Abstract
Background:
There is no conclusion about the most important contributor to the upswing of locally advanced colorectal cancer (LACRC) survival.
Methods:
Data from the Surveillance, Epidemiology, and End Results (SEER) database was extracted to identify colorectal adenocarcinoma cancer patients at stage II and III diagnosed in the two periods 1989–1990 and 2009–2010. The statistical methods included Pearson’s chi-squared test, log-rank test, Cox regression model and propensity score matching.
Results:
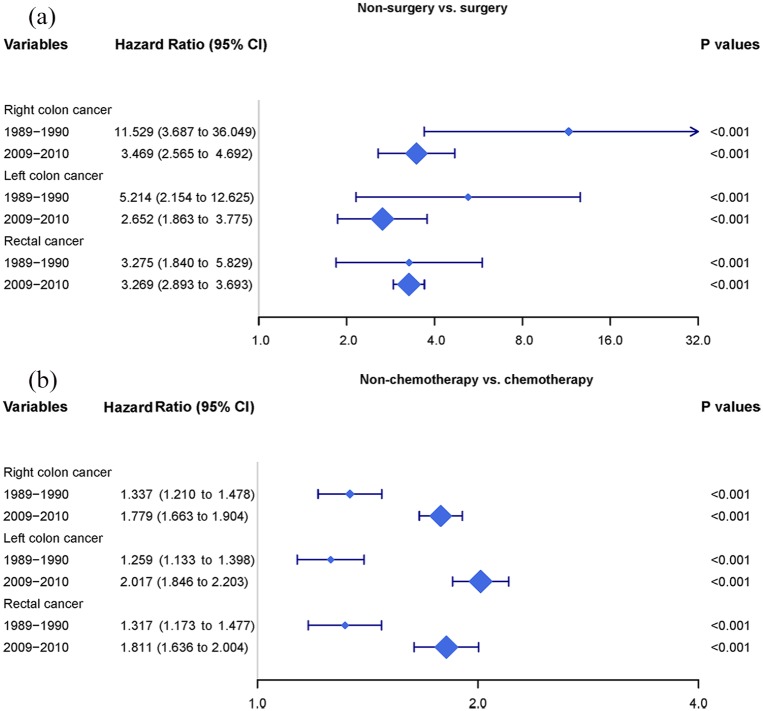
The Cox regression model showed that hazard ratio (HR) of non-surgery dropped from 11.529 to 3.469 in right colon cancer (RCC), 5.214 to 2.652 in left colon cancer (LCC) and 3.275 to 3.269 in rectal cancer (RC) from 1989–1990 to 2009–2010. The 95% confidence intervals (CIs) for surgical resection in 2009–2010 were narrower than those in 1989–1990. HR became greater in LACRC without chemotherapy (from 1.337 to 1.779 in RCC, 1.269 to 2.017 in LCC, 1.317 to 1.811 in RC). There was no overlapping about the 95% CI of chemotherapy between the two groups. The progress of surgery was not linked to the improvement of overall survival (OS) of RCC (p = 0.303) and RC (p = 0.660). Chemotherapy had a significant association with OS of all colorectal cancer (CRC) patients (p = 0.017 in RCC; p = 0.006 in LCC; p = 0.001 in RC).
Conclusions:
Advancements in chemotherapy regimen were the main contributor to the upswing of CRC survival. The improvements in surgery had a limited effect on improvements in CRC survival.
Keywords: adjuvant therapy, chemotherapy, locally advanced colorectal cancer, radiotherapy, surgery
Introduction
Colorectal cancer (CRC) is the third most common adult cancer in the world, with an estimated 1.8 million cases and 881,000 deaths annually by the GLOBOCAN estimate in 2018.1 With advances in treatment technology over the past few decades, the survival of patients with locally advanced colorectal cancer (LACRC) has improved significantly.
Treatment for locally advanced colorectal cancer includes surgical resection,2 chemotherapy3 and/or radiation therapy.4 Advances in surgical resection techniques are attributed to updated surgical equipment and concepts. Total mesorectal excision (TME) and complete mesocolic excision (CME) have become the consensus of all colorectal surgeons.5,6 In addition, application of laparoscopy and robot-assisted laparoscopy contribute to the refinement of CRC surgery.7,8 Adjuvant chemotherapy for LACRC patients with high-risk stage II and III cancer has substantially evolved over the past decades, concomitant with progress in marketing of oxaliplatin, irinotecan, cetuximab and bevacizumab, as well as the concept of neoadjuvant therapy.
The uptake of TME or CME combined with adjuvant oncological treatment for locally advanced rectal cancer has reduced local recurrence rates and improved long-term survival.9 However, which is the most important contributor to the upswing in CRC survival? There is no final conclusion yet. Exploration of this issue can provide research directions relating to CRC, or even all tumors, in the future.
Therefore, the aim of this study was to explore the main contributor to the upswing of survival in LACRC.
Materials and methods
Patients
Data in this retrospective analysis were extracted from the Surveillance, Epidemiology, and End Results (SEER) linked database. The SEER Program of the National Cancer Institute is an authoritative source of information on cancer incidence and survival in the USA that is updated annually. SEER currently collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 34.6% of the US population.10 The target population was limited to patients with stage II and III colorectal adenocarcinoma diagnosed in the periods 1989–1990 and 2009–2010, which includes 40,470 patients in total. All patients were followed for more than 5 years. Exclusion criteria were: (1) appendix tumor, (2) diagnosed at autopsy or on the death certificate. The final study sample contained 40,184 patients.
