Abstract
Background:
Attention-deficit/hyperactivity disorder (ADHD) is associated with working memory (WM) deficits. However, WM is a multi-process construct that can be impaired through several pathways, leaving the source of WM impairments in ADHD unresolved. In this study, we aim to replicate, in an independent sample, previously reported deficits in component processes of WM deficits in ADHD and expand to consider their implications for neurocognitive outcomes.
Methods:
In 119 children (7–14 years old, 85 with ADHD), we used electroencephalography measures to quantify component processes during performance of a spatial working memory task. We quantified stimulus encoding using alpha range (8–12Hz) power; vigilance by the P2 event-related potential to cues; and WM maintenance by occipital-alpha and frontal-theta (4–7Hz) power. These measures were evaluated against metrics of executive function, ADHD symptoms, and academic achievement.
Results:
Encoding alpha-power decreases and cue P2 amplitude were attenuated in ADHD, whereas occipital-alpha power during maintenance was significantly greater in ADHD, consistent with a compensatory response to weak encoding. Weak alpha modulation during encoding was associated with poorer reading comprehension and executive function, as well as enhanced ADHD symptoms. Previously reported effects in frontal-theta power failed to replicate.
Conclusions:
Stimulus encoding, a component process of WM coupled to alpha modulation, is impaired in ADHD, and, unlike WM maintenance or vigilance processes, has implications outside of the laboratory via a relationship with executive function, and, to a weaker extent, reading comprehension.
Keywords: ADHD, working memory, maintenance, visual attention, academic achievement, alpha oscillations, EEG
Introduction
Working memory (WM), the ability to transiently store and manipulate information in memory (Baddeley, 1986), is a core neurocognitive function affected in ADHD (Castellanos & Tannock, 2002). Group differences in behavioral performance on WM tasks consistently show medium-to-large effect sizes distinguishing ADHD from healthy youth (Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). While such impairments are compatible with fronto-striato-cerebellar dysfunction stemming from catecholaminergic dysregulation of prefrontal cortex (PFC; McCracken, 1991; Arnsten, 2006), WM is a multi-process construct that can be impaired through several pathways (e.g., vigilance, visual encoding, maintenance, content manipulation, retrieval), leaving the source of WM impairments in ADHD unresolved.
Evidence from brain oscillations suggests that attention processes play an important role in WM impairments in ADHD (Lenartowicz, Mazaheri, Jensen, & Loo, 2018). Modulation of power in the alpha band (8–12Hz) during stimulus processing, a marker of visual perception and attention (Foxe & Snyder, 2011), has been shown to be attenuated in both children (Lenartowicz et al., 2014; Mazaheri et al., 2010; Vollebregt, Zumer, Ter Huurne, Buitelaar, & Jensen, 2016) and adults with ADHD (Hasler et al., 2016; Missonnier et al., 2013; Ter Huurne et al., 2017). In the study of Lenartowicz et al. (2014), aberrant modulation of alpha was observed during the encoding phase of a WM task, and predicted both neural responses during WM maintenance and task performance, thus supporting the possibility that visual attention processes account for a portion of WM impairments. WM deficits can also arise secondary to a deficit of vigilance, via disruption of sustained attention (Biederman & Spencer, 1999; Sergeant, 2005). In ADHD, this hypothesis is supported by impairments in continuous performance tasks, such as variability in responding over time (Kofler et al., 2013). Lenartowicz et al. (2014) also reported attenuated neural responses to alerting cues. Assuming tonic activation establishes a physiological background against which phasic arousal takes place, the weakened cue response is a correlate of weakened vigilance.
In the present study, we expand on these findings in two ways. First, we aim to replicate deficits of encoding and vigilance in an independent sample. Second, we test the implications of these deficits for neurocognitive outcomes. Namely, deficits of executive function (of which WM is one) in ADHD have been associated with negative effects on academic achievement (reading, spelling, and math), educational attainment (repeating a grade, needing extra help, special education classes, learning disabilities) and IQ (Biederman et al., 2004; Biederman et al., 2006). To the extent that group differences in encoding or vigilance are sub-processes of WM that contribute meaningfully to WM deficits, then measures of these functions during WM should also predict academic achievement and executive function.
Methods
Participants
This study examines the baseline data of a subset of 246 children recruited from the UCLA community to participate in a clinical trial (Bilder et al., 2016; Loo et al., 2016; McCracken et al., 2016) (clinicaltrials.gov ID: NCT00429273). In a prior publication, we reported results based on 102 individuals from this dataset. Here we evaluated the remaining sample, in an independent replication test of the original result. Note that our final analyses were performed on 119 individuals. Of the 144 in the independent sample, five were excluded during our independent component analysis protocol (c.f., S2) and another 20 were excluded because task performance was less than our criterion of 60%. Full diagnostic details are provided in Supplemental Materials (c.f., S1) as well as in prior publications (Bilder et al., 2016; Loo et al., 2016; McCracken et al., 2016).
Behavioral and Cognitive Outcome Measures
Dimensional ratings of behavioral symptoms were obtained using the Child Behavior Checklist (CBCL) (Achenbach, 2000). Severity of ADHD symptoms was assessed using the Strengths and Weaknesses of ADHD symptoms and Normal (SWAN) Behavior Scale (Swanson et al., 2006). Estimated intelligence (IQ) was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI). Academic achievement was assessed using: Woodcock Johnson IV (WJ, word attack and letter-word identification subscales), Gray Oral Reading Test (GORT4), and the Wide Range Achievement Test (WRAT4, spelling and math subscales). Executive function was assessed using subtests of the Delis-Kaplan Executive Function System (DKEFS).
