Abstract
Background:
Helicobacter pylori is one of the most common human infections in the world and studies in Alaska Native people, as well as other Indigenous peoples, have shown a high prevalence of this gastric infection. This study was undertaken to determine the prevalence of H. pylori infection by urea breath test (UBT) and anti-H. pylori IgG among Alaskans living in four regions of the state and to identify factors associated with infection.
Methods:
A convenience sample of persons > 6 months old living in five rural and one urban Alaskan community were recruited from 1996 to 1997. Participants were asked about factors possibly associated with infection. Sera were collected and tested for anti-H. pylori IgG antibodies; a UBT was administered to participants > 5 years old.
Results:
We recruited 710 people of whom 571 (80%) were Alaska Native and 467 (66%) were from rural communities. Rural residents were more likely to be Alaska Native compared with urban residents (p<0.001). Of the 710 people, 699 (98%) had a serum sample analyzed and 634 (97%) persons > 5 years old had a UBT performed. H. pylori prevalence was 69% by UBT and 68% by anti-H. pylori IgG. Among those with a result for both tests, there was 94% concordance. Factors associated with H. pylori positivity were Alaska Native racial status, age ≥ 20 years, rural region of residence, living in a crowded home, and drinking water that was not piped or delivered.
Conclusions:
H. pylori prevalence is high in Alaska, especially in Alaska Native persons and rural residents. Concordance between UBT and serology was also high in this group. Two socioeconomic factors, crowding and drinking water that was not piped or delivered, were found to be associated with H. pylori positivity.
Keywords: Helicobacter pylori, Alaska, UBT, IgG antibody, prevalence
Introduction
Helicobacter pylori is one of the most common human infections with over 50% of persons infected in some countries (1). Numerous studies from around the world have shown that the prevalence of H. pylori infection is related to age, gender, ethnicity, and a variety of socioeconomic indicators (2–6); moreover, intrafamilial clustering also occurs (7–10). H. pylori colonization of the stomach results in an inflammatory response, gastritis, that can persist for decades (11), and infected persons have a 10 to 20% lifetime risk of developing peptic ulcer disease and a 1 to 2% lifetime risk of developing gastric cancer, particularly in populations with specific host-genetic risk (12–14). Because of this, H. pylori is characterized as a group I carcinogen by the International Agency for Research on Cancer (World Health Organization) (15).
Gastric cancer incidence and mortality is decreasing in many parts of the world, but this is not true for Alaska Native people for whom gastric cancer is the third most common cause of cancer-related death (16–18). Additionally, the gastric cancer mortality rate among Alaska Native people is more than three times higher than among U.S. whites (11.3 vs. 3.2 per 100,000 persons) (19). Past studies in Alaska have shown that H. pylori-infected persons have a higher risk of gastric cancer compared with non-infected persons and that 75% of Alaska Native people have H. pylori IgG antibodies (20, 21). This study was undertaken to determine the prevalence of H. pylori infection by both urea breath test (UBT) and anti-H. pylori IgG among Alaskans living in four regions of the state and to identify factors associated with infection.
Methods
Study Participants
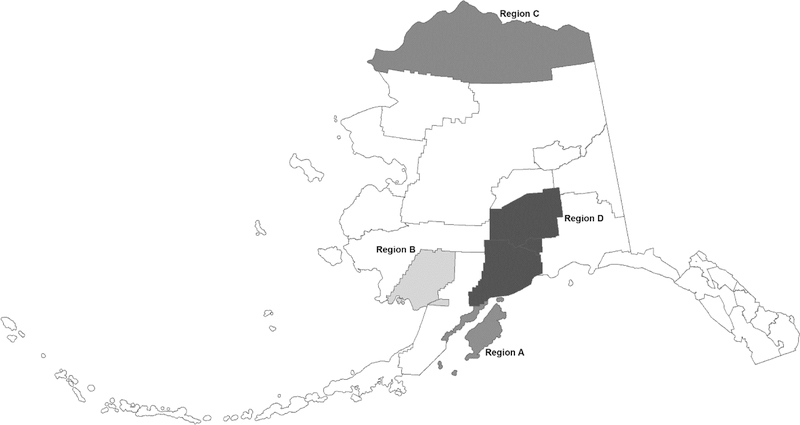
Persons living in five rural Alaska Native communities and one urban city were invited to participate. The six communities were selected to represent four geographic regions (Figure 1). Study approval was obtained from the regional health corporations and village leadership. The study was approved by Institutional Review Boards of the Centers for Disease Control and Prevention and the Alaska Area Indian Health Service.
