Abstract
The fruit fly Drosophila melanogaster has emerged as an ideal system in which to study 2-hydroxyglutarate (2HG) metabolism. Unlike many mammalian tissues and cell lines, which primarily accumulate d- or l-2HG as the result of genetic mutations or metabolic stress, Drosophila larvae accumulate high concentrations of l-2HG during normal larval growth. As a result, flies represent one of the few model systems that allows for studies of endogenous l-2HG metabolism. Moreover, the Drosophila genome not only encodes key enzymes involved in the synthesis and degradation of d-2HG, but the fly has also been used as to investigate the in vivo effects of oncogenic isocitrate dehydrogenase 1 and 2 (IDH1/2) mutations. All of these studies, however, rely on mass spectrometry-based methods to distinguish between the d- and l-2HG enantiomers. While such approaches are common among labs studying mammalian cell culture, few Drosophila studies have attempted to resolve and measure the individual 2HG enantiomers. Here we describe a highly reproducible gas chromatography-mass spectrometry (GC-MS)-based protocol that allows for quantitative measurements of both 2HG enantiomers in Drosophila homogenates.
Keywords: 2-Hydroxyglutarate, Drosophila, Gas chromatography-mass spectrometry, Oncometabolite, Metabolomics
1. Introduction
d- and l-2HG are commonly referred to as oncometabolites and primarily studied in the context of tumor growth and cancer cell proliferation [1–3]. The production of these compounds (Fig. 1), however, is not limited to cancer cells but also is produced by a variety of healthy tissues, such as the brain, testis, activated T-cells, and primary human cell cultures that have been exposed to hypoxia [4–10]. Such observations indicate that both 2HG enantiomers likely serve endogenous roles in normal cellular metabolism and physiology; however, nearly all 2HG studies rely on cell culture models, and relatively little is known about the functions of either d- or l-2HG in vivo. We recently discovered that the concentration of l-2HG exceeds 2 mM in larvae of the fruit fly Drosophila melanogaster, which is among the highest 2HG levels observed in healthy animal tissues [11]. Unlike human cells, however, which generate l-2HG in response to hypoxia and disruption of the electron transport chain [4, 5, 12, 13], Drosophila larvae generate this compound under standard growth conditions, indicating that fly metabolism is uniquely adapted to synthesize and accumulate l-2HG. Moreover, Drosophila also accumulates d-2HG, albeit at significantly low levels when compared with l-2HG [11], and the fly genome encodes the enzyme, d-2-hydroxyglutarate dehydrogenase (D2HGDH; FBgn0023507) [14], that degrades d-2HG. Finally, this system is also amenable to studies of the oncogenic IDH1/2 mutations [14], which result in aberrant d-2HG production. These studies demonstrate that Drosophila can be used to study nearly any aspect of 2HG metabolism and suggest that future studies in the fly will be essential for elucidating the mechanisms that control 2HG accumulation in vivo.
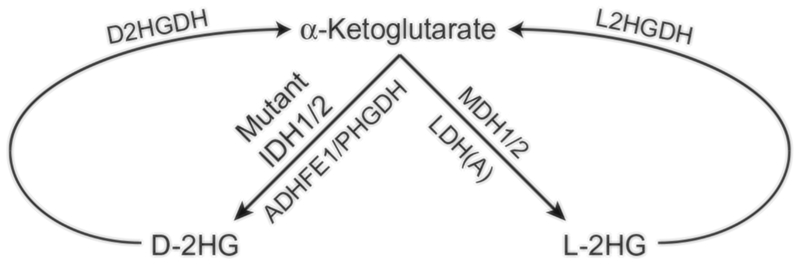
Fig. 1.
