4) | 7.1 (3.5) | 0.363 |
| MGQoL score | 18.5 (17.3) | 17.3 (18.7) | 20.0 (16.7) | 0.633 |
| Pyridostigmine treatment, n (%) | 12 (75%) | 7 (77.8) | 5 (71.4) | 1.000 |
| Pyridostigmine, mg/day | 178.1 (35.4) | 180 (30) | 175.7 (43.9) | 1.000 |
| Immunosuppressants, mg/day: | ||||
| Prednisone (n = 15) | 22.2 (24.7) | 21.1 (20.3) | 23.6 (31.2) | 0.957 |
| Azathioprine (n = 1) | 50 | 50 | 0 | |
| Tacrolimus (n = 10) | 3.4 (1.3) | 3.2 (0.8) | 3.6 (1.7) | 1.000 |
Data as mean ± SD unless otherwise stated.
AChR, acetylcholine receptor; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; IVIg, intravenous immunoglobulin; MG, myasthenia gravis; MGFA, Myasthenia Gravis Foundation of America; MGQoL, MG quality-of-life questionnaire; QMG, quantitative myasthenia gravis score; SD, standard deviation.
Table 3.
Type of surgical procedures.
| Surgery | Number of patients (%) |
|---|---|
| All patients | 47 (100) |
| Thymectomy | 16 (34.0) |
| Combined gastroscopy and colonoscopy | 9 (19.1) |
| Abdominal procedures | 7 (14.9) |
| Laparoscopic cholecystectomy | 5 |
| Adrenalectomy | 1 |
| Bariatric surgery | 1 |
| Cardiothoracic procedures | 5 (10.6) |
| Cardiac radiofrequency ablation | 2 |
| Mitral valve replacement | 1 |
| Thoracoscopic lobectomy | 1 |
| Metastatic pleural resection | 1 |
| Hysterectomy | 4 (8.5) |
| Maxillofacial procedures | 4 (8.5) |
| Mastoidectomy | 2 |
| Parotidectomy | 2 |
| Bladder tumour resection | 1 (2.1) |
| Total hip replacement | 1 (2.1) |
Table 4.
Perioperative data in all patients and in those undergoing thymectomy.
| Variables | Overall study population |
Thymectomy surgical procedures |
||||
|---|---|---|---|---|---|---|
| All patients (n = 47) |
IVIg (n = 25) |
Placebo (n = 22) |
All patients (n = 16) |
IVIg (n = 9) |
Placebo (n = 7) |
|
| Operation time, min | 120.7 (68.0) | 122.9 (68.7) | 118.2 (68.8) | 159.1 (52.1) | 166.7 (59.1) | 149.3 (43.9) |
| p = 0.749 | p = 0.832 | |||||
| Blood transfusion, n (%) | 1 (2.1) | 1 (4.2) | 0 | 1 (6.2) | 1 (11.1) | 0 |
| p = 1.000 | p = 0.601 | |||||
| Time in recovery room, h | 22.6 (42.2) | 19.9 (28.9) | 25.8 (54.1) | 42.6 (65.8) | 32.9 (40.1) | 55.2 (91.5) |
| p = 0.733 | p = 0.536 | |||||
| Length of hospital stay, days | 3.7 (3.7) | 3.2 (2.7) | 4.2 (4.5) | 5.4 (4.9) | 4.8 (3.3) | 6.3 (6.5) |
| p = 0.586 | p = 0.782 | |||||
Data as mean ± SD unless otherwise stated.
IVIg, intravenous immunoglobulin; SD, standard deviation.
In relation to the primary endpoint, one patient randomized to the placebo group presented with MC, which required non-invasive ventilation (but no reintubation) for 6 days. This 63-year-old woman had been diagnosed with MG 8 months previously and presented with a MC 3 months before surgery. Her BMI was 41.9 kg/m2. The chest CT identified the presence of a thymoma. She presented clinical signs of MC 2 h after leaving the operating room and undergoing extubation. A control CT confirmed bilateral pleural effusion, left basal atelectasis, left phrenic nerve palsy, anaemia, cardiac arrhythmia (atrial fibrillation), progressive decompensation of her diabetes, high blood pressure values and renal insufficiency. The patient was discharged home 28 days after surgery. The patient was randomized to the placebo group. MC was not recorded in any of the other study patients.
