Abstract
Background:
Nanoliposomal irinotecan (nal-IRI) plus 5-fluorouracil/leucovorin (5-FU/LV) is a novel treatment option for gemcitabine-pretreated metastatic pancreatic adenocarcinoma (PAC) patients, but real-world evidence is rare. Our aim was to determine the effectiveness and tolerability of this regimen in advanced PAC patients and to compare it with oxaliplatin plus fluoropyrimidines in the second-line setting after failure of gemcitabine.
Methods:
This is a retrospective single-center analysis of all patients who have been treated with nal-IRI plus 5-FU/LV. To compare its effectiveness with other second-line treatment options, all patients who had received oxaliplatin plus fluoropyrimidines after gemcitabine-based chemotherapy were also eligible for analysis.
Results:
Fifty-two patients were treated with nal-IRI plus 5-FU/LV between April 2016 and August 2018. The median progression-free survival (PFS) was 3.84 months and the median overall survival (OS) was 6.79 months. Median OS from the beginning of the treatment for advanced disease was 19.9 months. Median PFS in patients that received nal-IRI plus 5-FU/LV as second-line treatment after gemcitabine-based chemotherapy was 4.49 months whereas median PFS in a matched cohort of patients treated with oxaliplatin plus fluoropyrimidines was 3.44 months (p = 0.007). Between these two groups the median OS of patients with CA 19-9 levels above the statistical median (⩾772.8 kU/l) differed significantly (9.33 versus 6.18 months, p = 0.038).
Conclusion:
Our data confirms the effectiveness of nal-IRI plus 5-FU/LV treatment as a well-tolerated regimen in the treatment of advanced PAC and extends available data on its use as a second-line treatment option when compared with oxaliplatin plus fluoropyrimidines.
Keywords: 5-fluorouracil, liposomal irinotecan, oxaliplatin, pancreatic cancer, second-line treatment
Introduction
Pancreatic ductal adenocarcinoma (PAC) is a lethal disease with a devastating 5-year overall survival (OS) of approximately 7%. Although, just 4% of all malignant diseases are attributed to PAC, it is projected to become the second leading cause of cancer-related deaths in the United States before 2030.1 Since the introduction of the new chemotherapy regimens including albumin-bound paclitaxel (nab-paclitaxel) plus gemcitabine and FOLFIRINOX, after the gemcitabine monotherapy era, survival of patients with PAC has improved.2,3 This led to a change in the before rather theoretical debate about second-line treatment in the management of PAC and opened the clinical field for the exploration of continuum of care strategies. In 2015, there was the first approval of a second-line treatment option for patients with advanced PAC who have been previously treated with gemcitabine-based chemotherapy based upon the results of the phase III NAPOLI-1 trial.4 In this trial, 417 patients with metastatic PAC were randomized to three treatment arms and the combination treatment with nanoliposomal irinotecan (nal-IRI) and 5-fluorouracil/leucovorin (5-FU/LV) demonstrated superior survival compared with 5-FU/LV monotherapy (median OS of 6.1 versus 4.2 months; p = 0.012).
Real-world clinical data about safety, effectiveness, dosing schedules as well as integration in a continuum of care treatment algorithm is still rare and therefore important to expand our knowledge on the performance of this therapy. Thus, we aimed to summarize our clinical experience with the use of nal-IRI plus 5-FU/LV by this retrospective, single-institution analysis. Furthermore, we compared the clinical effectiveness of matched second-line treatments cohorts who received either the combination treatment of nal-IRI plus 5-FU/LV or oxaliplatin plus fluoropyrimidines, which is another available second-line treatment option in the management of patients with advanced PAC after failure of gemcitabine.5–7
Materials and methods
Subjects and study design
This is a single-center, retrospective, observational study including patients with histologically proven nonresectable PAC that was either locally advanced or metastasized who were treated at the Medical University of Vienna between January 2012 and August 2018. For the primary study cohort the data for all patients that received treatment with one or more doses of nal-IRI plus 5-FU/LV was retrieved. For the comparison of second-line systemic treatments the data for all patients who had received combination treatment with nal-IRI and 5-FU/LV or oxaliplatin and fluoropyrimidines as second-line treatment after gemcitabine-based first-line treatment was received. The electronic medical history was queried for patient demographics, performance status [Eastern Cooperative Oncology Group (ECOG)], date of diagnosis, date of advanced disease, diagnosis and carbohydrate antigen 19-9 (CA 19-9) level at baseline, bilirubin at baseline, prior treatment details and duration, treatment starting dose, treatment dose reductions, treatment duration, adverse events, and progression-free survival (PFS) and OS. All treatment-related adverse events were graded per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE V4.0). To assess effectiveness, the individual patient’s response was determined every 8–12 weeks by computed tomography (CT). Disease response was assessed using RECIST version 1.1 criteria. Response was evaluated by independent radiological review. Date of disease progression on treatment and date of death were recorded. Informed consent was obtained for data analysis. The here presented data analysis received prior approval by the ethical committee of the Medical University of Vienna (EK.No. 1806/2017) and was performed according to the Helsinki criteria for good scientific practice.