Figure 1.
Map of Alaska with study regions identified; Alaska 1996–1997.
Recruitment occurred during 1996 and 1997. A convenience sample of persons living in all six communities were recruited using public advertisements and personal contact with study staff. All residents >6 months old were eligible to enroll. Participants ≥18 years old provided written, informed consent whereas parents or legal guardians provided consent for participants <18 years old.
Data collection
The head of a household provided information about the number of household members, size of the house, and drinking water source(s) for the home. We obtained age, sex, ethnicity, education level, and water use behaviors for individual household members. We also asked about clinical information and medication use that could be related to an H. pylori infection. A chart review was not done to confirm the self-reported clinical information nor did we confirm whether or not the clinical diagnoses (gastritis, ulcer, gastric cancer, H. pylori infection) were determined by esophagogastroduodenoscopy.
From each participant, we collected a whole blood specimen in a standard serum separator tube. We centrifuged the specimens in the field, decanted the serum, and transported them on ice packs to Anchorage where they were frozen at −30oC until analysis. After all sera were collected, they were shipped to the CDC’s Foodborne and Diarrheal Diseases Laboratory in Atlanta where they were tested for H. pylori IgG antibodies with an enzyme-linked immunosorbent assay (ELISA) used previously in this population (21–23). This assay has a sensitivity of 92% and a specificity of 98% compared with upper endoscopy. A value of ≥0.8 was considered positive.
Each participant >5 years old underwent a urea breath test (UBT) for current H. pylori infection using the commercial Meretek UBT® (now called BreathTek UBT, Otsuka Pharmaceuticals) according to the manufacturer’s instructions. Discontinuation of medications was not a requirement for UBT testing. Breath samples were kept at room temperature and sent to the Meretek testing laboratory (Houston, TX) where they were assessed for 13CO2 by mass spectrophotometry. The amount of 13CO2 in each person’s baseline and 30-minute samples was compared and a ratio of >2.4 was considered positive.
Statistical analysis
Proportions were compared using a chi-squared or Fisher’s exact test as appropriate, and logistic regression was used to examine multiple factors simultaneously. All factors were examined in a univariate fashion to assess preliminary significance. In addition, multiple options for grouping categorical and ordinal variables were assessed. A backwards selection procedure was used beginning with a comprehensive model with all factors; the adjusted odds-ratios for all factors are reported. Logistic regression analyses were repeated treating household as a random effect with very similar results (not presented). Water use in a village was classified into two groups, “Piped and delivered water only” and “Other water sources”. The former included piped municipal water, well water, water delivered by municipal truck, and bottled water; the latter included water hauled from a municipal spigot and natural water sources such as that from rivers and lakes, or acquired from rain or melted snow and ice. Analyses were performed using Stata V10. P-values are two-sided and those less 0.05 are considered statistically significant.
Results
We recruited 710 people (age range, 2–92 years) of whom 382 (54%) were female and 571 (80%) were Alaska Native (Table 1). Participants from rural regions A-C were more likely to be Alaska Native than participants from urban region D (p<0.001; Figure 1). As compared with the 2000 census population for these six communities, study participants were more likely to be female and Alaska Native. This remains true when the rural and urban communities are analyzed separately. The most common clinical symptom related to a possible H. pylori infection was stomach pain, with 33% of participants reporting it. Diagnoses of gastritis, ulcer, gastric cancer, or a previous H. pylori infection were uncommon with 7%, 8%, 0.1%, and 1%, respectively, of persons reporting each (Table 1).
Table 1.
Characteristics of study participants; Alaska 1996–1997.
