Abstract
The stop-signal task (SST) is used to study action-stopping in the laboratory. In SSTs, the P3 event-related potential following stop-signals is considered to be a neural index of motor inhibition. However, a similar P3 deflection is often observed following infrequent events in non-inhibition tasks. Moreover, within SSTs, stop-signals are indeed infrequent events, presenting a systematic confound that hampers the interpretation of the stop-signal P3 (and other candidate neural indices of motor inhibition). Therefore, we performed two studies to test whether the stop-signal P3 is uniquely related to motor inhibition or reflects infrequency detection. In Study 1, participants completed the SST and a visually identical change-detection task requiring the detection of a task-relevant, frequent signal (but not motor inhibition). We observed a P3 associated with motor inhibition in the SST, but no such positivity in the change-detection task. In Study 2, we modified the change-detection task. Some task-relevant events were now infrequent, matching the frequency of stop-signals in the SST. These events indeed evoked a P3, though of smaller amplitude than the P3 in the SST. Independent component analysis suggested that stop-signal P3 and infrequency-P3 ERPs were non-independent and shared a common neural generator. Further analyses suggested that this common neural process likely reflects motor inhibition in both tasks: infrequent events in the change-detection task lead to a non-instructed, incidental slowing of motor responding, the degree of which was strongly correlated with P3 amplitude. These results have wide-reaching implications for the interpretation of neural signals in both stop-signal and infrequency/oddball-tasks.
Keywords: motor inhibition, infrequency detection, action-stopping, frontocentral P3, event-related potentials
Introduction
The abilities to detect environmental changes and stop ongoing actions when necessary are paramount to behaving flexibly and surviving in an ever-changing world. For example, if a person is about to step off a sidewalk to cross a street and sees a car speeding towards them, they are able to correct their actions by inhibiting the planned (and perhaps partially executed) action of stepping into the street. Notably, successful action-stopping depends both on motor inhibition as well as the detection of a stimulus indicating a change in behavior is necessary (such as the car in this example). Without attentional detection of the car, motor inhibition would never be initiated – perhaps with disastrous consequences.
In the laboratory setting, action-stopping may be studied using the stop-signal task (SST), a task that requires participants cancel ongoing motor responses (Verbruggen & Logan, 2008). Participants make responses to “go” stimuli, which are present on every trial, but must attempt to withhold responding on a subset of trials when they receive a “stop” stimulus shortly following the “go” stimulus. Logan and Cowan proposed a “horse-race model” of inhibition to explain trial outcomes in the stop-signal task (Logan & Cowan, 1984; Logan, Cowan, & Davis, 1984; Band et al., 2003). In this race model, the go and stop processes start with the onset of their respective stimuli and “race” one another to completion. Thus, the speed of an individual’s inhibitory process and the delay between the go stimulus and the stop signal (stop-signal delay, SSD) both factor into whether a given stop signal trial culminates in successful or failed stopping (see also Boucher et al., 2007).
The SST is a widely used tool for the study of motor inhibition, but research suggests that – similar to real-world scenarios – performance on the task relies on more factors than motor inhibition alone. In the above example of action-stopping, an oncoming car must be detected before the movement into the street can be inhibited. Indeed, action-stopping is a sequential process that involves several additional processes before the onset of inhibition. Before inhibition occurs, a stimulus (such as the stop signal) must be perceived by sensory systems and then attentionally detected. Research suggests that attentional processes influence stopping success and speed. Verbruggen and colleagues found that when participants were distracted by irrelevant stimuli during an inhibition task, their stopping was impaired (Verbruggen et al., 2014), but also that participants can “bias” their attentional system to improve stopping success (Elchlepp et al., 2016).
Naturally, participants in a laboratory study differ in their times to detect a stimulus (Drew & Vogel, 2008; Martens et al., 2006, Kanai & Rees, 2011), which will affect the time it takes for the stopping process to begin during a stop trial. This may not prove problematic in samples of healthy individuals, where one might assume time to signal detection is roughly equivalent. However, time to signal detection increases as a function of typical aging (Ratcliff et al., 2001), and in several neuropsychiatric diseases (Saccuzzo & Braff, 1981; Johannes et al., 2001). This is problematic because the stop-signal task is used to infer ostensible motor inhibition deficits in both aging and models of disease such as Parkinson’s (Obeso et al., 2011; Alegre et al., 2013; Gauggel et al., 2004) and ADHD (Oosterlaan et al., 1998; Schachar et al., 1995; Nigg 1999; Logan, & Sergeant, 1998), where deficits or group differences may not truly be constrained to differences in motor inhibition, but may in fact reflect attentional difficulties as well (Huster et al., 2013; Bekker et al., 2005; Kenemans et al., 2005). In line with this, recent research suggests that “trigger failures”, the failure to initiate the stopping process at all, can account for ostensible ‘inhibitory’ deficits in the stop-signal task, for example, in schizophrenia (Matzke, Love, & Heathcote, 2017; Matzke et al., 2017). These trigger failures may occur because of problems with triggering the inhibitory process (e.g., a lack of attentional detection of the stop-signal), and not necessarily inhibition itself. In light of these findings, understanding and quantifying the neural basis of perceptual and attentional contributions to action-stopping is crucial.
In electrophysiological studies of the stop-signal task, a frontocentral P3 (the stop-signal P3) event-related potential (ERP), is often used as a neural proxy of inhibition (Kok et al., 2004; Ramautar et al., 2004; Dimoska et al., 2006; Wessel & Aron, 2015; Bekker et al., 2005). In particular, the onset of this ERP after stop-signals shows significant differences between successful and failed stop-trials that are in line with predictions derived from the race model: the onset of the stop-signal P3 occurs earlier in successful vs. failed stop-trials, reflecting the fact that an earlier onset of the “stop”-side of the race will lead to more successful action-stopping. In large samples, the onset of this stop-signal P3 also positively correlates with SSRT, with participants showing slower stopping also showing later P3-onset (Wessel & Aron, 2015).
However, the interpretation of the stop-signal P3 as an index of motor inhibition is complicated by the fact that a P3 ERP with similar properties is also observed following infrequent or oddball events (Campanella et al., 2002; Debener et al., 2005; Garcia-Larrea et al., 1992). This is problematic given that stop signals are infrequent in the SST. Therefore, it is hitherto unclear whether the stop-signal P3 can be accounted for by the infrequency of the stop-signal in the SST. Furthermore, the neural underpinnings of infrequency detection and inhibition are difficult to separate. Inhibitory signaling during the SST is purportedly implemented via hyper-direct pathway signaling to the basal ganglia (Parent & Hazrati, 1993; Nambu, Tokuno, & Takada, 2002; Miocinovic et al., 2018; Wessel & Aron 2017), resulting in a net decrease of thalamic output (for recent reviews on stopping-related neural circuits, see Bari & Robbins, 2013; Kenemans, 2015; Schall & Godlove, 2012; and Zavala et al., 2015). Much controversy exists as to whether the right inferior frontal cortex (rIFC), a proposed cortical node involved in the recruitment of this circuit, has a role that is singularly related to inhibition of motor movements (Aron, Robbins, & Poldrack, 2014) or if it also may have a role in the attentional detection infrequent signals that do not explicitly signal inhibition (Erika-Florence, Leech, & Hampshire, 2014). Therefore, the use of electrophysiological measures to attempt to disentangle signals of infrequency detection and inhibition on the short-term scale during reactive stopping may help shed light on the complex interaction between these two processes, and perhaps their neural underpinnings.