In relation to the secondary endpoints, no statistically significant differences were found for the length of stay in the recovery room after surgery or the total length of hospital stay between patients treated with IVIg and those treated with placebo (Table 4). As shown in Figure 2, the length of hospital stay was similar in patients treated with IVIg and those treated with placebo in the overall study population and in the subset of patients undergoing thymectomy. Similar findings were observed regarding the length of stay in the postsurgical recovery room (Figure 3).
Figure 2.
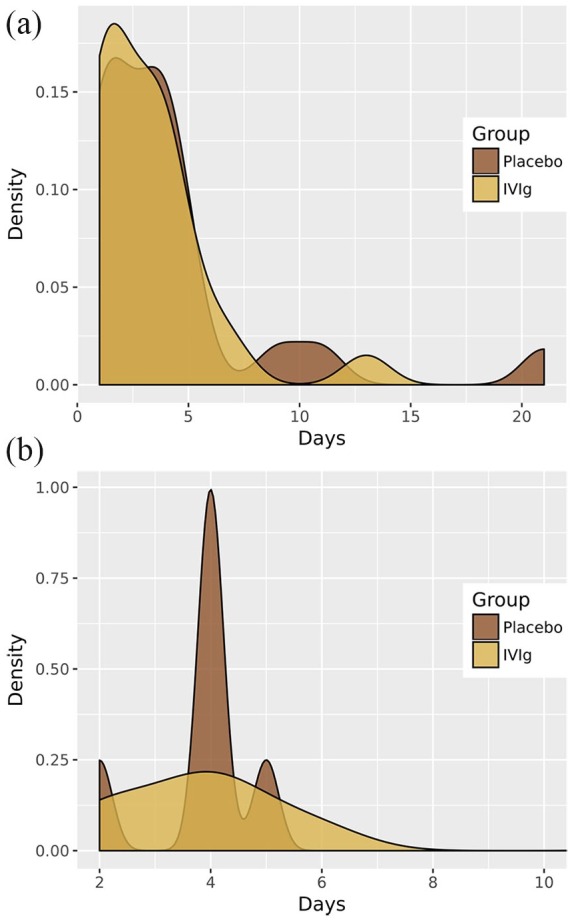
Effect of preoperative preparation with IVIg or placebo on hospital stay.
Effect of preoperative preparation with IVIg or placebo on the length of hospital stay in the overall study population of 47 patients (a) and in the subset of 16 patients undergoing thymectomy (b).
IVIg, intravenous immunoglobulin.
Figure 3.
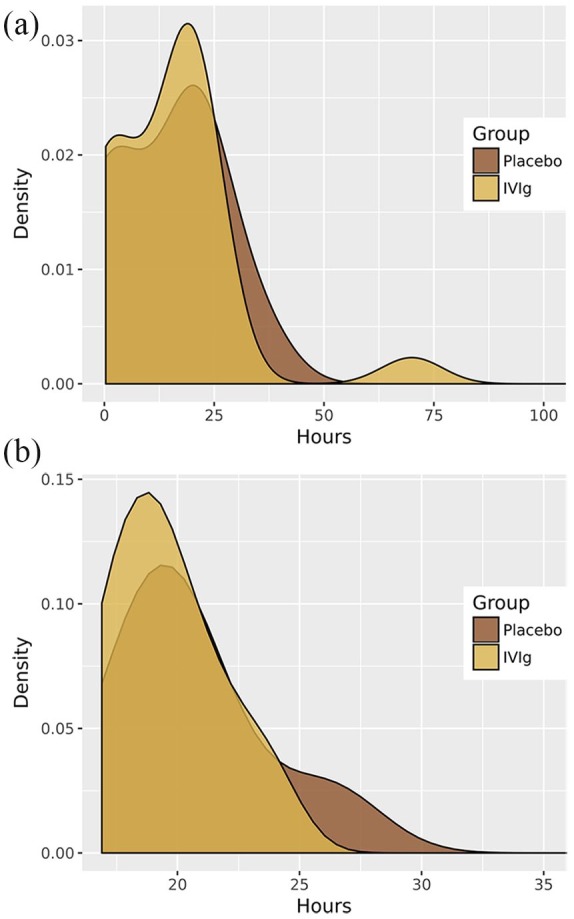
Effect of preoperative preparation with IVIg or placebo on stay in the postsurgical recovery room.