| Region | Region Aa n (%) |
Region Ba n (%) |
Region Ca n (%) |
Region D n (%) |
Total n (%) |
|---|---|---|---|---|---|
| Participants | 122 | 226 | 119 | 243 | 710 |
| Female | 54 (44) | 115 (51) | 76 (64) | 137 (56) | 382 (54) |
| Alaska Native | 116 (95) | 224 (99) | 105 (88) | 126 (52) | 571 (80) |
| Age | |||||
| <5 | 4 (3) | 17 (8) | 7 (6) | 12 (5) | 40 (6) |
| 5–9 | 16 (13) | 31 (14) | 16 (13) | 25 (10) | 88 (12) |
| 10–19 | 30 (25) | 44 (19) | 17 (14) | 59 (24) | 150 (21) |
| 20–34 | 13 (11) | 59 (26) | 20 (17) | 38 (16) | 130 (18) |
| 35–49 | 34 (28) | 42 (19) | 16 (13) | 67 (28) | 159 (22) |
| ≥50 | 25 (20) | 33 (15) | 43 (36) | 42 (17) | 143 (20) |
| Clinicalb,c | |||||
| Stomach pain | 38/122 (31) | 82/226 (36) | 42/119 (35) | 75/241 (31) | 237/708 (33) |
| Gastritis | 8/120 (7) | 12/212 (6) | 9/113 (8) | 21/239 (9) | 50/684 (7) |
| Ulcer | 16/121 (13) | 8/217 (4) | 6/115 (5) | 25/242 (10) | 55/695 (8) |
| Gastric cancer | 0/122 (0) | 0/218 (0) | 0/114 (0) | 1/243 (0.4) | 1/697 (0.1) |
| H. pylori | 0/121 (0) | 1/217 (0.5) | 2/111 (2) | 2/242 (1) | 5/691 (1) |
| Medicationsb,c,d | |||||
| Antacids | 55/122 (45) | 30/222 (14) | 31/117 (26) | 99/242 (41) | 215/703 (31) |
| PPIe | 0/121 (0) | 1/216 (0.5) | 1/109 (1) | 5/241 (2) | 7/687 (1) |
| H2 blocker | 16/120 (13) | 5/219 (2) | 9/111 (8) | 34/241 (14) | 64/691 (9) |
rural region
denominator differences are due to each participant’s ability and/or willingness to answer each question
self-reported; no chart review done
any use in the past 12 months
Proton pump inhibitor
We analyzed UBT samples from 634 (97%) of 655 eligible persons >5 years old. Six persons had indeterminate results and were not included in the final analysis. Characteristics of the remaining 628 persons are in Table 2. We analyzed 699 serum samples for IgG antibodies to H. pylori (Table 2). H. pylori prevalence was similar by UBT and anti-H. pylori IgG (69% and 68%, respectively). For persons with a result for both tests, there was 94% concordance between the two results.
Table 2.
Prevalence of Helicobacter pylori infection as determined by urea breath test and enzyme linked immunosorbent assay; Alaska 1996–1997.
| Characteristic | Urea Breath Test positive | H. pylori IgG positive | |||||
|---|---|---|---|---|---|---|---|
| Positive/total (%) | Univariate ORa (p value) | aORb (p value) | Positive/total (%) | Univariate OR (p value) | aOR (p value) | ||
| Overall | 433/628 (69) | 478/699 (68) | |||||
| Sex | Female | 240/347 (69) | Ref | Ref | 265/376 (70) | Ref | Ref |
| Male | 193/281 (69) | 0.98 (0.897) | 1.18 (0.470) | 213/323 (66) | 0.81 (0.199) | 0.87 (0.466) | |
| Race | Alaska Native | 409/500 (82) | 19.5 (<0.001) | 11.3 (<0.001) | 422/561 (75) | 10.5 (<0.001) | 7.20 (<0.001) |
| Non-Native | 24/128 (19) | Ref | Ref | 36/138 (26) | Ref | Ref | |
| Age | <5 | N/A | 14/37 (38) | 0.25 (<0.001) | 0.09 (<0.001) | ||
| 5–9 | 36/54 (67) | 0.83 (0.590) | 0.37 (0.029) | 55/85 (65) | 0.74 (0.313) | 0.36 (0.005) | |
| 10–19 | 98/147 (67) | 0.83 (0.467) | 0.43 (0.016) | 100/148 (68) | 0.85 (0.511) | 0.46 (0.017) | |
| 20–34 | 91/126 (72) | 1.08 (0.773) | 0.66 (0.266) | 96/129 (74) | 1.18 (0.544) | 0.93 (0.828) | |
| 35–49 | 107/158 (68) | 0.87 (0.586) | 1.09 (0.809) | 112/158 (71) | 0.99 (0.963) | 1.17 (0.623) | |
| ≥50 | 101/143 (71) | Ref | Ref | 101/142 (71) | Ref | Ref | |
| Grouped Age | <20 | 134/201 (67) | Ref | 169/270 (63) | Ref | ||
| ≥20 | 299/427 (70) | 1.17 (0.397) | 309/429 (72) | 1.54 (0.009) | |||
| Region | Region Ac | 81/114 (71) | 3.68 (<0.001) | 1.47 (0.185) | 94/122 (77) | 4.07 (<0.001) | 1.91 (0.026) |
