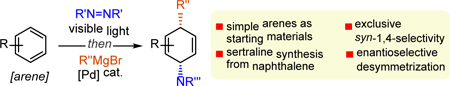
Abstract
A protocol for palladium-catalyzed dearomative functionalization of simple, non-activated arenes with Grignard reagents has been established. This one-pot method features a visible-light-mediated [4+2] cycloaddition between an arene and an arenophile, and subsequent palladium-catalyzed allylic substitution of the resulting cycloadduct with Grignard reagent. A variety of arenes and Grignard reagents can participate in this process, forming carboaminated products with exclusive syn-1,4-selectivity. Moreover, the dearomatized products are amenable to further elaborations, providing functionalized alicyclic motifs and pharmacophores. For example, naphthalene was converted to sertraline, one of the most prescribed antidepressants, in only four operations. Finally, this process could also be conducted in an enantioselective fashion, as demonstrated with the desymmetrization of naphthalene.
A novel dearomative process is described using arenophile-based chemistry and Pd catalysis. A range of arenes were readily converted into the corresponding syn-1,4-carboaminated products using Grignard reagents as nucleophiles. The synthetic value of this transformation was demonstrated by several elaborations of products, including short synthesis of sertraline from naphthalene.
Keywords: arene, arenophile, catalytic dearomatization, carboamination, photochemistry
Graphical Abstract
Aromatic compounds are often considered as precursors for the synthesis of alicyclic hydrocarbon frameworks, exemplified by the developments of numerous versatile dearomatization processes.[1] In addition to venerable dearomative reductions[2] and oxidative dearomatization of phenols[3], a wide range of dearomative transformations have been developed, enabling the construction of stereochemically complex cyclic structures that are otherwise difficult to prepare.[4] Thus, these processes have also provided significant advantages in expediting syntheses of natural products and bioactive compounds.[5] Nevertheless, despite these notable advancements in the field, there still remains several challenges to be addressed. For instance, the catalytic dearomatization of arenes has gained much attention in recent years,[6] whereas the application of such transformations has largely been limited to specific classes of activated arenes such as naphthols,[7] naphthylamines,[8] heteroarenes,[9] and arenes bearing benzylic heteroatoms.[10] Dearomative conversions of simple, non-activated arenes in a catalytic manner are rare, especially the variants which concurrently introduce functionality other than hydrogen.[11]
Recently, we have documented several dearomative functionalization methods based on in situ formation of arene–arenophile cycloadduct (I) via visible-light-mediated [4+2] cycloaddition,[12] and subsequent elaborations (Figure 1A).[13] Using N-methyl-1,2,4-triazoline-3,5-dione (MTAD, 1) as an arenophile, we have demonstrated transition metal-catalyzed substitutions of the resulting cycloadducts to access highly functionalized cyclohexane derivatives. Interestingly, the products obtained from Ni-[14] and Pd-catalyzed[15] nucleophilic substitutions exhibited complementary selectivity, providing exclusive access to anti-1,2- and syn-1,4-aminofunctionalized motifs, respectively (Figure 1A). The observed regiochemical outcomes are likely due to the difference in preferred structure of the cationic metal intermediates. Specifically, the Ni-complex forms the η5-cyclohexadienyl-like intermediate (II) with greater positive charge localized on the termini of the η5-system,[14a] while the Pd-complex adopts the η3-allyl-like structure (III) with incoming nucleophile reacting at the remote site from the urazole.[16] The observed stereoselectivity was in accordance with the general trends of transition metal-catalyzed allylic substitution.[17] For example, Grignard reagents were amenable to Ni-catalyzed anti-1,2-carboamination,[14] while Li-enolates[15a] and amines[15b] provided syn-1,4-aminofunctionalization under Pd-catalysis.
Figure 1.
