Abstract
Background:
Solitary chemosensory cells (SCCs) are rare epithelial cells enriched in nasal polyps and are the primary source of IL-25, an innate cytokine eliciting Th2 immune response. While it is proposed that SCCs are stimulated by antigens released by upper airway pathogens, the exogenous triggers of human SCCs remain elusive. We studied patients with noninvasive fungal rhinosinusitis to determine if extracts of Aspergillus fumigatus and Alternaria alternata stimulate SCC proliferation as an early event in type-2 inflammation.
Methods:
Multicolor flow cytometry, immunofluorescence, and ELISA were used to interrogate mucosa from patients with mycetomas and allergic fungal rhinosinusitis (AFRS) for SCCs and IL-25. Primary sinonasal epithelial cells from AFRS patients and non-inflamed inferior turbinates were stimulated with fungal extracts for 72 hours, and SCC population frequency as well as mitotic activity were quantified using flow cytometry.
Results:
SCCs producing IL-25 are enriched in inflamed mucosa compared to intra-patient non-inflamed control tissue (38.6% vs. 6.5%, p = 0.029). In cultured sinonasal epithelial cells from AFRS nasal polyps, Aspergillus f. and Alternaria a. stimulated higher SCC frequency compared to controls (27.4% vs. 10.6%, p = 0.002; 18.1% vs. 10.6%, p = 0.046), which led to increased IL-25 secretion in culture media (75.5 vs. 3.3 pg/mL, p < 0.001; 32.3 vs. 3.3 pg/mL, p = 0.007). Ki-67 expression was higher in SCCs grown in fungal stimulation conditions compared to controls.
Conclusion:
While fungal antigens are known to potentiate immune response through innate cytokines, including IL-25, the early expansion of SCCs in the presence of fungus has not been described. This early event in the pathogenesis of noninvasive fungal rhinosinusitis may represent a target for intervention.
Keywords: type-2 inflammation, mycetoma, allergic fungal rhinosinusitis, fungal antigens, solitary chemosensory cells, IL-25
Introduction
Fungi are ubiquitous in the environment and are common components in the normal sinonasal microbiome. However, in some immunocompetent individuals, fungi induce pathogenic hypersensitivity and inflammatory reactions in the nose and paranasal sinuses, collectively termed noninvasive fungal rhinosinusitis. Subtypes of noninvasive fungal disease include local fungal colonization, mycetoma (i.e., fungal ball), and allergic fungal rhinosinusitis (AFRS).1 The latter two entities are often characterized by extensive inflammation necessitating functional endoscopic sinus surgery to ventilate the paranasal sinuses in conjunction with adjuvant medical therapy.
Current investigation aims to elucidate how mucosal inflammation is triggered in patients with noninvasive fungal rhinosinusitis. While downstream immune cellular pathways are well studied, the contribution of epithelial cell dysfunction to fungus-mediated inflammation is incompletely understood. To investigate this question, we studied patients with mycetoma and AFRS. Mycetomas represent a uniquely isolated fungal infection often by Aspergillus species. Because of the anatomically confined nature, inflamed mucosa affected by the fungal ball can be compared to non-inflamed tissue as an intra-patient control. AFRS is characterized by nasal polyposis, thick eosinophilic mucus (i.e., allergic mucin), and elevated antifungal IgE. Studying AFRS may shed light on how sinonasal epithelium can influence a type-2 (Th2) inflammatory phenotype. While the contribution of downstream CD4+ Th2 cells2, group 2 innate lymphoid cells (ILC2s)3–5, Th17 cells6, B cells7, and mast cells8 is well studied, how epithelial cells initially trigger the type-2 inflammatory cascade in chronic rhinosinusitis is a relatively new frontier.
Recent studies by our group revealed that rare chemo-sensing epithelial cells, called solitary chemosensory cells (SCCs), are elevated in patients who have chronic rhinosinusitis with nasal polyposis (CRSwNP).9 Moreover, SCCs were found to be the primary source of IL-25, an important innate cytokine which elicits a downstream type-2 inflammatory response.5,9 While it is known that solitary chemosensory cells can detect substances in the mucosal environment10, the exact link between pathogens and detected antigenic substances remains elusive. Patients with noninvasive fungal disease represent an ideal population to investigate this question. Recent studies have revealed that fungal antigens may activate inflammatory cascades independently of the canonical antigen presenting pathways.11,12 Moreover, while the AFRS phenotype is considered a subset of CRSwNP13, the contribution of SCCs to the pathogenesis of this type-2 inflammatory condition has not been described. Therefore, we sought to determine how the epithelial compartment, in particular SCC biology, may be involved in chronic, noninvasive fungal rhinosinusitis.
