Abstract
Background & Aims:
Despite the high burden of hepatitis C virus (HCV) infection among people who inject drugs (PWID), uptake of interferon-based therapies has been extremely low. Increasing availability of direct-acting antiviral (DAA)-based therapies offers the possibility of rapid treatment expansion with the goal of controlling the HCV epidemic. We evaluated DAA-based treatment uptake among HCV-positive PWID in Vancouver after introduction of DAAs in the government drug formulary.
Methods:
Using data from three cohorts of PWID in Vancouver, Canada, we investigated factors associated with DAA-therapies uptake among participants with HCV between April 2015-November 2017.
Results:
Of 915 HCV-positive PWID, 611 (66.8%) were recent PWID and 369 (40.3%) had HIV coinfection. During the study period, 146 (16.0%) initiated DAA-therapies, a rate of 6.0 per 100 person-year, with higher initiation rates among non-recent PWID and an increasing trend over time. In multivariable analysis, HIV coinfection (Adjusted Odds Ratio [AOR] = 2.29, 95% Confidence Interval [CI]: 1.55–3.40), white race (AOR=1.56, 95%CI: 1.05–2.35), and engagement in HCV care (AOR=1.94, 95%CI: 1.31–2.90) were positively associated with DAA-therapies uptake, while high-risk drinking (AOR=0.47, 95%CI: 0.23–0.88) and daily crack use were negatively associated (AOR=0.41, 95%CI: 0.17–0.85). Among recent PWID, engagement in opioid agonist therapy emerged as an independent correlate of DAA uptake.
Conclusions:
Despite increases in HCV treatment uptake among PWID after the introduction of DAAs in our setting, disparities in access remain. Social-structural and behavioral barriers to HCV care should be addressed for the success of any HCV elimination strategy.
Keywords: Hepatitis C, people who inject drugs, direct-acting-antiviral, HIV
Lay summary:
Despite the high and increasing burden of hepatitis C (HCV) among people who inject drugs (PWID), treatment rates have been historically low. The increasing availability of highly effective treatments (i.e., direct acting antivirals [DAAs]) against HCV brought renew optimism to expand treatment to this population, and control the HCV epidemic. While or study found substantial increases in HCV treatment among PWID after the introduction of DAAs in British Columbia, Canada, non-white participants or those engaged in high-risk drinking or frequent crack use were less likely to have started these treatments, suggesting that disparities in access still remain.
Introduction
People who inject drugs (PWID) are a key population within the hepatitis C (HCV) epidemic. A recent systematic review estimated that there were approximately 15.6 million PWID globally in 2015, of whom over half (52.3%) had been exposed to HCV (i.e., anti-HCV positive).1 Although data on the prevalence of chronic HCV infection (i.e., HCV RNA positive) in different subpopulations is limited, available studies suggest that around 39% of PWID (corresponding to 6.1 million individuals) have chronic HCV compared to 1% of the general population.1 The high prevalence of chronic HCV among PWID, alongside the aging of this population, have resulted in a rising burden of HCV disease attributable to injection drug use. In 2013, it was estimated that injection drug use caused approximately 40% of total disability adjusted life-years (DALYs) due to HCV, almost double the rate than in 1993.2 Moreover, PWID presently account for approximately a quarter of new HCV infections, with recent HCV outbreaks documented among young injectors living in suburban areas in the United States (U.S.).3
Despite the high burden of HCV-related morbidity and mortality among PWID, and the importance of HCV sustained virological response (SVR) for optimal individual- (e.g., reduced disease progression and mortality)4,5 and population-level outcomes (e.g., decreased transmission),6,7 rates of HCV treatment uptake among this population have been historically low, ranging from 0.5 to 2.0 per 100 person-years (PY).8–11 While barriers underlying these low rates are multifactorial, key contributing factors have been the poor tolerability and low efficacy of interferon (IFN)-based therapies.12
In recent years, the increasing availability of highly effective and well-tolerated IFN-free, direct acting antiviral (DAA)-based therapies for HCV have brought new optimism in the prospects of overcoming some of the aforementioned barriers.7,12 While optimized access to HCV treatment among PWID will be critical in addressing high levels of HCV-related morbidity and mortality, as well as controlling the HCV epidemic, there is limited research on factors shaping uptake of DAA-based regimens among this population. Therefore, the objective of this study was to evaluate correlates of uptake of DAA-based therapies among PWID in Vancouver, Canada.