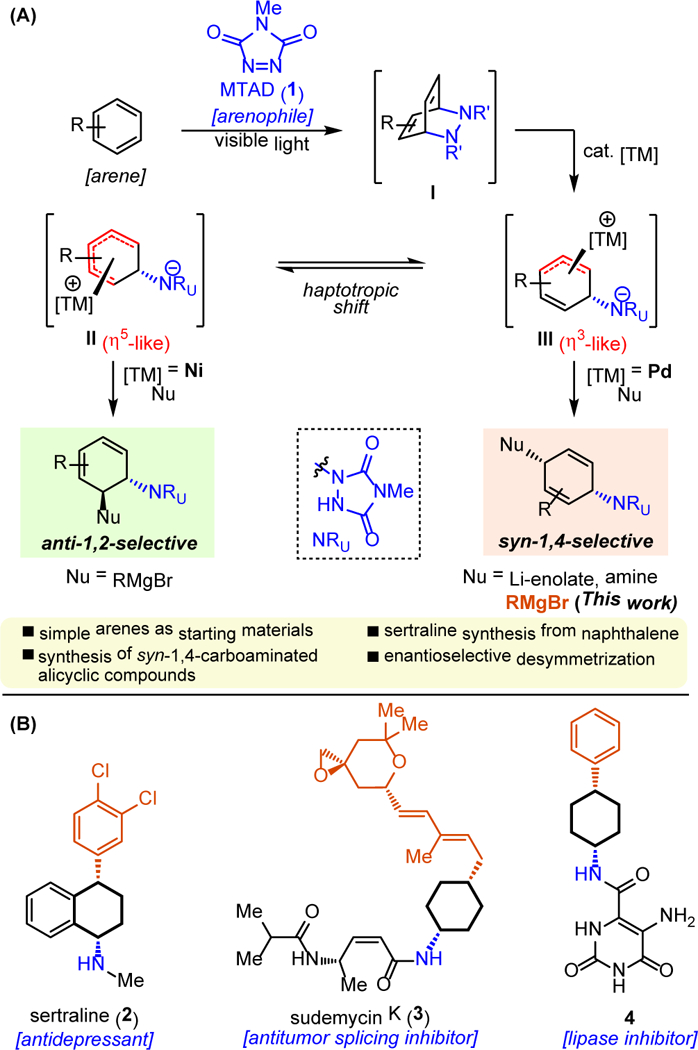
(A) Transition metal-catalyzed arenophile-mediated dearomative aminofunctionalizations. (B) Examples of bioactive compounds with syn-1,4-carboaminated cyclohexane scaffold.
To further extend the utility of these arenophile-mediated catalytic dearomative processes, we decided to test Grignard reagents in combination with Pd catalysis. Gratifyingly, preliminary experiments revealed complementary selectivity compared to Ni-catalysis, as Pd-derived carboaminated products were formed with the exclusive syn-1,4-selectivity. Importantly, this type of cyclohexane derivatives with syn-1,4-carboaminated pattern are commonly found in natural products and bioactive compounds, exemplified by the antidepressant sertraline (2),[18] antitumor splicing inhibitor sudemycin K (3),[19] and lipase inhibitor tetrahydropyrimidinedione 4 (Figure 1B).[20] Therefore, considering the general need to access these important structural motifs, we disclose herein the use of Grignard reagents in Pd-catalyzed dearomative carboaminations with simple arenes, which delivered products with syn-1,4-selectivity. A wide variety of commercially available and simple arenes, as well as Grignard reagents were amenable to this process. Moreover, the synthetic utility of the method was demonstrated by several elaborations of the resulting unsaturated products, as well as the concise synthesis of sertraline from naphthalene. Finally, the proof-of-concept was established for enantioselective desymmetrization using naphthalene or its MTAD cycloadduct.
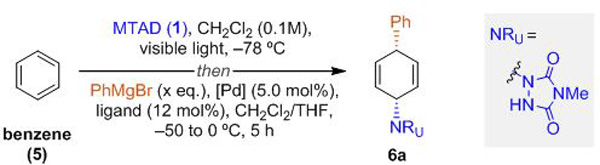
Our investigation began by employing benzene (5), MTAD (1), and phenylmagnesium bromide with different palladium complexes as catalysts (Table 1). Accordingly, when the cycloaddition was conducted in dichloromethane at cryogenic temperature (–78 ºC), followed by the addition of PhMgBr and Pd(PPh3)4 as the catalyst, and slow warming of the resulting mixture from to 0 ºC over 5 h, we observed formation of the syn-1,4-carboaminated product 6a in 35% yield (entry 1, see Table 2 for the X-ray of this compound). Importantly, other constitutional- and diastereoisomers were not observed by 1H NMR analysis of the crude mixture. This net-retentive stereochemical outcome was unexpected, considering that double inversion is generally observed in transition metal-catalyzed allylic substitutions with non-stabilized (hard) nucleophiles through transmetalation and subsequent inner-sphere reductive elimination.[17] Although, there are a few reports of net-retentive Pd-catalyzed allylic substitution with relatively hard nucleophiles,[21] to the best of our knowledge, there are no examples using Grignard reagents.[22]
Table 1.
Optimization of reaction conditions.[a]
 | ||||
|---|---|---|---|---|
| entry | x (eq.) | [Pd] | ligand | yield (%)[b] |
| 1 | 2.5 | Pd(PPh3)4 | – | 35 |
| 2 | 2.5 | Pd(dba)2 | dppf | <5 |
| 3 | 2.5 | Pd(dba)2 | P(p-FC6H4)3 | 34 |
| 4 | 2.5 | Pd(dba)2 | P(p-MeC6H4)3 | <5 |
| 5 | 2.5 | Pd(dba)2 | P(o-MeC6H4)3 | 63 |
| 6 | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 63 |
| 7[c] | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 61 |
| 8[c,d] | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 78 (75) |
Reaction conditions: benzene (5, 5.0 mmol, 10 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.1 M), visible light, –78 ºC; then addition of PhMgBr (3.0 M in Et2O), [Pd] catalyst in THF.
Determined by 1H NMR analysis relative to the internal standard. Isolated yield shown in parentheses.
CH2Cl2 (0.2 M).
Substitution step was conducted from –50 to 15 ºC over 10 h.