| Region Bc | 186/193 (96) | 39.9 (<0.001) | 13.4 (<0.001) | 187/220 (85) | 6.86 (<0.001) | 2.56 (0.001) | |
| Region Cc | 78/101 (77) | 5.09 (<0.001) | 2.49 (0.004) | 88/116 (76) | 3.81 (<0.001) | 1.99 (0.020) | |
| Region D | 88/220 (40) | Ref | Ref | 109/241 (45) | Ref | Ref | |
| Region type | Urban | 88/220 (40) | Ref | 109/241 (45) | Ref | ||
| Rural | 345/408 (85) | 8.21 (<0.001) | 369/458 (81) | 5.02 (<0.001) | |||
| # persons in household | ≥5 persons | 234/306 (76) | 2.02 (<0.001) | 0.96 (0.877) | 263/361 (73) | 1.54 (0.008) | 1.05 (0.847) |
| < 5 persons | 198/321 (62) | Ref | Ref | 214/337 (64) | Ref | Ref | |
| Household crowding | >1 person/room | 212/247 (86) | 4.41(<0.001) | 1.72 (0.064) | 235/287 (82) | 3.16 (<0.001) | 2.00 (0.009) |
| ≤1 person/room | 220/380 (58) | Ref | Ref | 242/411 (59) | Ref | Ref | |
| Education (among those ≥20 years) | ≤12 years | 207/245 (84) | 6.31 (<0.001) | 1.27 (0.511) | 204/248 (82) | 3.72 (<0.001) | 0.72 (0.362) |
| >12 years | 76/164 (46) | Ref | Ref | 91/164 (55) | Ref | Ref | |
OR = Odds Ratio
aOR = adjusted OR
rural region
Univariate analysis showed that H. pylori positivity as detected by UBT or anti-H. pylori IgG was associated with Alaska Native race, rural region of residence, being part of a large family, living in a more crowded house, and, among persons ≥20 years old, having ≤12 years of education (Table 2). Gender was not associated with H. pylori positivity using either test. In multivariate analysis, factors associated with H. pylori positivity were Alaska Native racial status, age ≥20 years old, and rural region of residence. Household crowding remained associated with seropositivity but not a positive UBT.
In the three rural regions where drinking water sources could vary, active H. pylori infection as detected by UBT was associated with drinking water source (Table 3). Persons having access to municipal piped or delivered sources of water had a lower prevalence of infection. Moreover, among persons <20 years old, access to municipal piped or delivered water was also associated with a lower prevalence of anti-H. pylori IgG.
Table 3.
Relationship of participant’s drinking water source with Helicobacter pylori infection status as determined by urea breath test and enzyme-linked immunosorbent assay, rural residents only; Alaska 1996–1997.
| Piped or Delivered Watera | Other Water Sourcesb | ORc (p value) | |
|---|---|---|---|
| Urea Breath Test positive | |||
| All Ages | 195/250 (78%) | 150/159 (94%) | 0.21 (<0.001) |
| Age <20 years | 55/80 (69%) | 46/48 (96%) | 0.10 (0.002) |
| Anti-H. pylori IgG positive | |||
| All Ages | 218/280 (78%) | 151/178 (85%) | 0.63 (0.067) |
| Age <20 years | 74/111 (67%) | 56/65 (86%) | 0.32 (0.006) |
piped municipal water, well water, water delivered by a municipal truck, bottled water
water hauled from a municipal spigot, natural water sources such as from rivers, lakes, rain, melted snow/ice
Odds Ratio
Discussion
Despite the high H. pylori seroprevalence (21) and high rates of gastric cancer in the Alaska Native people (19), few studies have been published investigating factors in this population that may be associated with infection. In this 1996–1997 study of H. pylori infection in four Alaska regions using UBT and serology, we found associations between H. pylori positivity and race, rural residence, and age ≥ 20 years old. Two socioeconomic factors, living in a crowded home and drinking water source, were also associated with infection. Prevalence was nearly 70% for both UBT and serology and concordance between the two tests was high, at 94%.