We hypothesized that solitary chemosensory cells in the sinonasal epithelium undergo hyperplasia and increase production of IL-25 in patients with noninvasive fungal rhinosinusitis. Here, we report that SCCs are enriched in both patients with mycetomas and AFRS. Additionally, data support that exposure of fungal extract derived from Aspergillus fumigatus and Alternaria alternata are able to skew in vitro epithelial cell cultures toward a greater chemo-sensing phenotype.
Methods
Patient population & Tissue Acquisition:
Tissue samples were obtained from patients recruited from the Department of Otorhinolaryngology – Head and Neck Surgery, Division of Rhinology, University of Pennsylvania, with informed consent and full approval of the Institutional Review Board. The study patient population met the diagnosis of mycetoma (n = 3), AFRS (n = 6), and chronic sinusitis without nasal polyps (CRSsNP) (n = 5) in accordance to clinical diagnostic standards.13–15 Selected patients were refractory to medical management for their sinus disease and were therefore undergoing functional endoscopic sinus surgery. Patients were excluded if they carried an existing diagnosis of systemic disease that involve nasal polyposis as a clinical manifestation, such as granulomatosis with polyangiitis, sarcoidosis, cystic fibrosis, and disorders of ciliary motility (e.g., primary ciliary dyskinesia) to avoid confounding factors in the pathogenesis of their phenotype or had received steroids or antibiotics in the 4 weeks preceding surgery.
Immunofluorescent staining
Tissue specimens (nasal polyps from AFRS patients, inflamed mucosa adjacent to mycetomas, and non-inflamed inferior turbinate mucosa) were rinsed 3 times in Dulbecco’s Modified-Eagle Medium (Lonza, Basel, Switzerland) media containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 250 ng/mL amphotericin B. Large bone fragments were removed, and samples were fixed in 4% paraformaldehyde at 4°C. Samples were embedded in paraffin wax. Five- to 7-mm sections were obtained, and tissue slices were mounted on positively charged slides (Thermo Fisher Scientific, Waltham, MA).
Immunofluorescence of tissue sections was performed after de-paraffinization with xylene and graded ethanol washes. Samples were blocked for 1 hour at room temperature in 10% donkey serum and 2% bovine serum albumin (BSA) with 0.1% Triton X-100 and then incubated in 10% donkey serum and 2% BSA with primary antibodies (Supplementary Table 1) at 4°C overnight. Specimens were rinsed with phosphate buffered saline (PBS) and incubated in 10% donkey serum and 2% BSA with secondary antibodies (Supplementary Table 1) at room temperature for 1 hour. Samples were serially washed with PBS. Before mounting, tissue sections were incubated in TrueBlack Autofluorescence Quencher (Biotium, Fremont, CA) for 3 minutes at room temperature. Tissue sections were again rinsed 3 times in PBS and mounted using EverBrite Mounting Medium with DAPI (Biotium, Fremont, Calif) with an overlying glass coverslip.
All imaging was performed with an Olympus FluoView confocal system attached to an Olympus IX81 confocal microscope (Olympus, Center Valley, PA), as previously described.23 Images were acquired with a 60× oil objective by using Type FF immersion oil (Cargill, Wayzata, MN) at 0.2-mm intervals, and image reconstruction was accomplished with Fiji software. Tissue images were processed with a median filter (pixel size 2). All comparative polyp and turbinate images were taken with the same microscope settings.
Human Sinonasal Epithelial Culture
Sinonasal mucosal specimens were acquired as described above, and enzymatically dissociated human sinonasal epithelial cells were obtained, as previously described.10,16 Cells were filtered through 40-mm filters (Corning, Corning, NY) and plated onto 75 cm2 U-shaped flasks (Corning, Corning, NY) and grown to confluence over 7 days in proliferation media (PneumaCult™-Ex Medium, StemCell Technologies, Vancouver, Canada) supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin. Cells were then trypsinized and 2.0 million cells of each patient sample were plated onto 75 cm2 U-shaped flasks into three conditions: untreated media, treated with 26 μg/mL Aspergillus fumigatus fungal antigen (Greer Laboratories, Lenoir, NC), or treated with 26 μg/mL Alternaria alternate fungal antigen (Greer Laboratories, Lenoir, NC). Cells were fed and stimulated daily for 72 hours in proliferation media. After 72-hours, cells were incubated with media supplemented with 5μg/mL Brefeldin A (Sigma-Aldrich, St. Louis, MO) for 5 hours to stop golgi-mediated release of IL-25 and then were harvested for analysis by flow cytometry.