Methods
Study setting
In the province of British Columbia (BC), public coverage for new DAAs began on March 25, 2015, with the inclusion of sofosbuvir and sofosbuvir/ledispavir in the government drug formulary.13 Subsequently, DAA coverage expanded to include dasabuvir/ombitasvir/paritaprevir/ritonavir in July 2015 (discontinued on March 2017),14,15 and sofosbuvir-velpatasvir, elbasvir-grazoprevir, asunaprevir, and daclastavir in March 2017.15 Glecaprevir/pibrentasvir was approved by Health Canada in August 2017, but it is still not publicly covered in BC. Up to March 2017, public funding for DAAs was restricted to individuals with liver fibrosis ≥F2 or extra-hepatic manifestations of HCV.13 After this date, eligibility criteria for public coverage expanded to include individuals with HIV or hepatis B coinfection or other specific comorbidities, regardless of their fibrosis level.15 Though not captured within the study period, in March 2018, DAA coverage in BC expanded to include all individuals with chronic HCV regardless of their fibrosis stage, and sofosbuvir-velpatasvir-voxilaprevir was added as a treatment option for DAA-treatment-experienced individuals.16
Data sources
Data for this study were drawn from three ongoing cohorts of people who use drugs with harmonized procedures for recruitment, follow-up and data collection, in Vancouver, Canada: the Vancouver Injection Drug Users Study (VIDUS); the AIDS Care Cohort to Evaluate exposure to Survival Services (ACCESS); and the At-Risk Youth Study (ARYS). VIDUS began recruitment in 1996, and ARYS and ACCESS in 2005. VIDUS consists of HIV-negative adults (≥ 18 years) who injected drugs in the month prior to enrolment, ACCESS of HIV-positive adults who used illicit drugs in the previous month, and ARYS of street-involved youth (14–26 years old at enrolment). Individuals are recruited through snowball sampling and extensive street outreach in the city’s Downtown Eastside and Downtown South neighborhoods, both urban areas with high levels of illicit drug use, marginalization and criminalization. Study procedures for the three studies have been described in detail previously.17–19 In brief, after providing written informed consent, at baseline, and semi-annually thereafter, participants completed an interviewer-administered questionnaire and provided blood for HIV/ HCV serological testing as appropriate. The questionnaire elicited data on socio-demographic characteristics, drug use patterns, health care utilization, and other relevant exposures. Participants received a $40 honorarium at each study visit. The VIDUS, ACCESS and ARY S studies have received approval by the University of British Columbia/Providence Health Care Research Ethics Board.
Study sample
The analytic sample for the current study was restricted to HCV-seropositive participants, with a history of injection drug use and self-reported presence of active HCV infection (i.e., answered “no” to the question: “Since you tested positive, have you been told by a doctor that you no longer have HCV?” before April 2015), who completed at least one study visit between April 2015 and November 2017. In the event of multiple observations for one individual, the most recent observation was used.
Measures
The primary outcome of interest was accumulated uptake of IFN-free DAA-based treatment, defined as responding “yes” to the question “Have you ever taken HCV treatment” and selected any (combination) of the following DAAs: sofosbuvir, ledispavir, sofosbuvir/ledispavir, daclastavir, asunaprevir, dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/gazoprevir, or sofosbuvir/velpatasvir, and did not select IFN.
We selected a range of explanatory variables that were hypothesized to influence uptake of HCV treatment among PWID.8–11 Socio-demographic characteristics included: age (per year older), sex (male versus female), self-reported race (white versus others), and HIV serostatus. We also considered substance use patterns, including high intensity (≥ daily versus < daily) illicit substance use (heroin injection, cocaine injection, non-medical use of opioid analgesics, crack use) and high-risk drinking as per the U.S. National Institute on Alcohol Abuse and Alcoholism (NIAAA)’s definition;20 as well as social-structural exposures, including place of residence (Downtown Eastside versus others), homelessness, employment, prohibited income generation (i.e., any report of sex work or illegal income generation such as theft, drug dealing, or street-based income sources), and incarceration. Access to HCV and addiction services, was explored through the following variables: being in HCV-specific care (e.g., ≥ 1 visit where HCV-related evaluations took place in the last six months); previous exposure to IFN-based therapy; being in addiction care (none versus opioid agonist therapy [OAT, i.e., methadone or buprenorphine/naloxone] versus other addiction treatments [i.e., detox, counselling, 12-step programs, residential treatment]). Among HIV-positive participants, we also considered use of antiretroviral therapy (ART). Except for the socio-demographic variables and previous exposure to HCV treatment, all other variables refer to the six-month period prior to the visit of interest.
Statistical analysis
First, we described characteristics of participants stratified by recent injection drug use (i.e., in the last six months) and DAA-based therapy uptake. Next, we used logistic regression to assess the bivariable relationship between each explanatory variable and uptake of DAA-based therapy. Finally, to determine the independent correlates of DAA-based therapy uptake we ran a fixed multivariable model containing all explanatory variables that were associated with the outcome at p<0.10 in bivariable analyses.
We conducted two sub-analyses, where we investigated correlates of DAA-based treatment (a) restricted to recent PWID and (b) stratified by HIV status. Given the small numbers, the latter was limited to assessing bivariable relationships. All analyses were conducted using R (Version 3.2.4), and all p-values were two-sided.