Table 2.
Grignard reagent scope for Pd-catalyzed dearomative syn-1,4-carboamination.[a]
 |
|---|
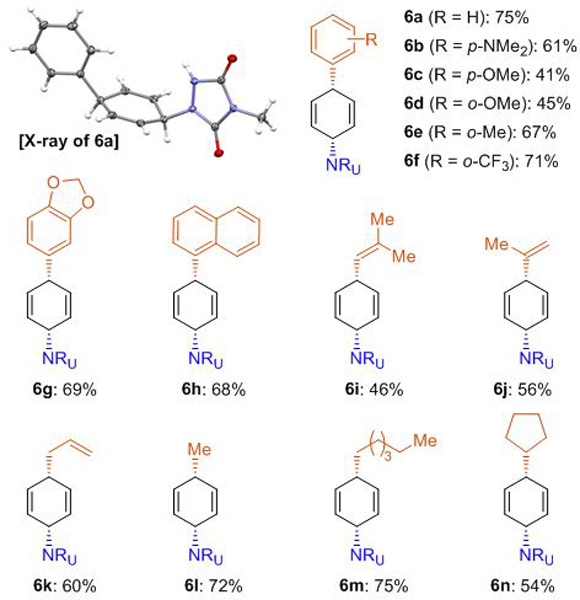 |
Reaction conditions: benzene (5, 5.0 mmol, 10 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.2 M), visible light, –78 ºC; then addition of Grignard reagents (1.5 eq. in Et2O or THF), Pd(dba)2/P(o-MeC6H4)3 (5.0 mol%/12 mol%) in THF, –50 to 15 ºC, 10 h. Yields of isolated product after column chromatography.
Further optimization of the reaction conditions revealed that monodentate phosphines in combination with Pd(dba)2 performed better that bidentate, with P(o-MeC6H4)3 being identified as an optimal ligand (entries 2–5, see Supporting Information for full details). With this result in hand, the reaction parameters were examined next. Accordingly, decreasing the amount of PhMgBr, or increasing the solvent concentration to 0.2 M had minimal effect on the reaction outcome (entries 6–7). On the other hand, increasing the reaction temperature during the substitution step from –50 to 15 ºC over 10 h, rather than up to 0 ºC over 5 h, had significant impact in improving the overall yield, as 75% isolated yield of the desired syn-1,4-carboaminated product 6a was obtained (entry 8). Importantly, 1H-NMR analysis of the crude reaction mixture did not reveal any detectable side-products.
With optimized conditions in hand, the scope of the Grignard reagent was evaluated with benzene as an archetypical nonactivated aromatic substrate (Table 2). Accordingly, a variety of mononuclear arylmagnesium bromides bearing both electron-rich and deficient functional groups, as well as sterically encumbering o-substituents were well tolerated (6a–6f). Also, other aryl Grignard reagents, such as 3,4-methylenedioxyphenyl- and 1-naphthylmagnesium bromide proved to be viable substrates (6g, 6h). In addition to arenes, vinyl groups with different degrees of substitution (6i, 6j), allyl- (6k), and alkyl motifs (6l–6n) could also be installed with this dearomative carboamination. Finally, in all cases, the products were obtained as a single constitutional- and diastereoisomer with exclusive syn-1,4-selectivity.
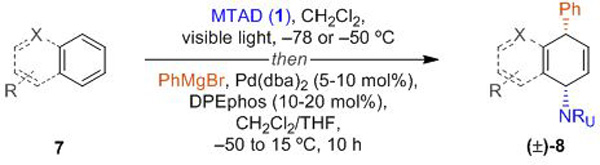
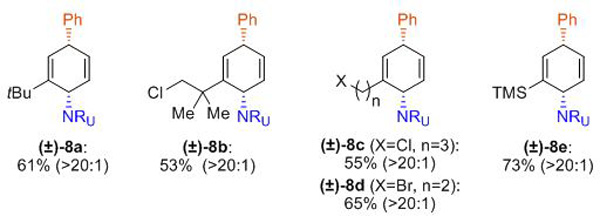
Next, we turned our attention towards the evaluation of the scope of the arenes (Table 3). In contrast to the optimized conditions for benzene, bis[(2-diphenylphosphino)phenyl] ether (DPEphos) turned out to be the most effective ligand for substituted arenes. Monosubstituted arenes bearing alkyl-, alkylhalide, or silyl substituents were well tolerated in this process, providing products as single constitutional isomers (8a–8e). Additionally, carboamination of naphthalene proceeded smoothly to form product 8f, of which the relative stereochemistry was unambiguously determined by an X-ray analysis. Several other polynuclear arenes such as mono- and disubstituted naphthalenes (8g–8i), as well bromoquinoline (8k), delivered desired products with high selectivities of constitutional isomers, albeit with diminished yields.[23] On the other hand, described carboamination of phenanthrene gave a mixture of two constitutional isomers (8j and 8j’).
Table 3.