Single-cell Tissue Preparation
Inflamed mucosa (n = 3 AFRS nasal polyps, n = 1 mucosa adjacent to mycetoma) and control mucosa from inferior turbinates (n = 4) were each taken from the same patient and prepared as previously decribed.5 Briefly, tissue was incubated for 5 hours at 37°C in Dulbecco’s Modified-Eagle Medium (Lonza, Basel, Switzerland) with 5μg/mL Brefeldin A (Sigma-Aldrich, St. Louis, MO). Tissue was enzymatically digested releasing single cells into suspension.5 Ethylenediamine tetra-acetic acid (EDTA) (Sigma-Aldrich, St. Louis, MO) at final concentration 2mM was added to quench the digestion. Debris was removed using a 40-μm cell strainer and red blood cells (RBCs) were removed using an RBC lysis buffer (Miltenyi Biotech, Bergisch Gladbach, Germany). Cells were serially washed in 2% BSA in PBS (Sigma-Aldrich, St. Louis, MO) for analysis by flow cytometry.
Cell Staining & Flow Cytometry
Single cell suspensions (each >1.0 million cells) were stained with viability dye Aqua at 1:1000 dilution (Thermofisher) for dead cell exclusion. Fc receptors were blocked with TruStain FcX TM (Biolegend). For each marker, fluorescence minus one (FMO) controls were also prepared. Isotype control antibodies were used in FMOs for GNAT3 and IL-25.
For SCCs, cell surface markers were stained using anti-EpCAM-PECy7 (eBiosciences) to mark epithelial cells and anti-CD45-eFlour450 (eBiosciences) to mark lymphocytes. After fixation and permeabilization, intracellular markers were stained overnight at 4°C using anti-DCLK1-AF647 (AbCAM), anti-GNAT3-PE (LS Biosciences), anti-IL25-FITC (Invitrogen). To determine mitotic activity, anti-Ki67 BV711 (BioLegend) was used as an intracellular stain. See Supplementary Table 2 for full antibody panel and dilutions used.
Antibody-labelled cell suspensions were analyzed on an LSR Fortessa TM using FACSDiva Software 8 (BD Biosciences). At least 500,000 live events were acquired per sample. Data were analyzed using FlowJo 10 (Treestar). Samples were FSC and SSC gating was used to identify singlets and exclude debris. Positive gates were determined using <1% of events on FMO samples. SCCs were identified as Singlets, Aqua Live, CD45-EpCAM+IL25+DCKL1+ GNAT3+ events and represented as a frequency of Aqua Live, EpCAM+ events. Comparisons of Ki-67 expression were then used to determine degree of cell division among the SCC subset.
ELISA Protein Analysis
Human IL-25 ELISA (Abexxa Biologics, Cambridge, MA) was performed according to the manufacturer’s instructions with 100 μL samples of media collected from epithelial cell cultures. Media was collected on day 7, at the time of cell harvest from the three different growth conditions: untreated, Aspergillus f. treated, and Alternaria a. treated. Culture media that was not exposed to cell culture served as blank optical density controls. Concentrations of soluble IL-25 (i.e., secreted IL-25) were determined by linear interpolation of measured optical densities using a standard curve.
Statistical Methods
Stata version 13 (StataCorp, College Station, TX) software was used for statistical analysis, with p < 0.05 considered statistically significant. Cell population frequencies were compared with Mann-Whitney test (upaired tissue samples data) and Kruskall-Wallis for (paired cell culture lines) to account for non-parametric data, as normality assumptions do not apply to flow cytometry data.