Results
Between April 2015 and November 2017, 2,256 HCV-positive PWID completed at least one study visit. Of these, 1,034 (45.8%) self-reported presence of active HCV infection, of whom 915 (88.5%) provided valid answers to the primary outcome, and thus were included in the present study. Selected socio-demographic characteristics of study participants, stratified by recent injection drug use, are reported in Table 1. The median age was 49 years (Interquartile range [IQR] 39–56), 560 (61.2%) were male, 493 (53.9%) were white, 369 (40.3%) were coinfected with HIV, and 611 (66.8%) self-reported recent injection drug use. The majority of nonwhite participants self-identified as Indigenous (n=345, 81.8%). Substance use varied from less than 5% for at least daily opioid analgesic use (21, 2.3%) to almost 25% for at least daily heroin injection (223, 24.4%). Fifteen percent of participants (n=137) reported high-risk drinking. Around half of the study population was enrolled in OAT programs (489, 53.4%) or receiving HCV-specific care (404, 44.2%), and 6.6% (n=60) reported ever receiving IFN-based HCV treatment.
Table 1.
Characteristics of 915 PWID with chronic HCV infection, stratified by recent injection drug use and DAA-uptake, Vancouver, Canada (April 2015 – November 2017)
| Characteristic, n (%) | Total (N = 915) | Recent PWID (n = 611) |
Non-recent PWID (n = 304) |
||
|---|---|---|---|---|---|
| DAA-uptake=Yes (n = 78) | DAA-uptake=No (n = 533) | DAA-uptake=Yes (n = 68) | DAA-uptake=No (n = 236) | ||
| Individual-level factors | |||||
| Age (median, IQR) | 49 (39–56) | 50 (45–57) | 46 (35–53) | 55 (48–59) | 53 (46–59) |
| Male sex | 560 (61.2) | 54 (69.2) | 315 (59.1) | 50 (73.5) | 141(59.7) |
| White race | 493 (53.9) | 50 (64.1) | 271 (50.8) | 45 (66.2) | 127 (53.8) |
| HIV-positive | 369 (40.3) | 50 (64.1) | 191 (35.8) | 39 (57.4) | 89 (37.7) |
| Substance use-related factors* | |||||
| ≥ Daily heroin injection | 223 (24.4) | 19 (24.4) | 204 (38.3) | NA | NA |
| ≥ Daily cocaine injection | 47 (5.1) | 5 (6.4) | 42 (7.9) | NA | NA |
| ≥ Daily crack use | 94 (10.3) | 7 (9.0) | 59 (11.1) | 1 (1.5) | 27 (11.4) |
| ≥ Daily opioid analgesic use | 21 (2.3) | 3 (3.8) | 18 (3.4) | 0 (0.0) | 0 (0.0) |
| Syringe sharing | 21 (2.3) | 3 (3.8) | 38 (3.4) | NA | NA |
| High-risk drinking | 137 (15.0) | 7 (9.0) | 79 (14.8) | 5 (7.4) | 46 (19.5) |
| Health care-related factors* | |||||
| In addiction care | |||||
| OAT | 489 (53.4) | 53 (67.9) | 292 (31.9) | 30 (44.1) | 114 (48.3) |
| Other modalities (e.g., detox, psychosocial, residential treatment) | 101 (11.0) | 7 (9.0) | 52 (9.8) | 30 (44.1) | 114 (48.3) |
| None | 320 (35.0) | 17 (21.8) | 188 (20.5) | 29 (42.6) | 86 (36.4) |
| In HCV-related care | 404 (44.2) | 44 (56.4) | 202 (37.9) | 50 (73.5) | 108 (45.8) |
| Ever received IFN-based therapy for HCV infection | 60 (6.6) | 3 (3.8) | 38 (7.1) | 5 (7.4) | 14 (5.9) |
| Other structural-level | |||||
| Homelessness | 145 (15.8) | 7 (9.0) | 111 (20.8) | 5 (7.4) | 22 (9.3) |
| Residency in the Downtown | 573 (62.6) | 49 (62.8) | 388 (72.8) | 27 (39.7) | 109 (46.2) |
| Employment | 203 (22.2) | 19 (24.4) | 98 (18.4) | 23 (33.8) | 63 (26.7) |
| Prohibited income generation | 386 (42.2) | 32 (41.0) | 301 (56.5) | 7 (10.3) | 46 (19.5) |
| Incarceration | 49 (5.4) | 2 (2.6) | 45 (8.4) | 0 (0.0) | 2 (0.8) |
DAA, direct-acting antiviral; OAT, opioid agonist therapy. NA, not applicable
Refers to the 6-month period prior to the interview
Wilcoxon rank sum test
Fisher’s exact test
Overall, 146 participants (16.0%) reported starting IFN-free DAA-based regimens during the study period, a rate of 6.0 per 100 PY (95% Confidence Interval [CI]: 5.0–7.0 per 100 PY). Treatment initiation rates were higher among non-recent PWID (8.4 per 100 PY, 95% CI 6.4–10.4 per 100 PY) than among recent PWID (4.8 per 100 PY, 95% CI 3.7–59 per 100 PY). As shown in Figure 1, there was an overall trend to increasing DAA uptake rates over time, mostly driven by a steady rise in treatment initiation rates among recent PWID.