H. pylori prevalence varies around the world. Recent reviews by Peleteiro et al. and Eusesbi et al. reported prevalence ranging from 13% in Finland and the United Kingdom to 94% in Nigeria (3, 24). Data collected from the 1999–2000 United States National Health and Nutrition Examination Surveys show an all-ages U.S. seroprevalence of 27% (25). The prevalence in our study, particularly among Alaska Native people, is similar to the prevalence reported in developing countries rather than the general U.S. population (3, 5, 24, 26). It is also high when compared with the prevalence among Aboriginal adults living in Arctic communities. In a review of six relevant studies of H. pylori prevalence among Aboriginal populations of Canada, Greenland, and Russia, Goodman et al. identified only two populations with a prevalence higher than the Alaska Natives in our study (27). One was from Arctic Russia in 1990 (prevalence 77%) and the other from Northern Manitoba in 1996 (prevalence 95%) (28, 29).
Similar to other populations, socioeconomic factors are likely the major reason for the high prevalence of H. pylori infection in Alaska (1, 30–35). Our finding that H. pylori infection is associated with living in a more crowded home is not surprising because crowding leads to close contact between household members and H. pylori transmission is mainly intrafamilial (1). Other studies have shown intrafamilial clustering of infections (7–10) and in a previous study conducted in Alaska, household family members’ infection status was associated with risk of becoming reinfected with H. pylori after successful eradication (36).
Water source has been identified as a possible risk factor for H. pylori infection in other epidemiologic studies (32, 37–40) and H. pylori organisms have been detected in a variety of water sources (40–43). We did not test water as part of this study; however, ingestion is not the only means of transmission potentially affected by water. Lee et al. showed that infrequent hand washing was associated with increased prevalence of H. pylori in Malaysia (44) suggesting that H. pylori infection could be related to inadequate hygiene practices. Previously, we have identified lack of in-home water service in Alaska to be a risk factor for reinfection with H. pylori (36) as well as for skin and respiratory infections (45–47). It is possible that the availability of clean water was a surrogate for the availability of water in general and that lack of water for hygiene contributed to transmission in the rural communities. More work is needed to better understand improvement in water source as a potential approach to preventing infection.
In this population H. pylori is typically acquired in childhood, thus it is not surprising we found increasing prevalence until adulthood. In persons >20 years old, prevalence appeared stable at about 70%. In contrast, other groups have found increasing prevalence past 20 years of age (6, 34, 35, 48–52). It is thought that the increase in prevalence in older age groups is due to a birth cohort effect in which childhood acquisition rates have decreased over time presumably due to improved sanitary, hygiene, and socioeconomic factors in subsequent generations of children. However, overall seroprevalence rates do not appear to be decreasing in rural Alaska. Prevalence in this 1996–1997 study was similar to that found in a serosurvey from 1980–1986 (21). This is an important observation because gastric cancer incidence remains high among Alaska Native people (16). Peleteiro et al. presented an analysis of gastric cancer incidence as it relates to H. pylori prevalence around the world, concluding that prevalence of infection was at least two-fold higher in countries with high gastric cancer incidence (24). High H. pylori prevalence in Alaska is likely at least partly responsible for the continued high incidence of gastric cancer among the Alaska Native people and, as there is no evidence of improvement, it is becoming increasingly important to develop strategies to address this disparity.
This is one of the few studies that tested for H. pylori infection in the same people using both UBT and serology; the 94% concordance is informative. Using serology to diagnose an H. pylori infection is not recommended in populations where infection is common (53). This is because antibodies circulate long after eradication, so many people who are no longer infected may have a positive test result, resulting in a low test specificity (54). However, a study by Bruden et al. showed that false positive serology results were associated with previous H. pylori treatment (55). In our study group, a previous H. pylori diagnosis was uncommon at study entry, so it is unlikely many participants had formerly been treated for the infection. Therefore, data from our study suggests that UBT and serology results are comparable when persons have not been previously treated for infection. We can have confidence comparing results across studies of untreated populations where prevalence of infection was determined using different methods.
This study has four important limitations. A cross-sectional survey that includes primarily adults is not a suitable method for understanding factors associated with an infection usually acquired during childhood, because factors may have changed over time. However, it is likely that many of the factors studied, related to living in rural, primarily Alaska Native communities, did not change for many persons between the time they acquired an H. pylori infection and the time of the study. The convenience sampling methodology we used, particularly in the urban region, may have resulted in a non-representative sample. Therefore, generalizations to the entire population should be made with caution. The water source used and reported at the time of the study may have been different from the water source used when the organism was acquired. Finally, the samples were collected and tested over 20 years ago and H. pylori prevalence may have changed during that time. However, numerous studies published since these data were collected show that H. pylori infection and gastric cancer continue to negatively affect the Alaska Native people (16, 36, 53, 56, 57). Thus, it is important these older data be published as a prevalence baseline as we continue to address this health disparity.