Task and Procedures
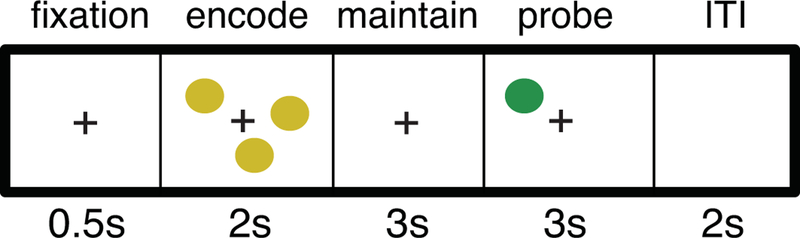
We used a computerized version of the Spatial Working Memory (SWM) Task (Glahn et al., 2002) to assess components of working memory (WM) (Fig.1). Trials began with a fixation cross presented for 500-msec, followed by an encoding display containing 1, 3, 5, or 7 yellow dots whose locations were to be remembered. The number of dots is a manipulation of load, with greater load expected to engage more WM. After 2-seconds, the screen turned blank and remained blank for a 3-sec maintenance interval. The probe was a single dot (3 sec); children indicated with a button press whether this probe stimulus was in a location previously shown (match) or not (non-match). Task outcome variables included accuracy, reaction time (RT), and standard deviation of reaction time (RTsd) as an index of response variability (see Table 1). The left and right arrow keys were assigned to match and non-match responses, respectively. Stimuli were presented on a Dell PC (Round Rock, TX) and responses were collected on a QWERTY keyboard, controlled by E-Prime Software (v1.1b5; Psychology Software Tools, Pittsburg, PA).
Figure 1.
In the spatial working memory task participants encode the spatial location of 1 or 3 (low load) or 5 or 7 (high load) dots. Following a maintenance interval, they must indicate if the probe dot occurs in the same or different location than any stimuli in the encoding stimulus.
Table 1.
Sample Characteristics
| ADHD | TD | Group Difference | |
|---|---|---|---|
| n | 85 | 34 | |
| Females | 24 (28.2%) | 13 (38.2%) | χ2= 1.13, p=.29 |
| Age | 10.5 yrs | 10.3 yrs | t=0.3, p=.73 |
| FSIQ | 103.3 | 109.6 | t=2.5, p=.02 |
| SWAN inattention | 14.0 | 36.0 | t=14.4, p<.001 |
| SWAN hyperactive | 20.6 | 37.4 | t=9.3, p<.001 |
| Task Performance | |||
| Accuracy | 74.1% | 80.2% | t=3.4, p=.001 |
| RT | 1357 ms | 1377 ms | t=0.4, p=.68 |
| RTsd | 430 ms | 405 ms | t=1.3, p=.19 |
Notes. SWAN=Strengths and Weaknesses of ADHD symptoms and Normal behavior rating scale (higher indicates fewer attention problems); FSIQ=Full Scale IQ; RT=reaction time; RTsd=response time standard deviation.
A training block preceded the testing session. In 8 trials, encoding and probe stimuli appeared side-by-side; in the next 8 trials the probe followed encoding without the maintenance interval; finally, 8 full trials were presented. A requirement of >60% accuracy during practice was required to continue to the two testing blocks, each containing 48 trials. In each block, there were equal numbers of trials for each load and match/no-match response type; the order of which was randomized within block. Each block lasted about 7 minutes, for a total testing time of approximately 17 minutes, including practice.
We analyzed four phases of the task: (1) fixation, as an index of how vigilant participants were in attending to the task; (2) encoding, as index of attention processes; (3) maintenance of the locations in memory as an index of WM maintenance; and (4) retrieval from working memory during probe. The retrieval phase provided an additional control on encoding effects. Namely, effects common to retrieval and encoding would be consistent with attention processes, whereas effects present at only one interval would be more likely consistent with the corresponding memory process (encoding or retrieval).
EEG Recording
While participants performed the SWM task, EEG recordings were collected using an Electrocap (Electro-cap International, Inc., Eaton, OH), containing 40 silver chloride electrodes positioned in accordance with the 10/20 System. Electrode impedances were brought below 10KΩ before task recording. Electrical signals were recorded using MANSCAN hardware and recording software (SAM Technology Inc., San Francisco, CA). EEG was recorded at 256 Hz with linked-ears reference. Electrode locations were recorded prior to the EEG session by measuring the pairwise distances between electrodes and landmarks (pre-auricular points and nasion), using Fowler calipers, and transformed within the MANSCAN software to 3-D spherical coordinates.
Behavioral Analysis
We analyzed task accuracy, RT and RTsd (for correct trials only) using a confirmatory repeated-measures ANOVA (SPSS, IBM Corporation, Somers, NY), following Lenartowicz et al., (2014). Each analysis included three factors. The between-subject factor of GROUP (ADHD vs. TD controls) tested for differences in performance between groups. The within-subject factor of LOAD (1 or 3 dots at low load vs. 5 or 7 dots at high load) was used to identify processes sensitive to WM demands. We also included the between-subjects factor of AGE to test for developmental changes. Two age subgroups were defined according to a median split in age: 7–10 years and 11–14 years, for consistency with our prior report. Gender was included as a covariate of no interest, to identify results independent of gender differences.
EEG Analysis
EEG processing was performed using custom MATLAB (The Mathworks, Inc.) scripts using functions from the EEGLAB (v.11.03.b) software (Delorme & Makeig, 2004). The EEG data from correct trials were high-pass filtered (>1 Hz), inspected for noisy electrodes, which were excluded from further analysis. The data were re-referenced to average reference. Within each subject, epochs of gross movements and muscle artifact were identified and removed if signal power in that epoch exceed the 85th percentile for >60% of the channels. The cleaned data were decomposed into source signals by independent component analysis (ICA, extended infomax algorithm, see S2 in Supplemental Materials for additional details) (Lee, Girolami, & Sejnowski, 1999). Each IC time course is thought to reflect a putative cortical source generator, associated with a single topography across electrodes. IC time courses were analyzed in lieu of channel data in all subsequent analyses (unless specifically noted), segmented into epochs time-locked to the onset of the encoding stimulus, beginning 1.6 s before and ending 8 s after stimulus presentation. Each 9.5-s epoch encompassed all phases of the SWM task trial. Features of interest were identified and extracted in each subject based on a priori criteria as follows.
To quantify vigilance, we extracted the amplitude of the P2, event-related potential (ERP) indexed to the fixation cue, from ICs with mid-frontal topography (D’Ardenne et al., 2012; Lenartowicz, Escobedo-Quiroz, & Cohen, 2010). P2 amplitudes were averaged across epochs and the mean voltage in the baseline preceding the fixation cue (−100 ms to 0 ms) was subtracted.