Results
Noninvasive fungal rhinosinusitis mucosa is enriched for IL25-producing SCCs
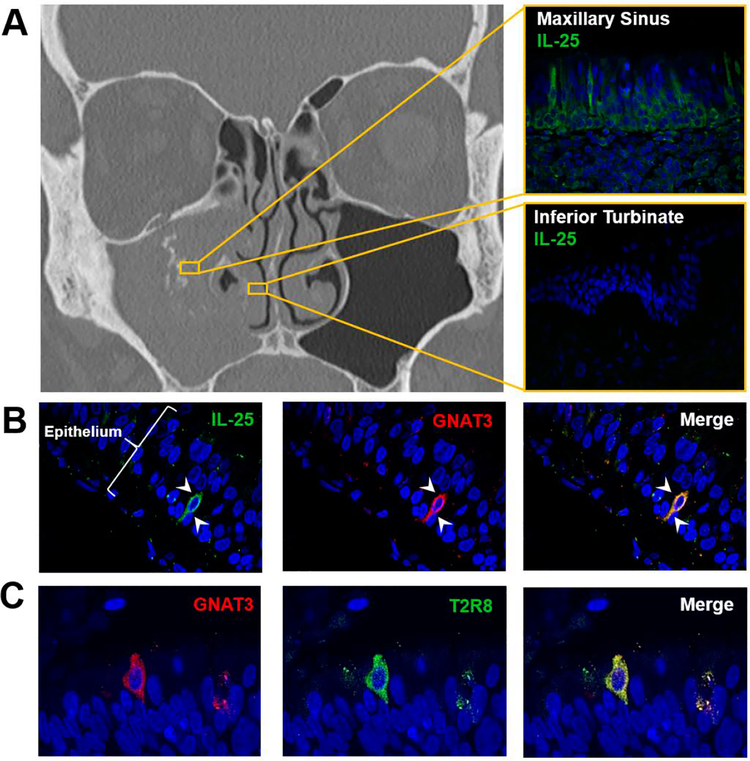
Immunofluorescence of inflamed sinus mucosa affected by mycetomas demonstrated discrete cellular expression of IL-25 (Figure 1A). Moreover, IL-25 was restricted to cells which also expressed gustucin, also called G protein subunit alpha transducin (GNAT3), a critical component of the taste conduction pathway and a specific marker for SCCs (Figure 2B). Of note, the IL-25 producing SCCs were limited to the affected sinus, with marked reduction of IL-25 expression in the epithelium of the ipsilateral non-inflamed inferior turbinate mucosa (Figure 1A). The observation that SCCs are enriched in the epithelium of the sinus harboring the fungal ball was consistent across all examined patients with mycetoma (n = 3). Given that SCCs are known to have a chemosensing (i.e., tasting) role, we also performed staining for bitter taste receptor, T2R8, which has been implicated in respiratory pathology.17 T2R8 also localized to SCCs in inflamed sinonasal mucosa (Figure 1C).
Figure 1:
(A) Computed tomographic scan of a patient with mycetoma of the right maxillary sinus. Immunofluorescent staining was performed on a tissue sample from the right maxillary sinus (top) and the inferior turbinate (bottom). (B-C) Representative staining images of inflamed mucosa from patients with mycetoma (n = 3 patients). DAPI blue, IL-25 green, T2R8 green, GNAT3 red. Arrow heads demonstrate solitary chemosensory cell.
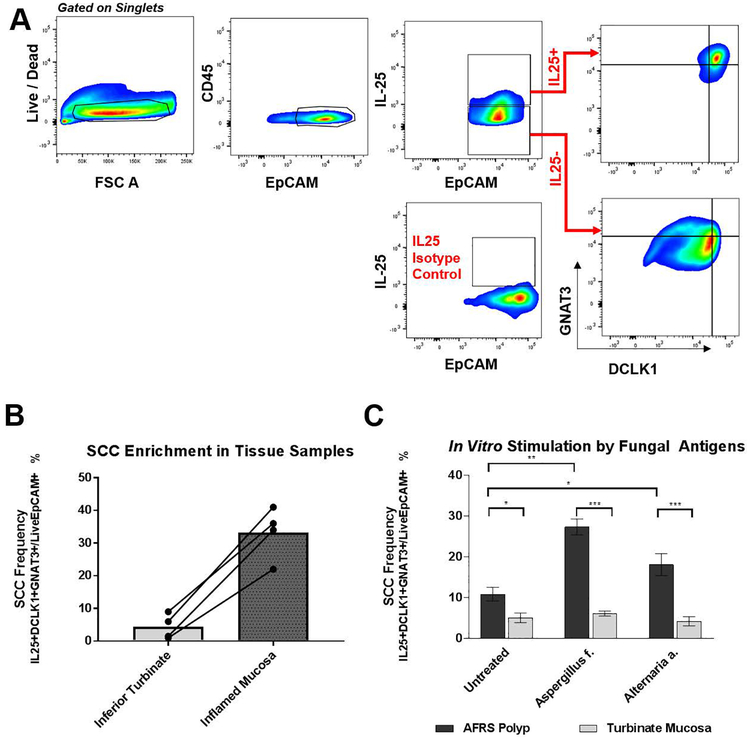
Figure 2:
(A) Representative gating strategy to identify SCCs in primary epithelial cells grown in culture (See Supplementary Figure 1 for gating strategy in tissue samples). (B) Frequency of SCCs in tissue samples among all epithelial cells quantified by flow cytometry (n = 3 AFRS patients; n = 1 mycetoma patients) compared to matched inferior turbinates. (C) Frequency of SCCs in primary sinonasal epithelial cultures stimulated for 72 hours with fungal antigens (n = 6 AFRS patients’ epithelium from nasal polyps; n = 4 patients’ epithelium from non-inflamed turbinates). Error bars represent standard error of mean. [***p <0.0005; **p<0.005; *p<0.05]
While immunofluorescence allows for direct visualization of upregulated cell types in their native architecture, we also sought to perform more rigorous quantification of cell populations in tissue samples using multicolor flow cytometry. Representative gating to identify SCCs is show in Figures Supplementary Figure 1. After isolating single cells and excluding dead cells, epithelial cells were identified as CD45-EpCAM+. The frequency of SCCs (identified as CD45-EpCAM+IL25+DCLK1+ GNAT3+ events) among all epithelial cells was found to be higher in inflamed mucosa compared to non-inflamed turbinate tissue (Figure 2B, 38.6% vs. 6.5%, p = 0.029). This findings corroborate the observation by flow cytometry that SCCs are enriched in the epithelium of patients with noninvasive fungal rhinosinusitis.
Fungal antigens stimulate SCC expansion in vitro