In conclusion, H. pylori prevalence is high in Alaska, especially in Alaska Native people. Concordance between UBT and serology was high which gives increased validity to the results and confidence in interpreting data between studies of untreated persons that use different testing methods. Socioeconomic factors associated with infection in Alaskans are living in a crowded home and drinking water from a less protected source. The prevalence in this study conducted in the late 1990s was similar to a seroprevalence study from the 1980s. This, combined with the stable prevalence in persons ≥ 20 years old in this study, indicate more recent birth cohorts may not be at decreased risk of H. pylori infection as seen in other populations around the world. This has health implications for addressing the high incidence of gastric cancer in the Alaska Native people. Because the socioeconomic conditions in rural Alaska are similar now as compared with 20 years ago, an updated prevalence study in a similar cohort could be considered to investigate whether or not prevalence continues to be stable.
Acknowledgements
We would like to acknowledge the staff at the Arctic Investigations Program, especially Helen Peters, Marilyn Getty, Carolyn Zanis, Rhonda Baisden, and Debra Parks for their work recruiting participants, processing specimens, and managing data. A special acknowledgement goes to Kenneth Peterson M.D., the study’s original principal investigator, who passed away tragically before the data could be published.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors have no conflict of interest to disclose.
References
- 1.Mentis A, Lehours P, Megraud F. Epidemiology and Diagnosis of Helicobacter pylori infection. Helicobacter 2015. September;20 Suppl 1:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Eshraghian A Epidemiology of Helicobacter pylori infection among the healthy population in Iran and countries of the Eastern Mediterranean Region: a systematic review of prevalence and risk factors. World J Gastroenterol 2014. December 14;20(46):17618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014. September;19 Suppl 1:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Laszewicz W, Iwanczak F, Iwanczak B. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Advances in medical sciences 2014. March;59(1):147–50. [DOI] [PubMed] [Google Scholar]
- 5.Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control 2013. February;24(2):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhsen K, Cohen D, Spungin-Bialik A, Shohat T. Seroprevalence, correlates and trends of Helicobacter pylori infection in the Israeli population. Epidemiol Infect 2012. July;140(7):1207–14. [DOI] [PubMed] [Google Scholar]
- 7.Roma E, Panayiotou J, Pachoula J, Kafritsa Y, Constantinidou C, Mentis A, et al. Intrafamilial spread of Helicobacter pylori infection in Greece. Journal of clinical gastroenterology 2009. September;43(8):711–5. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes DT, Fischbach LA, Goodman KJ, Phillips CV, Chen S, Broussard CS. Exposure to Helicobacter pylori-positive siblings and persistence of Helicobacter pylori infection in early childhood. J Pediatr Gastroenterol Nutr 2010. May;50(5):481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didelot X, Nell S, Yang I, Woltemate S, van der Merwe S, Suerbaum S. Genomic evolution and transmission of Helicobacter pylori in two South African families. Proc Natl Acad Sci U S A 2013. August 20;110(34):13880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaty HM, Kumagai T, Tanaka E, Ota H, Kiyosawa K, Graham DY, et al. Evidence from a nine-year birth cohort study in Japan of transmission pathways of Helicobacter pylori infection. J Clin Microbiol 2000. May;38(5):1971–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover TL. Helicobacter pylori Diversity and Gastric Cancer Risk. mBio 2016. January 26;7(1):e01869–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther 1995;9 Suppl 2:59–69. [PubMed] [Google Scholar]
- 13.Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther 1999. March;13 Suppl 1:3–11. [DOI] [PubMed] [Google Scholar]
- 14.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol 2011. February 1;173(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. In: A review of human carcinogens (vol. 100). 2012; p. 1–441. [PMC free article] [PubMed] [Google Scholar]
- 16.Carmack AM, Schade TL, Sallison I, Provost EM, Kelly JJ. Cancer in Alaska Native People: 1969–2013 The 45-Year Report 2015.