To quantify encoding, we computed power in the alpha frequency range (8–12Hz), from ICs with mid-occipital topography, during encoding (0–2000 ms) and, also, retrieval (5000–6000 ms). Alpha power decreases are interpreted as reflecting attention system (fronto-parietal cortex) engagement, supporting an interaction between visual cortex and storage systems. For each frequency and time point in an interval, the power was divided by the baseline (−600 to −100 ms) and log-transformed (10log10) to decibel (dB) units. These values were averaged across all frequencies and time points to produce a single value per subject per interval.
To quantify maintenance, we computed power in the alpha (8–12Hz, mid-occipital ICs) and theta (4–7Hz, mid-frontal ICs) frequencies during the maintenance interval – as both have been implicated in working memory maintenance with alpha representing a parieto-occipital contribution and theta representing a frontal contribution to WM maintenance. Alpha power increases, rather than decreases as in encoding, are expected during this period and are interpreted as inhibition of visual processing of inputs to minimize interference with stored information. Frontal theta is associated with support of storage operations. The power values were baseline normalized and converted to decibel units as for encoding features.
Group Analysis.
We performed confirmatory (Lenartowicz et al, 2014), repeated-measures ANOVAs for each phase of the WM trial (vigilance, encoding/retrieval, maintenance), using the same three independent factors as in the behavior analysis (GROUP, LOAD, AGE), as well as gender as a covariate of no interest. For validation and comparison with the ICA approach, all analyses were also performed on single channel data (c.f., S4 in Supplemental Materials). For group comparisons we report Cohen’s f effect size, recommended for analyses involving F tests or ANOVA models (Cohen, 1988). Small, medium and large f values are traditionally defined as .02, .15, and .35, respectively.
EEG Predictors of Neurocognitive Outcomes
To reduce dimensionality of outcome measures, we first performed a factor analysis (principal axis factor extraction). The analysis included symptom measures (SWAN & CBCL attention scales), academic achievement measures (WJ, GORT4, WRAT4), and DKEFS sub-tests targeting executive function. Model assumptions were tested using Kaiser-Meyer-Olkin Measure of Sampling Adequacy (>.6) and Bartlett’s Test of Sphericity (p<.001). The number of factors was selected based on the Screen test. Factors were rotated using Promax oblique rotation, selected based on the presence of factor correlations >.32 (Tabachnick & Fidell, 2007). For each of the resulting factors, we performed a confirmatory multiple regression (SPSS, IBM Corporation, Somers, NY) with predictors including each of the EEG features, and age as a continuous covariate of no interest. FSIQ and gender were also examined as covariates. Gender did not show any significant effects and is thus not further examined. FSIQ was significantly correlated with variables of interest (academic achievement, executive function) and did not change the outcome of the analysis, as such it was omitted from the final model (c.f., S6, Supplemental Materials). Individual coefficients were evaluated using t-tests. In Supplemental Materials, we also provide an exploratory regression of CBCL metrics on EEG indicators (c.f., S7) and first-order correlations of regression variables (c.f., S9).
Internal Consistency of EEG Predictors
Finally, for EEG predictors of neurocognitive outcomes, we evaluated internal consistency. For each EEG feature, we split each subject’s data into two halves, grouping odd-numbered trials into one half and even-numbered trials into the second half for the appropriate trial phase (fixation, encoding, maintenance) and load (low and high load), The two samples were then used to in calculation of Cronbach’s alpha for each measure.
Results
Demographics & Performance
There were no differences between children with and without ADHD in gender distribution (ADHD: 71.8% males, TD: 61.8% males, χ2(1, n=119)=1.13, p=.29) or age (ADHD: 10.24 yrs, TD: 10.52 yrs, t(117)=.34, p=.73) (Table 1). Children with ADHD had lower scores on tests of executive function (DKEFS), academic achievement (GORT, WJ, WRAT4) and showed more symptoms across neuropsychiatric dimensions assessed by the CBCL (c.f., S3, Supplemental Materials). Children with ADHD also had lower accuracy on the SWM task (F(1,115)=12.4, p<.001, Cohen’s f =.33), but did not differ in RT (F(1,115)=.77, p=.38, Cohen’s f =.08) or RTsd (F(1,115)=1.2, p=.3, Cohen’s f =.1). A main effect of load was significant for accuracy (F(1,115)=157.8, p<.001, Cohen’s f =1.2), with lower accuracy at high load than low load (71.6% vs 84.4%). Responses were faster (F(1,115)=102.6, p<.001, Cohen’s f =.95) and less variable (F(1,115)=11.1, p=.001, Cohen’s f =.30) at low load than high load (RT: 1263ms vs 1406ms; RTsd: 395ms vs 427ms). No other main effects or interactions were significant. A main effect of age (but not interactions), indicating better performance in older children, was significant across metrics: accuracy (F(1,115)=22.4, p<.001, Cohen’s f =.44) (74.2% vs 81.9%), RT (F(1,115)=60.7, p<.001, Cohen’s f =.73) (1485ms vs 1184ms), and RTsd (F(1,115)=12.2, p=.001, Cohen’s f =.33) (441ms vs 381ms).
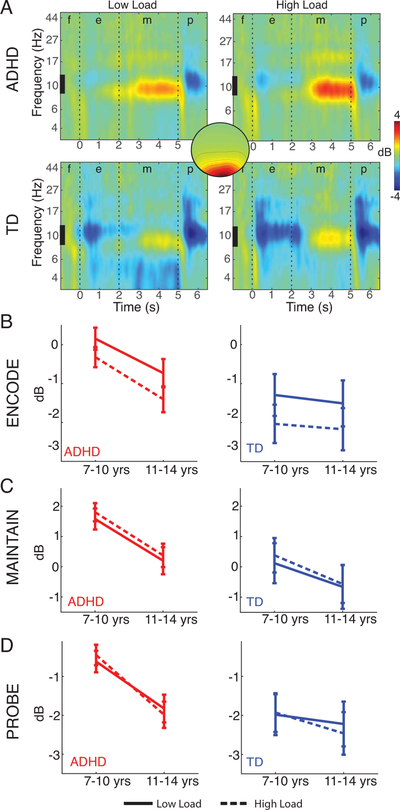
Encoding & Retrieval: Alpha power
Modulation of alpha power, shown in Figure 2, was strongly correlated across task phases (rencodeXprobe(119)=.82, p<.001), indicating a common mechanism (Cronbach’s alpha = .91).