Arene scope of Pd-catalyzed dearomative syn-1,4-carboamination.[a]
 |
|---|
 |
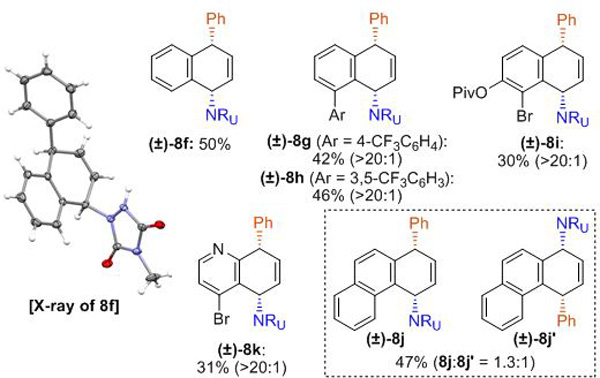 |
Reaction conditions for mononuclear arene: arene 7 (5.0 mmol, 10 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.2 M), visible light, –78 ºC; then addition of PhMgBr (1.5 eq., 3.0 M in Et2O), Pd(dba)2/DPEphos (5.0 mol%/10 mol%) in THF, –50 to 15 ºC, 10 h. Reaction conditions for polynuclear arene: arene 7 (1.0 mmol, 2.0 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.1 M), visible light, –50 ºC; then addition of PhMgBr (1.5 eq., 3.0 M in Et2O), Pd(dba)2/DPEphos (10 mol%/20 mol%) in THF, –50 to 15 ºC, 10 h. Yields of isolated product after column chromatography. Ratio of the constitutional isomers are determined by 1H NMR analysis of the crude reaction mixture (shown in parenthesis).
The described dearomative syn-1,4-carboamination provides novel access to a diverse array of functionalized alicyclic structures from simple arenes (Figure 2). For example, naphthalene-derived product 8f was successfully converted to the corresponding amine 9,[24] dihydronaphthalenes 10 and 11, or to ketone 12, and α–chloroketone 13 using standard chemistry. Moreover, this method also enabled facile preparation of compounds of medicinal significance. Specifically, we showcase the utility of this Grignard-mediated syn-1,4-carboamination by preparing the antidepressant agent sertraline (2) from naphthalene (Figure 3).[25] Thus, a dearomative carboamination with naphthalene and 3,4-dichlorophenylmagnesium bromide, followed by a chemoselective reduction of the alkene, provided sertraline precursor 15. At this stage, the urazole was fragmented into urea 16 by N-alkylation with α–bromoacetophenone and subsequent basic cleavage.[24] Finally, the reduction of the urea moiety with LiAl(OMe)3H[25b] provided the requisite amine functionality to complete the synthesis of sertraline (2). Importantly, by using this fragmentation/reduction maneuver, the arenophile moiety served as a masked equivalent of methylamine.
Figure 2.
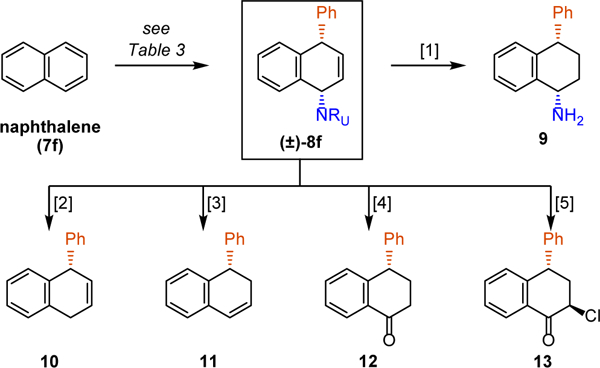
Diversification of product 8f. Reagents and conditions [1] (i) Rh/Al2O3 (cat.), H2, 96%; (ii) α–bromoacetophenone, K2CO3, 56%; (iii) KOH, 95%; [2] Li, NH3, 83%; [3] (i) Rh/Al2O3 (cat.), H2, 96%; (ii) HCl, 59%; [4] (i) Rh/Al2O3 (cat.), H2, 96%; (ii) tBuOCl, AcOH, 73%; [5] (i) Rh/Al2O3 (cat.), H2, 96%; (ii) tBuOCl, 68%.
Figure 3.
