Abstract
Background.
Patients with myotonic dystrophy (DM) are at high risk of brain cancer. This study described the spectrum of brain neoplasms in DM patients.
Methods.
Data from 1,119 DM patients identified from the National Swedish Patient Register between 1987 and 2007 were linked to the National Cancer and the Cause of Death Registers. We calculated standardized incidence ratios (SIR), and cumulative incidence to quantify the relative and absolute risks of brain neoplasms, and used the Kaplan-Meier estimator for survival analysis. Patient follow-up started at birth or the age at the start of Swedish cancer registration (January 1, 1958), and ended at the age of brain neoplasm diagnosis, death, or in December 31, 2007.
Results.
Twenty patients developed brain neoplasm during follow-up (median age=53, range=2–76 years, accounting for a five-fold excess risk of brain tumors during patient lifetime (Standardized incidence ratio, SIR=5.4 [95%CI=3.4–8.1, p=1×10−5]). Astrocytoma was the most common histological subtype (n=16, 80%), and almost all cases (n=19) developed after age 20. No statistically significant differences in gender-specific risks (SIR in men= 6.3, and in women=3.8, p-heterogeneity=0.46) were observed. After accounting for competing mortality related to DM, the cumulative incidence of brain neoplasms reached 2.9% (95%CI=1.8–4.7%) by age 70. Five-year survival after brain tumor diagnosis was 52% (95%CI=29–75%) overall (number at risk=8), and 34% (95% CI=26–47%) for malignant neoplasms (number at risk=5).
Conclusion.
Despite the high relative risk of DM-related brain tumors, the absolute risk is modest. Nonetheless, careful evaluation of DM patients with new central nervous system symptoms is warranted.
Keywords: Myotonic dystrophy, neoplasms, cancer, brain, incidence, mortality
INTRODUCTION
Myotonic dystrophy (DM) is an autosomal dominant, progressive, multisystem disorder which presents clinically with muscle weakness and myotonia [1]. Two disease subtypes have been identified: DM type1 (DM1) - caused by a CTG-repeat expansion in the dystrophia myotonica protein kinase (DMPK) gene [2–4], and DM type2 (DM2) -caused by a CCTG-repeat expansion in the CCHC-type zinc finger protein (CNBP) gene [5]. Other DM clinical manifestations include early-onset cataract, diabetes, pulmonary insufficiency, cardiac arrhythmias, neurocognitive abnormalities [1], and certain cancers [6–10]. Brain imaging studies mostly in DM1 patients, have documented various abnormalities including white matter lesions [11–14], dilated Virchow-Rabin spaces [15], cortical atrophy [14, 16], and scattered cerebral hypoperfusion [17]. The presence of these lesions has been correlated with disease severity [18], duration [15], CTG repeat size [19], and patient intellectual status [13]. Severe cognitive impairment are frequently observed in patients with congenital or childhood DM [20]. Thus, the brain is clearly one of several syndrome-related target organs in DM patients.
In our large population-based cohort of Swedish and Danish DM patients, statistically significant excess of all cancers combined, and specifically increased risks of cancers of the colon, ovary, endometrium and brain were identified [6]. The risk of developing brain cancer after being diagnosed with DM was five-fold higher than in the general population. Brain neoplasms are generally rare, and their survival outcome depends on tumor histology, grade, and patient age, with 5-year survival probabilities of 5% or less after a diagnosis with glioblastoma and as high as 90% for meningioma [21–23]. In the general population, gliomas are the most common type of brain tumors, accounting for approximately 45% of all cases and 80% of those with malignant disease; three-fourths of these are astrocytomas and glioblastomas [22]. Ionizing radiation [24] and a positive family history of brain tumors [25] are among the few risk factors identified. Brain cancer risk has been associated with multiple inherited cancer predisposition syndromes including Li-Fraumeni syndrome, Turcot syndrome (a variant of hereditary non-polyposis colorectal cancer), neurofibromatosis types 1 and 2, von Hippel Lindau syndrome, familial adenomatous polyposis, nevoid basal cell carcinoma syndrome, melanoma/astrocytoma syndrome and Cowden syndrome (reviewed in [26]). The exact carcinogenic mechanism underlying the brain cancer susceptibility in DM patients is unknown, but it has been attributed to an alternative splicing defect within the pre-mRNA of specific genes, as has been suggested as the basis for other DM-related phenotypes [27, 28].
This study aimed to describe the full spectrum of DM-related brain neoplasms, calculate the lifetime relative and absolute risks of brain tumors, and estimated survival probabilities after their diagnosis in DM patients.