Figure 2.
Mid-occipital alpha (8–12Hz) decreased in power during encoding and probe, but increased in power during maintenance (A). Alpha decreases, consistent with attention processes, were weaker in children with ADHD, whereas alpha increases during maintenance were stronger. Alpha power decreased with age (B-D). Load effects were only significant during the encoding phase (A, B). f=fixation, e=encoding, m=maintenance, p=probe.
Group Effects.
A main effect of GROUP was significant during encoding (F(1,101)=8.3, p=.005, Cohen’s f=.29). Alpha decreased less in children with than without ADHD (−0.6dB vs. −1.8dB) (Fig. 2a). A similar effect was observed during retrieval (F(1,101)=5.2, p<.002, Cohen’s f =.23) (−1.2dB vs. −2.2dB), consistent with the strong correlation in alpha during encoding and retrieval (i.e., the stimulus processing phases). These data suggest that visual attention processes are weakened in children with ADHD.
Age Effects.
A main effect of AGE was not significant during encoding (F(1,101)=1.9, p=.2, Cohen’s f =.14) (Young: −.87dB, Old: −1.45dB), but was significant during retrieval (F(1,101)=30.68, p<.001, Cohen’s f =.39) (Young: −1.25dB, Old: −2.13dB), where alpha power decreased with age (Fig. 2b,d). AGE did not show any significant interactions.
Load Effects.
The effect of LOAD was significant during encoding (F(1,101)=4.2, p=.04, Cohen’s f =.20) but not retrieval (F(1,101)=.11, p=.7, Cohen’s f =.03). During encoding, stronger alpha power decreases were present at high load compared to low load (Low Load: −.85dB, High-Load: −1.5dB). No interactions of load by group were significant.
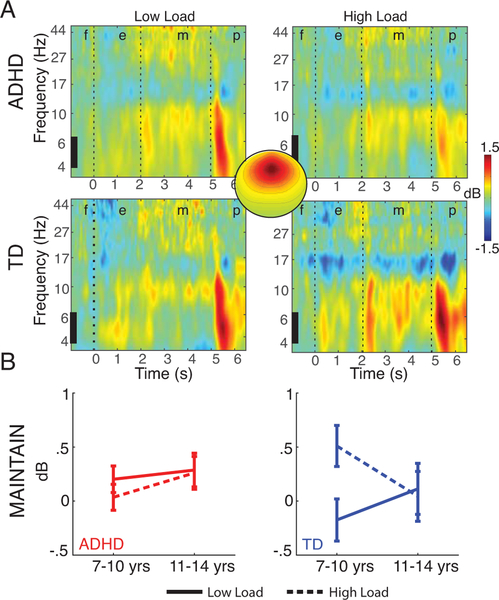
Maintenance: Theta & Alpha power
Occipital-alpha power during maintenance (Fig. 2a,c) was strongly correlated with alpha power during encoding (rencodeXmaintain(101)=.70, p<.001) and retrieval (rprobeXmaintain(101)=.63, p<.001) – suggesting common mechanisms (Cronbach’s alpha = .87 and .82 respectively). However, mid-occipital alpha and mid-frontal theta power (Fig. 3) during maintenance were not correlated to one another (r(101)=.05, p=.6).
Figure 3.
Mid-frontal theta (4–7Hz) increased in power during maintenance, but this effect did not differ by group or load (A), and did not show significant effects of age (B). f=fixation, e=encoding, m=maintenance, p=probe.
Group Effects.
An effect of GROUP was significant in occipital-alpha power (F(1,101)=5.5, p=.02, Cohen’s f =.23) but not frontal-theta power (F(1,110)=.3, p=.6). Alpha power during maintenance was elevated in children with ADHD (−.18dB vs. .99dB, Fig. 2a,c). If we consider weaker alpha modulation during encoding as resulting in a weaker memory trace, then elevated alpha power during maintenance may indicate compensatory occipito-parietal activities serving to inhibit visual input processing in order to protect this weaker trace.
Age Effects.
A main effect of AGE in occipital-alpha power was significant during maintenance (F(1,101)=49, p=.03, Cohen’s f=.22) as alpha power decreased with age during maintenance (Young: .97dB, Old: −.16dB, Fig. 2c). We did not find significant main effects of AGE in theta power during maintenance (F(1,110)=.1, p=.8, Cohen’s f =.03).
Load Effects.
Effects of LOAD, or its interactions, were not significant in neither alpha (F(1,101)=.52, p=.47, Cohen’s f =.07) nor theta power (F(1,110)=1.3, p=.26, Cohen’s f =.11)).