In the current study, we used independent component analysis (ICA) to attempt to separate neural signatures of inhibition from neural signatures of attentional detection of infrequent stimuli. ICA decomposition (Bell and Sejnowski, 1995) of EEG data is commonly used for removal of stereotypical data artifacts (such as blinks and saccades; Delorme et al., 2007), but also allows for the separation of meaningful independent neural processes or identification of common processes across experimental situations and tasks (Wessel, 2018; Onton, Delorme, & Makeig, 2005). By subjecting an EEG recording collected during multiple cognitive tasks to a common, combined ICA, one can identify neural signatures of target processes (e.g., the frontocentral P3) operationalized in one paradigm (e.g., the SST), isolate the independent component (IC) representing that neural signature, and examine the activation of that component across other behavioral experimental situations. With regards to the SST, once one identifies the component that accounts for the stop-signal P3 in the SST, one can test the same IC in a non-inhibition task of interest. If the IC shows significant activation during an event of interest in another task – we can conclude that the events in those two tasks (the stop-signal we used to identify the IC and the other event we tested the IC on) share a common process as indexed by that IC. The logic behind this method is discussed further in Wessel (2018) and the procedures detailed in the Method Section.
To summarize, two features of the SST complicate the interpretation of neural activity during successful stop trials as indices of inhibitory processes: 1) the SST requires attentional detection of task-relevant stimuli (stop-signals) to trigger motor inhibition, and 2) due to the infrequency of stop-signals in the context of the stop-signal task, motor inhibition and attentional detection of infrequency are invariably confounded. We therefore conducted two studies to attempt to tease inhibitory processes from those associated with the detection of task-relevant and/or infrequent events. In the first study, we collected behavioral and EEG data and conducted ICA to demonstrate that no frontocentral P3 is elicited when a frequent signal has to be attentionally detected. In the second study, we use the same approach to assess whether the P3 elicited by inhibition is separable from the P3 that is elicited when a to-be-detected signal is infrequent but does not signal inhibition.
Method
Participants
Study 1.
Twenty-four healthy adult participants were recruited via a research recruitment email sent out to the University of Iowa email-listserv. Participants were compensated $15 per hour for their time. The procedure was approved by the University of Iowa’s Institutional Review Board (#201511709). Due to hardware defects during data collection, six of the datasets could not be included in analyses and one additional dataset was excluded because the participant stopped successfully on fewer than 10% of stop trials, indicating a failure to follow the task instructions. These exclusions yielded a final count of seventeen participants included in analyses for Study 1 (6 male, 2 left-handed, mean age 22.8y ± 3.64).
Study 2.
Twenty healthy adult participants were recruited via a research recruitment email sent out to the University of Iowa listserv. Participants were compensated $15 per hour for their time. The procedures were approved under the same IRB protocol as Study 1. All twenty participants were included in all analyses for Study 2 (11 male, 1 left-handed, mean age 24.9y ± 4.48).
Behavioral task administration
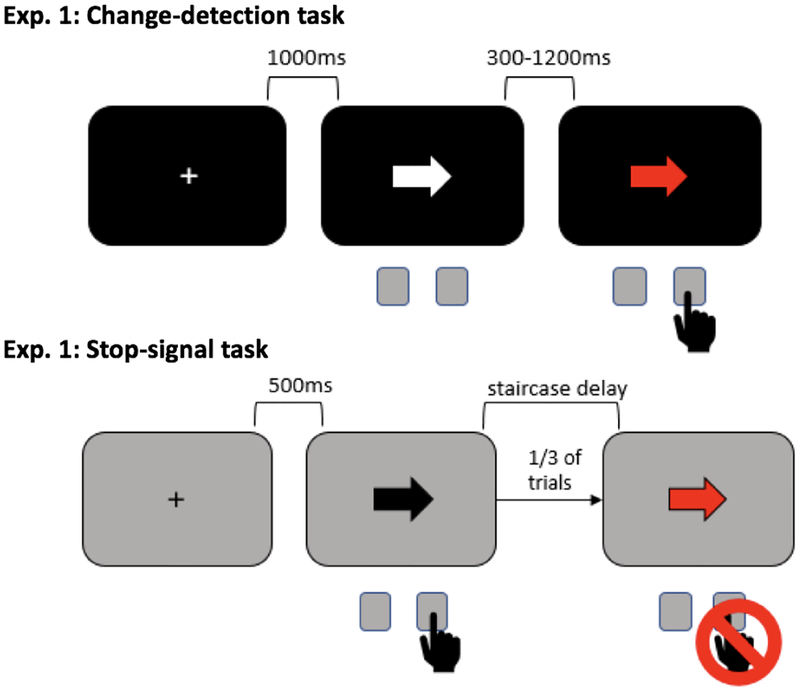
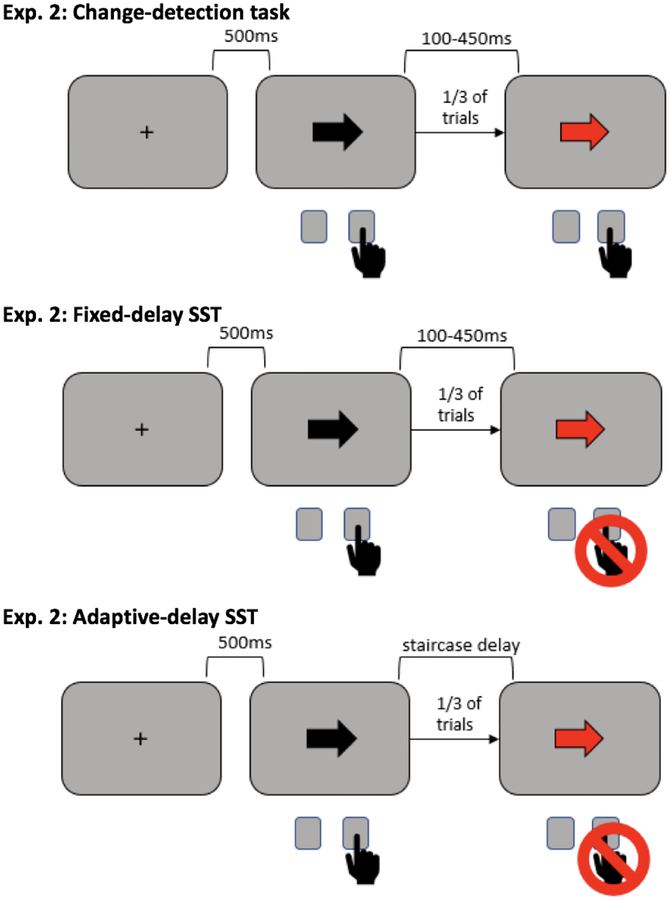
Behavioral paradigms for both studies were presented on a Linux desktop computer running Fedora, through Psychtoolbox (Brainard et al., 1997) on MATLAB 2015b (TheMathWorks, Natick, MA). Participants made left and right responses by pressing the “q” and “p” keys on a standard keyboard with their left and right index fingers, respectively. In Study 1, participants completed a visual change-detection task, followed by the SST. All participants completed the behavioral tasks in this order so they were not predisposed to inhibit their responses to the red arrows in the visual change detection task. In Study 2, Participants completed a slightly different visual change-detection task and two SSTs: one with a tracking algorithm for SSD and one with fixed SSDs that matched delays between first and second stimuli in the visual change-detection task exactly. Participants completed behavioral tasks in the following order: visual change-detection task, fixed-delay SST, adaptive-delay SST. (See Figures 1 & 2 for diagrams of all Study 1 & 2 tasks.)
Figure 1.
Behavioral tasks used in Study 1.
Figure 2.
Behavioral tasks used in Study 2.
Visual change-detection task
Study 1.
In the visual change-detection task, participants viewed a screen and saw white arrows appearing on a black background and turning red after a variable delay (300–1200ms). Participants were instructed to make a response when they detected the arrow had changed from white to red, pressing a left or right key to indicate the direction in which the arrow was pointing. Participants had one second to respond, after which they were given on-screen feedback that read “Too Slow!”. Each arrow was preceded by one second of fixation cross in the center of the screen. Total trial duration was set at 4.5s. Participants completed three blocks of 40 trials.