To further explore the mechanism of the observed SCC hyperplasia, we isolated sinonasal epithelial cells and stimulated the cell cultures using soluble fungal extracts derived from Aspergillus fumigatus and Alternaria alternata. Human epithelial cells were isolated from nasal polyps removed from AFRS patients and non-inflamed inferior turbinates. We hypothesized that progenitor basal cells from which SCCs proliferate and/or SCCs themselves will be stimulated to proliferate when grown in vitro in the presence of fungal antigens, thereby recapitulating the SCC hyperplasia we previously observed in vivo.
After 3 days of fungal antigen exposure, the frequency of SCCs in the AFRS nasal polyp epithelial cell lines was significantly elevated in the Aspergillus f. (27.4% vs 10.6%, p = 0.002) and the Alternaria a. (18.1% vs 10.6%, p = 0.046) conditions when compared to the untreated condition (Figure 2C). Moreover, in the inferior turbinate epithelial cultures, there was no observed statistically significant difference in the frequency of SCCs between untreated and the Aspergillus f. or Alternaria a. conditions (5.1%, 6.1%, 4.2%, respectively). There was, however, statistically higher populations of SCCs in the polyp-derived cell lines compared to turbinate-derived cell lines in all three conditions (untreated 10.6% vs. 5.1% p = 0.029; Aspergillus f. 27.4% vs. 6.1% p <0.0001; Alternaria a.18.1% vs. 4.2% p < 0.0001).
To ensure that the observed increase in SCCs was not influenced by paracrine signaling mediated by immune cells (i.e., sensitized antigen presenting cells, ILC2s, and granulocytes) anti-CD45 was included in the staining panel (Figure 2A). In each of the cell lines, there were no detectable immune cells (CD45+ events) as demonstrated in the representative flow panels in Figure 2A. Prior studies have also supported that the method of isolating primary epithelial cell lines utilized herein yields purely epithelial cell types.16
SCCs grown in the presence of fungal antigens are more mitotically active
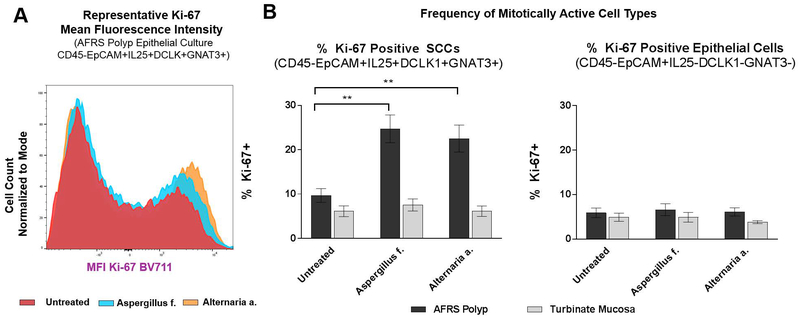
Given that fungal antigen exposure may in fact be affecting the differential survival of cell types rather than promoting a truly proliferative state, we examined by flow cytometry the expression of Ki-67, a marker of cell division. Among cultures from AFRS patients, the percent of Ki-67 positive (i.e., mitotically active) SCCs was elevated in both the Aspergillus f. and the Alternaria a. conditions compared to untreated (24.7% vs. 9.67%, p = 0.0016; 22.5 vs. 9.67%, p = 0.0037) (Figure 3A–B). There was no difference in proliferative characteristics in SCCs from inferior turbinate cell lines, regardless of stimulation status. However, SCCs from the AFRS patients were overall more mitotically active when compared to cells from the inferior turbinate (Figure 3B). Furthermore, to answer the question of whether fungal products are specifically affecting chemo-sensing cells and not other epithelial cell types, we quantified the frequency of Ki-67 positive events in the CD45-EpCAM+IL24-DCLK1-GNAT- population. There was no statistically significant difference in Ki-67 positive events in these cells (Figure 3B), further supporting that cell proliferation in the presence of fungal antigens is restricted to the SCC population.