Figure 1. Trends in DAA-based therapy uptake among PWID with chronic HCV infection, Vancouver, Canada (April 2015 - November 2017).
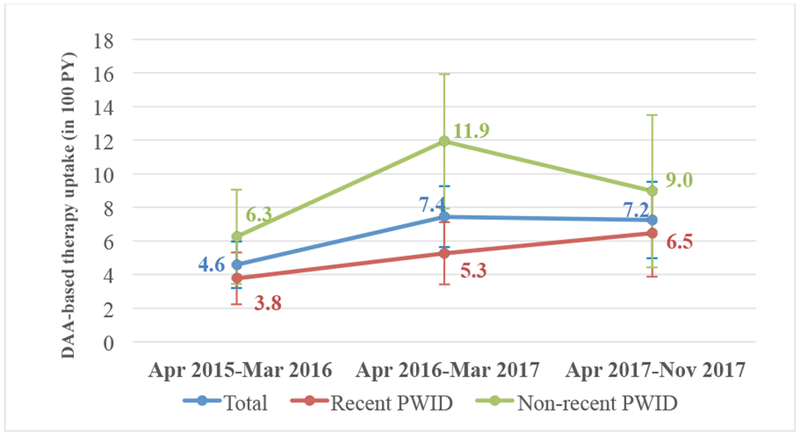
DAA, direct acting antiviral. PY, person years. PWID, people who inject drugs.
Table 2 presents the results of unadjusted and adjusted logistic regression analyses of factors associated with uptake of DAA-based regimens. In the final multivariable model, HIV-coinfected (Adjusted Odds Ratio [AOR] = 2.29, 95% CI: 1.55–3.40) those in HCV care (AOR=1.94, 95% CI: 1.31–2.90) and white participants (AOR=1.56, 95% CI 1.05–2.35) had increased odds of having initiated DAA-therapies, while those reporting high-risk drinking (AOR=0.47, 95% CI: 0.23–0.88) or at least daily crack use (AOR=0.41, 95% CI: 0.17–0.85) had decreased odds. When restricting the analysis to recent PWID, we found that in addition to HIV-coinfection and white race, engagement in OAT emerged as an independent positive correlate of DAA uptake (Table 3).
Table 2.
Unadjusted and adjusted logistic regression analyses of factors associated with DAA-based therapy uptake among PWID with chronic HCV infection (n=915), Vancouver, Canada (April 2015 – November 2017)
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (per year older) | 1.04 (1.02 – 1.06)† ‡ | 1.02 (1.00 – 1.04) |
| Male gender (yes vs. no) | 1.70 (1.16 – 2.52)† ‡ | 1.19 (0.78 – 1.84) |
| White race (yes vs. no) | 1.74 (1.21 – 2.52)† ‡ | 1.56 (1.05 – 2.35)‡ |
| HIV-positive (yes vs. no) | 2.72 (1.89 – 3.92)† ‡ | 2.29 (1.55 – 3.40)‡ |
| ≥ Daily heroin injection (yes vs. no)* | 0.41 (0.24 – 0.67)† ‡ | 0.63 (0.34 – 1.09) |
| ≥ Daily cocaine injection (yes vs. no)* | 0.61 (0.21 – 1.44) | |
| ≥ Daily crack use (yes vs. no)* | 0.46 (0.20 – 0.92)† ‡ | 0.41 (0.17 – 0.85)‡ |
| ≥ Daily opioid analgesic use (yes vs. no)* | 0.88 (0.20 – 2.63) | |
| Syringe sharing (yes vs. no)* | 0.87 (0.20 – 2.62) | |
| High-risk drinking (yes vs. no)* | 0.46 (0.24 – 0.83)† ‡ | 0.47 (0.23 – 0.88)‡ |
| In addiction care (reference: none)* | ||
| OAT | 1.22 (0.83 – 1.81) | |
| Other treatment modalities | 1.12 (0.59 – 2.05) | |
| Ever received IFN-based therapy for HCV infection | 0.80 (0.35 – 1.63) | |
| In HCV-related care (yes vs. no)* | 2.68 (1.86 – 3.89)† ‡ | 1.94 (1.31 – 2.90)‡ |
| Homelessness (yes vs. no)* | 0.43 (0.22 – 0.76)† ‡ | 0.80 (0.39 – 1.52) |
| Residency in the Downtown Eastside (yes vs. no)* | 0.59 (0.42 – 0.85)† ‡ | 0.80 (0.54 – 1.18) |
| Employment (yes vs. no)* | 1.53 (1.02 – 2.26)† ‡ | 1.47 (0.94 – 2.28) |
| Prohibited income generation (yes vs. no)* | 0.44 (0.30 – 0.65)† ‡ | 0.79 (0.51 – 1.23) |
| Incarceration (yes vs. no)* | 0.21 (0.04 – 0.70)† ‡ | 0.54 (0.08 – 1.97) |
DAA, direct-acting antiviral; OAT, opioid agonist therapy.