We selected the period 1989–1990 as a baseline for comparison because the management of LACRC started to evolve rapidly from the 1990s;9 we chose patients from the period 2009–2010 since these were the patients with the most recent with 5-year follow up. In 1989–1990 CRC was defined using the third edition AJCC staging. However, in 2009–2010 the sixth edition of the AJCC staging was adopted. Therefore, we re-staged the N stage according to the number of positive lymph nodes. We defined N1 as 1–3 lymph nodes positive and N2 as more than 4 lymph nodes positive.
Methods
Intergroup comparisons were analyzed using Pearson’s chi-squared test. The log-rank test was used to compare overall survival (OS) between different groups. A hazard ratio (HR) and a 95% confidence interval (CI) were evaluated by a single factor and a multivariate Cox proportional hazards regression model. Univariate analysis of variables with significant differences was included in the Cox regression model for multivariate analysis. In order to eliminate the influence of other variables, we conducted propensity score matching (PSM). Statistical analyses were performed with IBM SPSS statistics trial v. 25.0 (IBM, Armonk, NY, USA). All reported p values lower than 0.05 were considered significant.
Results
Patient characteristics
This study enrolled 40,184 patients, including 10,604 (26.39%) cases in 1989–1990 and 29,580 (73.61%) cases in 2009–2010. We found marked differences between 1989–1990 and 2009–2010. The proportion of male LACRC increased from 49.72% to 51.21%. Elderly patients (more than 70 years old) with LACRC decreased from 53.54% to 45.30%. The ethnic composition was also different. In addition, T stage, N stage and histologic grade were significantly different between the two groups.
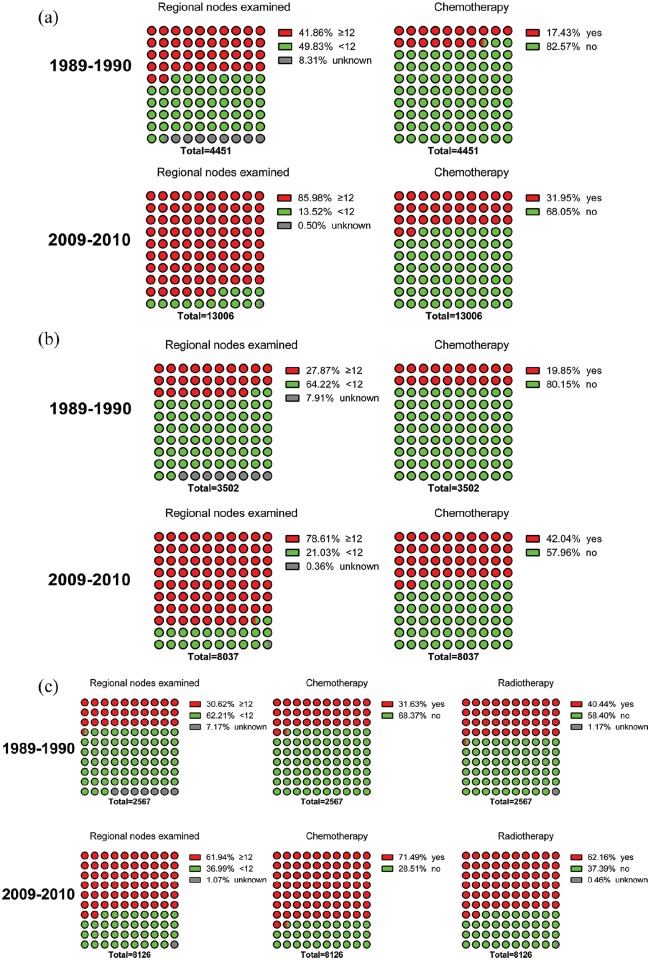
Importantly, there were significant differences in the rates of surgery, radiotherapy and chemotherapy between 1989–1990 and 2009–2010. The proportion of chemotherapy (from 21.64% to 45.58%) and radiotherapy (from 12.56% to 18.48%) increased significantly as the rate of surgery (from 99.56% to 96.73%) decreased from 1989–1990 to 2009–2010. The qualified number of regional nodes examined (RNE), an important indicator of the quality of surgery, soared from 35.00% to 77.29% (Table 1).
Table 1.
Characteristics of local advanced colorectal cancer.