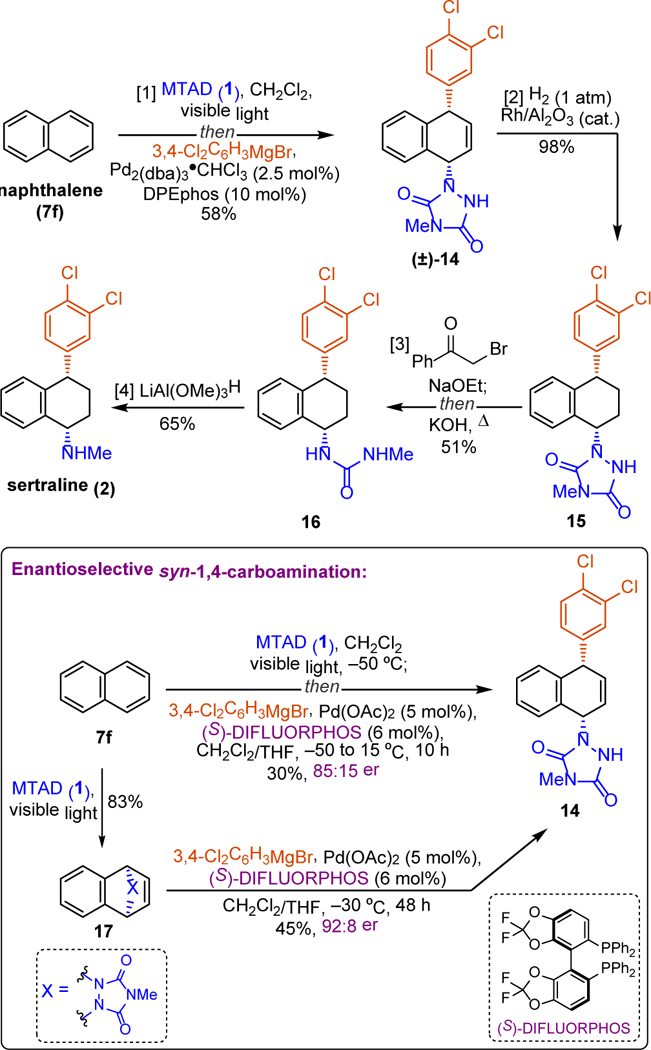
Synthesis of sertraline (2) from naphthalene (7f) and establishment of enantioselectivity of dearomative syn-1,4-carboamination.[a] Reagents and conditions: [1] naphthalene (7f, 1.0 mmol, 2.0 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.1 M), visible light, –50 ºC; then addition of PhMgBr (2.0 eq., 2.0 M in THF), Pd2(dba)3·CHCl3/DPEphos (10 mol%/20 mol%) in THF, –50 to 15 ºC, 10 h, 58%. [2] Rh/Al2O3 (cat.), H2, 98%. [3] α-bromoacetophenone, NaOEt, EtOH, then KOH, 51%. [4] LiAl(OMe)3H, 65%.
We have also examined if this dearomative carboamination process could be conducted in an enantioselective fashion (Figure 3, inset). Considering that the MTAD-naphthalene cycloadduct could undergo desymmetrization, a range of chiral ligands were evaluated under our standard conditions using 3,4-dichlorophenylmagnesium bromide as a nucleophile (see Supporting information for full details). The highest levels of enantioinduction were obtained with (S)-DIFLUORPHOS, delivering product 14 with 85:15 er, albeit with the modest yield. Though extensive optimization did not provide any further improvements, we did observe higher yield and selectivity when we carried out the carboamination as a two-step protocol. Accordingly, when the isolated naphthalene-derived cycloadduct 17 was employed directly, improvements in both the product yield and the enantiopurity were achieved (45% yield, 92:8 er).
In conclusion, we have developed a Pd-catalyzed dearomative syn-1,4-carboamination of simple arenes with Grignard reagents. Not only was the scope of Grignard nucleophiles broad, but a wide variety of mononuclear- and polynuclear arenes were also amenable to this process. The synthetic utility of the syn-1,4-carboaminated products was demonstrated through a series of product diversifications, showcasing how arenophile motif (urazole) could be converted into an amine, a ketone, or be reduced or eliminated. Importantly, the synthetic value of the reported method was highlighted by a concise synthesis of sertraline starting from the simple hydrocarbon naphthalene. Finally, also an asymmetric variant of this dearomatization strategy was established. Further mechanistic studies and applications of this transformation are ongoing and will be reported in due course.