Study 2.
The visual change-detection task in Study 2 differed from the change-detection task in Study 1 primarily in that responses were required before the onset of a change and changes were infrequent. Participants responded as fast as they could to black arrows on a gray background pointing left or right. Participants were instructed to press the “q” key if the arrow was pointing left, and the “p” key if the arrow was pointing right. On one-third of trials, a second, red arrow appeared after a variable delay once the participant had responded to the black arrow. Participants were instructed to make a second response when they saw a red arrow by pressing the space bar. The delays between black and red arrows following a response were set at 50ms increments between 100–450ms, divided equally and shuffled randomly among all trials (delays that matched exactly the SSDs in the SST of comparison). Each black arrow was preceded by 500ms of a black fixation cross in the center of the screen, and trial length was capped at 3s. If participants did not respond to black arrows within 1s, feedback of “Too Slow!” appeared on the screen. Participants completed four blocks of 72 trials.
Stop-signal task
Study 1.
In the SST, participants were instructed to respond to black arrows appearing on a gray background by responding with a left or right key to indicate the direction in which the arrow was pointing. On a subset of trials (33%), the arrow turned red after a delay. Participants were instructed to attempt to withhold their response when they saw the arrow turn red. A tracking algorithm with left and right response staircases was implemented to keep the probability of stopping at 50% for a given stop trial. The staircases began at 200ms, increased 50ms following a failed stop, and decreased 50ms following a successful stop. On go-only trials, participants had one second to respond, after which they were given on-screen feedback that read “Too Slow!”. Trial duration was capped at 3s and each arrow was preceded by 500ms of fixation. Participants completed five blocks of 60 trials.
Study 2.
The adaptive-delay SST was the same as in Study 1. In Study 2, we included second SST with fixed-value SSDs that matched the delays between black and red arrows in the change-detection task. This was done so we could be sure outcomes reflected the neural processes of interest and not the nature of the SST used - in other words, that results were not produced by having a fixed- versus adaptive-delay SST. The fixed-delay SST was the same as the adaptive SST except that SSDs were set at 50ms increments between 100 and 450ms, spread evenly throughout the trials and shuffled randomly.
EEG data collection
Study 1.
EEG data for Study 1 were collected using a 62-channel BrainVision EasyCap cap connected to two BrainVision MRplus amplifiers (BrainVision). Two additional electrodes were placed: one on the left canthus and the other directly beneath the left eye. The ground was placed at electrode Fz and the reference at electrode Pz. Recordings were digitized at a rate of 500 Hz.
Study 2.
EEG data for Study 2 were collected using a 64-channel BrainVision active electrode cap connected to a BrainVision actiCHamp active channel amplifier (BrainVision). The ground was placed at electrode Fz and the reference at electrode Pz. Recordings were digitized at a rate of 500 Hz.
EEG preprocessing
Studies 1 & 2.
Preprocessing procedures were identical for Studies 1 and 2. EEG data were preprocessed and analyzed using custom MATLAB (MATLAB 2015b, TheMathWorks) functions and the EEGLAB toolbox (Delorme and Makeig, 2004). All data, task code, and analyses scripts for both studies are available on the Open Science Framework (at https://osf.io/NUMRA/, DOI 10.17605). Once imported into MATLAB, datasets from all tasks within one study were merged and filtered using least-squares finite impulse response filters with a high-pass cutoff of 0.5 Hz and low-pass cutoff of 50 Hz. After filtering, the recording was visually inspected for non-stereotypical artifacts (such as muscle activation or intermittent electrode artifacts) and any 1 second segment of the recording found to contain an artifact was removed from the data. After manual inspection, the data were re-referenced to the common average, epoched into 1 second segments around events of interest (stimuli onsets), and subjected to temporal infomax independent component analysis (ICA) decomposition (Bell and Sejnowski, 1995) with extension to sub-Gaussian sources (Lee et al., 1999). The resulting ICA components were screened for artifact components (such as blinks and saccades) using outlier statistics. Identified blink and saccade components were removed from the data.
ERPs
Studies 1 & 2.
Stimulus-locked event-related potentials (ERPs) were created for go trials (time-locked to the go signal), successful stop trials (time-locked to the go and stop signals), and failed stop trials (time-locked to the go and stop signals) in all versions of the SST used in Studies 1 and 2. For both visual change-detection tasks, ERPs were created time-locked to the first and second stimuli (when a second stimuli was presented). These ERPs were time-locked to stimulus presentation and averaged across epochs set at 300ms before stimulus onset to 700ms following stimulus onset. All ERPs were baseline corrected using a baseline time period of 100ms before stimulus onset to stimulus onset.
Selection of prototype inhibition component
Studies 1 & 2.
For each participant, one ICA component was selected that represented the frontocentral P3 in the SST. This ERP has been shown to be a reliable index of inhibition (Kok et al., 2004, Wessel & Aron, 2015). IC selection was achieved algorithmically in several steps. First, every IC for a given participant was subjected to a topographical criterion test. ICs that displayed maximal activation of their weights over frontocentral electrodes (Fz, FCz, Cz, FC1, FC2, C1, C2) were selected as candidate inhibition ICs. The weights of these candidate ICs projected into channel space were used to create an ERP of the time range of interest (100–450ms following stop-signal onset) for the difference between successful and failed stop trials. Each of these IC ERPs was correlated with the overall channel-space ERP wave of the difference between successful and failed stop trials averaged across all stop trials in the SST for that participant (again at average of Fz, FCz, Cz, FC1, FC2, C1, C2). The one IC that had the highest significant correlation coefficient was selected as the “prototype” frontocentral P3 IC. For more information on the logic of this procedure see the Introduction section or Wessel 2018.
Statistical analyses
Studies 1 & 2.
All analyses were performed on the post-exclusion participant samples described under the “Participants” sections for Studies 1 and 2. To assess for significant differences between P3 amplitude on successful versus failed stop trials, we ran t-tests between the trial-wise ERP vectors (see “ERP creation” section) for successful and failed stop trials. This analysis was conducted three times: on the ERPs created with data containing all ICA components, on ERPs created with data containing only the identified inhibition component (see “Selection of prototype inhibition component” section), and the data containing all ICA components except for the identified inhibition IC. Resulting p-value vectors from these t-tests were then corrected for multiple comparisons with a FDR procedure at a threshold of p < .05 (Benjamini and Hochberg, 1995). Then, to quantify onset of P3 for each participant, we started by selecting four groups of trials: successful stop trials, failed stop trials, and pools of matched go trials for both successful and failed stop trials. Matched go trials were obtained for each successful and failed stop trial by selecting a go-only trial with the same assigned SSD as a given stop trial, selected to be as close in time as possible to the stop trial of interest (meaning the SSD in the staircase would be close if not identical to the SSD during the stop trial). We found that go trials selected as matches for failed stops did not significantly differ from the go trials not selected in mean RT (t = 1.39, p = .18). However, we did find that successful stop-matched go trials were significantly slower than the mean RT of non-matched go trials (t = 3.57, p = .002, which aligns with findings from Bissett and Logan (2012) showing slowing on post-successful-stop trials. To confirm this RT difference would not confound our test of ERP onset latency, we conducted multiple comparisons t-tests on all data points (FDR corrected p < .05) to confirm there were no significant differences between the amplitude of ERPs for stop-matched go trials and all other go trials. Monte-Carlo t-tests (10,000 iterations) were performed on these pools of stop trials between successful stops and paired go trials and between failed stops and paired go trials. The resulting t-value vectors were used to quantify the onset for failed and successful stop trials separately. We identified the peak of the P3 by identifying the maximum value in the vector within the window of interest (200–500ms following the stop-signal) and classified this point as P3 peak, then worked backwards to find the first significant deflection point in that block of significant values. This first significant deflection point (where p < .01) was identified as P3 onset.