| Characteristics | 1989–1990 (n = 10,604) | 2009–2010 (n = 29,580) | p value |
|---|---|---|---|
| Gender | 0.008 | ||
| Male | 5272 (49.72%) | 15,148 (51.21%) | |
| Female | 5332 (50.28%) | 14,432 (48.79%) | |
| Age (years) | <0.001 | ||
| ⩽50 | 722 (6.81%) | 3665 (12.39%) | |
| 51–70 | 4205 (39.65%) | 12,516 (42.31%) | |
| >70 | 5677 (53.54%) | 13,399 (45.30%) | |
| Race | <0.001 | ||
| White | 9224 (86.99%) | 23,586 (79.74%) | |
| Black | 748 (7.05%) | 3341 (11.29%) | |
| Other | 630 (5.94%) | 2572 (8.70%) | |
| Unknown | 2 (0.02%) | 81 (0.27%) | |
| Primary tumor location | 0.209 | ||
| Right colon | 4451 (41.97%) | 13,006 (43.97%) | |
| Left colon | 3502 (33.03%) | 8037 (27.17%) | |
| Rectum | 2567 (24.21%) | 8126 (27.47%) | |
| Unknown | 84 (0.79%) | 411 (1.39%) | |
| Histologic grade | <0.001 | ||
| Well/moderately differentiated | 7923 (74.72%) | 22,590 (76.37%) | |
| Poor differentiated/undifferentiated | 1829 (17.25%) | 5965 (20.17%) | |
| Unknown | 852 (8.03%) | 1025 (3.47%) | |
| T staging | <0.001 | ||
| T0–3 | 8553 (80.66%) | 25,153 (85.03%) | |
| T4 | 2011 (18.96%) | 4353 (14.72%) | |
| Unknown | 40 (0.38%) | 74 (0.25%) | |
| N staging | <0.001 | ||
| N0 | 6065 (57.20%) | 14,603 (49.37%) | |
| N1 | 2998 (28.27%) | 10,106 (34.16%) | |
| N2 | 1207 (11.38%) | 4871 (16.47%) | |
| Unknown | 334 (3.15%) | 0 (0.00%) | |
| Surgery | <0.001 | ||
| Yes | 10,557 (99.56%) | 28,614 (96.73%) | |
| No | 47 (0.04%) | 889 (3.01%) | |
| Unknown | 0 (0.00%) | 77 (0.26%) | |
| Radiotherapy | <0.001 | ||
| Yes | 1332 (12.56%) | 5467 (18.48%) | |
| No | 9213 (86.88%) | 24,051 (81.31%) | |
| Unknown | 59 (0.56%) | 62 (0.21%) | |
| Chemotherapy | <0.001 | ||
| Yes | 2295 (21.64%) | 13,483 (45.58%) | |
| No | 8309 (78.36%) | 16,097 (54.42%) | |
| Regional nodes examined | <0.001 | ||
| <12 | 6106 (57.58%) | 6531 (22.08%) | |
| ⩾12 | 3658 (35.00%) | 22,863 (77.29%) | |
| Unknown | 840 (7.92%) | 186 (0.63%) |
Survival analysis
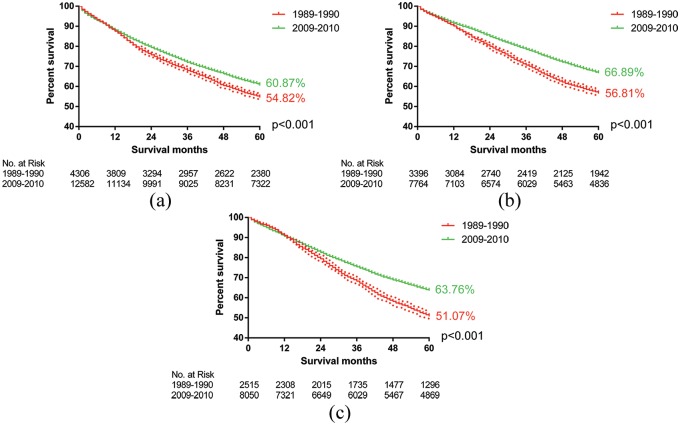
The OS of patients with LACRC improved significantly due to advances in surgery combined with adjuvant therapy in the period between 1989–1990 and 2009–2010. The 5-year survival rate increased from 54.82% to 60.87% (p < 0.001, Figure 1(a)), 56.81% to 66.89% (p < 0.001, Figure 1(b)) and 51.07% to 63.76% (p < 0.001, Figure 1(c)) in right colon cancer (RCC), left colon cancer (LCC) and rectal cancer (RC) respectively. Meanwhile, LACRC patients undergoing chemotherapy increased by 14.52% (RCC, Figure 2(a)), 22.19% (LCC, Figure 2(b)) and 39.86% (RC, Figure 2(c)). Moreover, the proportion of radiotherapy grew from 37.39% to 58.40% in RC patients. There was also a significant increase in the number of RNE. The qualified ratio rose by 44.12% (RCC, Figure 2(a)), 50.74% (LCC, Figure 2(b)) and 31.32% (RC, Figure 2(c)).
Figure 1.
The log-rank test showed that the overall survival of patients with locally advanced colorectal cancer improved significantly due to the advances in surgery combined with adjuvant therapy. (a) The 5-year survival rate increased from 54.82% to 60.87% (p < 0.001) in right colon cancer; (b) the 5-year survival rate increased from 56.81% to 66.89% (p < 0.001) in left colon cancer; and (c) the 5-year survival rate increased from 51.07% to 63.76% (p < 0.001) in rectal cancer.
Figure 2.
The ratio of chemotherapy (radiotherapy) and qualified regional nodes examined (RNE) in colorectal cancer patients. (a) Patients undergoing chemotherapy increased by 14.52% and the ratio of qualified RNE, which was ⩾12, increased by 44.12% in right colon cancer. (b) Patients undergoing chemotherapy increased by 22.19% and the ratio of qualified RNE increased by 50.74% in left colon cancer. (C) Patients undergoing chemotherapy increased by 39.86%, the proportion of radiotherapy increased by 21.72% and the ratio of qualified RNE increased by 31.32% in rectal cancer.
Cox regression model
Age, pathological grade, T stage, N stage, surgery, chemotherapy and RNE were important prognostic factors in both LACRC of 1989–1990 and 2009–2010. Also, several new poor prognostic factors emerged in the cases of 2009–2010, including black people in RCC (p < 0.001), and men in LCC (p < 0.001) and RC (p < 0.001). Although used as a prognostic factor, radiotherapy was a risk factor in RCC patients in 2009–2010 (HR: 0.754, p = 0.015).