Descriptive statistics were calculated as mean, median, or percentages as appropriate. PFS was calculated from the time of first treatment administration to disease progression or death. OS was calculated from time of first treatment administration to death. OS and PFS were depicted by Kaplan–Meier plots. For subgroup comparisons of these variables, the log rank test and the Breslow test (in the case of nonconstant hazard ratios) were used, respectively. The assessment of constant and nonconstant hazard ratios was based on a graphical approach. Multiple Cox regression analysis was used to investigate the influence of covariates on OS. No formal sample size calculation has been performed because of the retrospective character of the study. Statistical analysis was performed using the open-source R statistical software package, version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism Software Prism 7 for Windows, Version 7.03, 20 February 2017 (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
A total of 52 patients with advanced PAC were identified who received treatment with nal-IRI plus 5-FU/LV at our institution between April 2016 and August 2018. Table 1 lists patient and tumor characteristics of the entire nal-IRI plus 5-FU/LV study cohort.
Table 1.
Characteristics of patients and tumors of the whole study cohort that received nal-IRI plus 5-FU/LV.
| n = 52 (%) | |
|---|---|
| Median age at diagnosis of advanced disease (years, range) | 64.6 (58.9–73.1) |
| Gender | |
| Male | 20 (38) |
| Female | 32 (62) |
| Stage at beginning of treatment with nal-IRI + 5-FU/LV | |
| Locally advanced unresectable diseases | 4 (8) |
| Metastatic | 48 (92) |
| CA 19-9 levels in kU/l at onset of treatment with nal-IRI + 5-FU/LV | |
| within normal range | 11 (21) |
| above normal range | 38 (73) |
| n/a | 3 (6) |
| Median CA 19-9 levels in kU/l at onset of treatment with nal-IRI + 5-FU/LV (range) | 399 (30–4730) |
| Site of metastatic disease | |
| Liver | 36 (69) |
| Lung | 9 (17) |
| Peritoneal | 13 (25) |
| Other | 7 (13) |
| Number of metastatic sites | |
| 0 | 4 (8) |
| 1 | 33 (63) |
| 2 | 13 (25) |
| ⩾3 | 2 (4) |
| ECOG performance status | |
| 0 | 21 (40) |
| 1 | 26 (50) |
| 2 | 1 (2) |
| n/a | 4 (8) |
| Prior lines of advanced disease therapy | |
| 0 | 1 (2) |
| 1 | 30 (58) |
| 2 | 14 (27) |
| 3 or more | 7 (13) |
ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9; n/a, not available; nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
The median age was 64.6 years [interquartile range (IQR) 58.9–73.1 years]. The majority of patients presented with metastatic disease at the start of treatment with nal-IRI plus 5-FU/LV and only four patients (8%) had locally advanced disease that was technically not resectable. CA 19-9 levels at the time of initiation of treatment were elevated in 38 patients (73%) and median CA 19-9 levels were 399 kU/l (IQR 30–4730 kU/l). Liver was the predominant site of metastatic spread (69%), followed by peritoneum (25%) and lungs (17%). Nearly two thirds of all patients (63%) had one organ affected from metastasis, whereas 15 patients (29%) had two or more metastatic sites. Most of the patients (90%) had an ECOG performance status of 0 or 1, whereas only one patient (2%) had an ECOG performance status of 2. In four patients (8%) data was not available.
A total of 60% of patients received nal-IRI plus 5-FU/LV before third-line treatment and the other 40% of patients received this treatment after second-line treatment. Concerning the pre nal-IRI systemic treatment, there was one patient (2%) who received nal-IRI due to onset of new metastatic lesions after previous adjuvant treatment with gemcitabine. All other patients experienced disease progression under a gemcitabine-based chemotherapy in a previous treatment line for advanced disease. For a detailed list of administered chemotherapies, we refer the reader to Table 2.
Table 2.
Administered chemotherapies before nal-IRI treatment.
| Line of nal-IRI treatment | 2nd line n = 30 (%) |
3rd line n = 14 (%) |
4th line n = 6 (%) |
5th line n = 1 (%) |
|---|---|---|---|---|
| Previous therapy | ||||
| Gemcitabine | 30 (100) | 14 (100) | 6 (100) | 1 (100) |
| Nab-paclitaxel | 25 (83) | 10 (71) | 4 (67) | 1 (100) |
| Oxaliplatin | 0 (0) | 10 (71) | 5 (83) | 1 (100) |
| Irinotecan | 0 (0) | 3 (21) | 4 (67) | 1 (100) |
| Fluoropyrimidines | 0 (0) | 11 (79) | 5 (83) | 1 (100) |
| Chemoradiation | 0 (0)) | 1 (7) | 2 (33) | 0 (0) |
| Erlotinib | 0 (0) | 1 (7) | 1 (17) | 0 (0) |
nal-IRI, nanoliposomal irinotecan.