Effect of preoperative preparation with IVIg or placebo on the length of stay in the postsurgical recovery room in the overall study population of 47 patients (a) and in the subset of 16 patients undergoing thymectomy (b).
IVIg, intravenous immunoglobulin.
The mean QMG score before surgery was 6.3 points (SD ± 3.7). There was a deterioration of the QMG score on the first day after surgery of less than 2 points (mean 7.9, SD ± 4.0), returning to baseline values on the postoperative day 4. Changes in the QMG score in the two study groups were similar (Figure 4). Intergroup differences in the QMG score over the 14 days of the study compared with baseline were not statistically significant.
Figure 4.
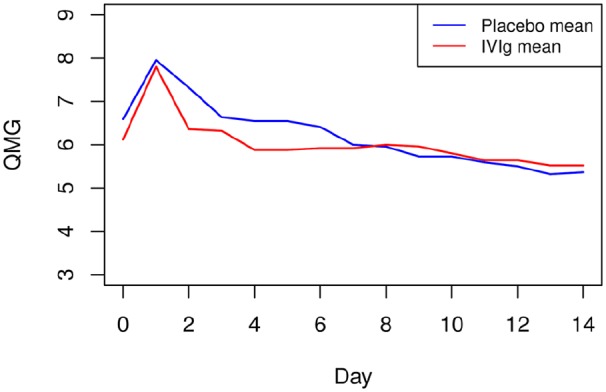
Changes in mean QMG score.
Changes in mean QMG score in the overall study population of 47 patients according to preoperative preparation with IVIg or placebo.
IVIg, intravenous immunoglobulin; QMG, quantitative myasthenia gravis.
Finally, in the logistic regression model, none of the variables analysed (preoperative QMG score, duration of MG, preoperative FVC and operative time) were predictors of MC in either the overall study population or in the subset of thymectomized patients.
Discussion
The lifetime prevalence of acute episodes of respiratory muscle weakness in MG patients that are severe enough to require intubation and mechanical ventilation is approximately 20–30%.33 A significant number of these MCs occur in the context of surgical procedures, particularly thymectomies, and often lead to prolonged postoperative intubation and prolongation in the intensive care unit and extended hospitalization. A number of risk factors for MC have been reported, including chronic MG (more than 6 years), pre-existing respiratory disease, large doses of pyridostigmine, severe bulbar weakness, thymoma, major postoperative complications, and marginal preoperative vital capacity.8,34–36 Furthermore, weaning patients with MC from mechanical ventilation is often difficult, and the ideal time for extubation is uncertain. It has been shown that older age, male sex, history of previous crisis, development of pulmonary complications, in particular atelectasis, and intubation for more than 10 days are associated with extubation failure.6,37 Moreover, extubation failure leads to poorer outcomes.38
The first-line therapies for the treatment of MC are IVIg and PLEX.39,40 Although there is some evidence to suggest PLEX may be more effective than IVIg in the treatment of MC,41 other studies have found these treatments to be equally effective.20,42 In addition, PLEX has been used to prepare patients for thymectomy and has been shown to improve postoperative outcomes.18 Isolated reports have described cases of IVIg as also being able to prevent MC.23,43 However, there have been no randomized controlled and double-blind trials to examine the effectiveness of preoperative treatment with IVIg in preventing MC in surgical MG patients. Although they were obtained in a limited number of patients, the results reported here are therefore new and clinically relevant.