SSRT calculation
Adaptive-delay SST.
Stop-signal reaction time (SSRT) in the SST for the adaptive SST in both studies was estimated using the integration method. For each delay staircase (left and right hand) each unique SSD was used to calculate p-inhibit, the likelihood of a response being made if a stop-signal was presented at that SSD. Then, the corresponding go RT was extracted from the ranked distribution of all go RTs from correct go-only trials and the SSD subtracted from this value to yield a SSD-specific SSRT. Following this calculation for all SSDs, the two most frequent SSDs for a given staircase (the two SSDs around which the adaptive delay staircase converged) were selected and their SSRTs averaged to yield an SSRT for the entire staircase. The SSRTs of both staircases were then averaged to yield the SSRT for that particular participant.
Fixed-delay SST.
SSRT for the fixed-delay SST in Study 2 was calculated the same as for the adaptive-delay SSTs, but with one difference. Because, by the nature of the fixed-delay SST, there are no two SSDs around which an adaptive algorithm converges, integrated SSRTs at all SSDs were included in the final average to yield an overall SSRT for each participant.
Results
Study 1
Visual change-detection task behavioral results.
In the visual change-detection task of Study 1, participants responded to this imperative stimulus (the red arrow) with a mean reaction time of 334.91ms (SD = 39.73ms). Mean accuracy for this response was 99% (SD = 1.5%), with response direction errors and early responses constituting inaccurate trials, suggesting that participants were paying attention and performing this task appropriately.
Stop-signal task behavioral results.
During the SST, participants responded on go-only trials with a mean reaction time of 486.96ms (SD = 124.64ms). On failed stop trials the mean reaction time was 432.29ms (SD = 106.70ms). As confirmed by a t-test the distributions of go and failed RTs conform to the race model, with the failed RTs being significantly faster (p < .001, t = −6.80, df = 16, d = .47). Average SSRT was estimated at 249.39ms (SD = 55.23ms) using the integration method. This task converged at a mean p-inhibit of .50 (SD = .03, range of .43-.55), confirming the effectiveness of the staircase algorithm.
Data segmentation.
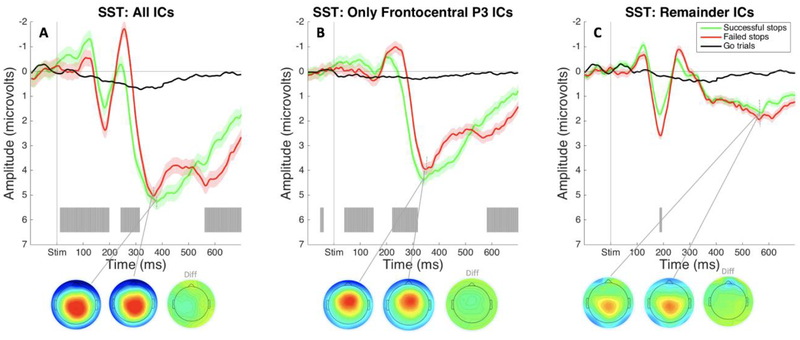
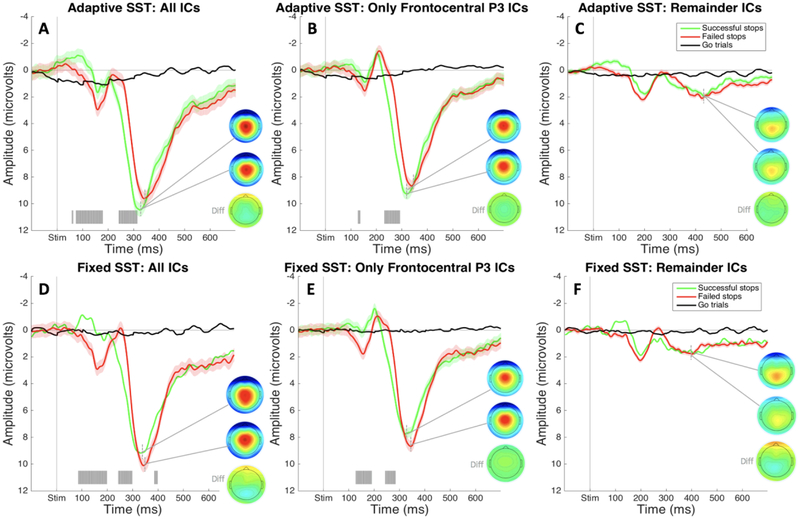
As described in the Method section, ICA was used to isolate the IC that accounts for the frontocentral P3 ERP during the SST for each participant. We examined our electrophysiological data both with and without this IC present. In the following, we report results from channel-space data reconstructed from: a) all non-artifact ICs including the frontocentral P3-IC (referred to hereafter as “All ICs”), b) only the activation of the identified frontocentral P3-IC(referred to hereafter as the “frontocentral P3 IC”), and c) all non-artifact ICs besides the frontocentral P3-IC (referred to hereafter as “remainder IC’s”).
Event-related potentials: SST.
ERPs were calculated using average voltage over Cz and FCz electrodes during the time range of interest. The ERP from all-IC data showed typical P3 frontocentral positivity for the SST. There was a large frontocentral positivity peaking shortly after 300ms following the stop-signal on stop-signal trials. There was a significant difference in amplitude between activation on successful and failed stop trials beginning at 242ms post-stimulus that resulted from the delay in onset of P3 on failed stop trials (as opposed to larger amplitude in the failed stop ERP compared to the successful stop ERP; see Figure 3A). We saw the same pattern when we looked at the frontocentral P3 IC, suggesting we accurately identified the IC for each participant that accounts for this inhibition-related neural activity (see Figure 3B). The ERP created from remainder-IC data showed no significant positivity in the early P3 time range. Though some late, posterior P3b-like positivity remained, there were no significant differences between successful and failed stop trials, which confirmed that no inhibition-specific positivity remained in those ICs (see Figure 3C).
Figure 3.
ERPs from the SST in Study 1 time-locked to stop-signal onset and plotted over average of Cz and FCz electrodes. Gray shading indicates significant differences between successful and failed stop trials (FDR corrected at p <. 05). A) ERPs and P3 peak topography for failed and successful stop trials plotted during the same time range on matched go trials. B) ERPs and peak P3 topography for successful and failed stop trials and matched-go trials plotted using only the selected Frontocentral P3 IC. C) ERPs and peak P3 topography for successful and failed stop trials and matched-go trials plotted using all independent components besides the selected Frontocentral P3 IC.
P3 onset in SST.
The onset of the P3 IC was quantified for both successful and failed stop trials using iterative permutation testing (described in detail in the Statistical Analyses subsection of the Method section). For the data from our frontocentral P3 IC, a t-test on the resulting p-value vectors from Monte-Carlo t-tests of failed and successful stops versus matched go trials indicated significantly earlier P3 onset in successful stop trials (p = .01, t = −2.60, df = 16, d =.46, one-tailed).
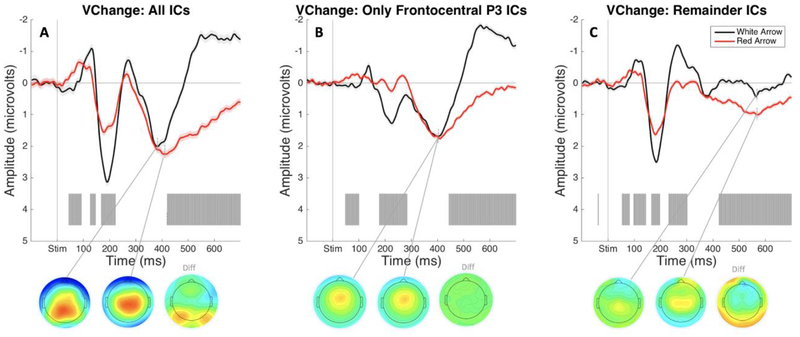
Event-related potentials: change-detection task.