Dosing schedules and dose reductions
Nearly all patients (98%) started with the full recommended dose of 80 mg/m² nal-IRI. The majority of patients with a nal-IRI dose reduction (86%) required only one dose reduction; two patients (14%) had two dose reductions. The average dose of patients with at least one dose reduction was 65 mg/m², which is an average relative dose of 81%. A total of 14 patients (27%) received a reduced dose of 56–64 mg/m² and two patients (6%) subsequently received a reduced dose of 34–40 mg/m². The main reason for dose reduction was diarrhea (50%), followed by nausea (15%) as well as fatigue, emesis, thrombocytopenia, neutropenia, and mucositis (each 7%). Details are listed in Table 3.
Table 3.
Dosing and dose reductions.
| n = 52 (%) | |
|---|---|
| Starting nal-IRI dose (mg/m²) | |
| 80 | 51 (98) |
| 56 | 1 (2) |
| Dose reductions | n = 14 (%) |
| 0 | 38 (73) |
| 1 | 12 (23) |
| 2 | 2 (4) |
| Reason for dose reduction | |
| Diarrhea | 7 (50) |
| Nausea | 2 (15) |
| Vomiting | 1 (7) |
| Fatigue | 1 (7) |
| Emesis | 1 (7) |
| Thrombocytopenia | 1 (7) |
| Neutropenia | 1 (7) |
| Mucositis | 1 (7) |
| Reduced doses | |
| 56–64 mg/m² | 14 (27) |
| 34–40 mg/m² | 2 (4) |
| Average overall dose (mg/m²) (% of maximal administrable dose) | 581 (96) |
| Average dose of patients with at least one dose reduction (mg/m²) (% of recommended dose) | 65 (81) |
nal-IRI, nanoliposomal irinotecan.
Effectiveness
Median PFS for the entire cohort was 3.84 months and the median OS was 6.79 months. Two patients had a complete response (4%) and eight patients a partial response (15%) according to RECIST 1.1 criteria. The overall response rate (ORR) was 19.2%. Fourteen patients (27%) had stable disease (SD). Together, with the responding patients this resulted in a disease control rate (DCR) of 46.2%. A total of 27 patients (52%) experienced immediate disease progression during nal-IRI plus 5-FU/LV and in one patient (2%) data concerning response to therapy was not available. The median OS from beginning of first-line systemic treatment for the entire patient cohort was 19.9 months. For the Kaplan–Meier curves we refer the reader to Figure 1 and for details to Table 4.