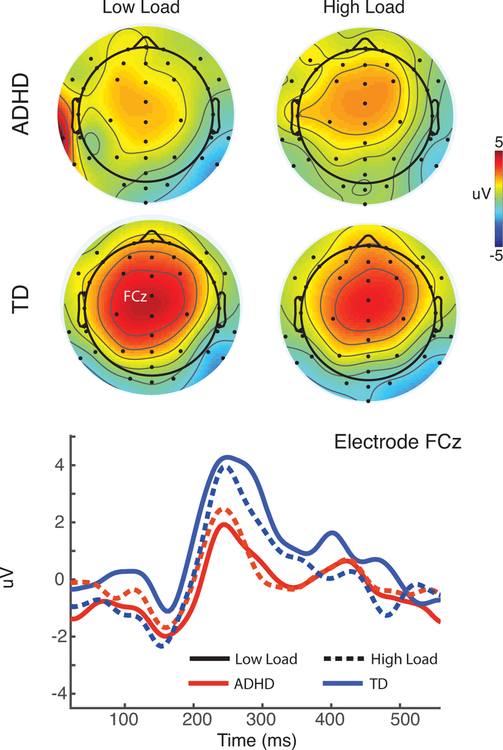
Vigilance: P2 amplitude
In the analysis of mid-frontal P2 amplitude, we did not find any significant effects of load, group or diagnosis. However, because the topography of the mid-frontal IC solution appeared to be different than that of the P2 (Fig. 4), consistent with a different cortical source (c.f., S5 in Supplemental materials), we repeated this analysis in frontal channels. At channel FCz, a main effect of group showed a near-significant trend (F(1,110)=3.6, p=.06, Cohen’s f =.18). Children with ADHD had a lower amplitude P2 during fixation (2.5uV) than TD children (3.9uV). There was a significant interaction between age and group (F(1,110)=4.3, p=.04, Cohen’s f =.20), occurring because the group effect was significant in younger (F(1,67)=9.4, p=.003, Cohen’s f =.38) but not older children (F(1,42)=0.01, p=.99). P2 was not significantly correlated with alpha during stimulus processing (rencoding(119)=−.14, p=.11, rprobe(119)=−.14, p=.13), but was negatively correlated with maintenance alpha (r(119)=−.26, p=.01). It was not correlated with maintenance theta (r(119)=.06, p=.53).
Figure 4.
The fixation cue produced a significant positivity (P2) over central scalp, that was weakened in ADHD relative to TD, consistent with decreased vigilance across trials. This effect was eliminated with age. The P2 did not show effects of load (consistent with load being unknown during the fixation cue).
EEG Prediction of Academic Achievement, Executive Function & Symptoms
The result of the factor analysis (FA) is shown in Table 2. The FA produced four factors (accounting for 73% of variance), with the solution meeting assumptions of both sampling (Kaiser-Meyer-Olkin Measure of Sampling Adequacy=.74) and sphericity (Bartlett’s Test χ2(136)=1768, p<.001). Factor 1 (37.6% variance) identified an “ADHD symptom” dimension, it loaded most strongly (>.75) on SWAN symptoms and CBCL attention scales, with loadings for other measures not exceeding .24. Factor 2 (17.6% variance) captured an “Executive Function” dimension, as it loaded most strongly (>.76) on DKEFS color-word interference performance, with the next highest loading (.39) on DKEFS trails, but less strongly on DKEFS verbal fluency (.28). Factor 3 (10.9% variance), identified a “Basic Reading” skills dimension as it loaded more strongly on reading fluency/rate/accuracy, spelling and sound-letter/word-letter decoding (>.71), than on math ability (.19) or reading comprehension (−.11). In complement, factor 4 (7.0% variance), identified a “Reading Comprehension & Math” dimension, as it loaded most strongly (1.1) on reading comprehension, followed by overall reading ability (.58) and math ability (.34). This factor also loaded on DKEFS Trails task suggesting shared variance with this executive function test.
Table 2.
Rotated Factor Loadings
| Measure | ADHD Symptoms | Executive Function | Basic Reading | Reading Comprehension & Math |
|---|---|---|---|---|
| SWAN inattention | .79 | .16 | −.08 | .08 |
| SWAN hyperactivity | .75 | .08 | −.07 | .06 |
| CBCL Attention Problems | −.86 | .03 | −.12 | .06 |
| CBCL ADHD Symptoms | −.91 | .06 | −.05 | .07 |
| DKEFS Trails (number-letter switch) | .05 | .39 | −.05 | .36 |
| DKEFS VF (switch) | .04 | .28 | −.01 | .14 |
| DKEFS CW (color-word) | .06 | .77 | −.09 | −.07 |
| DKEFS CW (switch) | .02 | .76 | .02 | −.14 |
| GORT (rate) | −.06 | .25 | .71 | .08 |
| GORT (accuracy) | −.02 | .16 | .87 | −.12 |
| GORT (fluency) | −.09 | .26 | .88 | −.06 |
| GORT (comprehension) | −.03 | −.14 | −.11 | 1.1 |
| GORT (oral reading index) | −.08 | .09 | .49 | .58 |
| WRAT4 spelling | .00 | .04 | .82 | −.04 |
| WRAT4 math | .24 | .08 | .19 | .34 |
| WJ (word attack) | .00 | −.30 | .96 | .06 |
| WJ (letter-word ID) | .16 | −.34 | .85 | −.10 |
Notes: CBCL=Child Behavior Checklist; DKEFS=Delis-Kaplan Executive Functions System [VF=verbal fluency; CW=color-word interference]; GORT=Gray Oral Reading Test; SWAN=Strengths and Weaknesses of ADHD symptoms and Normal behavior rating scale (higher indicates fewer attention problems); WJ=Woodcock Johnson IV Achievement Test; WRAT=Wide Range Achievement Test.
The prediction of these factor dimensions by EEG measures is summarized in Table 3. Since alpha measures were highly correlated across task phases, we initially included in the model only alpha power from encoding, as it is sequentially the first cognitive operation of the trial. However, because maintenance processes are theoretically distinct from stimulus processing during encoding, their EEG indicators could capture unique variance. For instance, the occipito-parietal attention network is likely to be engaged in both encoding and in maintenance (consistent with a strong correlation between phases), but during maintenance it would also be engaged in maintenance-specific storage operations that, if impaired in ADHD, should also show a correlation with symptoms. To test this idea, we regressed maintenance alpha power on encoding alpha power and used the residual from this regression to represent unique maintenance alpha variance in the multiple regression (Table 3).
Table 3.