Refers to the 6-month period prior to the interview
p<0.10 and included in the multivariable model
p<0.05
Table 3.
Unadjusted and adjusted logistic regression analyses of factors associated with DAA-based therapy uptake among recent PWID with chronic HCV infection (n=611), Vancouver, Canada (April 2015 – November 2017)
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (per year older) | 1.05 (1.02 – 1.07)† ‡ | 1.02 (0.99 – 1.05) |
| Male gender (yes vs. no) | 1.56 (0.95 – 2.64)† | 1.38 (0.79 – 2.44) |
| White race (yes vs. no) | 1.73 (1.06 – 2.86)† ‡ | 1.73 (1.02 – 3.00)‡ |
| HIV-positive (yes vs. no) | 3.18 (1.95 – 5.97)† ‡ | 2.65 (1.57 – 4.57)‡ |
| ≥ Daily heroin injection (yes vs. no)* | 0.52 (0.29 – 0.88)† ‡ | 0.71 (0.38 – 1.27) |
| ≥ Daily cocaine injection (yes vs. no)* | 0.80 (0.27 – 1.92) | |
| ≥ Daily crack use (yes vs. no)* | 0.79 (0.32 – 1.70) | |
| ≥ Daily opioid analgesic use (yes vs. no)* | 1.14 (0.26 – 3.48) | |
| Syringe sharing (yes vs. no)* | 1.14 (0.26 – 3.48) | |
| High-risk drinking (yes vs. no)* | 0.57 (0.23 – 1.20) | |
| In addiction care (reference: none)* | ||
| OAT | 2.01 (1.15 – 3.67)† ‡ | 2.00 (1.11 – 3.78)‡ |
| Other treatment modalities | 1.49 (0.55 – 3.65) | 1.44 (0.51 – 3.72) |
| Ever received IFN-based therapy for HCV infection | 0.52 (0.12 – 1.49) | |
| In HCV-related care (yes vs. no)* | 2.12 (1.32 – 3.45)† ‡ | 1.44 (0.86 – 2.43) |
| Homelessness (yes vs. no)* | 0.37 (0.15 – 0.78)† ‡ | 0.74 (0.28 – 1.69) |
| Residency in the Downtown Eastside (yes vs. no)* | 0.63 (0.39 – 1.05)† | 0.76 (0.45 – 1.31) |
| Employment (yes vs. no)* | 1.43 (0.80 – 2.47) | |
| Prohibited income generation (yes vs. no)* | 0.54 (0.33 – 0.87)† ‡ | 0.78 (0.46 – 1.31) |
| Incarceration (yes vs. no)* | 0.29 (0.05 – 0.96)† ‡ | 0.50 (0.08 – 1.86) |
DAA, direct-acting antiviral; OAT, opioid agonist therapy.
Refers to the 6-month period prior to the interview
p<0.10 and included in the multivariable model
p<0.05
Our second sub-analysis revealed that 57 (10.5%) of the 544 HIV-negative participants initiated DAA-based therapy during the study period (a rate of 3.9 per 100 PY, 95%CI 2.9–5.0 per 100 PY), while among the 369 HIV co-infected participants, 89 (24.1%) did so (a rate of 9.0 per 100 PY, 95%CI 7.2–10.9 per 100 PY). Positive and negative correlates of DAA-based therapy uptake in stratified bivariable logistic regression analyses were overall similar to those of the main analysis (data not shown). Of note, among HIV-positive participants, being on ART was associated with an almost 8-fold higher odds of DAA-based therapy uptake (OR = 7.80, 95% CI: 4.26–15.30).
Discussion
This study demonstrated high rates of DAA-based HCV treatment uptake among PWID in Vancouver during the first thirty-two months of introduction of second-generation DAAs in BC’s drug formulary, with an increasing trend over time. Although DAA uptake rates were higher among non-recent PWID than recent PWID, there was a steady increase in treatment initiation rates over time among the latter group. Altogether, DAA initiation rates observed in this study lie somewhere between the 3.0 per 100 PY documented among PWID in Amsterdam21 and the 22.0 per 100 PY for Needle and Syringe Program’s attendees in Australia, during the first year of DAA availability in those settings.22 Importantly, these treatment uptake rates are substantially higher than those observed in the IFN-era in Vancouver and other high-income settings (<2.0 per 100 PY).8–11 Our analysis also found that, in particular, HIV-coinfected participants (and specially those on ART) were more likely to have initiated DAA-based therapy. While DAA-based therapies appeared to have removed some of the barriers to HCV treatment uptake for PWID in our setting, gaps in access and disparities within this population still remain. Indeed, although almost half of the population reported being in engaged in HCV-specific care, only one in seven actually started treatment. In addition, non-white participants, or those reporting high-risk drinking or frequent crack use were less likely to have accessed DAA-based therapies.