Supplementary Material
Acknowledgements
Financial support for this work was provided by the University of Illinois, the National Science Foundation (CAREER Award No. CHE-1654110), the NIH/National Institute of General Medical Sciences (R01 GM122891), and the donors of the American Chemical Society Petroleum Research Fund (PRF#57175-DNI1). D.S. is an Alfred P. Sloan Fellow. M.O. thanks the Honjo International Scholarship Foundation. Solvias AG is acknowledged for a generous gift of chiral ligands. We also thank Dr. D. Olson and Dr. L. Zhu for NMR spectroscopic assistance, Dr. D. L. Gray and Dr. T. Woods for X-ray crystallographic analysis assistance, and F. Sun for mass spectrometric assistance.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].For recent reviews on synthetic utility of dearomatization chemistry, see: a) Pape AR, Kaliappan KP, Kündig EP, Chem. Rev. 2000, 100, 2917–2940; [DOI] [PubMed] [Google Scholar]; b) Rosillo M, Dominguez G, Pérez-Castells J, Chem. Soc. Rev. 2007, 36, 1589–1604; [DOI] [PubMed] [Google Scholar]; c) Zhuo C-X, Zhang W, You S-L, Angew. Chem. Int. Ed. 2012, 51, 12662–12686; Angew. Chem. 2012, 124, 12834–12858; [DOI] [PubMed] [Google Scholar]; d) Remy R, Bochet CG, Chem. Rev. 2016, 116, 9816–9849; [DOI] [PubMed] [Google Scholar]; e) Liebov BK, Harman WD, Chem. Rev. 2017, 117, 13721–13755; [DOI] [PubMed] [Google Scholar]; f) Wertjes WC, Southgate EH, Sarlah D, Chem. Soc. Rev. 2018, 47, 7996–8017. [DOI] [PubMed] [Google Scholar]
- [2].For selected reviews on dearomative reductions, see: a) Rabideau PW, Marcinow Z, Org. React. 1992, 42, 1; [Google Scholar]; b) Foubelo F, Yus, M. M Reduction/Hydrogenation of Aromatic Rings, In Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds, (Ed.: Mortier J), Wiley, Hoboken, 2015, 337–364; [Google Scholar]; c) Stanislaus A, Cooper BH, Catal. Rev. Sci. Eng. 1994, 36, 75–123; [Google Scholar]; d) Wang D-S, Chen Q-A, Lu S-M, Zhou Y-G, Chem. Rev. 2012, 112, 2557–2590. [DOI] [PubMed] [Google Scholar]
- [3].L. Pouységu, D. Deffieux, S. Quideau, S. Tetrahedron 2010, 66, 2235–2261. For recent examples: a) M. Uyanik N Sasakura M Mizuno M; Ishihara K ACS Catal. 2017, 7, 872–876; [Google Scholar]; b) Uyanik M, Mutsuga T, Ishihara K, Angew. Chem. Int. Ed. 2017, 56, 3956–3960; Angew. Chem. 2017, 129, 4014–4018. [DOI] [PubMed] [Google Scholar]
- [4].For recent examples, see: a) Phipps RJ, Toste FD, J. Am. Chem. Soc. 2013, 135, 1268–1271; [DOI] [PubMed] [Google Scholar]; b) Wiesenfeldt MP, Nairoukh Z, Li W, Glorius F, Science 2017, 357, 908–912; [DOI] [PubMed] [Google Scholar]; c) Nakayama H, Harada S, Kono M, Nemoto T, J. Am. Chem. Soc. 2017, 139, 10188–10191; [DOI] [PubMed] [Google Scholar]; d) Farndon JJ, Ma X, Bower JF, J. Am. Chem. Soc. 2017, 139, 14005–14008; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Good SN, Sharpe RJ, Johnson JS, J. Am. Chem. Soc. 2017, 139, 12422–12425; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) James MJ, Schwarz JL, Strieth-Kalthoff F, Wibbeling B, Glorius F, J. Am. Chem. Soc. 2018, 140, 8624–8626. [DOI] [PubMed] [Google Scholar]
- [5].Roche SP, Porco JA Jr., Angew. Chem. Int. Ed. 2011, 50, 4068–4093; Angew. Chem. 2011, 123, 4154–4179; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Zheng C, You S-L, Chem, 2016, 1, 830–857; [Google Scholar]; b) Asymmetric Dearomatization Reactions. Ed. S.-L. You, Wiley-VCH, 2016, 1–346. Also see reference 1c. [Google Scholar]
- [7].For selected recent examples involving dearomatization of naphthols and phenols, see: a) Oguma T, Katsuki T, J. Am. Chem. Soc. 2012, 134, 20017–20020; [DOI] [PubMed] [Google Scholar]; b) Zhuo C-X, You S.-Li., Angew. Chem. Int. Ed. 2013, 52, 10056–10059; Angew. Chem. 2013, 125, 10240–10243; [Google Scholar]; c) Zheng J, Wang S-B, Zheng C, You S-L, J. Am. Chem. Soc. 2015, 137, 4880–4883; [DOI] [PubMed] [Google Scholar]; d) Wang S-G, Yin Q, Zhuo C-X, You S-L, Angew. Chem. Int. Ed. 2015, 54, 647–650; Angew. Chem. 2015, 127, 657–660; [DOI] [PubMed] [Google Scholar]; e) Du K, Guo P, Chen Y, Cao Z, Wang Z, Tang W, Angew. Chem. Int. Ed. 2015, 54, 3033–3037; Angew. Chem. 2015, 127, 3076–3079; [DOI] [PubMed] [Google Scholar]; f) Xu R-Q, Yang P, Tu H-F, Wang S-G, You S-L, Angew. Chem. Int. Ed. 2016, 55, 15137–15141; Angew. Chem. 2016, 128, 15361–15365; [DOI] [PubMed] [Google Scholar]; g) Wang D-C, Xie M-S, Guo H-M, Qu G-R, Zhang M-C, You S-L, Angew. Chem. Int. Ed. 2016, 55, 14111–14115; Angew. Chem. 2016, 128, 14317–14321; [DOI] [PubMed] [Google Scholar]; h) Zuo Z, Wang H, Fan L, Liu J, Wang Y, Luan X, Angew. Chem. Int. Ed. 2017, 56, 2767–2771; Angew. Chem. 2017, 129, 2811–2815; i) Tan B, Bai L, Ding P, Liu J, Wang Y, Luan X, Angew. Chem. Int. Ed. 2019, 58, 1474–1478; Angew. Chem. 2019, 131, 1488–1492. [Google Scholar]