Multiple Regression: EEG Predictors of Outcome Variables
| tβ (regression coefficient) | Model Fit | ||||||
|---|---|---|---|---|---|---|---|
| Alphae | Alpham⊥e | Theta | P2 | Age | R2adj | F | |
| Symptom Factora | −2.1* | 0.5 | 0.3 | 0.6 | −0.4 | .01 | 1.1 |
| Executive Function Factor | −2.1* | 3.0** | 0.2 | 0.6 | 1.2 | .13 | 3.4** |
| Basic Reading Factor | 0.6 | 1.7 | −0.3 | −1.0 | −0.5 | .01 | .53 |
| Reading Comprehension/Math Factor | −2.1* | 1.1 | −0.5 | 0.6 | −1.1 | .01 | .34 |
| Task Accuracy | −2.6** | 0.1 | 1.3 | 0.6 | 4.3*** | .30 | 9.5*** |
| Task Reaction Time | 0.6 | −0.3 | −0.6 | 0.1 | −7.9*** | .44 | 16.9*** |
| Task Reaction Time SD | 0.9 | −0.1 | −0.03 | 1.0 | −4.5*** | .19 | 5.7*** |
| Internal Consistency (αCronbach’s)b | .85/.80 | .82/.84 | .43/.33 | .24/.35 | |||
Notes.
p<.1,
p<.05,
p<.01,
p<.001.
SD=standard deviation.
Higher score indicates fewer attention problems, thus greater alpha decrease during encoding, is associated with fewer ADHD symptoms).
Cronbach’s alpha for each measure calculated on comparison of odd-numbered trials with even-numbered trials within each participant and is reported for low-load/high-load conditions.
Alpha power during encoding was negatively associated with several outcome measures. Namely, a weakening of the typically observed alpha power decreases during encoding was significantly associated with lower task accuracy on the spatial working memory task, elevated ADHD symptoms (factor1), lower scores on executive function tests (factor 2), and weaker reading comprehension/math scores (factor 4), but was not associated with basic reading skills (factor 3). Supplemental analyses (c.f., S7 in Supplemental Materials), revealed that alpha power during encoding was not associated with other symptom scales of the CBCL, suggesting specificity of this indicator to the attention-related outcomes noted above.
Independent of the encoding effects, elevated alpha power during maintenance was associated positively with higher executive function scores (factor 2), but not with symptoms. This finding confirms the hypothesis that there exist maintenance mechanisms contributing to alpha power that are independent from those during encoding alpha, but not that these mechanisms are impaired in ADHD. Rather, the positive correlation with executive function implies that, when group differences are eliminated, increases in alpha power during maintenance are associated with better executive functioning. This finding bolsters the interpretation that higher alpha power during maintenance is a compensatory response.
The P2 and theta variables were not significantly associated with any of the outcome measures. Finally, task performance but not outcome factor dimensions, were predicted by age, with better performance associated with increasing age.
Internal Consistency of Alpha, Theta and P2
Cronbach’s alpha for each metric included in the multiple regression analyses are reported in Table 3 (bottom). Encoding alpha power and orthogonalized alpha power during maintenance had high internal consistency (Cronbach’s alpha > .8), whereas frontal theta and P2 had low internal consistency (Cronbach’s alpha < .45).
Discussion
This study replicates, in an independent sample, previously observed ADHD deficits in encoding and vigilance. It fails to replicate previously observed differences in frontal theta power during maintenance or associated age effects (see S8, Supplemental Materials for sample differences). Additionally, EEG correlates of encoding, but not of maintenance or vigilance, predicted several domains of academic achievement, ADHD symptoms, and executive function, suggesting that attention processes may play an important role in accounting for WM-related neurocognitive outcomes.
Alpha Power & Attentional Health
The decreases in alpha power during encoding and retrieval reflects attentional processes (Foxe & Snyder, 2011; Jensen & Mazaheri, 2010), thought to be critical in lending the visual system access to storage systems (Klimesch, 2012). In complement, increases in alpha power such as during maintenance in the Sternberg task, or during distractor processing in selective attention, are thought to serve an inhibitory role, blocking visual inputs from being processed and/or stored. Evidence from neurophysiological recordings (Bollimunta, Mo, Schroeder, & Ding, 2011) and TMS studies (Capotosto, Babiloni, Romani, & Corbetta, 2009; Romei, Gross, & Thut, 2010), indicate that the neural substrates of alpha modulation include occipito-parietal, attention system interactions. The disruption of alpha power modulation in ADHD, at the group level, can therefore be interpreted as a disruption of attention processes. Moreover, the significant negative correlation between power decreases during encoding and increases during maintenance that we observed, suggest that weak encoding of to-be-stored content, results in compensatory inhibitory effects on visual processing during maintenance – perhaps in an attempt to protect the weakly stored information from interfering visual inputs.
This hypothesis is gaining momentum. A recent review (Lenartowicz et al., 2018) identified 12 published studies since 2009, spanning adult and child samples, to document weakened alpha power modulation during encoding in tasks of cued selective attention (Mazaheri et al., 2010; Yordanova, Kolev, & Rothenberger, 2013), visual interference (i.e., flanker)(Hasler et al., 2016; Heinrich et al., 2014; Mazaheri et al., 2014), spatial working memory (Gomarus, Wijers, Minderaa, & Althaus, 2009; Lenartowicz et al., 2014; Lenartowicz et al., 2016; Missonnier et al., 2013), as well as spatial visual attention (Ter Huurne et al., 2017; ter Huurne et al., 2013; Vollebregt et al., 2016). Consistent with our results, alpha power deficits have been associated with inattentive symptoms, suggesting that this metric is sensitive to typical behavioral dysfunction in ADHD (Gomarus et al., 2009; Lenartowicz et al., 2014; Mazaheri et al., 2014; Ter Huurne et al., 2017).
Clinical Implications for ADHD
Our findings of a relationship between alpha power and neurocognitive outcome measures expands on this interpretation by outlining the scope of influence of visual attention outside of the laboratory. The association of alpha power with reading comprehension, math ability, and ADHD symptoms implies that poor encoding, not maintenance, during SWM contributes to impairments in academics. This is consistent with an absence of evidence to support a maintenance deficit in our study, including no difference in the frontal theta metric during maintenance, and a strong relationship between maintenance and encoding alpha, suggesting that the latter accounts for the former. Furthermore, the associations between alpha power and executive functions supports the possibility that attention deficits mediate executive function deficits in at least some cases (Biederman et al., 2004; Biederman et al., 2006; Loo et al., 2007). The broader consequence of attention processes mediating WM deficits, would be potential variability, not only in WM performance, but also treatment efficacy and long-term outcome in WM within the ADHD population. For instance, training an individual with an encoding deficit on a WM-maintenance computerized training regimen (e.g., Cogmed; Shinaver, Entwistle, & Soderqvist, 2014) would not be expected to be effective, whereas training an individual with a WM maintenance deficit would be effective.