In line with other research,13,23,24 this study showed that HIV co-infection was positively associated with DAA treatment uptake. The higher uptake of DAA-based therapies among HIV-positive participants may reflect treatment coverage restrictions during most of the study period to patients with advanced liver fibrosis—typically more prevalent among HIV/HCV coinfected populations compared to HCV-monoinfected individuals25,26—as well as further expansion to all HIV/HCV co-infected individuals independent of fibrosis stage in March 2017. An alternative explanation might be that many HIV/HCV coinfected PWID are already in care for another chronic condition, which may facilitate referral for assessment of liver fibrosis and consideration for HCV treatment. This is supported by our data indicating a higher proportion of HIV-positive PWID being engaged in HCV care (a strong positive correlate of DAA uptake) compared to HCV-monoinfected participants, as well as higher likelihood of receipt of DAAs among HIVpositive PWID on ART.
Our sub-analysis also showed that engagement in OAT was associated with DAA uptake among recent PWID. HCV treatment of active injectors (and prevention of reinfection) will be essential to curb the HCV epidemic. As such, this finding is encouraging particularly given the well-known benefits of OAT to reduce the risk of HCV acquisition.27 Collectively, our findings support the need for a multidisciplinary approach, including integrated models of HIV, HCV, and addiction care for the successful delivery of HCV care for PWID.28
Our study also found that non-white PWID had lower odds of initiating DAA-based therapies. These results are consistent with studies showing lower DAA treatment uptake rates among nonwhites in the U.S.,23,24,29 as well as among Indigenous people in Canada8,30 and Australia.31 This is concerning given higher HCV prevalence and incidence rates among Indigenous populations relative to non-indigenous populations.32,33 Studies conducted in the U.S. have documented how racial/ethnic disparities in DAA treatment uptake may be a reflection of difficulties in accessing costly medications given typically lower socio-economic status among non-whites.23,24,29 However, in a setting with public coverage of DAAs for eligible patients as ours, other factors may better explain differences in treatment access between white and non-white participants. Among these, a growing body of evidence has highlighted how the dual stigma that PWID of Indigenous ancestry face when trying to access health care can result in delaying or forgoing needed care altogether.34,35 Collectively, these findings underscore the need for safe and culturally-appropriate health services to optimize engagement in HCV care and improve health outcomes among Indigenous people.
Finally, the present analysis indicated that certain patterns of substance use (i.e., daily crack use and high-risk drinking) were associated with reduced likelihood of DAAs uptake. While alcohol use disorder was a relative contraindication to IFN-based therapies,36 current Canadian guidelines recommend that “all individuals with chronic HCV infection should be considered candidates for [IFN-free DAA-based] antiviral therapy”37 In addition, and in contrast to other jurisdictions,38,39 a review of reimbursement criteria for DAA-based therapy in Canada found no restrictions related to drug or alcohol use.40 Therefore, in the absence of formal exclusion criteria, the reduced likelihood of DAA treatment initiation among PWID with high-intensity drinking or crack use may be partially explained by concerns among healthcare providers about non-adherence among poly-substance users.41 Given the lack of evidence to support such concerns,6 withholding effective HCV treatments from PWID who engage in poly-substance use may not only be considered unethical but also a poor public health strategy. Indeed, given the well-known synergistic effects of alcohol and HCV on liver disease progression, there is a strong rationale to treat and cure HCV infection among individuals with heavy alcohol use to decrease liver-related morbidity and mortality, as well as healthcare related costs.42,43 Likewise, while data on the effects of crack on liver disease is limited and inconclusive,44,45 given the higher risk of HCV infection among this population,44 achieving and sustaining SVR may prevent onward HCV transmission.6 Accordingly, efforts are needed to facilitate and ensure equitable access to DAAs for substance using populations and optimize the individual and public-health benefits of these treatments. These should range from HCV and addiction training opportunities to health care providers working with PWID, to integration of addiction and HCV services in primary care settings, to peer-led interventions to support engagement of PWID with HCV and addiction health services.12,46
There are a number of limitations to this study. First, our sample was not randomly selected, and thus our results may be subject to selection bias. Specifically, findings from this study may not be generalizable to all HCV-positive PWID in Vancouver or other settings with different health care systems. Second, our definition of chronic HCV infection was based on a positive HCV-Ab test and self-reported presence of active infection. In the absence of systematic access to confirmatory RNA-testing, our study sample may have been overestimated. Third, many measures of this analysis relied on self-reported data which may be prone to social-desirability and recall biases. However, past research has indicated PWID’ reports to be reliable.47 Finally, despite the use of multivariable modelling techniques we cannot exclude the possibility of unmeasured confounding. In particular, although studies suggest that approximately 40% of HCV-positive PWID may have significant liver fibrosis,48given the lack of information on liver fibrosis among our study participants (the main eligibility criteria for public coverage of DAAs in BC for most of the study period), we were not able to distinguish its impact on the associations between other factors and DAA-treatment uptake.