MATERIALS & METHODS
Study participants
We identified all patients with a DM discharge diagnosis (the International Classification of Diseases (ICD) 9th version=359C or ICD-10=G711) in the hospital inpatient or outpatient records of the National Swedish Patient Register between January 1, 1987 and December 31, 2007 (n=1,121), as described in previously [6, 9]. Briefly, the National Swedish Patient Register started in 1964 by collecting inpatient records, and reached complete hospitalization coverage in 1987; outpatient records were added in the year 2000 [29]. Patient hospital data were linked to the National Cancer Register to identify cancer diagnosis, and to the Cause of Death Register to ascertain patient vital status and date of death. Benign or malignant brain neoplasms were identified using ICD-7 code 193.0. For this analysis, we only included primary tumors identified through the pathology anatomic diagnostic [PAD] codes.
The study was conducted in accordance to the Declaration of Helsinki ethical standard. The requirement for informed consent was waived because we did not have direct contact with study subjects, or access to identifiable information. An exemption from the National Institute of Health (NIH) Institutional Review Board review was obtained from the National Institutes of Health Office of Human Subjects Research because we used existing data without personal identifiers.
Statistical Analysis
Study follow-up started at birth, or age on January 1, 1958 (start of cancer registration) for those born prior to 1958. Follow-up ended at the age of diagnosis of a brain tumor, death, or the age on December 31, 2007. Two patients were excluded from the analysis because they died at the start of follow-up.
To evaluate the lifetime risk of developing a brain tumor in DM patients, we calculated standardized incidence ratios (SIRs), and 95% confidence intervals (CIs) for primary brain neoplasms, overall, and stratified by: sex, age at cancer diagnosis (0–19, 20–59 and ≥60 years), and age at first DM discharge diagnosis (<20 and ≥20 years). The SIR was defined as the ratio between the observed numbers of brain neoplasms versus those expected. Expected numbers were calculated by applying age-, sex-, and calendar time–specific population incidence rates from the Swedish National Cancer Register to the person-years observed among DM cases. For these analyses, we used SEER*Stat software version 8.1.5 (seer.cancer.gov/seerstat), and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA)
We estimated the cumulative incidence of brain tumors accounting for competing risk events identified as death from a non-brain cancer cause non-parametrically using (STATA stcompet [30]) (Stata, release 13; StataCorp, College Station, TX, USA). We used the Kaplan-Meier method (SPSS software version 21; Armonk, NY: IBM Corp.) to estimate the 3- and 5-year overall survival after the diagnosis of a brain neoplasm in DM patients.
RESULTS
Patient Characteristics:
The study included 1,119 DM patients who contributed 45,183 person-years of follow-up, of whom 20 had a diagnosis of a primary brain tumor in the National Cancer Register. DM patients developed brain tumors at a median age of 53 years (range=2–76 years), and 55% (n=11) were males. Almost all patients who developed a brain neoplasm (n=19/20) had their first DM diagnosis at age ≥20 years, none had a co-diagnosis of severe cognitive impairment (one of the congenital DM hallmarks), and one patient had multiple independent primary cancers (ovary, brain, and endometrium). Histologically, 16 patients had astrocytoma (PAD codes 475 and 476; 4 were low-grade, and 12 high-grade), 2 patients had meningeal tumors (meningioma: PAD code 461, and meningeal sarcoma: PAD code 466), and 2 patients had a brain tumor not otherwise specified (NOS) (not specified malignant or benign: PAD code 991, malignant NOS: PAD code 996). Table 1 summarizes the characteristics of DM patients who developed brain tumors by tumor characteristic.