Figure 1.
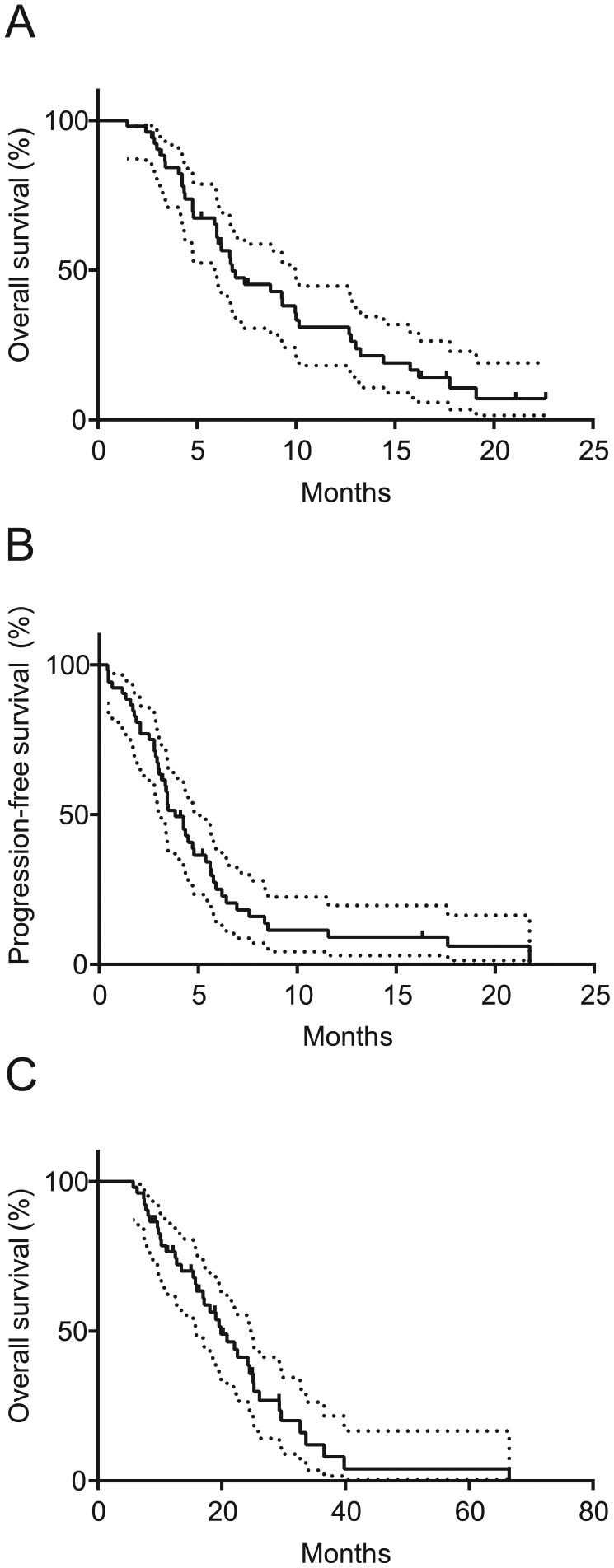
Kaplan–Meier survival analysis, overall cohort of patients who were treated with nal-IRI plus 5- FU/LV.
Overall survival and progression-free survival of all patients who were treated with nal-IRI plus 5-FU/LV as second-line treatment (A, B) and from start of first-line advanced disease treatment (C).
nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
Table 4.
Overall effectiveness and response to treatment with nal-IRI plus 5-FU/LV.
| n = 52 (%) | |
|---|---|
| PFS (median, months) | 3.84 |
| OS (median, months) | 6.79 |
| Response rate | |
| Complete response | 2 (4) |
| Partial response | 8 (15) |
| Stable disease | 14 (27) |
| Progressive disease | 27 (52) |
| Not evaluable | 1 (2) |
| Overall response rate (%) | 19.2 |
| Disease control rate (%) | 46.2 |
PFS, progression-free survival; OS, overall survival; nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
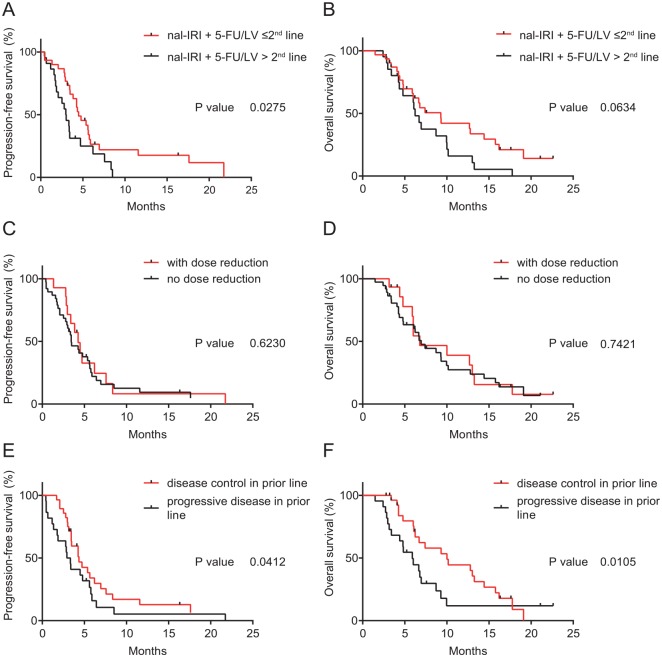
When comparing patients who received nal-IRI plus 5-FU/LV in an earlier treatment line (at most second line) opposed to in a later line (third or later line), a significant correlation with PFS but not OS was observed (Figure 2A and B). Median PFS was 4.49 and 3 months [p = 0.0275; hazard ratio (HR) 0.53, 95% confidence interval (CI) 0.28–1.01]. Dose reductions did not result in significant differences in PFS or OS (Figure 2C and D). Patients who had at least one dose reduction compared with none had a median PFS of 4.26 and 3.44 months (p = 0.6230; HR 0.86, 95% CI 0.46–1.6) and a median OS of 6.95 and 6.79 months (p = 0.7421; HR 0.89, 95% CI 0.46–1.7). A disease control (response or stable disease) in the last treatment line prior to nal-IRI plus 5-FU/LV therapy was significantly associated with PFS and OS (Figure 2E and F). Patients who experienced disease control in the prior treatment line compared with nonresponding patients had a median PFS of 4.36 versus 3.13 months (p = 0.0412; HR 0.65, 95% CI 0.35–1.20). The median OS was 10.0 versus 5.9 months (p = 0.0105; HR 0.60, 95% CI 0.31–1.17).