A schematic diagram illustrating the metabolic reactions that generate 2HG from α-ketoglutarate. l-2HG can be synthesized by either lactate dehydrogenase (LDH(A)) or malate dehydrogenase 1 or 2 (MDH1/2). d-2HG can be synthesized by d-3-phosphoglycerate dehydrogenase (PHGDH), alcohol dehydrogenase iron-containing protein 1 (ADHFE1), or mutant isocitrate dehydrogenase 1 and 2 (IDH1/2). Both d- and l-2HG can be converted back to α-ketoglutarate by d-2HG dehydrogenase (D2HGDH) and l-2HG dehydrogenase (L2HGDH), respectively
In order to facilitate additional 2HG studies in Drosophila, here we provide a detailed version of the GC-MS-based protocol that we’ve previously used to measure both d- and l-2HG [11]. This method, which is adapted from an established protocol used to measure d- and l-2HG in human bodily fluids [15], provides the user with detailed instruction on how to reproducibly extract 2HG from Drosophila homogenates and quantitatively measure each molecule. Moreover, the two-step derivatization protocol coupled with GC-MS allows for d- and l-2HG to be easily separated and quantified in a reproducible manner (Fig. 2).
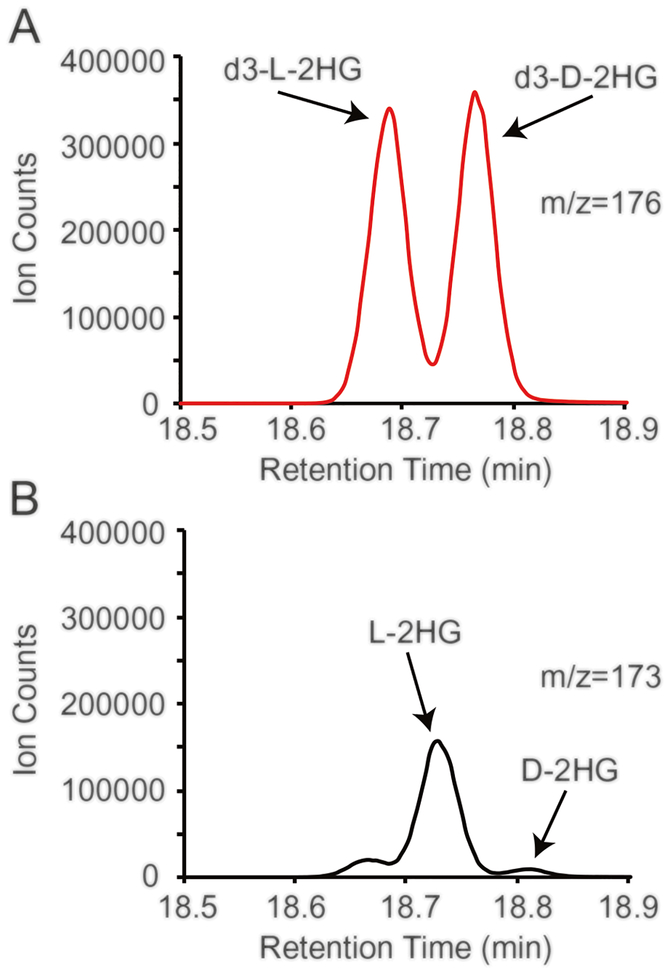
Fig. 2.
A representative annotated spectrum generated from a Drosophila larval sample. Peaks representing either (a) the deuterated d- and l-2HG standard (m/z = 176) or (b) endogenous d- and l-2HG levels (m/z = 173)
2. Materials
2.1. Sample Collection
Molasses agar plates (35 × 10 mm): Mix 115 mL molasses, 29 g agar, and 700 mL of H2O in a 2 L flask. Boil on a hot plate for 15 min while stirring at 350 rpm. Cool to 70 °C. Add 25 mL of acid mix (20 mL of 85% phosphoric acid and 209 mL propionic acid in 1 L of H2O; store in the dark) and 10 mL of 10% p-hydroxy-benzoic acid methyl ester in 95% ethanol. Pour the molasses agar into the lid and bottom of a 35 × 10 mm plastic tissue culture dish (see Note 1).
Yeast paste: Mix 5 g of baker’s yeast with 7 mL of water. The exact ratio will depend of the brand of yeast. The final mixture should have the creamy consistency (see Note 2).
Mating bottles: Use a 22-gauge needle to poke holes in a 6 oz. plastic Drosophila stock bottle. The egg-laying caps should fit snuggly in the mouth of the bottle and can be secured in place using laboratory tape.
Phosphate buffer saline (pH 7.4): For 1 L of 1× stock, dissolve 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 in 800 mL of H2O. Adjust pH to 7.4 with HCl. Add H2O to 1 L.
1.5 mL microfuge tubes (see Note 3).