This is the first prospective study to evaluate the protective capacity of IVIg against postoperative MC in patients with MG who are going to undergo surgical procedures under general anaesthesia. In addition to thymectomy, this is also the first time that other major and high-risk surgical procedures (cardiothoracic and abdominal surgery), as well as low-risk interventions (gynaecological, urological, orthopaedic and endoscopic procedures) have been evaluated. Our study was designed following the recommendations for clinical research standards27 and the international consensus guidance for the management of MG of the MGFA.44 Only one case of MC occurred in this study, in a patient assigned to the placebo group, and none of the patients treated preoperatively with IVIg developed MC. Given the baseline characteristics of the study population, it therefore seems that in MG subjects with preoperative QMG score < 8 and preoperative FVC > 70%, no MC can be expected, regardless of age, MGFA class at onset, BMI, anti-AChR antibody level, and dosage of pyridostigmine or other immunosuppressant drugs. On the other hand, we were unable to identify predictors of postoperative MC, such as vital capacity < 80%, disease duration < 3 months, or the recently reported bulbar symptoms immediately before thymectomy45 in a study that developed a scoring system to predict the possibility of postoperative MC. We also failed to identify other risk factors for MC shown in other studies, such as high BMI, high doses of pyridostigmine, anti-AChR titres, thymoma, history of MC, dysphagia, bulbar symptoms, or intraoperative blood loss. Pretreatment with IVIg administered before thymectomy and other surgical procedures may consequently be omitted, based on our results for the placebo and IVIg groups. It is possible that the high cost of IVIg and potentially secondary effects of high-dose IVIg are not justified, provided that MG patients have a preoperative QMG score < 8, good vital capacity, and the surgical procedure takes place in hospitals with MG units and experienced neurologists, anaesthetists, and surgeons experienced in the medical and surgical care of MG patients.
The number of thymectomies performed during the study period of 16 procedures is substantially higher than in other studies of the outcome of thymectomy. In the MGTX study11 36 sites and 6 years were required to recruit 66 patients treated with thymectomy, with a ratio of 0.3 patients/centre/year. In our single-centre study, 16 patients underwent thymectomy over a 4-year period, with a ratio of 4 patients/centre/year. Evaluation of the preparation regimes for surgical procedures other than thymectomy was also a distinctive feature of the study.
The main limitations of this study were that the MG patients at baseline were in good condition, with QMG scores and vital capacity close to normal values. Before surgery, MG patients must achieve a postintervention status14 that is close to minimal manifestations or improvement. The fact that only one patient presented with MC means that statistical conclusions cannot be drawn, and logistic regression analysis with rare events also fails to provide a solution.
Taken together, our findings indicate that preoperative treatment with IVIg to prevent MC does not appear to be justified in well-controlled MG patients undergoing surgical procedures with general anaesthesia in high-volume MG centres. Plasmapheresis is also an effective treatment for MC, but no controlled studies have been performed to compare IVIg and plasmapheresis.46,47 On the other hand, use of IVIg as a maintenance therapy or as a steroid-sparing agent has been studied to a limited extent.47 Better knowledge of the main cells and pathways involved in the immune network of MG have led to emerging target-specific biologics with promising clinical relevance for treating early, acute and chronic MG and patients with refractory disease.47–49
Footnotes
Authors’ note: This paper was presented at the Clinical Trials Plenary Session of the 71st Annual Meeting of the American Academy of Neurology, Philadelphia, USA, 4–10 May 2019.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a Fondo de Investigación Sanitaria (FIS-FEDER) (grants PI16-01673 and PI19/00593) and an Interlaken Research Award Programme (2012-12094537).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Josep Gamez  https://orcid.org/0000-0003-3127-7486
https://orcid.org/0000-0003-3127-7486
Contributor Information
Josep Gamez, Department of Neurology, Vall d’Hebron University Hospital, Vall d’Hebron Research Institute (VHRI), European Reference Network on Rare Neuromuscular Diseases (ERN EURO-NMD), Department of Medicine, Universitat Autònoma de Barcelona. Passeig de la Vall d’Hebron 119-129, Barcelona E-08035, Spain.
María Salvadó, Department of Neurology, Vall d’Hebron University Hospital, Barcelona, Spain; Department of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain.
Francesc Carmona, Department of Genetics, Microbiology and Statistics, University of Barcelona, Barcelona, Spain.
Miriam de Nadal, Department of Anesthesiology and Intensive Care, Universitat Autònoma de Barcelona, Barcelona, Spain.
Laura Romero, Department of Thoracic Surgery, Vall d’Hebron University Hospital, Barcelona, Spain.
Daniel Ruiz, Department of Anesthesiology and Intensive Care, Universitat Autònoma de Barcelona, Barcelona, Spain.
Alberto Jáuregui, Department of Thoracic Surgery, Vall d’Hebron University Hospital, Barcelona, Spain.
Olga Martínez, Department of Anesthesiology and Intensive Care, Universitat Autònoma de Barcelona, Barcelona, Spain.
Javier Pérez, Department of Thoracic Surgery, Vall d’Hebron University Hospital, Barcelona, Spain.
Pilar Suñé, Department of Hospital Pharmacy, Vall d’Hebron University Hospital, Barcelona, Spain.