Table1:
Characteristics of the DM patients with a diagnosis of brain tumor
| Benign or low grade tumorsa (N=5) |
Malignant tumors (N=14) |
|
|---|---|---|
| N (%) | ||
| Sex | ||
| Male | 2 (40) | 9 (64.3) |
| Female | 3 (60) | 5 (35.7) |
| Age at first DM diagnosis | ||
| 0–29 | 1 (20) | 1 (7.1) |
| 30–54 | 3 (60) | 4 (28.6) |
| ≥55 | 1 (20) | 9 (64.3) |
| Calendar year at first DM diagnosis | ||
| 1987–1993 | 0 (0) | 3 (21.5) |
| 1994–2000 | 1 (20) | 5 (35.7) |
| 2001–2007 | 4 (80) | 6 (42.8) |
| Age at brain tumor diagnosis | ||
| 0–19 | 1 (20) | 0 (0) |
| 20–49 | 3 (60) | 4 (28.6) |
| 50–64 | 1 (20) | 5 (35.7) |
| ≥65 | 0 (0) | 5 (35.7) |
| Calendar year at brain tumor diagnosis | ||
| 1958–1986 | 0 (0) | 1 (7.1) |
| 1987–1993 | 2 (40) | 2 (14.3) |
| 1994–2000 | 1 (20) | 8 (57.1) |
| 2001–2007 | 2 (40) | 3 (21.5) |
| Died during follow-up | ||
| Yes | 0 (0) | 11 (78.6) |
| No | 5 (100) | 3 (21.4) |
One patient was excluded because the PAD code did not specify if the brain tumor was benign or malignant
Lifetime relative and absolute risk of brain neoplasms in DM patients:
Compared with the Swedish general population, DM-related brain tumor risk was 5-fold higher than the general population (SIR=5.4, 95%CI=3.4–8.1, p=1×10−5). No statistically significant differences by gender were noted (SIR: males=6.3, 95%CI=3.2–11.3; females=3.8, 95% CI=1.8–6.9), p-heterogeneity=0.46). The risk of brain tumors in DM patients increased with age (SIR=2.6, 95% CI=0.1–12.6 in <20 years old; SIR=4.0, 95% CI=1.5–8.9 in 20–59 years old, and SIR= 8.8, 95% CI= 3.6–18.4 in patients ≥60 years), a difference that was not statistically significant (p-heterogeneity=0.46). Similarly, the brain tumor risk was not statistically different between patients who had their first DM diagnosis at <20 or ≥ 20 years old (SIR=2.5, 95% CI=0.1–12.3 vs. 5.7, 95% CI=3.6–8.7, p- heterogeneity=0.46) (Table 2).
Table 2:
Standardized incidence ratios of brain tumors in Swedish DM patients
| Observed | Expected | SIR (95% CI) | P value | |
|---|---|---|---|---|
| Overall | 20 | 3.7 | 5.4 (3.4–8.1 | 1×10−5 |
| Age in years | ||||
| 0–19 | 1 | 0.39 | 2.6 (0.1–12.6) | 0.4 |
| 20–59 | 5 | 1.25 | 4.0 (1.5–8.9) | 0.01 |
| ≥60 | 6 | 0.68 | 8.8 (3.6–18.4) | 8×10−5 |
| Sex | ||||
| Male | 11 | 1.7 | 6.5 (3.4–11.2) | <1×10−5 |
| Female | 9 | 2.4 | 3.8 (1.8–6.9) | 0.001 |
| Age at first DM diagnosis | ||||
| <20 years | 1 | 0.4 | 2.5 (0.1–12) | 0.4 |
| ≥ 20 years | 19 | 3.3 | 5.7 (3.5–8.7) | <1×10−5 |
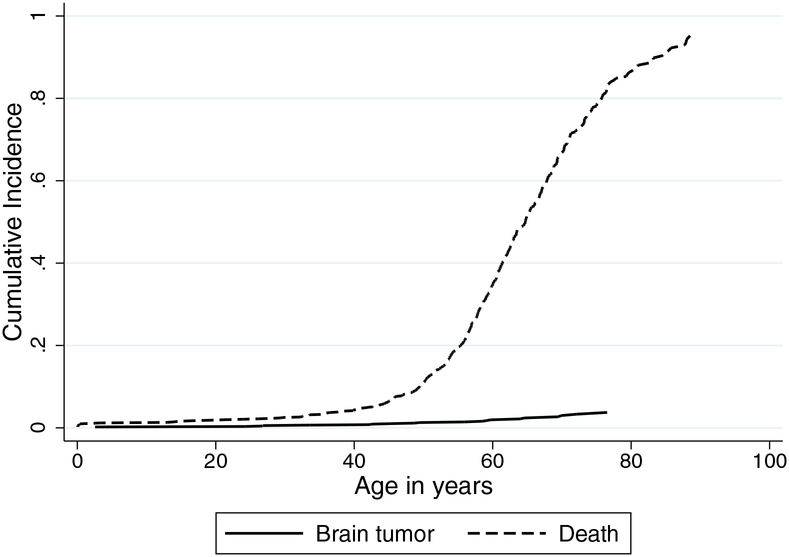
The cumulative incidence of brain tumors in DM patients accounting for competing mortality was: 1.3% (95%CI=0.6–2.3%) by age 50 years, 1.9% (95%CI=1.1–3.2%) by age 60, and 2.9% (95%CI=1.8–4.7%) by age 70 (Figure 1).