- 17.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer 2009. August 1;125(3):666–73. [DOI] [PubMed] [Google Scholar]
- 18.Peleteiro B, Severo M, La Vecchia C, Lunet N. Model-based patterns in stomach cancer mortality worldwide. Eur J Cancer Prev 2014. November;23(6):524–31. [DOI] [PubMed] [Google Scholar]
- 19.Kelly JJ, Schade TL, Starkey BM, White S, Ashokkumar R, Lanier AP. Cancer in Alaska Native People 1969–2008 40 year report 2012.
- 20.Keck JW, Miernyk KM, Bulkow LR, Kelly JJ, McMahon BJ, Sacco F, et al. Helicobacter pylori infection and markers of gastric cancer risk in Alaska Native persons: a retrospective case-control study. Canadian journal of gastroenterology & hepatology 2014. June;28(6):305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkinson AJ, Gold BD, Bulkow L, Wainwright RB, Swaminathan B, Khanna B, et al. High prevalence of Helicobacter pylori in the Alaska Native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol 2000. November;7(6):885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold BD, Khanna B, Huang LM, Lee CY, Banatvala N. Helicobacter pylori acquisition in infancy after decline of maternal passive immunity. Pediatr Res 1997;41(5):641–6. [DOI] [PubMed] [Google Scholar]
- 23.Khanna B, Cutler A, Israel NR, Perry M, Lastovica A, Fields PI, et al. Use caution with serologic testing for Helicobacter pylori infection in children. J Infect Dis 1998;178(2):460–5. [DOI] [PubMed] [Google Scholar]
- 24.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014. August;59(8):1698–709. [DOI] [PubMed] [Google Scholar]
- 25.Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol 2006. January 15;163(2):127–34. [DOI] [PubMed] [Google Scholar]
- 26.Khedmat H, Karbasi-Afshar R, Agah S, Taheri S. Helicobacter pylori Infection in the general population: A Middle Eastern perspective. Caspian journal of internal medicine 2013. Fall;4(4):745–53. [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman KJ, Jacobson K, Veldhuyzen van Zanten S. Helicobacter pylori infection in Canadian and related Arctic Aboriginal populations. Canadian Journal of Gastroenterology 2008;22(3):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reshetnikov OV, Nikitin YP, Kholmogortsev MV, Kurilovich SA, Pycllik OA. Helicobacter pylori in a Chukotka Native male population. International Journal of Circumpolar Health 1998;57 Suppl 1:293–5. [PubMed] [Google Scholar]
- 29.Bernstein CN, McKeown I, Embil JM, Blanchard JF, Dawood M, Kabani A, et al. Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer-associated hospitalizations in a Canadian Indian population. Digestive Diseases & Sciences 1999;44(4):668–74. [DOI] [PubMed] [Google Scholar]
- 30.Ueda M, Kikuchi S, Kasugai T, Shunichi T, Miyake C. Helicobacter pylori risk associated with childhood home environment. Cancer Sci 2003. October;94(10):914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanafi MI, Mohamed AM. Helicobacter pylori infection: seroprevalence and predictors among healthy individuals in Al Madinah, Saudi Arabia. The Journal of the Egyptian Public Health Association 2013. April;88(1):40–5. [DOI] [PubMed] [Google Scholar]
- 32.Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet 1991. June 22;337(8756):1503–6. [DOI] [PubMed] [Google Scholar]
- 33.World Gastroenterology Organisation Global Guideline: Helicobacter pylori in developing countries. Journal of clinical gastroenterology 2011. May-Jun;45(5):383–8. [DOI] [PubMed] [Google Scholar]
- 34.Nouraie M, Latifi-Navid S, Rezvan H, Radmard AR, Maghsudlu M, Zaer-Rezaii H, et al. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter 2009. February;14(1):40–6. [DOI] [PubMed] [Google Scholar]
- 35.Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce MG, Bruden DL, Morris JM, Reasonover AL, Sacco F, Hurlburt D, et al. Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect 2015. April;143(6):1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breckan RK, Paulssen EJ, Asfeldt AM, Kvamme JM, Straume B, Florholmen J. The All-Age Prevalence of Helicobacter pylori Infection and Potential Transmission Routes. A Population-Based Study. Helicobacter 2016. May 12. [DOI] [PubMed]