Interestingly, HR of non-surgery dropped from 11.529 to 3.469 in RCC, 5.214 to 2.652 in LCC and 3.275 to 3.269 in RC. Meanwhile, the 95% CIs for surgical resection in 2009–2010 were narrower than those in 1989–1990 (Figure 3(a)). Conversely, the HR became greater in LACRC without chemotherapy (from 1.337 to 1.779 in RCC, from 1.269 to 2.017 in LCC, from 1.317 to 1.811 in RC). There was no overlap about the 95% CI of chemotherapy between the two groups (Figure 3(b)) (Tables 2–4).
Figure 3.
Forest plots for the Cox regression model. (a) Non-surgery versus surgery. Hazard ratio (HR) of non-surgery dropped from 11.529 to 3.469 in right colon cancer; 5.214 to 2.652 in left colon cancer; and 3.275 to 3.269 in rectal cancer. (B) Non-chemotherapy versus chemotherapy. The HR became greater in locally advanced colorectal cancer without chemotherapy (from 1.337 to 1.779 in right colon cancer, from 1.269 to 2.017 in left colon cancer, from 1.317 to 1.811 in rectal cancer).
Table 2.
Multivariate analysis of survival months in right colon cancer patients.
| Variables | 1989–1990 |
2009–2010 |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | <0.001 | <0.001 | ||
| 51–70 versus ⩽50 | 2.834 (2.280–3.524) | <0.001 | 1.396 (1.208–1.614) | <0.001 |
| >70 versus ⩽50 | 7.015 (5.639–8.727) | <0.001 | 2.991 (2.599–3.442) | <0.001 |
| 51–70 versus >70 | 0.404 (0.373–0.438) | <0.001 | 0.466 (0.437–0.496) | <0.001 |
| Race | 0.050 | <0.001 | ||
| Black versus white | 1.141 (1.002–1.300) | 0.047 | 1.152 (1.060–1.252) | 0.001 |
| Other versus white | 0.900 (0.767–1.056) | 0.196 | 0.832 (0.742–0.933) | 0.002 |
| Black versus other | 1.268 (1.039–1.548) | 0.020 | 1.363 (1.193–1.557) | <0.001 |
| Histologic grade | ||||
| Poor/undifferentiated versus well/moderately differentiated | 1.111 (1.027–1.203) | 0.009 | 1.218 (1.150–1.291) | <0.001 |
| T staging | ||||
| T4 versus T0–3 | 1.142 (1.050–1.242) | <0.001 | 1.816 (1.701–1.938) | <0.001 |
| N staging | <0.001 | <0.001 | ||
| N1 versus N0 | 1.311 (1.210–1.421) | <0.001 | 1.592 (1.492–1.699) | <0.001 |
| N2 versus N0 | 2.258 (2.021–2.522) | <0.001 | 2.823 (2.619–3.042) | <0.001 |
| N1 versus N2 | 0.581 (0.517–0.652) | <0.001 | 0.555 (0.516–0.597) | <0.001 |
| Surgery | ||||
| No versus Yes | 11.529 (3.687–36.049) | <0.001 | 3.469 (2.565–4.692) | <0.001 |
| Chemotherapy | ||||
| No versus Yes | 1.337 (1.210–1.478) | <0.001 | 1.779 (1.663–1.904) | <0.001 |
| Radiotherapy | ||||
| No versus Yes | NA | NA | 0.754 (0.593–0.959) | 0.015 |
| Regional nodes examined | ||||
| <12 versus ⩾12 | 1.341 (1.252–1.437) | <0.001 | 1.524 (1.420–1.637) | <0.001 |
NA, not applicable.
Table 3.
Multivariate analysis of survival months in left colon cancer patients.
| Variables | 1989–1990 |
2009–2010 |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | <0.001 | <0.001 | ||
| 51–70 versus ⩽50 | 1.762 (1.456–2.134) | <0.001 | 1.296 (1.126–1.492) | <0.001 |
| >70 versus s50 | 4.180 (3.445–5.073) | <0.001 | 2.903 (2.529–3.333) | <0.001 |
| 51–70 versus >70 | 0.404 (0.373–0.438) | <0.001 | 0.446 (0.412–0.484) | <0.001 |
| Gender | ||||
| Male versus female | NA | NA | 1.182 (1.100–1.271) | <0.001 |
| Race | 0.204 | |||
| Black versus white | 1.040 (0.895–1.209) | 0.609 | NA | NA |
| Other versus white | 0.875 (0.748–1.024) | 0.096 | NA | NA |
| Black versus other | 1.188 (0.964–1.465) | 0.106 | NA | NA |
| Histologic grade | ||||
| Poor/undifferentiated versus well/moderately differentiated | 1.170 (1.039–1.316) | 0.009 | 1.270 (1.157–1.393) | <0.001 |
| T staging | ||||
| T4 versus T0–3 | 1.142 (1.050–1.242) | <0.001 | 1.953 (1.788–2.134) | <0.001 |
| N staging | <0.001 | <0.001 | ||
| N1 versus N0 | 1.271 (1.163–1.389) | <0.001 | 1.406 (1.289–1.533) | <0.001 |
| N2 versus N0 | 1.731 (1.513–1.981) | <0.001 | 2.495 (2.254–2.762) | <0.001 |
| N1 versus N2 | 0.734 (0.639–0.843) | <0.001 | 0.563 (0.510–0.623) | <0.001 |
| Surgery | ||||
| No versus Yes | 5.214 (2.154–12.625) | <0.001 | 2.652 (1.863–3.775) | <0.001 |
| Chemotherapy | ||||
| No versus Yes | 1.259 (1.133–1.398) | <0.001 | 2.017 (1.846–2.203) | <0.001 |
| Regional nodes examined | ||||
| <12 versus ⩾12 | 1.162 (1.068–1.264) | <0.001 | 1.536 (1.415–1.669) | <0.001 |
NA, not applicable.