Figure 2.
Kaplan–Meier survival analysis, subgroup analysis of patients who were treated with nal-IRI plus 5- FU/LV.
Progression-free survival and overall survival of patients who were treated with nal-IRI plus 5-FU/LV at most second line versus third or later line (A, B), with no dose reduction versus with dose reduction (C, D), with a disease control in the treatment line before nal-IRI plus 5-FU/LV chemotherapy versus progressive disease in the previous treatment line (E, F).
nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
Safety
The most common treatment-related adverse events in patients receiving nal-IRI plus 5-FU/LV were anemia, diarrhea, nausea, fatigue, anorexia, and neutropenia. There was only one case with a nonfebrile grade 4 neutropenia. All other higher-grade adverse events were grade 3. Consistent with the toxicity profile of nal-IRI, the majority of these grade 3 adverse events were gastrointestinal symptoms (Table 5).
Table 5.
Adverse events and serious (grade 3 or 4) adverse events reported.
| Toxicities | Any grade (%) | Grade 3/4 (%) |
|---|---|---|
| Nausea | 23 (40) | 6 (12) |
| Vomiting | 9 (17) | 4 (8) |
| Diarrhea | 23 (40) | 1 (2) |
| Fatigue | 19 (37) | 1 (2) |
| Anorexia | 18 (35) | 0 (0) |
| Thrombocytopenia | 14 (27) | 2 (4) |
| Neutropenia | 19 (37) | 3 (6) |
| Anemia | 47 (90) | 3 (6) |
Effectiveness of second-line treatment with nal-IRI plus 5-FU/LV after gemcitabine-based chemotherapy
To compare the effectiveness of second-line treatments, all patients who received second-line treatment after a gemcitabine-based first line chemotherapy in our cancer center between January 2012 and August 2018 were analyzed. From a total of 101 patients, the two largest cohorts were treated with either nal-IRI plus 5-FU/LV (referred to going forward as nal-IRI second line group, n = 30) or oxaliplatin plus fluoropyrimidines (referred to going forward as oxaliplatin second-line group, n = 31). Baseline characteristics were balanced between the two groups, including age, disease stage, CA 19-9 levels as well as the number of metastatic sites. In both groups liver was the predominant metastatic site (67% and 71%). In the nal-IRI second-line group, the peritoneum was the second most common site of metastasis (30%), whereas in the oxaliplatin second-line group, the lung was the second most common (32%). In both groups the majority of patients received nab-paclitaxel plus gemcitabine as first-line treatment (83% in the nal-IRI second-line group versus 97% in the oxaliplatin second-line group; Table 6). Mean disease assessment intervals were nearly identical between the two groups. From start of the therapy and first reassessment, mean values were 2.65 ± 0.64 (SD) months versus 2.67 ± 0.68 months and from first and second reassessment these were 2.83 ± 0.73 months versus 2.69 ± 0.76 months. There was no statistically significant difference between these intervals (Kolmogorov–Smirnov test, p = 0.26 and 0.24).
Table 6.
Characteristics of patients and tumors in second-line treatment with either nal-IRI plus 5-FU/LV or oxaliplatin plus fluoropyrimidines after failure of first-line gemcitabine-based chemotherapy.