María Deu, Department of Thoracic Surgery, Vall d’Hebron University Hospital, Barcelona, Spain.
References
- 1. Ahmed S, Kirmani JF, Janjua A, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7: 129–141. [DOI] [PubMed] [Google Scholar]
- 2. Liu Z, Yao S, Zhou Q, et al. Predictors of extubation outcomes following myasthenic crisis. J Int Med Res 2016; 44: 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care. Semin Neurol 2004; 24: 75–81. [DOI] [PubMed] [Google Scholar]
- 4. Juel VC. Myasthenic crisis: smoother sailing ahead. Eur J Neurol 2009; 16: 775–756. [DOI] [PubMed] [Google Scholar]
- 5. Vacca VM. Myasthenia gravis and myasthenic crisis. Nurs Crit Care 2017; 12: 38–46. [Google Scholar]
- 6. Seneviratne J, Mandrekar J, Wijdicks EF, et al. Predictors of extubation failure in myasthenic crisis. Arch Neurol 2008; 65: 929–933. [DOI] [PubMed] [Google Scholar]
- 7. López-Cano M, Ponseti-Bosch JM, Espin-Basany E, et al. Clinical and pathologic predictors of outcome in thymoma-associated myasthenia gravis. Ann Thorac Surg 2003; 76: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 8. Xue L, Wang L, Dong J, et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur J Cardiothorac Surg 2017; 52: 692–697. [DOI] [PubMed] [Google Scholar]
- 9. Alshekhlee A, Miles JD, Katirji B, et al. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009; 72: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 10. Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2000; 55: 7–15. [DOI] [PubMed] [Google Scholar]
- 11. Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016; 375: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gamez J, Ponseti JM. Thymectomy for non-thymomatous myasthenia gravis: the end of controversy, albeit fifty years later. J Xianga Med 2017; 2: 4. [Google Scholar]
- 13. Howard JF., Jr. Myasthenia gravis–a summary. Clinical overview of MG, http://www.myasthenia.org/HealthProfessionals/ClinicalOverviewofMG.aspx. (accessed 25 March 2019).
- 14. Myasthenia Gravis Foundation of America. Thymectomy, http://www.myasthenia.org/LinkClick.aspx?fileticket=BIVoreOXJGo%3D. (accessed 25 March 2019).
- 15. Kas J, Kiss D, Simon V, et al. Decade-long experience with surgical therapy of myasthenia gravis: early complications in 324 transsternal thymectomies. Ann Thorac Surg 2001; 72: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 16. D’Empaire G, Hoaglin DC, Perlo VP, et al. Effect of prethymectomy plasma exchange on postoperative respiratory function in myasthenia gravis. J Thorac Cardiovasc Surg 1985; 89: 592–596. [PubMed] [Google Scholar]
- 17. Seggia JC, Abreu P, Takatani M. Plasmapheresis as preparatory method for thymectomy in myasthenia gravis. Arq Neuropsiquiatr 1995; 53: 411–415. [DOI] [PubMed] [Google Scholar]
- 18. Sarkar BK, Sengupta P, Sarkar UN. Surgical outcome in thymic tumors with myasthenia gravis after plasmapheresis–a comparative study. Interact Cardiovasc Thorac Surg 2008; 7: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 19. El-Bawab H, Hajjar W, Rafay M, et al. Plasmapheresis before thymectomy in myasthenia gravis: routine versus selective protocols. Eur J Cardiothorac Surg 2009; 35: 329–397. [DOI] [PubMed] [Google Scholar]
- 20. Gajdos P, Chevret S, Clair B, et al. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol 1997; 41: 789–796. [DOI] [PubMed] [Google Scholar]
- 21. Rønager J, Ravnborg M, Hermansen I, et al. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artif Organs 2001; 25: 967–973. [DOI] [PubMed] [Google Scholar]
- 22. Barth D, Nabavi Nouri M, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011; 76: 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhawan PS, Goodman BP, Harper CM, et al. IVIG versus PLEX in the treatment of worsening myasthenia gravis: what is the evidence? A critically appraised topic. Neurologist 2015; 19: 145–148. [DOI] [PubMed] [Google Scholar]
- 24. Pérez Nellar J, Domínguez AM, Llorens-Figueroa JA, et al. [A comparative study of intravenous immunoglobulin and plasmapheresis preoperatively in myasthenia.] Rev Neurol 2001; 33: 413–416. [PubMed] [Google Scholar]