The ERPs created for the frontocentral P3 IC following both black and red arrows in the visual change-detection task showed greatly reduced positivity in the P3 time range, and no significant differences in amplitude in the same range following their respective stimulus (see Figure 4). Though there was some positivity peaking shortly after 400ms following stimulus onset, this deflection was smaller than early deflections and resembled the more posterior P3b in terms of deflection morphology and topography. ERPs with all ICs or with all remainder ICs also did not show any positive deflection resembling frontocentral P3. Taken together, this suggests that, 1) we successfully created a task that requires attentional detection of a stimulus change but that did not require inhibition, and 2) attentional detection of a task-relevant, frequent stimulus alone is not sufficient to elicit a frontocentral P3.
Figure 4.
ERPs from the visual change-detection task in Study 1 time locked to the onset of white and red arrows, respectively, plotted using an average of Cz and FCz electrodes. Gray shading indicates a significant difference between ERP responses to white and red arrows (FDR corrected at p < .05). A) ERPs and P3 peak topographies following white versus red arrow stimulus onset. B) ERPs and P3 peak topographies following white versus red arrow stimulus onset plotted using only the selected Frontocentral P3 IC. C) ERPs and P3 peak topographies following white versus red arrow stimulus onset. B) ERPs and P3 peak topographies following white versus red arrow stimulus onset plotted using all independent components besides the selected Frontocentral P3 IC.
Study 2
Visual change-detection task behavioral results.
In the visual change-detection task of Study 2, trials were classified as inaccurate if any of the following were true: participants pressed the space bar too early during a red arrow trial, participants pressed the space bar during a black arrow-only trial, participants did not make a black arrow response on a black-arrow only trial, or participants failed to make both responses to black and red arrows on a trial containing both arrows. Following these criteria, participants were made accurate responses to 99% (SD = 1%) of black arrows and 99% (SD = .4%) of red arrows, indicating participants were paying attention and performing the task correctly.
Participants responded to the frequent black arrow with a mean reaction time of 410.24ms (SD = 42.30ms) and responded to the infrequent red arrow with a mean reaction time of 461.73ms (SD = 61.18ms). A t-test between reaction times to black and red arrows showed significantly slower responses made to the red arrows (p < .001, t = −5.54, df = 19, d = .98). Further parsing of response times to black arrows revealed that participants responded significantly more slowly to black arrows on trials when red arrows were also present (mean was 417.56ms; SD = 47.19ms) compared to when they were absent (mean was 406.93ms; SD = 40.30ms) (p < .001, t = .3.95, df = 19, d = .24).
Fixed-delay stop-signal task behavioral results.
During the fixed-delay SST, participants responded on go-only trials with a mean reaction time of 522.73ms (SD = 58.29ms). On failed stop trials the mean reaction time 457.92ms (SD = 48.50ms). As confirmed by a t-test (p < .001, t = −11.93, df = 19, d = 1.21) the distributions of go and failed RTs conformed to the race model, with the failed RTs being significantly faster. Average SSRT was estimated at 249.04ms (SD = 32.04ms) using the integration method. This task converged at a mean p-inhibit .52 (SD = .15, range of .33-.96). The large range in the stopping success rate is not surprising, given the fixed SSDs on this task. Some participants would have needed a shorter or longer SSD than programmed in the task to achieve an ideal .50 accuracy on stopping trials.
Adaptive-delay stop-signal task behavioral results.
During the adaptive-delay SST, participants responded on go-only trials with a mean reaction time of 513.68ms (SD = 64.20ms). On failed stop trials the mean reaction time was 453.42ms (SD = 61.38ms). As confirmed by a t-test (p < .001, t = −9.68, df = 19, d = .96) the distributions of go and failed RTs conformed to the race model, with the failed RTs being significantly faster. Average SSRT was estimated at 243.90ms (SD = 34.48ms) using the integration method. This task converged at a mean p-inhibit of .49 (SD = .02, range of .43-.53), confirming the effectiveness of the staircase algorithm.
Event-related potentials: SST.
t-tests between amplitude on trials of interest (failed and successful stop trials) did not reveal any significant differences in ERP amplitude obtained from the adaptive- versus fixed-delay version of the SST (FDR corrected to p < .05). Because of this evidence that the frontocentral ERP deflections for different trial types did not significantly differ between versions of the SST, we will describe them together here (though failed and successful stop ERPs for both tasks can be seen plotted together in Figure 5). As with Study 1, the SST was used to identify our frontocentral P3 IC based on a stereotyped positivity following the stop-signal on stop trials. The P3 was present in the data containing all ICs, and there was a significant amplitude difference between successful and failed stop trials. Moreover, this amplitude difference was accounted for by later P3 onset on failed stop trials (see Figure 5A & 5D). This significantly earlier onset was present in the IC we isolated and identified as the frontocentral P3 IC (see Figure 5B & 5E). Similar to Study 1 results, the ERPs for remainder IC’s showed a small, residual P3-type positivity that resembled a later, more posterior P3b, but there were no longer significant differences between neural activity on failed and successful stop trials (see Figure 5C & 5F).
Figure 5.
ERPs from the SSTs in Study 2 time-locked to stop-signal onset and plotted over average of Cz and FCz electrodes. Gray shading indicates significant differences between successful and failed stop trials (FDR corrected at p <. 05). A) ERPs and P3 peak topography for failed and successful stop trials from the adaptive-delay SST plotted during the same time range on matched go trials. B) ERPs and peak P3 topography for successful and failed stop trials and matched-go trials from the adaptive-delay SST plotted using only the selected P3a independent component. C) ERPs and peak P3 for successful and failed stop trials and matched-go trials from the adaptive-delay SST plotted using all independent components besides the selected Frontocentral P3 IC. D) ERPs and P3 peak topography for failed and successful stop trials from the adaptive-delay SST plotted during the same time range on matched go trials. E) ERPs and peak P3 topography for successful and failed stop trials and matched-go trials from the adaptive-delay SST plotted using only the selected Frontocentral P3 IC. F) ERPs and peak P3 for successful and failed stop trials and matched-go trials from the adaptive-delay SST plotted using all independent components besides the selected Frontocentral P3 IC.
P3 onset in SST.
For the frontocentral P3 IC in our adaptive-delay SST, a visual inspection of plotted ERPs showed an earlier onset of P3 in successful versus failed stop. Onset of the frontocentral P3 IC was quantified for both successful and failed stop trials using iterative permutation testing (described in detail in the Statistical Analyses subsection of the Method section). For the data from our frontocentral P3 IC, a t-test on the resulting p-value vectors from Monte-Carlo t-tests of failed and successful stops versus matched go trials indicated significantly earlier P3 onset in successful stop trials in both the fixed-delay (p = .034, t = 1.94, df = 19, d = .30, one-tailed) and adaptive-delay SST (p = .039, t = 1.86, df = 19, d = .45, one-tailed).
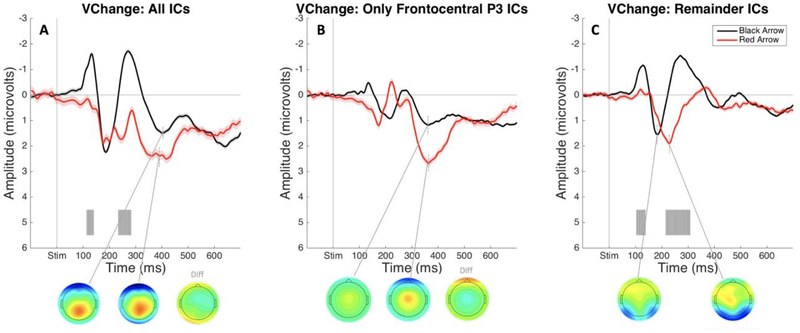
Event-related potentials: change-detection task.