| nal-IRI + 5-FU/LV n = 30 |
Oxaliplatin + fluoropyrimidines n = 31 |
|
|---|---|---|
| Median age at beginning of second-line therapy (years, range) | 66 (59–73) | 66 (59–73) |
| Gender | ||
| Male | 12 (40) | 15 (48) |
| Female | 18 (60) | 16 (52) |
| Stage at beginning of second-line systemic treatment | ||
| Locally advanced (unresectable disease) | 2 (7) | 0 (0) |
| Metastatic | 28 (93) | 31 (100) |
| CA 19-9 levels (kU/l) at onset of second-line treatment | ||
| Within normal range | 4 (13) | 1 (3) |
| Above normal range | 24 (80) | 26 (84) |
| n/a | 2 (7) | 4 (13) |
| Median CA 19-9 levels (kU/l) at onset of second-line treatment (range) | 706 (91–3733) | 989 (123–8198) |
| Site of metastatic disease | ||
| Liver | 20 (67) | 22 (71) |
| Lung | 2 (7) | 10 (32) |
| Peritoneal | 9 (30) | 4 (13) |
| Other | 5 (17) | 3 (10) |
| Number of metastatic sites | ||
| 0 | 2 (7) | 0 (0) |
| 1 | 20 (67) | 23 (74) |
| 2 | 8 (26) | 8 (26) |
| ECOG performance status | ||
| 0/1 | 28 (93) | 28 (90) |
| 2 | 0 (0) | 2 (6) |
| n/a | 2 (7) | 1 (4) |
| Prior systemic treatment regiment | ||
| Gemcitabine plus nab-paclitaxel | 25 (83) | 30 (97) |
| Gemcitabine mono | 5 (17) | 1 (3) |
ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9; n/a, not available; nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
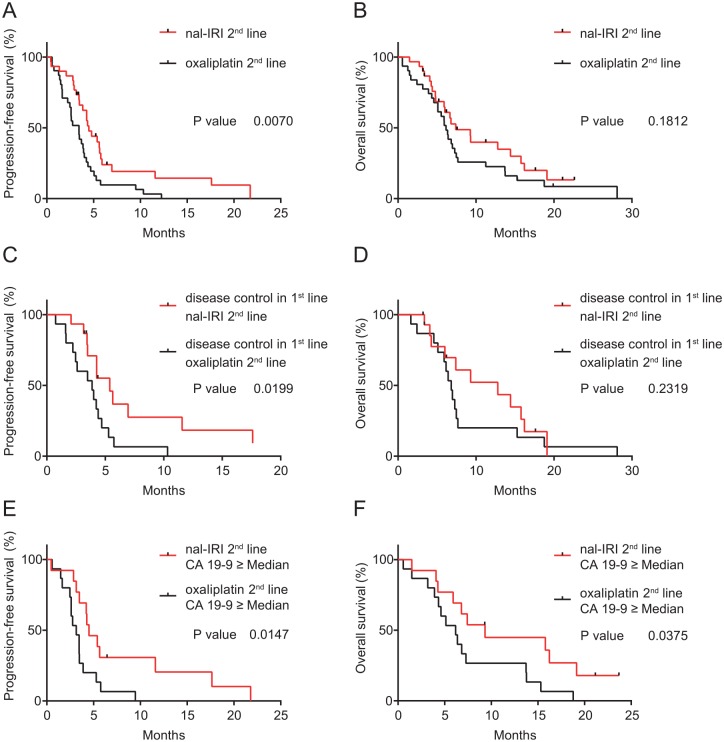
When comparing the effectiveness of these two treatments in second line after previous gemcitabine-based chemotherapy, there was a statistically significant difference in PFS, but not OS. Median PFS in the nal-IRI 2nd line group was 4.49 months and 3.44 months (p = 0.0070; HR 0.47, 95% CI 0.27–0.81) and the median OS was 7.41 months and 6.16 months (p = 0.1812; HR 0.68, 95% CI 0.39–1.20); see Figure 3A and B.
Figure 3.
Kaplan–Meier analysis, comparison of second-line cohorts nal-IRI plus 5-FU/LV and oxaliplatin plus fluoropyrimidines.
Progression-free survival and overall survival of patients who were treated with nal-IRI plus 5-FU/LV versus oxaliplatin plus fluoropyrimidines as second-line treatment after previous gemcitabine-based chemotherapy (A, B), with disease control in the first-line treatment versus with progressive disease (C, D) and with CA 19- 9 levels ⩾ median at start of second-line treatment (E, F).
nal-IRI, nanoliposomal irinotecan; 5-FU/LV, 5-fluorouracil/leucovorin.
To identify subgroups that were associated with survival, response to the first-line chemotherapy and CA 19-9 levels before initiation of second-line chemotherapy were analyzed. The response to first-line chemotherapy was significantly associated with PFS but again not with OS. Patients whose disease was stable or responded to gemcitabine-based first-line chemotherapy, who were treated with nal-IRI plus 5-FU/LV had a median PFS of 5.38 months compared with 3.87 months in patients with disease control in first-line therapy that received oxaliplatin plus fluoropyrimidines (p = 0.0199; HR 0.4242, 95% CI 0.19–0.94); see Figure 3C and D. However, PFS in patients who had progressive disease in first-line gemcitabine-based chemotherapy was not significantly different when compared with patients who were treated with nal-IRI plus 5-FU/LV or with oxaliplatin plus fluoropyrimidines (median PFS 4.64 versus 2.69 months, p = 0.3061; HR 0.67, 95% CI 0.31–1.47). Patients with CA 19-9 levels greater than or equal to the statistical median (⩾772.8 kU/l) differed also significantly in survival between these two different second-line treatments. The median PFS and OS was 4.50 (nal-IRI plus 5-FU/LV) versus 3.15 months (p = 0.0147; HR 0.35, 95% CI 0.15–0.81) and 9.33 versus 6.18 months (p = 0.0375; HR 0.41, 95% CI 0.18–0.95) The proportion of patients without progression at 4 months, where all patients still on treatment had already had their first disease reassessment, was higher when they were treated with nal-IRI plus 5-FU/LV in the comparison between overall cohorts (60% versus 35%) and subgroups according to prior disease progression in first line and high CA 19-9 levels (67% versus 53% and 77% versus 27%); see Figure 3E and F. Results for effectiveness are shown in Table 7.