2 mL screw cap tubes containing 1.4 mm ceramic beads (see Note 4).
Analytical balance capable of measuring 0.01 mg (see Note 5).
Liquid nitrogen.
2.2. Sample Processing
Benchtop cooler pre-chilled to −20 °C.
90% methanol: Dilute methanol using H2O. Store at −20 °C. Only use HPLC-grade reagents.
R,S-[2,3,3-2H3]-2-hydroxyglutarate standard.
Bead mill homogenizer (see Note 6).
Vacuum centrifuge with attached vapor trap.
2.3. Chiral Derivatization
R-2-butanol.
12 N hydrochloric acid.
1.5 mL microfuge tubes.
H2O (HPLC grade).
Hexane (HPLC grade).
Anhydrous pyridine (see Note 7).
Acetic anhydride.
Autosampler vials and caps: use a 150 μL glass insert.
2.4. GC-MS Analysis
GC-MS equipped with a 30 m Phenomenex ZB5–5 MSi column (or equivalent column) and an autosampler.
2.5. Data Analysis
A software package capable of analyzing the resulting spectra and integrating the peaks associated with d- and l-2HG. This software is usually provided by the manufacturer of the GC-MS (e.g., MassHunter from Agilent).
3. Methods
l-2HG levels vary dramatically during the course of the Drosophila life cycle (i.e., l-2HG levels will drop tenfold during the last 24 h of the third larval instar). Therefore, all samples collected during this analysis must be precisely staged. Only use HPLC-grade reagents when preparing samples for GC-MS analysis. Conduct all derivatization steps in a fume hood. Follow all waste disposal regulations when disposing of organic solvents and contaminated solid waste.
3.1. Sample Collection
Separately collect males and virgin females of the desired genotypes. Age these animals for 3–5 days in standard food vials (see Note 8).
Set up crosses of males and virgin females in mating bottles. Collect eggs on a molasses agar plate with ~1.5 g of yeast paste spread on the surface (egg-laying cap). Replace the egg-laying cap at least once a day for 3 days. Discard the egg-laying caps collected during this time (see Note 9).
After Day 3, place a fresh egg-laying cap into the mating cage. After 2 h, replace the egg-laying cap. Discard the first egg-laying cap used for the day (see Note 10).
Remove the second egg-laying cap after a defined period of time (usually 2 or 4 h, depending on the desired level of synchronization) and place inside a larger 60 × 15 mm dish. This step can be repeated multiple times during a single day.
Embryos are aged to the desired stage in a 25 °C incubator.
If collecting embryos, use a paintbrush to gently transfer 200 embryos onto a piece of PBS-soaked black filter paper that has been placed in the bottom a 35 mm tissue culture plate. Remove any large debris with a paintbrush. Tilt the culture dish and use a 1 mL pipette to slowly rinse the embryos with PBS (pH 7.4). This step will remove any yeast or agar stuck to the outside of the embryos. The embryos should remain in place during the rinse step. Remove the excess PBS from the bottom corner of the plate and discard prior to placing flat on the benchtop. Use the paintbrush to transfer the embryos to a pre-tared 2 mL screw cap tube containing 1.4 mm ceramic beads (see Notes 11 and 12). Record the mass of the sample and tubes using an analytical balance (see Note 5). Freeze samples in liquid nitrogen.
If collecting larvae, use a paintbrush to transfer synchronized populations of larvae (see Note 13) into a 1.5 mL microfuge tube (see Note 3). Individual samples should remain on ice (see Note 14). Typical larval sample sizes for this assay are 50 mid-first instar larvae (mid-L1), 25 mid-second instar larvae (mid-L2), and 10 mid-third instar larvae (mid-L3). Wash the samples three times with ice-cold PBS. Briefly centrifuge the sample at 2000 × g for 1 min, and remove all PBS from the sample tube with a 200 μL pipette. Freeze in liquid nitrogen. Remove the tube from the liquid nitrogen, and sharply pound the lid against the benchtop to dislodge the larval pellet (see Note 3). Quickly pour the larval pellet into a pre-tared 2 mL screw cap tube. Record the mass and immediately return the tube to liquid nitrogen (see Note 15).