- 38.Baingana RK, Kiboko Enyaru J, Davidsson L. Helicobacter pylori infection in pregnant women in four districts of Uganda: role of geographic location, education and water sources. BMC public health 2014;14:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravelomanana L, Imbert P, Kalach N, Ramarovavy G, Richard V, Carod JF, et al. Helicobacter pylori infection in children in Madagascar: risk factors for acquisition. Tropical gastroenterology : official journal of the Digestive Diseases Foundation 2013. Oct-Dec;34(4):244–51. [DOI] [PubMed] [Google Scholar]
- 40.Aziz RK, Khalifa MM, Sharaf RR. Contaminated water as a source of Helicobacter pylori infection: A review. Journal of advanced research 2015. July;6(4):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santiago P, Moreno Y, Ferrus MA. Identification of Viable Helicobacter pylori in Drinking Water Supplies by Cultural and Molecular Techniques. Helicobacter 2015. August;20(4):252–9. [DOI] [PubMed] [Google Scholar]
- 42.Ranjbar R, Khamesipour F, Jonaidi-Jafari N, Rahimi E. Helicobacter pylori in bottled mineral water: genotyping and antimicrobial resistance properties. BMC Microbiol 2016;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tirodimos I, Bobos M, Kazakos E, Haidich AB, Dardavessis T, Kostopoulos I, et al. Molecular detection of Helicobacter pylori in a large Mediterranean river, by direct viable count fluorescent in situ hybridization (DVC-FISH). Journal of water and health 2014. December;12(4):868–73. [DOI] [PubMed] [Google Scholar]
- 44.Lee YY, Ismail AW, Mustaffa N, Musa KI, Majid NA, Choo KE, et al. Sociocultural and dietary practices among Malay subjects in the north-eastern region of Peninsular Malaysia: a region of low prevalence of Helicobacter pylori infection. Helicobacter 2012. February;17(1):54–61. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy TW, Ritter T, Holman RC, Bruden DL, Yorita KL, Bulkow L, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. American journal of public health 2008. November;98(11):2072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J 2010. March;29(3):251–6. [DOI] [PubMed] [Google Scholar]
- 47.Thomas TK, Ritter T, Bruden D, Bruce M, Byrd K, Goldberger R, et al. Impact of providing in-home water service on the rates of infectious diseases: results from four communities in Western Alaska. Journal of water and health 2016;14(1):132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuzela L, Oltman M, Sutka J, Zacharova B, Nagy M. Epidemiology of Helicobacter pylori infection in the Slovak Republic. Hepatogastroenterology 2012. May;59(115):754–6. [DOI] [PubMed] [Google Scholar]
- 49.Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014. April;19(2):105–10. [DOI] [PubMed] [Google Scholar]
- 50.McDonald AM, Sarfati D, Baker MG, Blakely T. Trends in Helicobacter pylori infection among Maori, Pacific, and European Birth cohorts in New Zealand. Helicobacter 2015. April;20(2):139–45. [DOI] [PubMed] [Google Scholar]
- 51.van Blankenstein M, van Vuuren AJ, Looman CW, Ouwendijk M, Kuipers EJ. The prevalence of Helicobacter pylori infection in the Netherlands. Scand J Gastroenterol 2013. July;48(7):794–800. [DOI] [PubMed] [Google Scholar]
- 52.Leja M, Cine E, Rudzite D, Vilkoite I, Huttunen T, Daugule I, et al. Prevalence of Helicobacter pylori infection and atrophic gastritis in Latvia. Eur J Gastroenterol Hepatol 2012. December;24(12):1410–7. [DOI] [PubMed] [Google Scholar]
- 53.McMahon BJ, Bruce MG, Koch A, Goodman KJ, Tsukanov V, Mulvad G, et al. The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: Expert Commentary. Epidemiol Infect 2016. January;144(2):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miernyk KM, Bruden DL, Bruce MG, McMahon BJ, Hennessy TW, Peters HV, et al. Dynamics of Helicobacter pylori-specific immunoglobulin G for 2 years after successful eradication of Helicobacter pylori infection in an American Indian and Alaska Native population. Clin Vaccine Immunol 2007. January;14(1):85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruden DL, Bruce MG, Miernyk KM, Morris J, Hurlburt D, Hennessy TW, et al. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J Gastroenterol 2011. November 14;17(42):4682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tveit AH, Bruce MG, Bruden DL, Morris J, Reasonover A, Hurlburt DA, et al. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol 2011. October;49(10):3638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baggett HC, Parkinson AJ, Muth PT, Gold BD, Gessner BD. Endemic iron deficiency associated with Helicobacter pylori infection among school-aged children in Alaska. Pediatrics 2006. March;117(3):e396–404. [DOI] [PubMed] [Google Scholar]