This hypothesis is particularly relevant to recent findings suggesting that past efforts to train WM have been inconsistent because of their focus on training the maintenance (primary memory) component of WM, rather than the ability to manipulate content in WM (secondary memory), which is proposed to correspond to the executive component of WM impaired in ADHD (Chacko et al., 2014; Gibson et al., 2011; Rapport, Orban, Kofler, & Friedman, 2013). Consistent with these reports, our results suggest that WM maintenance is not the process that is critically impaired in WM deficits in ADHD. However, since our task did not include a manipulation component, it is not possible to assess if the attention processes impaired in our sample are analogous to the executive processes associated with WM manipulation. Such an outcome is feasible given similarity of the cognitive processes associated with WM manipulation and alpha power decreases, both of which involve interaction between attention and stored information systems, and similarity in neural systems, as both involve fronto-parieto-occipital interactions. If so, alpha power modulation during encoding should also predict WM manipulation ability.
Limitations
The presented results have several notable limitations. As mentioned above, we tested only three WM components (vigilance, maintenance, encoding), and thus cannot speak to a critical target in WM deficits in ADHD, namely, content manipulation. The task also tested only recognition, not recall, which may explain the sparsity of load effects. Our results, however, offer a novel prediction regarding alpha modulation being a correlate of WM manipulation. Furthermore, our study does not assess causal relationships between the studied components and so it is not possible to evaluate if the encoding deficits observed are causally responsible for neurocognitive outcomes. They are unlikely to be the sole predictors of neurocognitive outcomes given the small effect sizes (Cohen’s d=.2) of the regression coefficients. However, while the effects are small, they are meaningful, and, as measures, internally consistent (Cronbach’s alpha > .8). Encoding processes during WM were impaired in ADHD, were coupled to alpha power modulation, and, unlike WM maintenance or vigilance processes, had implications outside of the laboratory via their relationship to academic performance and executive function.
Supplementary Material
Appendix S1. Sample diagnostics.
Appendix S2. Independent Component Analysis.
Appendix S3. Sample characteristics.
Appendix S4. Single electrode event-related spectral analyses.
Appendix S5. Dipole analysis P2.
Appendix S6. Full-scale IQ covariate in EEG prediction of academic achievement, executive function & symptoms.
Appendix S7. EEG prediction of CBCL scales.
Appendix S8. Sample differences.
Appendix S9. Variable correlations.
Key points.
Working memory, impaired in ADHD, has been associated with distinct component process including encoding, maintenance and vigilance.
Replicating earlier findings in an independent sample, the largest effects were present during encoding, in weakened alpha-range (8–12Hz) oscillations, a correlate of attention processes.
Alpha power predicted reading comprehension subscales of academic achievement, ADHD symptoms and executive function, highlighting the importance of attention to neurocognitive outcomes.
Previously reported deficits in frontal theta power, during working memory maintenance, were not replicated, whereas deficits in vigilance were seen only among younger but not older children.
Acknowledgements
This research was supported by a Klingenstein Third Generation Foundation ADHD Fellowship (AL), the Tennenbaum Family Center for the Biology of Creativity (RMB), and by the National Institutes of Health grants MH092829 (SKL), NS047293 (SM), and P50 MH-077248 (JTM). The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting information
Additional Supporting Information may be found online in the Supporting Information section at the end of this article.
Conflict of interest statement: No conflicts declared.
References
- Achenbach TM (2000). Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry. [Google Scholar]
- Baddeley A (1986). Working Memory. New York: Oxford University Press. [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, … Faraone SV (2004). Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol, 72(5), 757–766. doi: 10.1037/0022-006X.72.5.757 [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty C, Fried R, Fontanella J, Doyle AE, Seidman LJ, & Faraone SV (2006). Impact of psychometrically defined deficits of executive functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry, 163(10), 1730–1738. doi: 10.1176/ajp.2006.163.10.1730 [DOI] [PubMed] [Google Scholar]
- Biederman J, & Spencer T (1999). Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry, 46(9), 1234–1242. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Loo SK, McGough JJ, Whelan F, Hellemann G, Sugar C, … McCracken JT (2016). Cognitive Effects of Stimulant, Guanfacine, and Combined Treatment in Child and Adolescent Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry, 55(8), 667–673. doi: 10.1016/j.jaac.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, & Ding M (2011). Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci, 31(13), 4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, & Buitelaar JK (2005). Executive functioning in adult ADHD: a meta-analytic review. Psychological Medicine, 35(8), 1097–1108. doi:Doi 10.1017/S003329170500499x [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, & Corbetta M (2009). Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci, 29(18), 5863–5872. doi:29/18/5863 [pii] 10.1523/JNEUROSCI.0539-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience, 3(8), 617–628. doi:Doi 10.1038/Nrn896 [DOI] [PubMed] [Google Scholar]
- Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A, … Ramon M (2014). A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. J Child Psychol Psychiatry, 55(3), 247–255. doi: 10.1111/jcpp.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic. [Google Scholar]
- D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, & Cohen JD (2012). Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences of the United States of America, 109(49), 19900–19909. doi:Doi 10.1073/Pnas.1116727109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. doi:Doi 10.1016/J.Jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, & Snyder AC (2011). The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol, 2, 154. doi: 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BS, Gondoli DM, Johnson AC, Steeger CM, Dobrzenski BA, & Morrissey RA (2011). Component analysis of verbal versus spatial working memory training in adolescents with ADHD: a randomized, controlled trial. Child Neuropsychol, 17(6), 546–563. doi: 10.1080/09297049.2010.551186 [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, … Cannon TD (2002). Maintenance and manipulation in spatial working memory: Dissociations in the prefrontal cortex. NeuroImage, 17(1), 201–213. doi:Doi 10.1006/Nimg.2002.1161 [DOI] [PubMed] [Google Scholar]