Table 4.
Multivariate analysis of survival months in rectal cancer patients.
| Variables | 1989–1990 |
2009–2010 |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age(years) | <0.001 | <0.001 | ||
| 51–70 versus ⩽50 | 2.047 (1.683–2.489) | <0.001 | 1.397 (1.231–1.585) | <0.001 |
| >70 versus ⩽50 | 4.251 (3.473–5.203) | <0.001 | 2.874 (2.530–3.265) | <0.001 |
| 51–70 versus >70 | 0.482 (0.437–0.531) | <0.001 | 0.486 (0.448–0.527) | <0.001 |
| Gender | ||||
| Male versus female | NA | NA | 1.150 (1.067–1.240) | <0.001 |
| Histologic grade | ||||
| Poor/undifferentiated versus well/moderately differentiated | 1.166 (1.035–1.313) | 0.012 | 1.399(1.275–1.535) | <0.001 |
| T staging | ||||
| T4 versus T0–3 | 1.364 (1.202–1.548) | <0.001 | 1.992 (1.806–2.196) | <0.001 |
| N staging | <0.001 | <0.001 | ||
| N1 versus N0 | 1.266 (1.142–1.403) | <0.001 | 1.308 (1.201–1.424) | <0.001 |
| N2 versus N0 | 1.792 (1.561–2.057) | <0.001 | 2.067 (1.868–2.288) | <0.001 |
| N1 versus N2 | 0.706 (0.613–0.814) | <0.001 | 0.633 (0.572–0.700) | <0.001 |
| Surgery | ||||
| No versus Yes | 3.275 (1.840–5.829) | <0.001 | 3.269 (2.893–3.693) | <0.001 |
| Chemotherapy | ||||
| No versus Yes | 1.317 (1.173–1.477) | <0.001 | 1.811 (1.636–2.004) | <0.001 |
| Radiotherapy | ||||
| No versus Yes | 1.008 (0.907–1.121) | 0.878 | 0.935 (0.847–1.032) | 0.184 |
| Regional nodes examined | ||||
| <12 versus ⩾12 | 1.192 (1.082–1.312) | <0.001 | 1.328 (1.219–1.448) | <0.001 |
NA, not applicable.
The impact of surgical advancement on survival
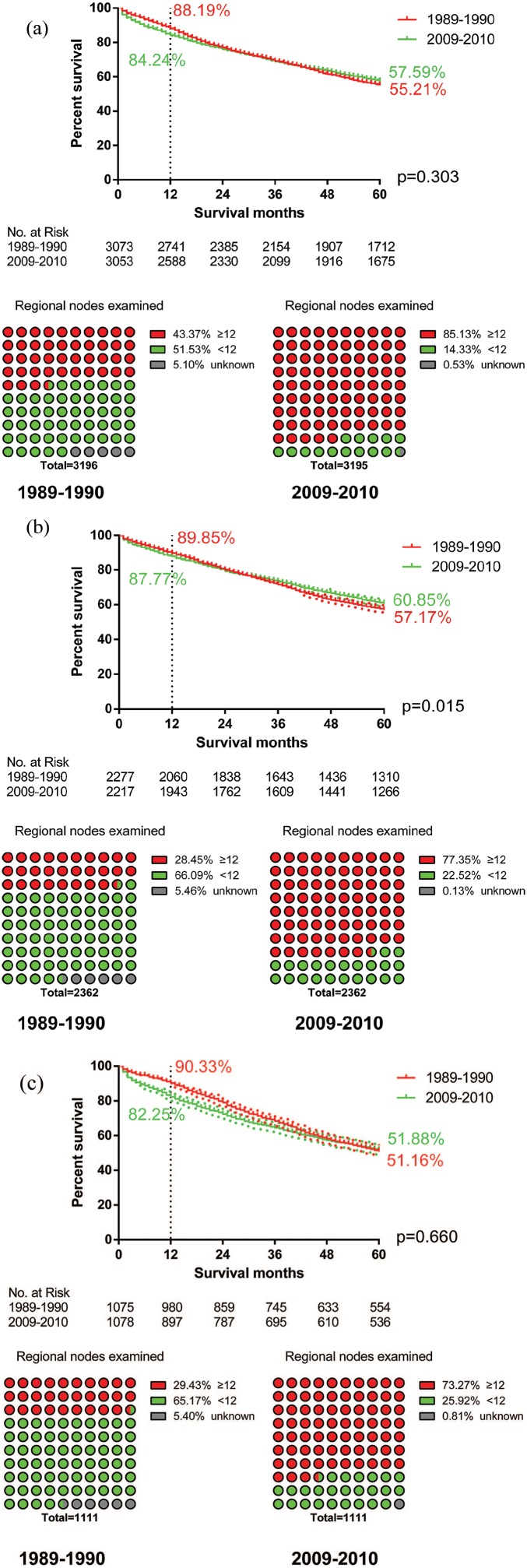
We screened patients who underwent surgery without adjuvant therapy. In order to eliminate the influence of the other variables, PSM was conducted for an analysis of variables, including age, gender, race, differentiation and T and N stage (Supplementary Tables 1–3). The number of regional nodes examined did not match between the two groups, which can reflect the quality of surgery. We found that the surgical advancement was associated with the qualified rate of regional nodes, which improved by 41.76%, 48.90% and 43.84% in RCC, LCC and RC respectively. The log-rank test showed that OS of LCC was significantly increased with the development of surgical techniques (p = 0.015) (Figure 4(b)). However, there was no significant effect of surgical advancement on the overall survival of RCC (p = 0.303, Figure 4(a)) and RC (p = 0.660, Figure 4(c)). Moreover, the 1-year survival rate of colorectal patients in 2009–2010 was lower than that in 1989–1990 (RCC, 88.19% versus 84.24%; LCC, 89.85% versus 87.77%; RC, 90.33% versus 82.25%).