Table 7.
Comparison of overall effectiveness and response rates between the oxaliplatin and nal-IRI second line cohorts.
| Entire second-line cohorts | ||
|---|---|---|
| nal-IRI plus 5-FU/LV n = 30 |
Oxaliplatin + fluoropyrimidines n = 31 |
|
| PFS (median, months) | 4.49 | 3.44 |
| OS (median, months) | 7.41 | 6.16 |
| Response rate | ||
| Complete response | 2 (7) | 0 (0) |
| Partial response | 5 (16) | 2 (6) |
| Stable disease | 11 (27) | 11 (36) |
| Progressive disease | 12 (40) | 17 (55) |
| Not evaluable | 0 (0) | 1 (3) |
| Overall response rate (%) | 23.3 | 6 |
| Disease control rate (%) | 60 | 42 |
| Subgroups among second-line cohorts | ||
| PFS, disease control in first line (median, months) | 5.38 | 3.87 |
| PFS, no disease control in first line (median, months) | 4.64 | 2.69 |
| PFS, CA 19-9 ⩾ median (median, months) | 4.50 | 3.15 |
| OS, CA 19-9 ⩾ median (median, months) | 9.33 | 6.18 |
PFS, progression-free survival; OS, overall survival; nal-IRI, nanoliposomal irinotecan; CA 19-9, carbohydrate antigen 19-9; 5-FU/LV, 5-fluorouracil/leucovorin.
Discussion
New effective first- and second-line treatment options in advanced PAC have been introduced in the clinic within the last few years.2–7 These have invigorated the debate about the optimal sequencing strategy for the management of this difficult-to-treat malignancy. The aim of this study was to address fields of uncertainty that medical oncologists worldwide face on a daily basis in the treatment of patients with advanced PAC when having to choose between different treatment options. Reports about post-approval effectiveness and tolerability of treatment with nal-IRI plus 5-FU/LV are still scarce and therefore researchers are urged to expand the experience for this treatment option. This is also the first analysis to compare treatment with nal-IRI plus 5-FU/LV with another commonly used second-line treatment option, oxaliplatin plus fluoropyrimidines, after failure of first-line gemcitabine-based chemotherapy.
Our cohort of patients who have been treated with nal-IRI plus 5-FU/LV matches well to the cohort studied in the NAPOLI-1 trial.4 This includes performance status (90% of all patients in our study had an ECOG performance status of 0 or 1 compared with 91% of patients with a Karnofsky index of 100, 90, or 80 in the NAPOLI-1 trial) as well as previous treatment lines (58% with one prior treatment line compared with 53%, 40% with two or more prior treatment lines compared with 34%). Our data concerning safety and tolerability is also comparable with the data reported in the NAPOLI-1 trial, although we observed decreased rates for diarrhea and vomiting (40% versus 59%, 17% versus 52%) as well as for other serious adverse events. The lower rates for gastrointestinal toxicities might be related to the vigorous pre-emptive administration of antiemetic and antidiarrheal drugs at our center.
Concerning the clinical outcome, results for effectiveness in our study are comparable with those reported in the NAPOLI-1 trial (median PFS 3.84 versus 3.1 months, median OS 6.79 versus 6.1 months). Another recently published real-world data study from Glassman et al. reported a median PFS of 2.9 months and median OS of 5.3 months.8 A crossover comparison between the clinical outcome from the overall study cohorts of our and the former mentioned study is not permissible because the study populations were different. Notably, the proportion of patients who received nal-IRI plus 5-FU/LV in second line was higher in our cohort (60% versus 43%).
With respect to dose modification, we have not observed a significant association between dose reductions and clinical outcome. This is in line with the updated results from the NAPOLI-1 study and the results reported by Glassman et al., where the authors reported that survival between patients with or without a dose reduction did not differ significantly.9 This demonstrates that appropriate dose modification of nal-IRI plus 5-FU/LV due to adverse events may not unfavorably affect outcomes. Prospective clinical trials should be undertaken to address different dosing strategies.
We also observed a significantly longer PFS in patients who received nal-IRI plus 5-FU/LV in second line compared with patients who received this treatment after second line. For OS there was a trend towards longer survival if this treatment was administered before third line, but the difference did not reach statistical significance. This is line with the results from the study of Glassman et al. and use of nal-IRI in the frontline setting is currently under clinical investigation (ClinicalTrials.gov identifier: NCT02551991).