In summary, this study found high and increasing HCV treatment uptake rates (approximately 6.0 per 100 PY) among PWID during the first thirty-two months of public coverage of DAAs in BC. Of note, modelling studies based on Vancouver data (estimated 65% HCV chronic prevalence among PWID) suggest that scaling up DAA-based therapies to 8.0 per 100 PWID annually, could halve prevalence of chronic HCV among this population within fifteen years.7 While this is encouraging, specific subgroups of this marginalized population were less likely to have initiated DAAs, including some of whom are at increased risk of disease progression (e.g., individuals with high-risk drinking behaviours) or transmission (e.g., frequent crack users). Even with unrestricted access to efficacious and safe DAA-based therapies, World Health Organization HCV elimination targets49 will not be achieved unless we address behavioural and social-structural barriers to HCV care.
Acknowledgments:
The authors thank the study participants, as well as current and past researchers and staff.
Financial support: This work was supported by the US National Institute on Drug Abuse (NIDA) (U01-DA038886 and U01-DA021525). MES is supported by a Michael Smith Foundation for Health Research (MSFHR)/St Paul’s Foundation Scholar Award. M-JM is supported by NIDA (U01-DA021525), and CIHR New Investigator and MSFHR Scholar Awards. EW is supported by a Tier 1 Canada Research Chair in Inner City Medicine. LT is supported by a MSFHR Scholar Award. KH is supported by the St. Paul’s Foundation, and CIHR New Investigator and MSFHR Scholar Awards. KD is supported by MSFHR/St. Paul’s Hospital Foundation Scholar Award and CIHR New Investigator Awards.
List of abbreviations:
- PWID
People who inject drugs
- HCV
Hepatitis C
- DALYs
Disability adjusted life-years
- SVR
Sustained virological response
- PY
Person-year
- IFN
Interferon
- DAA
Direct acting antiviral
- BC
British Columbia
- NIAAA
U.S. National Institute on Alcohol Abuse and Alcoholism
- OAT
Opioid agonist therapy
- ART
Antiretroviral therapy
- AOR
Adjusted odds ratio
- CI
Confidence interval
Footnotes
Conflict of interest: The University of British Columbia has received unstructured funding from NG Biomed, Ltd. to support M-JM. All other authors declare no conflict of interests.
References
- 1.Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degenhardt L, Charlson F, Stanaway J, et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(12):1385–1398. [DOI] [PubMed] [Google Scholar]
- 3.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. [DOI] [PubMed] [Google Scholar]
- 5.Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61(5):730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grebely J, Hajarizadeh B, Dore GJ. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol. 2017;14(11):641–651. [DOI] [PubMed] [Google Scholar]
- 7.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alavi M, Raffa JD, Deans GD, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver Int. 2014;34(8):1198–1206. [DOI] [PubMed] [Google Scholar]
- 9.Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999-2011. J Viral Hepat. 2014;21(3):198–207. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young S, Wood E, Milloy MJ, et al. Hepatitis C Cascade of Care among People Who Inject Drugs in Vancouver, Canada. Subst Abus. 2018:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 2014;104:62–72. [DOI] [PubMed] [Google Scholar]
- 13.Janjua NZ, Islam N, Wong J, et al. Shift in disparities in hepatitis C treatment from interferon to DAA era: A population-based cohort study. J Viral Hepat. 2017;24(8):624–630. [DOI] [PubMed] [Google Scholar]
- 14.Drug Coverage Decision for B.C. Pharmacare. July 28, 2015. https://fmdb.hlth.gov.bc.ca/AttachmentRetrievalServlet?sub=7346.
- 15.Drug Coverage Decision for B.C. Pharmacare. March 21, 2017. https://fmdb.hlth.gov.bc.ca/AttachmentRetrievalServlet?sub=7353.
- 16.Drug Coverage Decision for B.C. Pharmacare. March 13, 2018. https://fmdb.hlth.gov.bc.ca/AttachmentRetrievalServlet?sub=8283.
- 17.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. [DOI] [PubMed] [Google Scholar]
- 18.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. [DOI] [PubMed] [Google Scholar]
- 19.Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderatebinge-drinking. Accessed May 17, 2018.