Figure 4.
The impact of surgical advancement on survival. (a) The overall survival of right colon cancer patients did not improve significantly (p = 0.303). The 1-year survival rate of right colon cancer patients dropped from 88.19% to 84.24%. The rate of qualified RNE increased by 41.76% in right colon cancer. (b) OS of left colon cancer patients was significantly increased (p = 0.015). The 1-year survival rate of left colon cancer patients dropped from 89.85% to 87.77%. The rate of qualified RNE increased by 48.90% in left colon cancer. (c) The overall survival of rectal cancer did not improve significantly (p = 0.660). The 1-year survival rate of rectal cancer patients dropped from 90.33% to 82.25%. The rate of qualified RNE increased by 43.84% in rectal cancer.
The impact of advancement of adjuvant therapy on survival
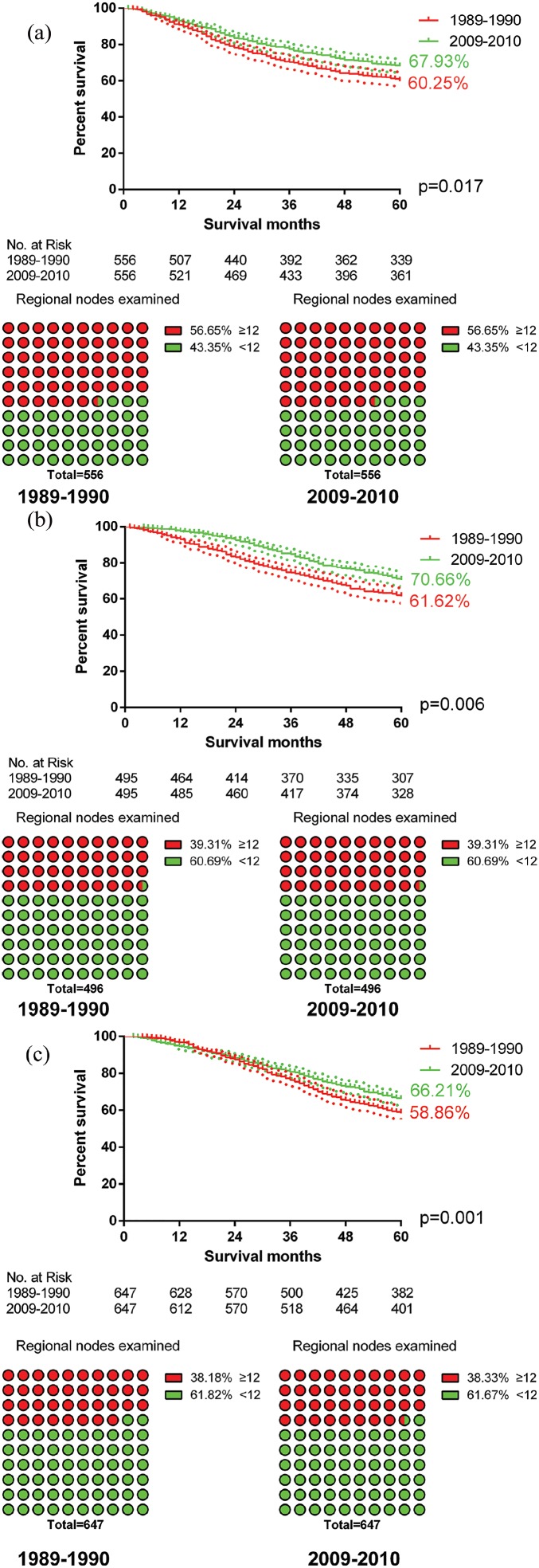
Patients treated with both surgery and chemotherapy were selected for PSM. The variables for PSM consisted of age, gender, race, differentiation, T stage, N stage, radiotherapy and the number of RNE (Supplementary Tables 4–6). A higher likelihood of improved OS occurred in all colorectal cancers after completion of updated adjuvant therapy compared to the patients with the old version of adjuvant therapy (p = 0.017 in RCC, Figure 5(a); p = 0.006 in LCC, Figure 5(b); p = 0.001 in RC, Figure 5(c)).
Figure 5.
The impact of chemotherapy advancements on survival. (a) Overall survival (OS) of right colon cancer patients increased from 60.25% to 67.93% (p = 0.017); (b) OS of left colon cancer patients increased from 61.62% to 70.66% (p = 0.006); and (c) OS of rectal cancer patients increased from 58.86% to 66.21% (p = 0.001).
For exploration of the impact of radiotherapy on the survival of RC patients, those receiving radiotherapy were the target population. The variables for PSM were age, gender, race, differentiation, T stage, N stage, chemotherapy, surgery and the number of RNE (Supplementary Table 7). Adjuvant radiotherapy was associated with an increased OS from 57.54% to 67.36% (p = 0.001, Figure 6).