Figure 3:
(A) Representative mean fluorescent intensity measured by flow cytometry of Ki-67 expression among SCCs in epithelial cultures grown under three conditions. (B) Frequency of Ki-67 positive SCCs and non-SCC epithelial cells in primary sinonasal cell cultures stimulated for 72 hours with fungal antigens (n = 6 AFRS patients’ epithelium from nasal polyps; n = 4 patients’ epithelium from non-inflamed turbinates). Error bars represent standard error of mean. [***p <0.0005; **p<0.005; *p<0.05]
SCCs secrete more IL-25 in presence of fungal antigens
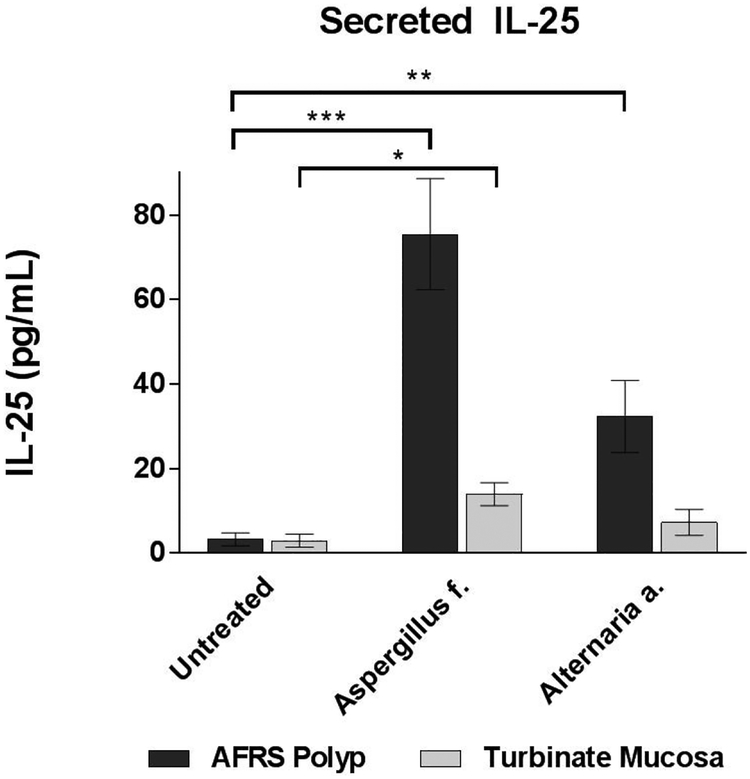
In addition to demonstrating increased frequency of SCCs, the functional status of IL-25 production by SCCs was determined by ELISA. After 7 days of either fungal exposure or no treatment, the apical media of epithelial cultures was assayed for levels of soluble IL-25. As shown in Figure 4, secreted IL-25 was higher in AFRS polyp cultures exposed to Aspergillus f. and Alternaria a. compared to untreated cultures (75.5 vs. 3.3 pg/mL, p < 0.0001; 32.3 vs. 3.3 pg/mL, p = 0.007). Moreover, when comparing between the treatment groups, the level of IL-25 was higher in cultures exposed to Aspergillus f. compared to Alternaria a. (75.5 vs. 32.3 pg/mL, p = 0.023). Among cultures derived of non-inflamed turbinate mucosa, IL-25 was only significantly greater in the Aspergillus f. compared to control (14.0 vs. 3.1 pg/mL, p = 0.006). However, there was no difference between Alternaria a. and control groups. These data demonstrate that exposure of fungal products to sinonasal epithelium promotes SCC proliferation and increased production of IL-25, particularly in polyp epithelium.