If collecting pupae or adults (see Note 16), place 15 individuals into a pre-tared 2 mL screw cap tube containing 1.4 mm ceramic beads (see Note 12). Record the mass of the sample and freeze the sample in liquid nitrogen.
Samples can be stored at −80 °C for 3 months (see Note 17).
3.2. Sample Processing
Remove the samples from the −80 °C freezer and transfer into a benchtop cooler that was pre-chilled to −20 °C (see Note 18).
Add 800 μL of 90% methanol (pre-chilled to −20 °C) and 5 μL of 1 mg/mL R,S-[2,3,3-2H3]-2-hydroxyglutarate to the 2 mL bead tubes that contain the sample (see Note 19).
Add 800 μL of 90% methanol (pre-chilled to −20 °C) and 5 μL of 1 mg/mL R,S-[2,3,3-2H3]-2-hydroxyglutarate to an empty 2 mL bead tube. This sample will serve as a negative control.
Homogenize the samples in bead mill homogenizer (see Notes 6 and 20).
Return the homogenized samples to the benchtop cooler and place in a −20 °C freezer for at least 1 h. A white precipitate should form in the homogenized sample.
Centrifuge the samples in a 4 °C cold room (or a refrigerated centrifuge) for 5 min at 20,000 × g.
Carefully remove the samples from centrifuge, and return to the benchtop cooler. Be careful to not disturb the precipitate.
Transfer 600 μL of supernatant from the bead tube to a 1.5 mL microfuge tube. Do not disturb the precipitate. If debris from the precipitate is released into the supernatant, repeat step 6. If necessary, the transferred supernatant can be centrifuged at maximum speed in the 1.5 mL microfuge tube and transferred again to a new tube (see Note 21).
Dry the samples in a vacuum centrifuge overnight at room temperature. If necessary, the samples can be stored at −80 °C following this step.
3.3. Chiral Derivatization
Remove the samples from the vacuum centrifuge and place into a microfuge tube rack. Move the sample tubes to a fume hood (see Note 22).
Add 50 μL of R-2-butanol and 5 μL of 12 N HCl (37−38%) to the dried samples.
Place the sample tubes into a thermomixer. Incubate the sample tubes at 90 °C for 3 h at 300 rpm (see Note 23).
Remove the samples from the thermomixer and place into a microfuge tube rack. Allow the samples to cool to room temperature.
Extract the modified 2HG by adding 200 μL of water and 500 μL of hexane to the sample. Collect the organic phase and place in a new 1.5 mL microfuge tube (the organic phase is the top layer). Add an additional 500 μL of hexane to the sample. Again, collect the organic phase and combine with the organic phase from the first extraction.
Dry the sample with a vacuum centrifuge for 30 min at room temperature.
Remove the dried samples from the vacuum centrifuge and place into a microfuge tube rack. Move the sample tubes to a fume hood, and add 60 μL of anhydrous pyridine and 60 μL of acetic anhydride (see Note 7).
Place the sample tubes into a thermomixer. Incubate the sample tubes at 80 °C for 1 h at 300 rpm (see Note 24).
Dry the sample with a vacuum centrifuge.
Resuspend in 60 μL of hexane.
Transfer 50 μL of resuspended sample into an autosampler vial with a 150 μL glass insert.
3.4. GC-MS Analysis
Set the flow rate of the helium carrier gas to 1 mL/min.
Set the inlet temperature to 250 °C.
- Set the GC-MS to execute the following temperature gradient:
- Initial temperature of 95 °C with a hold of 1 min.
- Increase to 110 °C at a rate of 40 °C per min. Hold for 2 min.
- Increase to 200 °C at a rate of 5 °C per min.
- Increase to 330 °C at the rate of 40 °C/min with a final hold of 4 min.
Set a solvent delay of 5 min (see Note 25).
Randomize the sample injection order.
Set the detector to selectively monitor ions with a m/z ratio of 173 (2HG) and 176 (2H3-2HG standard).
Inject 1 μL of the derivatized sample into the GC-MS (split ratio of 5:1; see Note 26).
3.5. Data Analysis
Integrate the peak area for d- and l-2HG with m/z = 173; the retention time of l-2HG is a little earlier than that of d-2HG. Denote the integration value as Atarget.