Figure 6.
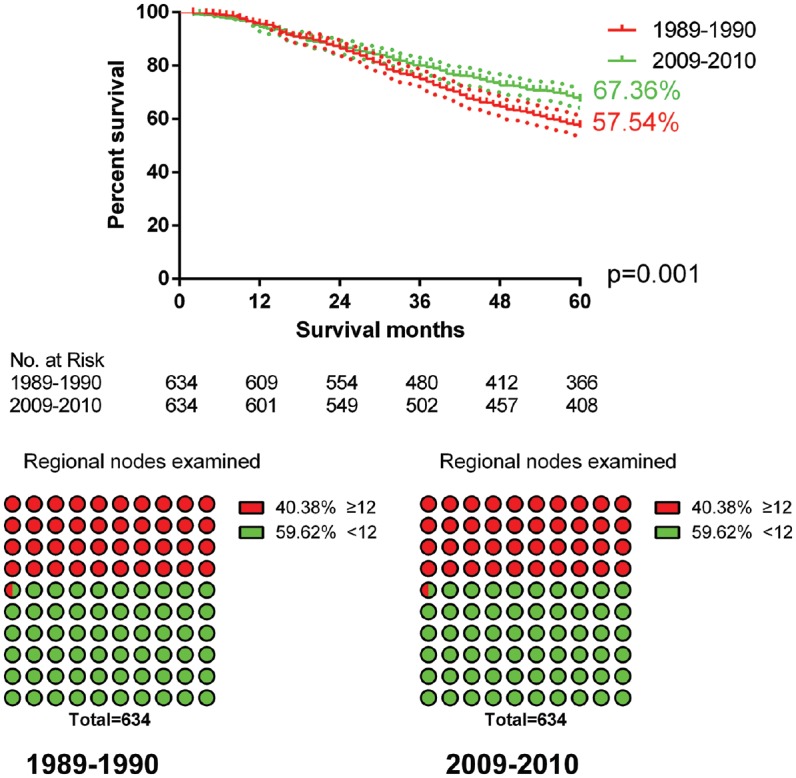
The impact of radiotherapy advancements on survival. Overall survival increased from 57.54% to 67.34% (p = 0.001).
Discussion
To the best of our knowledge, this study was the first to look into the main reason for the improvement of survival in LACRC. We selected patients with LACRC in the periods 1989–1990 and 2009–2010, explored the relative importance of prognostic factors by a Cox regression model, and compared the effects of surgery and adjuvant therapy on survival after PSM. We believe that research on the progress of treatment can be fundamental to guiding the improvement of current treatment options. Also, successful experience in CRC treatment can be regarded as a reference for other tumors.
Although decreasing, the HR of non-surgical treatment was still the highest among various treatment methods. Therefore, it is still undoubted that surgery is the first-choice treatment for CRC. Colorectal surgery had also seen tremendous developments in the two decades. The qualified number of RNE reached 77.29% in 2009–2010. Moreover, a narrow range of 95% CI in 2009–2010 suggested that colorectal surgeons reached some consensus on the methods and scope of surgical resection, like TME and CME. Unfortunately, patient survival of RCC and RC did not improve significantly with advances in surgery, while LCC patients may benefit from CME and/or advanced equipment. Although many researchers reported that laparoscopic colectomy, which was widely used in the field of colorectal surgery in 2009–2010, significantly improves the short-term outcomes of patients,11–14 the short-term survival rate in 2009–2010 was lower than that in 1989–1990. Therefore, surgeons need to pay more attention to the short-term survival rate after surgery in future research, especially for patients who need surgery only, even though the scope of surgical resection can be considered to be appropriately restricted.
TME was proposed by Heald and colleagues in 198215 and has become the standard for surgery of RC after more than 20 years of practice.16 Owing to the successful experience of TME, CME was quickly recognized by colorectal surgeons, and was initially introduced in 2009.17,18 Therefore, both colon and rectal cancer can benefit from advances in surgical equipment, but the revolutionary concept was only proposed for the treatment of colon cancer between 1989–1990 and 2009–2010. The values of HR and 95% CI for RC surgery varied minimally in our Cox regression model from 1989–1990 to 2009–2010; on the contrary, the change was huge in colon cancer. Therefore, we considered that advances in surgical equipment may be beneficial to the stability of operations, but the revolutionary surgical concept was the real engine for surgical progress.
More and more attention to adjuvant therapy is paid in modern medicine. The proportion of LACRC patients receiving chemotherapy and/or radiotherapy in 2009–2010 was almost double that in 1989–1990. Advancements in chemotherapy regimen had a significant association with OS of CRC patients. The main chemotherapy regimen for CRC was 5-FU/leucovorin in the 1990s.19 FOLFOX (oxaliplatin/5-FU/leucovorin) has become the first-line treatment for CRC in the 21st century.20 We found that there was no intersection about the 95% CIs of chemotherapy between the two groups. Meanwhile, OS of LACRC patients who underwent surgery with chemotherapy improved significantly (p = 0.017 in RCC; p = 0.006 in LCC; p = 0.001 in RC) after PSM, suggesting that the advancements in chemotherapy regimen are the root cause of the improvement in CRC survival.
Further investigations to explore the effects of radiotherapy on survival of CRC are needed. Although the OS of patients with RC who received radiotherapy in 2009–2010 was better than that in 1989–1990, the effects of chemotherapy cannot be ruled out. And radiotherapy cannot serve as a good prognostic factor in the Cox regression model. Specifically, patients who underwent radiotherapy had worse survival than those who did not undergo radiotherapy in RCC. Therefore, we tend to believe that radiotherapy alone cannot improve the RC survival. But we also cannot ignore the effect of radiotherapy on sphincter preservation in low rectal cancer.