The ERPs time-locked to black and red arrow onset containing all ICs showed a notable positive deflection in the P3 time range following infrequent red arrow stimuli that was not present following black arrows (see Figure 6A). Critically, the frontocentral P3 IC showed a substantial numerical increase in amplitude following red arrows (see Figure 6B). These differences between black and red arrow conditions during this time range in the frontocentral P3 IC data did not hold up to FDR correction across the entire epoch time range. The ERPs plotted with remainder IC’s showed no positivity in the P3 time range (see Figure 6C).
Figure 6.
ERPs from the visual change-detection task in Study 2 time locked to the onset of black (frequent stimulus) and red arrows (infrequent stimulus), respectively, plotted using an average of Cz and FCz electrodes. Gray shading indicates a significant difference between ERP responses to black and red arrows (FDR corrected at p < .05). A) ERPs and P3 peak topographies following black versus red arrow stimulus onset. B) ERPs and P3 peak topographies following black versus red arrow stimulus onset plotted using only the selected Frontocentral P3 IC. C) ERPs and P3 peak topographies following black versus red arrow stimulus onset. B) ERPs and P3 peak topographies following black versus red arrow stimulus onset plotted using all independent components besides the selected Frontocentral P3 IC.
Interestingly, the P3 elicited by detection of an infrequent stimulus alone appeared to be accounted for by the same IC as the stop-signal P3. This would suggest that any P3 amplitude that is accounted for in the SST by infrequency detection summed together in the same IC as a P3 elicited by motor inhibition, and is supported by the observation that the P3 elicited by infrequency alone in the change-detection task was smaller than the P3 related to both inhibition and infrequency detection in the SST.
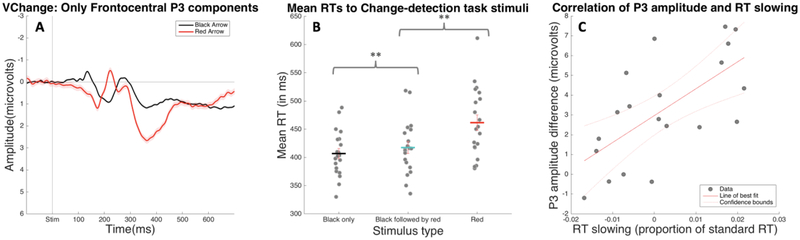
Exploratory analysis: Correlation of infrequency-related P3 and RT.
Our results showed that the IC representing the stop-signal P3 also showed a positive deflection following infrequent events that did not require stopping (i.e., following red arrows in the change detection task). Furthermore, we found that the presence of red arrows slowed the reaction times to the black arrow, indicating the possible presence of motor inhibition caused by the infrequent event. This pattern of results echoes behavioral findings from previous research also including “double-response” paradigms that are used to compare stimulus detection to the processes in the SST (Elchlepp et al., 2016; Hampshire et al., 2010; Verbruggen et al., 2010). To explore the possibility that the P3 elicited in the Study 2 change-detection task reflects the presence of incidental motor inhibition in that task, we conducted an additional post-hoc, exploratory analysis to assess whether the amplitude of the infrequency-related positivity in the frontocentral P3 IC correlated significantly with reaction time slowing on infrequent trials in the change detection task. For each participant, we found the peak amplitude of their infrequency-elicited P3 by quantifying the maximal amplitude value of each participant’s average ERP following red arrows (for the frontocentral P3 IC only) during the 100ms window surrounding the peak of the all-subjects mean ERP. After finding each subject’s peak red-arrow P3 amplitude, we took the amplitude of the black-arrow-locked ERP at the corresponding time and subtracted that value from the red-arrow P3 peak amplitude (see Figure 7A). This resulting value represented the P3 peak amplitude difference between the two ERPs. We calculated RT slowing on red arrow-present trials for each participant by taking mean RT to black arrows on red arrow-present trials and subtracting the mean RT of black arrow-only trials, dividing the resulting value by the mean RT of black arrow-only trials. (See Figure 7B for distribution of RT means to these stimuli.) Finally, we tested the correlation between our estimates of peak amplitude difference and proportion of reaction time slowing. We found a strong, significant correlation between peak amplitude of P3 elicited in response to infrequent red arrows with slowing in RT to black arrows on trials where infrequent red arrows were shown (p = .003, r = .66), suggesting this behavioral outcome is very likely brought about by the same neural mechanism that projects the frontocentral P3 deflection on the scalp (see Figure 7C).
Figure 7.
Analysis of the correlation between Frontocentral P3 peak amplitude and RT slowing in Study 2’s visual change-detection task. A) ERPs in response to black and red arrow onsets, plotted only using the activation of the Frontocentral P3 IC over the average of Cz and FCz. B) Mean RT to black arrows on black arrow-only trials, mean RT to black arrows on trials where both arrow colors were present, and mean RT to red arrows. Mean RT to black arrows on trials where red arrows were also present was significantly longer than the mean RT to black arrows on black arrow-only trials (** designates a significant difference as shown by t-test at p < .001). C) Scatter plot of participant-wise Frontocentral P3 amplitude difference and black arrow RT slowing (calculated as a proportion of the black arrow-only RT) with line of best fit.
Discussion
In the current study, we aimed to investigate the differential contributions of infrequency detection and motor inhibition to the frontocentral P3. We found that stimulus detection only led to a P3 wave when the events to be detected were infrequent within the context of the task (just like stop-signals in most variants of the SST). Moreover, ICA revealed that the P3 produced by stop-signals and the P3 produced by infrequent events that demand attentional detection (but no motor inhibition) were likely generated by the same neural source.
This raises the question of which psychological process this P3 reflects. We propose that it indeed reflects motor inhibition, largely based on two observations. First, its amplitude was much increased in the stop-signal task, which demands both infrequency detection and motor inhibition, compared to the change detection task, which solely demands infrequency detection. Therefore, frontocentral P3 amplitude is – at a minimum – clearly moderated by the inhibitory demand. Second, the P3 amplitude in the change-detection task was highly correlated with the incidental, non-instructed slowing of motor emission that occurred during this task. Therefore, we propose that the frontocentral P3 indeed indexes the activity of a generic motor inhibition system, which is actively recruited to fully cancel an impending response in the case of the stop-signal task, and which is incidentally recruited by all infrequent events (even those that do not explicitly demand motor inhibition). This is in line with the finding that rare or unexpected events have inhibitory effects on the motor system, even when inhibitory control is not instructed within the context of the experimental task (Wessel & Aron, 2013; Wessel 2018; Novembre et al., 2018).
A potential alternative explanation is that P3 amplitude differences may reflect response-locked activity associated with a button-press (Salisbury et al., 2001). However, this explanation cannot account for the data pattern across both tasks, since the P3 in the stop-signal task showed no differences between the condition with a button press (failed stop-trials) compared to the condition without a button press.
P3 as a signature of inhibitory control
Our finding that the frontocentral P3 indexes motor inhibition in both oddball/infrequency and stop-signal tasks is in line with prior findings. In the SST, P3 amplitude increases as stop-signal frequency decreases (Ramautar et al., 2004), and the same is true in Go/NoGo tasks (Donkers & van Boxtel, 2004; Nieuwenhuis et al., 2003). We propose that this occurs in the SST because the reduced relative frequency of No-Go/stop-stimuli increases the prepotency of the go-response, thereby increasing inhibitory demand (Wessel, 2018). Similarly, in non-inhibition tasks involving infrequent events, P3 amplitude increases as oddball stimuli become less frequent (Smith et al., 2008). We propose that this occurs in these task designs because the lower relative frequency of an event makes the event more unexpected. Unexpected events induce motor inhibition, and the more unexpected an event, the stronger this inhibitory effect (Wessel & Aron, 2013, 2017; Wessel, in press). Moreover, the unexpectedness of an event has been shown to directly influence the amplitude of the P3 (Wessel, 2017), which we propose to reflect an increase in incidental motor inhibition. Indeed, in our current study, the amplitude of the P3 in the change-detection task was related to RT slowing after infrequent events. Our data shows the same component that is sensitive to stimulus infrequency is also sensitive to the need to inhibit when stimulus infrequency is held constant. We observed a larger P3 in our SST than we did in our change-detection task, which we propose resulted from the combination of the demand to inhibit a prepotent response and the attentional detection of an infrequent stimulus. We conclude that the frontocentral P3 indeed reflects the activity of a generic mechanism for motor inhibition, and that a larger amplitude P3 indicates higher demands on inhibitory control. This is in line with proposals from Polich (2007), who suggests that the P3 in inhibition paradigms like the SST and the P3 occurring following oddball-type stimuli are likely different versions of the same underlying inhibition-related ERP component.