- 21.van Santen DK, van der Helm JJ, Lindenburg K, Schim van der Loeff M, Prins M. HIV and hepatitis C treatment uptake among people who use drugs participating in the Amsterdam Cohort Studies, 1985-2015. Int J Drug Policy. 2017;47:95–101. [DOI] [PubMed] [Google Scholar]
- 22.Heard S, Iversen J, Geddes L, Maher L. Australian Needle Syringe Program Survey National Data Report 2013-2017: Prevalence of HIV, HCV and injecting and sexual behaviour among NSP attendees. Sidney, Australia: Kirby Institute; 2018: https://kirby.unsw.edu.au. Accessed July 17, 2018. [Google Scholar]
- 23.Marcus JL, Hurley LB, Chamberland S, et al. Disparities in Initiation of Direct-Acting Antiviral Agents for Hepatitis C Virus Infection in an Insured Population. Public Health Rep. 2018:33354918772059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spradling PR, Xing J, Rupp LB, et al. Uptake of and Factors Associated With Directacting Antiviral Therapy Among Patients in the Chronic Hepatitis Cohort Study, 2014 to 2015. J Clin Gastroenterol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Re V, 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virusmonoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham SM, Mankhambo L, Phiri A, et al. Impact of human immunodeficiency virus infection on the etiology and outcome of severe pneumonia in Malawian children. Pediatr Infect Dis J. 2011;30(1):33–38. [DOI] [PubMed] [Google Scholar]
- 27.Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57 Suppl 2:S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanwal F, Kramer JR, El-Serag HB, et al. Race and Gender Differences in the Use of Direct Acting Antiviral Agents for Hepatitis C Virus. Clin Infect Dis. 2016;63(3):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeed S, Strumpf EC, Moodie EE, et al. Disparities in direct acting antivirals uptake in HIV-hepatitis C co-infected populations in Canada. J Int AIDS Soc. 2017;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alavi M, Micallef M, Fortier E, et al. Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: the ETHOS study. J Viral Hepat. 2015;22(11):914–925. [DOI] [PubMed] [Google Scholar]
- 32.Rempel JD, Uhanova J. Hepatitis C virus in American Indian/Alaskan Native and Aboriginal peoples of North America. Viruses. 2012;4(12):3912–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lelutiu-Weinberger C, Pouget ER, Des Jarlais DD, et al. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Soc Sci Med. 2009;68(3):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman A, Fleming K, Markwick N, et al. “They treated me like crap and I know it was because I was Native”: The healthcare experiences of Aboriginal peoples living in Vancouver’s inner city. Soc Sci Med. 2017;178:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brener L, Wilson H, Jackson LC, Johnson P, Saunders V, Treloar C. Experiences of diagnosis, care and treatment among Aboriginal people living with hepatitis C. Aust N Z J Public Health. 2016;40 Suppl 1:S59–64. [DOI] [PubMed] [Google Scholar]
- 36.Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol. 2012;26(6):359–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah H, Bilodeau M, Burak KW, et al. The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver. CMAJ. 2018;190(22):E677–E687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall AD, Cunningham EB, Nielsen S, et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol. 2018;3(2):125–133. [DOI] [PubMed] [Google Scholar]
- 39.Ooka K, Connolly JJ, Lim JK. Medicaid Reimbursement for Oral Direct Antiviral Agents for the Treatment of Chronic Hepatitis C. Am J Gastroenterol. 2017;112(6):828–832. [DOI] [PubMed] [Google Scholar]
- 40.Marshall AD, Saeed S, Barrett L, et al. Restrictions for reimbursement of direct-acting antiviral treatment for hepatitis C virus infection in Canada: a descriptive study. CMAJ Open. 2016;4(4):E605–E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA. Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Subst Use Misuse. 2016;51(9):1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punzalan CS, Bukong TN, Szabo G. Alcoholic hepatitis and HCV interactions in the modulation of liver disease. J Viral Hepat. 2015;22(10):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393–401. [DOI] [PubMed] [Google Scholar]
- 44.Butler AJ, Rehm J, Fischer B. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depend. 2017;180:401–416. [DOI] [PubMed] [Google Scholar]
- 45.Martel-Laferriere V, Nitulescu R, Cox J, et al. Cocaine/crack use is not associated with fibrosis progression measured by AST-to-Platelet Ratio Index in HIV-HCV co-infected patients: a cohort study. BMC Infect Dis. 2017;17(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34–46. [DOI] [PubMed] [Google Scholar]
- 47.Langendam MW, van Haastrecht HJ, van Ameijden EJ. The validity of drug users’ selfreports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol. 1999;28(3):514–520. [DOI] [PubMed] [Google Scholar]
- 48.Mehta SH, Kirk GD, Astemborski J, Sulkowski MS, Afdhal NH, Thomas DL. Stability of liver fibrosis among HCV-infected injection drug users. Antivir Ther. 2012;17(5):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis. Geneva: World Health Organization;June 2016. [Google Scholar]