The interesting findings of this study include: (1) although advancements in surgical treatment had not significantly prolonged the survival of CRC, surgeons should explore a more appropriate area of surgical resection and improve short-term outcomes without affecting the long-term survival of LACRC; (2) effective drugs are the key to cancer treatment since chemotherapy is the main contributor to the progress in treatment of CRC; (3) oncologists should consider whether the administration of radiotherapy can be abandoned for patients with mid/low rectal cancer if radiotherapy does not affect sphincter preservation. Access to only retrospective data was the main limitation of this study.
Conclusion
Advancements of chemotherapy regimen were the main contributor to the upswing in CRC survival. The improvements in surgery had a limited effect on improvements in CRC survival. The short-term survival of LACRC patients in 2009–2010 was even lower than that in 1989–1990.
Supplemental Material
Supplemental material, Supplementary_table_1_Characteristics_of_local_advanced_right_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_2_Characteristics_of_local_advanced_left_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_3_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_4_Characteristics_of_local_advanced_right_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_5_Characteristics_of_local_advanced_left_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_6_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplementary_table_7_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors.
Footnotes
Author contributions: The first author, Yuqiang Li, contributed mainly to this article.
Ethics statement: This retrospective study was approved by the Medical Ethics Committee of Xiangya Hospital, Central South University with Approval No. 201903130. Patients’ informed consent was waived because of the retrospective nature of the study design.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Contract grant sponsor: The Nature Scientific Foundation of China; Contract grant number: 81702956.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Yuqiang Li  https://orcid.org/0000-0003-1517-3687
https://orcid.org/0000-0003-1517-3687
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yuqiang Li, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; Department of General Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Lilan Zhao, Department of General Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Cenap Güngör, Department of General Visceral and Thoracic Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Fengbo Tan, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Zhongyi Zhou, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Chenglong Li, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Xiangping Song, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Dan Wang, Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Qian Pei, Department of Gastrointestinal Surgery and National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan, P.R. China.
Wenxue Liu, Department of Cardiology and National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan, P.R. China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Kim NK, Kim YW, Han YD, et al. Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy, surgical technique, and outcomes. Surg Oncol 2016; 25: 252–262. [DOI] [PubMed] [Google Scholar]
- 3. Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg 2017; 152: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren Y, Fleischmann D, Foygel K, et al. Antiangiogenic and radiation therapy: early effects on in vivo computed tomography perfusion parameters in human colon cancer xenografts in mice. Invest Radiol 2012; 47: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miskovic D, Foster J, Agha A, et al. Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg 2015; 261: 716–722. [DOI] [PubMed] [Google Scholar]
- 6. Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015; 16: 161–168. [DOI] [PubMed] [Google Scholar]
- 7. van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013; 14: 210–218. [DOI] [PubMed] [Google Scholar]
- 8. Mushtaq HH, Shah SK, Agarwal AK. The current role of robotics in colorectal surgery. Curr Gastroenterol Rep 2019; 21: 11. [DOI] [PubMed] [Google Scholar]
- 9. Brown KGM, Solomon MJ. Progress and future direction in the management of advanced colorectal cancer. Br J Surg 2018; 105: 615–617. [DOI] [PubMed] [Google Scholar]
- 10. Pei JP, Zhang CD, Fan YC, et al. Comparison of different lymph node staging systems in patients with resectable colorectal cancer. Front Oncol 2018; 8: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer abstracted from: Nelson H, Sargent D, Wieand HS, et al. ; for the Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004; 350: 2050–2059. Cancer Treat Rev 2004; 30: 707–709. [DOI] [PubMed] [Google Scholar]
- 12. Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002; 359: 2224–2229. [DOI] [PubMed] [Google Scholar]
- 13. Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005; 6: 477–484. [DOI] [PubMed] [Google Scholar]
- 14. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; 365: 1718–1726. [DOI] [PubMed] [Google Scholar]
- 15. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg 1982; 69: 613–616. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20: 207–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation – technical notes and outcome. Colorectal Dis 2009; 11: 354–364; discussion 64–65. [DOI] [PubMed] [Google Scholar]
- 18. West NP, Hohenberger W, Weber K, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010; 28: 272–278. [DOI] [PubMed] [Google Scholar]
- 19. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate – advanced colorectal cancer meta-analysis project. J Clin Oncol 1992; 10: 896–903. [DOI] [PubMed] [Google Scholar]
- 20. Adjuvant chemotherapy with oxaliplatin, in combination with fluorouracil plus leucovorin prolongs disease-free survival, but causes more adverse events in people with stage II or III colon cancer abstracted from: Andre T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators: oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–2351. Cancer Treat Rev 2004; 30: 711–713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_table_1_Characteristics_of_local_advanced_right_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_2_Characteristics_of_local_advanced_left_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_3_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_4_Characteristics_of_local_advanced_right_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_5_Characteristics_of_local_advanced_left_colon_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_6_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_table_7_Characteristics_of_local_advanced_rectal_cancer_after_PSM for The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database by Yuqiang Li, Lilan Zhao, Cenap Güngör, Fengbo Tan, Zhongyi Zhou, Chenglong Li, Xiangping Song, Dan Wang, Qian Pei and Wenxue Liu in Therapeutic Advances in Gastroenterology