- Gomarus HK, Wijers AA, Minderaa RB, & Althaus M (2009). Do children with ADHD and/or PDD-NOS differ in reactivity of alpha/theta ERD/ERS to manipulations of cognitive load and stimulus relevance? Clin Neurophysiol, 120(1), 73–79. doi: 10.1016/j.clinph.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Hasler R, Perroud N, Meziane HB, Herrmann F, Prada P, Giannakopoulos P, & Deiber MP (2016). Attention-related EEG markers in adult ADHD. Neuropsychologia, 87, 120–133. doi: 10.1016/j.neuropsychologia.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Heinrich H, Busch K, Studer P, Erbe K, Moll GH, & Kratz O (2014). EEG spectral analysis of attention in ADHD: implications for neurofeedback training? Front Hum Neurosci, 8, 611. doi: 10.3389/fnhum.2014.00611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, & Mazaheri A (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci, 4, 186. doi: 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci, 16(12), 606–617. doi: 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, & Kolomeyer EG (2013). Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin Psychol Rev, 33(6), 795–811. doi: 10.1016/j.cpr.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Lee TW, Girolami M, & Sejnowski TJ (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computation, 11(2), 417–441. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Delorme A, Walshaw PD, Cho AL, Bilder RM, McGough JJ, … Loo SK (2014). Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J Neurosci, 34(4), 1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Escobedo-Quiroz R, & Cohen JD (2010). Updating of context in working memory: An event-related potential study. Cognitive Affective & Behavioral Neuroscience, 10(2), 298–315. doi:Doi 10.3758/Cabn.10.2.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Lu S, Rodriguez C, Lau EP, Walshaw PD, McCracken JT, … Loo SK (2016). Alpha desynchronization and fronto-parietal connectivity during spatial working memory encoding deficits in ADHD: A simultaneous EEG-fMRI study. Neuroimage Clin, 11, 210–223. doi: 10.1016/j.nicl.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Mazaheri A, Jensen O, & Loo SK (2018). Aberrant Modulation of Brain Oscillatory Activity and Attentional Impairment in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 3(1), 19–29. doi: 10.1016/j.bpsc.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Bilder RM, Cho AL, Sturm A, Cowen J, Walshaw P, … McCracken JT (2016). Effects of d-Methylphenidate, Guanfacine, and Their Combination on Electroencephalogram Resting State Spectral Power in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry, 55(8), 674–682 e671. doi: 10.1016/j.jaac.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Humphrey LA, Tapio T, Moilanen IK, McGough JJ, McCracken JT, … Smalley SL (2007). Executive functioning among Finnish adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 46(12), 1594–1604. doi: 10.1097/chi.0b013e3181575014 [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS, & Corbett BA (2010). Functional Disconnection of Frontal Cortex and Visual Cortex in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 67(7), 617–623. doi:Doi 10.1016/J.Biopsych.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Fassbender C, Coffey-Corina S, Hartanto TA, Schweitzer JB, & Mangun GR (2014). Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol Psychiatry, 76(5), 422–429. doi: 10.1016/j.biopsych.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, McGough JJ, Loo SK, Levitt J, Del’Homme M, Cowen J, … Bilder RM (2016). Combined Stimulant and Guanfacine Administration in Attention-Deficit/Hyperactivity Disorder: A Controlled, Comparative Study. J Am Acad Child Adolesc Psychiatry, 55(8), 657–666 e651. doi: 10.1016/j.jaac.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missonnier P, Hasler R, Perroud N, Herrmann FR, Millet P, Richiardi J, … Baud P (2013). EEG anomalies in adult ADHD subjects performing a working memory task. Neuroscience, 241, 135–146. doi: 10.1016/j.neuroscience.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev, 33(8), 1237–1252. doi: 10.1016/j.cpr.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, & Thut G (2010). On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci, 30(25), 8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA (2005). Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biological Psychiatry, 57(11), 1248–1255. doi:Doi 10.1016/J.Bps.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Shinaver CS 3rd, Entwistle PC, & Soderqvist S (2014). Cogmed WM training: reviewing the reviews. Appl Neuropsychol Child, 3(3), 163–172. doi: 10.1080/21622965.2013.875314 [DOI] [PubMed] [Google Scholar]
- Swanson J, Schuck S, Mann M, Carlson C, Hartman K, Sergeant J, … McClearly R (2006). Categorical and dimensional definitions and evaluations of symptoms of ADHD: the SNAP and SWAN rating scales. University of California, Irvine. [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2007). Using multivariate statistics, 5th ed. Boston, MA: Allyn & Bacon/Pearson Education. [Google Scholar]
- Ter Huurne N, Lozano-Soldevilla D, Onnink M, Kan C, Buitelaar J, & Jensen O (2017). Diminished modulation of preparatory sensorimotor mu rhythm predicts attention-deficit/hyperactivity disorder severity. Psychol Med, 47(11), 1947–1956. doi: 10.1017/S0033291717000332 [DOI] [PubMed] [Google Scholar]
- ter Huurne N, Onnink M, Kan C, Franke B, Buitelaar J, & Jensen O (2013). Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol Psychiatry, 74(3), 227–233. doi: 10.1016/j.biopsych.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Vollebregt MA, Zumer JM, Ter Huurne N, Buitelaar JK, & Jensen O (2016). Posterior alpha oscillations reflect attentional problems in boys with Attention Deficit Hyperactivity Disorder. Clin Neurophysiol, 127(5), 2182–2191. doi: 10.1016/j.clinph.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry, 57(11), 1336–1346. doi: 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, & Rothenberger A (2013). Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder. Suppl Clin Neurophysiol, 62, 289–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Sample diagnostics.
Appendix S2. Independent Component Analysis.
Appendix S3. Sample characteristics.
Appendix S4. Single electrode event-related spectral analyses.
Appendix S5. Dipole analysis P2.
Appendix S6. Full-scale IQ covariate in EEG prediction of academic achievement, executive function & symptoms.
Appendix S7. EEG prediction of CBCL scales.
Appendix S8. Sample differences.
Appendix S9. Variable correlations.