In the current study, we used ICA to show that the P3 observed in the SST and change-detection task reflect the same underlying neural process. The P3 elicited by infrequent stimuli alone was notably smaller than the P3 elicited by infrequent stop-signals in the SST. If this were a case of two P3-waveforms signifying two different neural processes (one motor inhibition, one infrequency detection) and summing together in the SST to create a larger P3 in channel space, we would expect to observe these ERPs represented in separate ICs, which was not the case. Because ICA assumes individual IC’s originate from independent sources, this finding suggests that the stop-signal P3 and infrequency-related P3 likely emanate from a common underlying neural generator – meaning that they do in fact reflect the same neural process (Onton et al., 2005; Wessel, 2018). We believe a candidate for this common neural generator is the fronto-basal ganglia (FBg) network for global inhibition, which represents the core of a recently proposed model of inhibitory control following unexpected events (reviewed in Wessel & Aron, 2017). This model proposes that the same neural circuit underlying instructed, reactive stopping (such as in the SST) is recruited following multiple types of unexpected events in the environment. Evidence outlined in Wessel and Aron suggests that this circuit brings about global inhibition following action errors, unexpected action outcomes, and unexpected perceptual events (i.e., novel stimuli). It is not surprising that the same neural circuit may act to bring about global inhibition following infrequent (oddball) stimuli because they are, in a sense, unexpected perceptual events. While they are not entirely unexpected (as their occurrence in the task is announced and part of the task instruction), these events are unexpected on a trial-by-trial basis because they are less likely to occur on a given trial than not.
Previous research provides some preliminary evidence for the activation of the FBg system following infrequent stimuli. Motor slowing is one marker of FBg activation following unexpected events, set forth by Wessel and Aron (2017). In Woodward et al. (1991), reaction times to oddball events were slower than reaction times to standard events, a finding we observed also in our change-detection task. A second marker of FBg activation is broad skeletomotor inhibition, often evaluated by quantification of motor-evoked potential (MEP) suppression during transcranial magnetic stimulation (TMS). To our knowledge, no study has investigated the effects of infrequent events on MEP amplitude. However, Valsecchi and colleagues (2009) report inhibition of microsaccades following infrequent events in proportion to stimulus infrequency. This inhibition of microsaccades following infrequent events also correlated with P3 amplitude (Valsecchi et al., 2009). In a meta-analysis conducted using fMRI stop-signal task data, Levy and Wagner (2011) found that tasks requiring inhibition and tasks eliciting reflexive-reorienting to oddball-type stimuli activated a common cluster in ventrolateral prefrontal cortex (VLPFC; although they found common activation of right inferior frontal junction and not rIFC). Moving forward, more research is needed to establish evidence for the direct and causal interaction of FBg-system signatures following infrequent stimuli, such as simultaneous EEG/MEP studies that could establish direct links between motor slowing, MEP suppression, and the frontocentral P3 ERP.
Relevance of findings to research using the SST
As we have demonstrated here, it is not possible to separate neural indices of motor inhibition and attentional detection of infrequent signals, at least in our studies, because both processes are reflected in a common IC. This becomes a concern when we consider the use of the SST to assess motor inhibition or impulsivity in populations we know to have attentional deficits, such as individuals with ADHD. If the SST used in such research has stop-signals that are infrequent, deficits in the attentional realm may be indistinguishable from deficits in the motor inhibition realm when the events that require both occur together, because attentional detection of that infrequent signal will be required before inhibition begins. Furthermore, it appears that infrequent events themselves elicit incidental inhibitory control, adding amplitude to frontocentral P3 that is not related to task-instructed inhibition, per say. Future research that utilizes the SST might consider task designs in which the stop signal is not infrequent. If the population of interest has normal attentional capacities, this may be unnecessary. On the other hand, when the SST is implemented to study patient populations or it is crucial to study a pure inhibitory process, steps should be taken during task design to remove the confound created by the presence of infrequent stop-signals.
Concluding remarks
We here report evidence from two studies showing that infrequency detection and motor inhibition in the stop-signal task are inextricably linked. This is because infrequent events (such as stop-signals in most common stop-signal task designs) invoke activity in the neural generator underlying the frontocentral P3, the purported signature of motor inhibition in that task. In line with the role of this P3 as an index of a generic mechanism for motor inhibition, the positivity observed in this ERP on infrequent events that do not explicitly demand inhibitory control was directly related to the degree of incidental reaction time slowing incurred by such infrequent events. Therefore, we conclude that the frontocentral P3 reflects the activity of a generic mechanism for motor inhibition, which can be explicitly recruited to stop actions in task like the stop-signal task, and which is incidentally recruited whenever behaviorally relevant infrequent events occur.
Table 1.
Summary of behavioral Means and Standard Deviations in Study 1
| Task | Outcome variable | M (SD) |
|---|---|---|
| Stop-signal | GoRTs | 486.96 (124.64) |
| Failed stop RTs | 432.29 (106.70) | |
| Stop accuracy | .50 (.03) | |
| SSRTi | 249.39 (55.23) | |
| Change-detection | Red arrow RTs | 334.91 (39.73) |
Table 2.
Summary of behavioral Means and Standard Deviations in Study 2
| Task | Outcome variable | M (SD) |
|---|---|---|
| Adaptive-delay SST | GoRTs | 513.68 (64.20) |
| Failed stop RTs | 453.42 (61.38) | |
| Stop accuracy | .49 (.02) | |
| SSRTi | 243.90 (34.48) | |
| Fixed-delay SST | GoRTs | 522.73 (58.29) |
| Failed stop RTs | 457.92 (48.50) | |
| Stop accuracy | .52 (.15) | |
| SSRTi | 249.04 (32.04) | |
| Change-detection | Black arrow RTs | 410.23 (42.30) |
| Red arrow RTs | 461.73 (61.18) |
Highlights.