- [8].For selected recent examples of dearomatization of naphthylamine, see: a) García-Fortanet J, Kessler F, Buchwald SL, J. Am. Chem. Soc. 2009, 131, 6676–6677; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shibuya T, Noguchi K, Tanaka K, Angew. Chem. Int. Ed. 2012, 51, 6219–6222; Angew. Chem. 2012, 124, 6323–6326. [DOI] [PubMed] [Google Scholar]
- [9].For example, see: a) Trost BM, Quancard J, J. Am. Chem. Soc. 2006, 128, 6314–6315; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fernández-Ibáñez MÁ, Marciá B, Pizzuti MG, Minnaard A, Feringa BL, Angew. Chem., Int. Ed. 2009, 48, 9339–9341; Angew. Chem. 2009, 121, 9503–9505; [DOI] [PubMed] [Google Scholar]; c) Wu Q-F, He H, Liu W-B, You S-L, J. Am. Chem. Soc. 2010, 132, 11418–11419; [DOI] [PubMed] [Google Scholar]; d) Zhu S, MacMillan DWC, J. Am. Chem. Soc, 2012, 134, 10815–10818; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wu J, Tang W, Pettman A, Xiao J, Adv. Synth. Catal. 2013, 355, 35–40; [Google Scholar]; f) Kubota K; Watanabe Y; Hayama K; Ito H J. Am. Chem. Soc. 2016, 138, 4338–4341; [DOI] [PubMed] [Google Scholar]; g) Bertuzzi G; Sinisi A; Caruana L; Mazzanti A; Fochi M; Bernardi L ACS Catal. 2016, 6, 6473; [Google Scholar]; h) Panda S, Ready JM, J. Am. Chem. Soc. 2017, 139, 6038–6041; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Wang Y; Zheng C; You S-L Angew. Chem., Int. Ed. 2017, 56, 15093–15097; Angew. Chem. 2017, 129, 15289–15293; [Google Scholar]; j) Liu R-R, Wang Y-G, Li Y-L, Huang B-B, Liang R-X, Jia Y-X, Angew. Chem. Int. Ed. 2017, 56, 7475–7478; Angew. Chem. 2017, 129, 7583–7586; [DOI] [PubMed] [Google Scholar]; k) Bai L, Liu J, Hu W, Li K, Wang Y, Luan X, Angew. Chem. Int. Ed. 2018, 57, 5151–5155; Angew. Chem. 2018, 130, 5245–5155; [DOI] [PubMed] [Google Scholar]; l) Xia Z-L, Zheng C, Wang S-G, You S-L, Angew. Chem. Int. Ed. 2018, 57, 2653–2656; Angew. Chem. 2018, 130, 2683–2686; [DOI] [PubMed] [Google Scholar]; m) Gribble MW Jr., Guo S, Buchwald SL, J. Am. Chem. Soc. 2018, 140, 5057–5060; [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Grozavu A, Hepburn HB, Smith PJ, Potukuchi HK, Lindsay-Scott PJ, Donohoe TJ, Nat. Chem, 2019, 11, 242–247. [DOI] [PubMed] [Google Scholar]; For recent reviews, see: a) Q. Ding, X. Zhou, R. Fan, Org. Biomol. Chem. 2014, 12, 4807–4815; b) S. P. Roche, J.-J. Y. Tendoung, B. Tréguier, Tetrahedron, 2015, 71, 3549–3591. [Google Scholar]
- [10].Selected recent examples of dearomatization of arenes bearing benzylic leaving group: a) Bao M, Nakamura H, Yamamoto Y, J. Am. Chem. Soc. 2001, 123, 759–760; [DOI] [PubMed] [Google Scholar]; b) Lu S, Xu Z, Bao M, Yamamoto Y, Angew. Chem. Int. Ed. 2008, 47, 4366–4369; Angew. Chem. 2008, 120, 4438–4441; [DOI] [PubMed] [Google Scholar]; c) Peng B, Zhang S, Yu X, Feng X, Bao M, Org. Lett. 2011, 13, 5402–5405; [DOI] [PubMed] [Google Scholar]; d) Zhang S, Yu X, Feng X, Yamamoto Y, Bao M, Chem. Commun. 2015, 51, 3842–3845; [DOI] [PubMed] [Google Scholar]; (e) Komatsuda M, Muto K, Yamaguchi J, Org. Lett. 2018, 20, 4354–4357. [DOI] [PubMed] [Google Scholar]
- [11].For a recent example for stoichiometric dearomatizative functionalization of nonactivated arenes, see: Wilson KB, Myers JT, Nedzbala HS, Combee LA, Sabat M, Harman WD, J. Am. Chem. Soc. 2017, 139, 11401–11412. For recent reviews on this topic, see Refs. 1a and 1e. [DOI] [PubMed] [Google Scholar]
- [12].a) Hamrock SV, Sheridan RS, J. Am. Chem. Soc. 1989, 111, 9247–9249; [Google Scholar]; b) Southgate EH, Pospech J, Fu J, Holycross DR, Sarlah D, Nat. Chem. 2016, 8, 922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Okumura M, Sarlah D, Synlett, 2018, 29, 845–855. [Google Scholar]
- [14].a) Hernandez LW, Pospech J, Klöeckner U, Bingham TW, Sarlah D J. Am. Chem. Soc. 2017, 139, 15656–15659; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hernandez LW, Klöckner U, Pospech J, Hauss L, Sarlah D, J. Am. Chem. Soc. 2018, 140, 4503–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Okumura M, Shved AS, Sarlah D, J. Am. Chem. Soc. 2017, 139, 17787–17790; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wertjes WC, Okumura M, Sarlah D, J. Am. Chem. Soc. 2019, 141, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Observation of different regioselectivity in Ni- and Pd-catalyzed allylic substitution with Grignard reagents has been reported, see: Hayashi T, Konishi M, Yokota K, Kumada M, J. Chem. Soc., Chem. Commun, 1981, 313–314. [Google Scholar]
- [17].Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis in Topics in Organometallic Chemistry, Vol. 38, Ed. U. Kazmaier, Springer, Heidelberg, 2012, pp. 1–63.