Figure 4:
IL-25 secreted by epithelial cells into culture media detected by ELISA. (n = 6 AFRS patients’ epithelium from nasal polyps; n = 4 patients’ epithelium from non-inflamed turbinates). Error bars represent standard error of mean. [***p <0.0005; **p<0.005; *p<0.05]
Discussion
Noninvasive fungal rhinosinusitis represents a complex and overlapping group of diseases in which the sinonasal mucosa exhibits an inflammatory response to fungal elements. The literature on its pathogenesis is confounded by controversy over inclusion criteria defining each of the disease entities.18 Historically, studies have focused on the involvement of immune cell hypersensitivity and pro-inflammatory cytokines in AFRS, while literature on mycetoma has focused on ciliary dysfunction. Here, we report that solitary chemosensory cells may be playing an upstream role in the detection of fungal antigens in both AFRS and mycetoma. The finding that SCCs expand, and thereby increase local production of IL-25, a critical innate cytokine implicated in upper airway disease19–22, demonstrates a link between epithelial and immune dysfunction contributing to inflammation.
Strides have been made in gut physiology towards establishing the relationship among pathogens, chemosensing epithelial cells, and immune function. Recently, Scheider et al. and Nadjsombati et al. demonstrated that succinate, a metabolic byproduct of certain gut helminths and trichomonad protists, stimulates expansion of chemosensing cells in murine intestinal epithelium.23,24 A similar phenomenon may be occurring in the human upper airway. Fungal antigens may be detected by solitary chemosensory cells, leading to local cellular expansion and upregulation of innate cytokines. This study demonstrates a near 6-fold increase in SCC frequency in inflamed tissue of noninvasive fungal rhinosinusitis patients. Moreover, using a minimal in vitro cell culture model, we demonstrate that SCCs expand in primary sinonasal epithelial cells derived from AFRS patients when grown in the presence of fungal antigens. Of note, future studies are needed to investigate whether fungal products exert a direct effect on SCCs causing cell proliferation or an indirect effect whereby a secondary signal, such as another alarmin or cytokine, is released in response to fungus, which ultimately causes SCCs to expand.
Regardless of whether the effect of fungal products is direct or indirect, however, the mechanism by which the SCC detects a change in the mucosal environment represents a frontier of investigation. At its essence, the biological role of the solitary chemosensory cells is to survey the extracellular environment. While we demonstrate that T2R8 is expressed on solitary chemosensory cells, we have not established the link between taste receptors on SCCs and fungal antigens. Indeed, fungal antigenic extracts are biochemically diverse concoctions comprised of small molecule metabolites as well as proteinaceous and carbohydrate macromolecules. Therefore, future studies on the exact ligand-receptor relationship may lead to the identification of more targeted interventions for patients. Undoubtedly, a limitation of our study is the incredible heterogeneity in patients with noninvasive fungal rhinosinusitis. As future studies further delineate endotypes, we can better hone in on how various environmental and host factors differentially contribute to the clinical phenotype of sinonasal inflammation.
Conclusion
Fungal elements have been known to stimulate downstream immune cells; however, dysregulation at the level of epithelial cells is relatively understudied. We demonstrate that SCCs are enriched in inflamed mucosa of patients with noninvasive fungal rhinosinusitis, namely mycetoma and allergic fungal rhinosinusitis. Additionally, SCCs proliferate when stimulated with fungal antigens derived from Aspergillus fumigatus and Alternaria alternata. With the need for more targeted intervention, this early event in the inflammatory cascade represents a novel potential target for intervention.
Supplementary Material
Supplementary Table 1: Immunofluorescent staining antibodies
Supplementary Table 2: Multicolor flow cytometry antibody panel
Supplementary Figure 1: Representative gating strategy to identify SCCs in tissue sample (AFRS nasal polyp).
Funding:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880 (NNP) as well as by R01DC013588 (to N.A.C.), GM083204–08A1, UO1AI125940 and R01AI095289 (to DRH), a pilot grant from the University of Pennsylvania Department of Otorhinolaryngology Head and Neck Surgery (to NAC and DRH), and a philanthropic donation from the R.L.G. Foundation, Inc to N.A.C. The content is solely the responsibility of the authors and does not necessarily represent the offices views of the National Institutes of Health.
Footnotes
Podium Presentation at the American Rhinologic Society at the Academy, Atlanta, GA, October 6, 2018.