- 25. Huang CS, Hsu HS, Kao KP, et al. Intravenous immunoglobulin in the preparation of thymectomy for myasthenia gravis. Acta Neurol Scand 2003; 108: 136–138. [DOI] [PubMed] [Google Scholar]
- 26. Alipour-Faz A, Shojaei M, Peyvandi H, et al. A comparison between IVIG and plasma exchange as preparations before thymectomy in myasthenia gravis patients. Acta Neurol Belg 2017; 117: 245–249. [DOI] [PubMed] [Google Scholar]
- 27. Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology 2000; 55: 16–23. [DOI] [PubMed] [Google Scholar]
- 28. Dalakas MC. Intravenous immune globulin therapy for neurologic diseases. Ann Intern Med 1997; 126: 721–730. [DOI] [PubMed] [Google Scholar]
- 29. Kuitwaard K, Van Doorn PA, Vermeulen M, et al. Serum IgG levels in IV immunoglobulin treated chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 2013; 84: 859–861. [DOI] [PubMed] [Google Scholar]
- 30. Berger M, Jolles S, Orange JS, et al. Bioavailability of IgG administered by the subcutaneous route. J Clin Immunol 2013; 33: 984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tortorici MA, Lawo JP, Weide R, et al. Privigen® has similar pharmacokinetic properties in primary and secondary immune deficiency. Int Immunopharmacol 2019; 66: 119–126. [DOI] [PubMed] [Google Scholar]
- 32. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80; 27–38. [Google Scholar]
- 33. Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102: 97–107. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe A, Watanabe T, Obama T, et al. Prognostic factors for myasthenic crisis after transsternal thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 2004; 127: 868–876. [DOI] [PubMed] [Google Scholar]
- 35. Chu XY, Xue ZQ, Wang RW, et al. Predictors of postoperative myasthenic crisis in patients with myasthenia gravis after thymectomy. Chin Med (Engl) 2011; 124: 1246–1250. [PubMed] [Google Scholar]
- 36. Gamez J. Intravenous immunoglobulin for preparing myasthenia gravis patients for thymectomy and other surgical procedures preventing myasthenic crisis. Clin Exp Immunol 2014; 178: 134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rabinstein AA, Mueller-Kronast N. Risk of extubation failure in patients with myasthenic crisis. Neurocrit Care 2005; 3: 213–215. [DOI] [PubMed] [Google Scholar]
- 38. Wu JY, Kuo PH, Fan PC, et al. The role of non-invasive ventilation and factors predicting extubation outcome in myasthenic crisis. Neurocrit Care 2009; 10: 35–42. [DOI] [PubMed] [Google Scholar]
- 39. Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261: 127–133. [DOI] [PubMed] [Google Scholar]
- 40. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist 2011; 1: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qureshi AI, Choudhry MA, Akbar MS, et al. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999; 52: 629–632. [DOI] [PubMed] [Google Scholar]
- 42. Murthy JM, Meena AK, Chowdary GV, et al. Myasthenic crisis: clinical features, complications and mortality. Neurol India 2005; 53: 37–40. [DOI] [PubMed] [Google Scholar]
- 43. Jensen P, Bril V. A comparison of the effectiveness of intravenous immunoglobulin and plasma exchange as preoperative therapy of myasthenia gravis. J Clin Neuromusc Dis 2008; 9: 352–355. [DOI] [PubMed] [Google Scholar]
- 44. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis. Executive summary. Neurology 2016; 87: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanai T, Uzawa A, Sato Y, et al. A clinical predictive score for postoperative myasthenic crisis. Ann Neurol 2017; 82: 841–849. [DOI] [PubMed] [Google Scholar]
- 46. Gotterer L, Li Y. Maintenance immunosuppression in myasthenia gravis. J Neurol Sci 2016; 369: 294–302. [DOI] [PubMed] [Google Scholar]
- 47. Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol 2019; 15: 113–124. [DOI] [PubMed] [Google Scholar]
- 48. Behin A, Le Panse R. New pathways and therapeutic targets in autoimmune myasthenia gravis. J Neuromuscul Dis 2018; 5: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers 2019; 5: 30. [DOI] [PubMed] [Google Scholar]