Integrate the peak areas for internal standard (R,S-[2,3,3-2H3]-2-hydroxyglutarate) with m/z = 176. Add the integration values together for the two internal standard peaks. Denote the sum value as Ainternal.
- The concentration (μg/mg body mass) of l- or d-2HG in samples:
where M is the sample pellet mass with unit of mg.
4. Notes
Prior to pouring the agar, be sure that both the lid and bottom of the tissue culture plate fit snuggly in the mating bottle. Some brands do fit snuggly and allow flies to escape. We find that both the lids and bottoms of Falcon plates (catalog # 353001) work well for this purpose.
Be sure to use the same brand and strain of yeast for all experiments. We use Fleischmann’s Active Dry Yeast #2192; however, a variety of brands can be used for this purpose.
Frozen larval pellets are difficult to dislodge from some brands of microfuge tubes. Prior to collecting a large number of samples, be sure to determine if larval pellets can be easily removed from the brand of tube used for collection. We recommend 1.5 mL Eppendorf Flex-Tubes due to the ease with which we can dislodge pellets.
Some 2 mL screw cap tubes will disintegrate when used in a bead mill homogenizer. Be sure that the screw cap tubes used for sample collection can withstand the homogenization step.
Analytical balances that are only capable of measuring 0.1 mg are insufficient for conducting this analysis.
Insect cuticles are robust and require a large amount of force to destroy quickly. Moreover, samples must be homogenized while frozen because endogenous metabolic enzymes will reactivate in thawed tissue. Therefore, samples must be homogenized using an instrument capable of completely destroying a frozen sample in less than 1 min. We recommend the Omni Beadruptor 24.
Anhydrous pyridine should be stored in a desiccator and the vial flushed with an inert gas (e.g., nitrogen or argon) following every use.
l-2HG levels in l-2-hydroxyglutarate dehydrogenase (dL2HGDH; FBgn0032729) mutant larvae are sensitive to the maternal genotype. F1 progeny from dL2HGDH/+ heterozygous mothers exhibit lower l-2HG levels than those born from dL2HGDH homozygous mutants. As a result, studies based on the dL2HGDH mutations should only use homozygous mutant adults for generating offspring.
Egg-laying caps can be replaced by inverting the mating bottle and tapping the top against the benchtop. Quickly remove the old egg-laying cap and replace with a new one.
This step ensures that females are not holding their eggs. Since l-2HG levels undergo dramatic changes during embryogenesis, the inadvertent collection of asynchronous embryo samples will result in unreliable measurements.
Embryos do not need to be dechorionated. The bead mill homogenizer will destroy intact embryos.
Be careful that all beads remain in the tube during this transfer.
L3 larvae must be carefully synchronized because l-2HG levels drop precipitously during the latter half of this developmental stage. We recommend resynchronizing larvae at the L2–L3 molt. Methods for synchronizing larvae have been previously described [16, 17].
Sample should remain on ice for a maximum of 30 min. If necessary, wash and freeze subsets of samples.
Do not collect larvae in 2 mL bead tubes. The residual wash buffer will remain attached to the beads, resulting in inaccurate mass measurements and GC-MS artifacts.
While white prepupae only exhibit basal levels of d- and l-2HG, the abundance of these molecules during most of pupal development remains undefined. Therefore, any studies of 2HG metabolism must first determine how d- and l-2HG change in precisely staged pupal samples.
2HG appears to degrade in samples stored at −80 °C. Therefore, all samples should be processed within 3 months of collection.
This section of the protocol is optimized to quench metabolism and must be performed in an efficient and careful manner. Be sure all solutions are prepared in advance and chilled to −20 °C. Do not allow samples to thaw prior to homogenization in methanol.
We recommend using positive-placement pipettes for all steps involving the transfer of organic solvents.
Rapid homogenization will heat the sample. We recommend that the bead mill homogenizer is stored and used in a 4 °C controlled temperature room.
If sufficient sample material was collected at the beginning of the analysis (>15 mg), individual samples can be split in half immediately following this centrifugation step. One half of the sample is then derivatized and analyzed as described in the remainder of the protocol. Meanwhile, the other half of the sample can then be analyzed using a previously described metabolomic protocol that relies on derivatization by methoxylamine hydrochloride and N-methyl-N-trimethylsilyltrifluoracetamide [11–13]. This dual approach provides for quantitative measurements of d- and l-2HG while also allowing for a more extensive metabolomic comparison of the samples. In theory, samples derivatized as described in Subheading 3.3 could also be analyzed for other metabolites; however, there is no standard library currently available for this method.