We tested whether P3 in the stop-signal task can be explained by attentional detection
Detecting frequent events did not elicit frontocentral P3, but infrequent events did
Infrequency-P3 could be attributed to the same independent source as stop-signal P3
Infrequent events induced incidental response slowing, correlated with P3 amplitude
Acknowledgements
The authors would like to thank the following individuals for help with data collection: Cailey Parker, Kylie Dolan, Carly Ryder, Isabella Dutra, Alec Mather, Nathan Chalkley, and Brynne Dochtermann. This research was supported by funding from the National Institute of Health (R01 NS102201 to JRW and T32 GM108540 to DAW), the National Science Foundation (NSF CAREER 1752355 to JRW), and the Roy J. Carver Foundation (to JRW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, … & Obeso JA (2013). The subthalamic nucleus is involved in successful inhibition in the stop-signal task: A local field potential study in Parkinson’s disease. Experimental Neurology, 239, 1–12. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, and Poldrack RA (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci 18, 177–185. doi: 10.1016/j.tics.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, & Logan GD (2003). Horse-race model simulations of the stop-signal procedure. Acta Psychologica, 112(2), 105–142. [DOI] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Overtoom CC, Kenemans JL, Kooij JJ, De Noord I, Buitelaar JEEA, & Verbaten MN (2005). Stopping and changing in adults with ADHD. Psychological medicine, 35(6), 807–816. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Hoeksma MR, Talsma D, & Verbaten MN (2005). The pure electrophysiology of stopping. International journal of psychophysiology, 55(2), 191–198. [DOI] [PubMed] [Google Scholar]
- Bell AJ, & Sejnowski TJ (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, & Schall JD (2007). Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychological Review, 114(2), 376. [DOI] [PubMed] [Google Scholar]
- Brainard DH, & Vision S (1997). The psychophysics toolbox. Spatial vision, 10, 433–436. [PubMed] [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelinck M, & Guerit JM (2002). Discrimination of emotional facial expressions in a visual oddball task: an ERP study. Biological Psychology, 59(3), 171–186. [DOI] [PubMed] [Google Scholar]
- Debener S, Makeig S, Delorme A, & Engel AK (2005). What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cognitive Brain Research, 22(3), 309–321. [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, & Makeig S (2007). Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage, 34(4), 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, & Barry RJ (2006). The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain and Cognition, 62(2), 98–112. [DOI] [PubMed] [Google Scholar]
- Donkers FC, & van Boxtel GJ (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition, 56(2), 165–176. [DOI] [PubMed] [Google Scholar]
- Drew T, & Vogel EK (2008). Neural measures of individual differences in selecting and tracking multiple moving objects. Journal of Neuroscience, 28(16), 4183–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchlepp H, Lavric A, Chambers CD, & Verbruggen F (2016). Proactive inhibitory control: A general biasing account. Cognitive Psychology, 86, 27–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R, and Hampshire A (2014). A functional network perspective on response inhibition and attentional control. Nat. Commun 5:4073. doi: 10.1038/ncomms5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Larrea L, Lukaszewicz AC, & Mauguiére F (1992). Revisiting the oddball paradigm. Non-target vs neutral stimuli and the evaluation of ERP attentional effects. Neuropsychologia, 30(8), 723–741. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, & Feghoff TA (2004). Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 75(4), 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, & Herrmann CS (2013). Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International Journal of Psychophysiology, 87(3), 217–233. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, … & Dietrich DE (2001). Discrepant target detection and action monitoring in obsessive–compulsive disorder. Psychiatry Research: Neuroimaging, 108(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Kanai R, & Rees G (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12(4), 231. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Bekker EM, Lijffijt M, Overtoom CCE, Jonkman LM, & Verbaten MN (2005). Attention deficit and impulsivity: selecting, shifting, and stopping. International Journal of Psychophysiology, 58(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Kenemans JL (2015). Specific proactive and generic reactive inhibition. Neuroscience & Biobehavioral Reviews, 56, 115–126. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, & Ridderinkhof KR (2004). ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology, 41(1), 9–20. [DOI] [PubMed] [Google Scholar]
- Lee TW, Lewicki MS, & Sejnowski TJ (1999). Unsupervised classification with non-Gaussian mixture models using ICA. In Advances in neural information processing systems (pp. 508–514). [Google Scholar]
- Levy BJ, & Wagner AD (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224(1), 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, & Cowan WB (1984). On the ability to inhibit thought and action: A theory of an act of control. Psychological Review, 91(3), 295. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276. [DOI] [PubMed] [Google Scholar]
- Martens S, Munneke J, Smid H, & Johnson A (2006). Quick minds don’t blink: Electrophysiological correlates of individual differences in attentional selection. Journal of Cognitive Neuroscience, 18(9), 1423–1438. [DOI] [PubMed] [Google Scholar]
- Matzke D, Love J, & Heathcote A (2017). A Bayesian approach for estimating the probability of trigger failures in the stop-signal paradigm. Behavior Research Methods, 49(1), 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Hughes M, Badcock JC, Michie P, & Heathcote A (2017). Failures of cognitive control or attention? The case of stop-signal deficits in schizophrenia. Attention, Perception, & Psychophysics, 79(4), 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Chen W, Isbaine F, Willie JT, Ostrem JL, & Starr PA (2018). Cortical potentials evoked by subthalamic stimulation demonstrate a short latency hyperdirect pathway in humans. The Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, and Takada M (2002). Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci. Res 43, 111–117. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, and Behavioral Neuroscience, 3(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Nigg JT (1999). The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM–IV combined type, extension, and qualification. Journal of Abnormal Child Psychology, 27(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Novembre G, Pawar VM, Bufacchi RJ, Kilintari M, Srinivasan MA, Rothwell JC, … & Iannetti GD (2018). Saliency detection as a reactive process: unexpected sensory events evoke cortico-muscular coupling. Journal of Neuroscience, 2474–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Casabona E, Bringas ML, Alvarez M, Alvarez L, … & Jahanshahi M (2011). Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Experimental Brain Research, 212(3), 371–384. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, & Makeig S (2005). Frontal midline EEG dynamics during working memory. Neuroimage, 27(2), 341–356. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, & Sergeant JA (1998). Response inhibition in AD/HD, CD, comorbid AD/HD+ CD, anxious, and control children: A meta-analysis of studies with the stop task. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 39(3), 411–425. [PubMed] [Google Scholar]
- Parent A, and Hazrati LN (1993). Anatomical aspects of information processing in primate basal ganglia. Trends Neurosci. 16, 111–116. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, & Ridderinkhof KR (2004). Effects of stop-signal probability in the stop-signal paradigm: The N2/P3 complex further validated. Brain and Cognition, 56(2), 234–252. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, & Braff DL (1981). Early information processing deficit in schizophrenia: New findings using schizophrenic subgroups and manic control subjects. Archives of General Psychiatry, 38(2), 175–179. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Rutherford B, Shenton ME, & McCarley RW (2001). Button-pressing affects P300 amplitude and scalp topography. Clinical Neurophysiology, 112(9), 1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, & Logan G (1995). Deficient inhibitory control in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 23(4), 411–437. [DOI] [PubMed] [Google Scholar]
- Schall JD, & Godlove DC (2012). Current advances and pressing problems in studies of stopping. Current opinion in neurobiology, 22(6), 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, & Barry RJ (2008). Movement-related potentials in the Go/NoGo task: The P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology, 119(3), 704–714. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Dimigen O, Kliegl R, Sommer W, & Turatto M (2009). Microsaccadic inhibition and P300 enhancement in a visual oddball task. Psychophysiology, 46(3), 635–644. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2008). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12(11), 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2009). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience & Biobehavioral Reviews, 33(5), 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, & Chambers CD (2010). Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences, 107(31), 13966–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Stevens T, & Chambers CD (2014). Proactive and reactive stopping when distracted: An attentional account. Journal of Experimental Psychology: Human Perception and Performance, 40(4), 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33(47), 18481–18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2015). It’s not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology, 52(4), 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR (2016). A neural mechanism for surprise-related interruptions of visuospatial working memory. Cerebral Cortex, 28(1), 199–212. [DOI] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2017). On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron, 93(2), 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR (2018). Testing multiple psychological processes for common neural mechanisms using EEG and independent component analysis. Brain Topography, 31(1), 90–100. [DOI] [PubMed] [Google Scholar]
- Wessel JR (2018). Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology, 55(3). [DOI] [PubMed] [Google Scholar]
- Wessel JR (2018). Surprise: A More Realistic Framework for Studying Action Stopping?. Trends in cognitive sciences, 22(9), 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Brown WS, Marsh JT, & Dawson ME (1991). Probing the time-course of the auditory oddball P3 with secondary reaction time. Psychophysiology, 28(6), 609–618. [DOI] [PubMed] [Google Scholar]
- Zavala B, Zaghloul K, & Brown P (2015). The subthalamic nucleus, oscillations, and conflict. Movement Disorders, 30(3), 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]