The category 1 recommendation by the current treatment guidelines from the National Comprehensive Cancer Network (NCCN) is limited to nal-IRI plus 5-FU/LV as second-line treatment after first-line treatment with a gemcitabine-based chemotherapy.10 Other options include the use of oxaliplatin plus fluoropyrimidines, but are only supported by conflicting results from clinical phase III trials (CONKO-01, CONKO-003, and PANCREOX).5–7 The role of the classical irinotecan in combination with 5-FU/LV is even less examined and is extrapolated from the experience in other gastrointestinal malignancies.11 In a phase II trial, 61 patients with advanced PAC were randomized to folinic acid–fluorouracil–irinotecan (FOLFIRI) or folinic acid–fluorouracil–oxaliplatin (FOLFOX), but no difference in survival was found.12 A direct comparison of the new chemotherapy regimen nal-IRI with oxaliplatin or classical irinotecan however is still lacking. Therefore, an intriguing finding of our study is the survival difference between patients who received treatment with nal-IRI plus 5-FU/LV compared with oxaliplatin plus fluoropyrimidines in the second-line setting after previous gemcitabine-based chemotherapy. Given that high baseline CA 19-9 serum levels represent an independent factor for poor prognosis, our observation is of particular significance when choosing between these two different treatments.13 Conclusion about superior effectiveness cannot be drawn. However, in respect of the encouraging real-world experience with nal-IRI plus 5-FU/LV in an unselected patient population gives further evidence that this treatment option is a preferable choice as second-line treatment after gemcitabine-based therapy.
Our study has several limitations including its retrospective design, conduction in a single treatment center, inclusion of patients who crossed over treatment arms and the fact that the choice of a second regimen was at the discretion of the treating physician. However, all patients who were treated in this academic center with nal-IRI plus 5-FU/LV or oxaliplatin plus fluoropyrimidines in the second-line setting after gemcitabine-based chemotherapy were included in this analysis, without any selection bias. Furthermore, the relatively small sample size limits the interpretation of subgroup analysis. Crossover from nal-IRI to oxaliplatin treatment or vice versa could also have an impact on the results for OS, which should be considered when interpreting the observed differences in median OS in the subgroup analysis of patients with high CA 19-9 levels. A possible variability in disease assessment intervals can also have an influence on the results for PFS, which can be problematic if this is used as a comparative endpoint. In our study, the disease assessment intervals were nearly identical and there was no statistically significant difference between the intervals. Results for response rates are also superior for the treatment with nal-IRI plus 5-FU/LV in the second-line setting compared with oxaliplatin treatment. This is why we assume that in our study the observed differences for PFS between different treatment groups are not caused by a possible variability in disease assessment intervals.
The effectiveness of nal-IRI plus 5-FU/LV in our study is encouraging. Prospective randomized trials are now urged to validate the findings of our analysis, especially when considering our results for the head-to-head comparison between the different second-line treatment cohorts.
Acknowledgments
We thank Ernst Fladnitzer for helping with the data acquisition concerning chemotherapy doses, Julia Stefanie Brunner for formatting of figures, and Omar Sharif for assistance with English language editing. Biostatistical support was provided by ASOKLIF (Arbeitsgruppe zur Systemoptimierung klinischer Forschungsprojekte).
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: GWP has received Advisory Board or Speaker fees from Celgene, Shire, and Halozyme, Servier, Taiho, Bayer, Merck, Amgen, Lilly, BMS and Sanofi. MK received travel support from Merck, Bayer, Bristol-Myers Squibb and Roche and has participated in advisory board meetings from Bayer. All other authors declare no conflicts of interest.
ORCID iD: Markus Kieler  https://orcid.org/0000-0002-4082-5731
https://orcid.org/0000-0002-4082-5731
Contributor Information
Markus Kieler, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Austria.
Matthias Unseld, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Austria.
Daniela Bianconi, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Austria.
Werner Scheithauer, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Austria.
Gerald W. Prager, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Waehringer Guertel 18–20, 1090 Vienna, Austria.
References
- 1. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 5. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011; 47: 1676–1681. [DOI] [PubMed] [Google Scholar]
- 6. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 7. Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 8. Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018; 18: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang-Gillam A, Hubner R, Mirakhur B, et al. Dose modifications of liposomal irinotecan (nal-IRI) + 5-fluorouracil/leucovorin (5-FU/LV) in NAPOLI-1: impact on efficacy. J Clin Oncol 2018; 36: 388–388. [Google Scholar]
- 10. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 1028–1061. [DOI] [PubMed] [Google Scholar]
- 11. Gebbia V, Maiello E, Giuliani F, et al. Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol 2010; 33: 461–464. [DOI] [PubMed] [Google Scholar]
- 12. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009; 101: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008; 9: 132–138. [DOI] [PubMed] [Google Scholar]