If the samples were stored at −80 °C, place the unopened tubes in a vacuum centrifuge. Dry the outside of the sample tube for 30 min at room temperature. This step will remove and condense water from the outside of the tube.
Heating can dislodge the lid of microfuge tubes. Be sure that the tube lid is securely fastened throughout the 3-h incubation. Occasionally check to make sure that the sample tube lid does not become dislodged as the temperature reaches 90 °C.
Be sure that the microfuge tube lid is securely fastened throughout the 1 h incubation. Occasionally check to make sure that the sample tube lid does not become dislodged as the temperature reaches 80 °C. Limit the samples’ exposure to water vapor.
The time can be changed according to the GC-MS system.
This protocol is optimized for the sample sizes described herein. 2 μL of sample can be injected if the intensity of the peaks is too low.
Acknowledgment
J.M.T. is supported by a R35 Maximizing Investigators’ Research Award (MIRA; 1R35GM119557) from the National Institute of General Medical Sciences of the National Institutes of Health.
References
- 1.Losman JA, Kaelin WG Jr (2013) What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev 27(8):836–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye D, Guan KL, Xiong Y (2018) Metabolism, activity, and targeting of D- and L-2-hydroxyglutarates. Trends Cancer 4(2):151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra P et al. (2018) ADHFE1 is a breast cancer oncogene and induces metabolic reprogramming. J Clin Invest 128(1):323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldham WM et al. (2015) Hypoxia-mediated increases in l-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab 22(2):291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intlekofer AM et al. (2015) Hypoxia induces production of L-2-hydroxyglutarate. Cell Metab 22(2):304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyrakis PA et al. (2016) S-2-hydroxyglutarate regulates CD8 + T-lymphocyte fate. Nature 540(7632):236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan J et al. (2015) Human phosphoglycerate dehydrogenase produces the oncometabolite D-2-hydroxyglutarate. ACS Chem Biol 10(2):510–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng X et al. (2016) Lactate dehydrogenase C 13 produces S-2-hydroxyglutarate in mouse testis. ACS Chem Biol 11(9):2420–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Struys EA et al. (2005) Kinetic characterization of human hydroxyacid-oxoacid transhydroge-14 nase: relevance to D-2-hydroxyglutaric and gamma-hydroxybutyric acidurias. J Inherit Metab Dis 28(6):921–930 [DOI] [PubMed] [Google Scholar]
- 10.Becker-Kettern J et al. (2016) Saccharomyces 15 cerevisiae Forms D-2-hydroxyglutarate and couples its degradation to D-lactate formation via a cytosolic transhydrogenase. J Biol Chem 291(12):6036–6058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H et al. (2017) Drosophila larvae synthesize 16 the putative oncometabolite L-2-hydroxyglutarate during normal developmental growth. Proc Natl Acad Sci U S A 114(6):1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinecke CJ et al. (2012) Metabolomics of urinary organic acids in respiratory chain deficiencies in children. Metabolomics 8(2):264–283 [Google Scholar]
- 13.Nadtochiy SM et al. (2016) Acidic pH Is a metabolic switch for 2-hydroxyglutarate generation and signaling. J Biol Chem 291(38):20188–20197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitman ZJ et al. (2015) Genetic dissection of leukemia-associated IDH1 and IDH2 mutants and D-2-hydroxyglutarate in Drosophila. Blood 125(2):336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson KM et al. (1993) Stable-isotope dilution analysis of D- and L-2-hydroxyglutaric acid: application to the detection and prenatal diagnosis of D- and L-2-hydroxyglutaric acidemias. Pediatr Res 34(3):277–280 [DOI] [PubMed] [Google Scholar]
- 16.Ashburner M, Bownes M, Abrahamssen N, Gilman C (2005) Drosophila: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, pp 121–184 [Google Scholar]
- 17.Li H, Tennessen JM (2017) Methods for studying the metabolic basis of Drosophila development. Wiley Interdiscip Rev Dev Biol 6(5):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]