- [18].Welch WM, Kraska AR, Sarges R, Koe BK, J. Med. Chem. 1984, 27, 1508–1515. [DOI] [PubMed] [Google Scholar]
- [19].Makowski K, Vigevani L, Albericio F, Valcarcel J, Alvarez M, ACS Chem. Biol, 2017, 12, 163–173 [DOI] [PubMed] [Google Scholar]
- [20].Hu CH, Wang TC, Qiao JX, Haque L, Chen A. Y.a., Taylor DS, Ying X, Onorato JM, Galella M, Shen H, Huang CS, Toussaint N, Li Y-X, Abell L, Adam LP, Gordon D, Wexler RR, Finlay HJ, Bioorg. Med. Chem. Lett, 2018, 28, 3721–3725. [DOI] [PubMed] [Google Scholar]
- [21].a) Kurosawa H, Ogoshi S, Kawasaki Y, Murai S, Miyoshi M, Ikeda I, J. Am. Chem. Soc, 1990, 112, 2813–2814; [Google Scholar]; b) Kurosawa H, Kajimaru H, Ogoshi S, Yoneda H, Miki K, Kasai N, Murai S, Ikeda I, J. Am. Chem. Soc, 1992, 114, 8417–8424; d [Google Scholar]; c) Trost BM, Thaisrivongs DA, J. Am. Chem. Soc, 2008, 130, 14092–14093; [DOI] [PubMed] [Google Scholar]; d) Trost BM, Thaisrivongs DA, J. Am. Chem. Soc, 2009, 131, 12056–12057; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Trost BM, Thaisrivongs DA, Hartwig J, J. Am. Chem. Soc, 2011, 133, 12439–12441; [DOI] [PubMed] [Google Scholar]; f) Zhang J, Stanciu C, Wang B, Hussain MM, Da C-S, Carroll PJ, Dreher SD, Walsh PJ, J. Am. Chem. Soc. 2011, 133, 20552–20560; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Sha S-C, Zhang J, Carroll CPJ, Dreher SD, Walsh PJ, J. Am. Chem. Soc. 2013, 135, 17602–17609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].For recent examples of unexpected stereochemical outcomes in palladium-catalyzed allylic substitutions, see: Oliveira MT, Audisio D, Niyomchon S, Maulide N, ChemCatChem, 2013, 5, 1239–1247, and references therein. For computational analysis of the origin of stereoselectivity in oxidative addition of 3-chloro-cyclopentene to Pd(0), see: Wolf LM, Thiel W, J. Org. Chem. 2014, 79, 12136–12147. [Google Scholar]
- [23].Lower yields and high regioselectivity could arise from the catalyst control or the instability of the one of the constitutional isomeric products, which readily decomposed during aqueous work-up. Although we have not been able to directly detect these products in crude mixtures, similar paradigm was observed using amines as the nucleophiles (see Ref. 14b).
- [24].Adam W, Pastor A, Wirth T, Org. Lett. 2000, 2, 1295–1298. [DOI] [PubMed] [Google Scholar]
- [25].For selected examples involving synthesis of sertraline, see: a) Lautens M, Rovis T, J. Org. Chem. 1997, 62, 5246–5247; [Google Scholar]; b) Lautens M, Rovis T, Tetraheron 1999, 55, 8967–8976; [Google Scholar]; c) Vukics K, Fodor T, Fischer J, Fellegvari I, Levai S, Org. Proc. Res. Dev, 2002, 6, 82–85; [Google Scholar]; d) Poremba KE, Kadunce NT, Suzuki N, Cherney AH, Reisman SE, J. Am. Chem. Soc. 2017, 139, 5684–5687; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Clark JR, Feng K, Sookezian A, White MC, Nat. Chem, 2018, 10, 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