Disclosures: Authors have nothing to disclose
References
- 1.Rodrigues J, Caruthers C, Azmeh R, Dykewicz MS, Slavin RG, Knutsen AP. The spectrum of allergic fungal diseases of the upper and lower airways. Expert Rev Clin Immunol. 2016;12(5):531–550. doi: 10.1586/1744666X.2016.1142874. [DOI] [PubMed] [Google Scholar]
- 2.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23(3):281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 3.Padro Dietz C, Luong A. Innate Lymphoid Cells: The Innate Counterpart to T Helper Cells. Adv Otorhinolaryngol. 2016;79:58–68. doi: 10.1159/000445130. [DOI] [PubMed] [Google Scholar]
- 4.Poposki JA, Klingler AI, Tan BK, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immunity, Inflamm Dis. 2017;(Ilc):233–243. doi: 10.1002/iid3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel NN, Kohanski MA, Maina IW, et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2018;00(0):1–7. doi: 10.1002/alr.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai G, Das S, Ansari MA, et al. Phenotypic and functional profile of Th17 and Treg cells in allergic fungal sinusitis. Int Immunopharmacol. 2018;57. doi: 10.1016/j.intimp.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Collins M, Nair S, Smith W, Kette F, Gillis D, Wormald PJ. Role of local immunoglobulin E production in the pathophysiology of noninvasive fungal sinusitis. Laryngoscope. 2004;114(7):1242–1246. doi: 10.1097/00005537-200407000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Tyler MA, Russell CB, Smith DE, et al. Large-scale gene expression profiling reveals distinct type 2 inflammatory patterns in chronic rhinosinusitis subtypes. J Allergy Clin Immunol. 2017;139(3):1061–1064.e4. doi: 10.1016/j.jaci.2016.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohanski MA, Workman AD, Patel NN, et al. Solitary Chemosensory Cells are a Primary Epithelial Source of Interleukin-25 in Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol. May 2018. doi: 10.1016/j.jaci.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3):1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamad M Innate and adaptive antifungal immune responses: Partners on an equal footing. Mycoses. 2012;55(3):205–217. doi: 10.1111/j.1439-0507.2011.02078.x. [DOI] [PubMed] [Google Scholar]
- 12.Piehler D, Eschke M, Schulze B, et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol. 2016;9(4):937–949. doi: 10.1038/mi.2015.106. [DOI] [PubMed] [Google Scholar]
- 13.Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinology. 2012;(20):1–136. doi: 10.4193/Rhino50E2. [DOI] [PubMed] [Google Scholar]
- 14.Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. Int Forum Allergy Rhinol. 2016;6:S3–S21. doi: 10.1002/alr.21694. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119(9):1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan M, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol. 2007;21(3):373–377. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 17.Grassin-Delyle S, Abrial C, Fayad-Kobeissi S, et al. The expression and relaxant effect of bitter taste receptors in human bronchi. Respir Res. 2013;14(1). doi: 10.1186/1465-9921-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykewicz MS, Rodrigues JM, Slavin RG. Allergic fungal rhinosinusitis. J Allergy Clin Immunol. 2018;142(2):341–351. doi: 10.1016/j.jaci.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Ozturan A, Eyigor H, Eyigor M, et al. The role of IL-25 and IL-33 in chronic rhinosinusitis with or without nasal polyps. Eur Arch Oto-Rhino-Laryngology. 2017;274(1):283–288. doi: 10.1007/s00405-016-4260-6. [DOI] [PubMed] [Google Scholar]
- 20.Hong H, Chen F, Sun Y, et al. Nasal IL-25 predicts the response to oral-corticosteroid in chronic rhinosinusitis with nasal polyps (CRSwNP). J Allergy Clin Immunol. 2018;0(0):7–9. doi: 10.1016/j.jaci.2017.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Hong H-Y, Chen F-H, Sun Y-Q, et al. Local IL-25 contributes to Th2-biased inflammatory profiles in nasal polyps. Allergy. August 2017. doi: 10.1111/all.13267. [DOI] [PubMed] [Google Scholar]
- 22.Lam M, Hull L, Imrie A, et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29(3):175–181. doi: 10.2500/ajra.2015.29.4176. [DOI] [PubMed] [Google Scholar]
- 23.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity. 2018;49(1):33–41.e7. doi: 10.1016/j.immuni.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider C, O’Leary CE, von Moltke J, et al. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Immunofluorescent staining antibodies
Supplementary Table 2: Multicolor flow cytometry antibody panel
Supplementary Figure 1: Representative gating strategy to identify SCCs in tissue sample (AFRS nasal polyp).