Figure 1:
Lifetime cumulative incidence of brain tumors and death in patients with myotonic dystrophy
Survival patterns after the diagnosis of brain tumor:
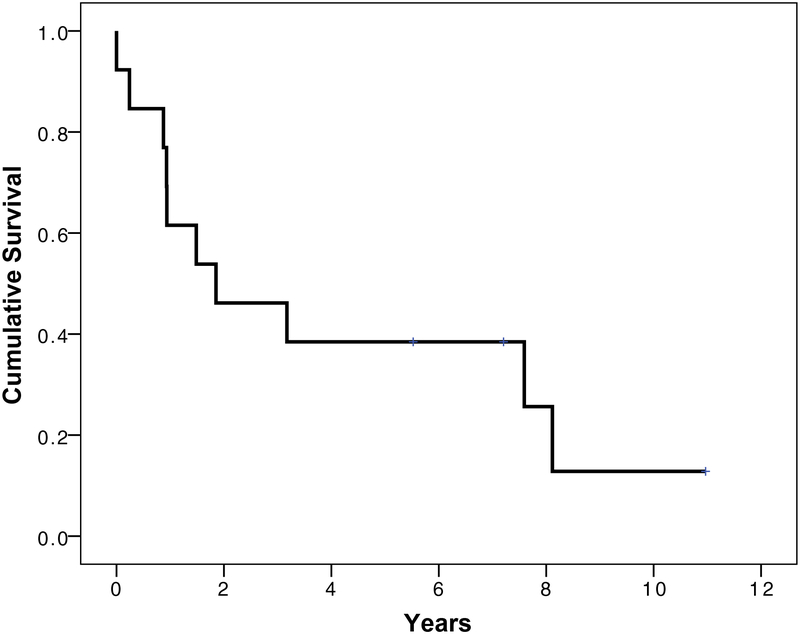
The 3- and 5-year overall survival probabilities after brain tumor diagnosis in DM patients were: 65% (95% CI= 43–86%), and 52% (95%CI=29–75%), respectively (Figure 2). When restricted to patients with malignant tumors (n=14; 12 high grade astrocytoma, 1 meningiosarcoma, and 1 malignant NOS), the 3 and 5-year survival probabilities were: 43% (95% CI=18–68%), and 34% (95% CI=26–47%), respectively.
Figure 2:
Survival patterns in myotonic dystrophy patients after brain tumor diagnosis
DISCUSSION
Our study, based on updated follow-up of a Swedish DM cohort, and including all brain tumors regardless of the date of the first recorded DM clinical diagnosis, confirmed that brain tumors are part of the DM syndrome phenotype. Similar to our previous observation [6] (based on 7 cases - 2 of which were from Sweden - and estimates restricted to events occurring after DM diagnosis), our current analysis showed a significantly elevated risk of DM-related brain neoplasms (SIR=5.4, 95%CI=3.4–8.1) based on 20 cases observed). Two reports published subsequent to ours have confirmed the significant excesses of cancers in DM patients, but these small studies identified brain cancer in only 1 of 307 patients and 0 of 109 patients, respectively [7, 8]. Both were under-powered to detect an excess risk of rare malignancies like those originating in the brain.
Our data indicate that brain neoplasms occur primarily in adult DM patients; only one of the 20 cases occurred prior to age 20. The risk increased with age and peaked in patients aged 60 or older, consistent with published literature of incidence rate of brain tumors in the general population by age categories across Europe [23]. The histopathology was known for 18 of the 20 brain tumors observed in this cohort, and it did not differ from that seen in the general population.
Several studies have suggested a possible excess risk of cutaneous melanoma in DM patients [6–8, 10]. A genetic link between cutaneous melanoma and tumors of the nervous system has been described in familial cutaneous melanoma studies [OMIM #155755] [31, 32]. Of note, none of the brain tumor patients in our study had cutaneous melanoma.
While the relative risk for developing brain tumors among DM patients was substantial and statistically significant, with a five-fold increase relative to that observed in the Swedish general population, the absolute risk was small, approximately 2% by age 60. Consequently, only a small number of DM patients will suffer this outcome, and brain tumors will contribute only a small additional morbidity and mortality burden to DM patients. There is no brain tumor screening modality that has been proven to reduce mortality; coupled with the low absolute brain tumor risks, we cannot recommend that active screening for brain tumors be employed in DM patients. However, careful evaluation of new symptoms suggestive of brain cancer is still warranted. Our analysis suggests a possible better survival for DM-related brain tumor cases (2-year survival probability= 100% for patients with benign or low grade tumors and 50% for patients with high grade neoplasms, respectively), than their population-based Swedish counterparts (2-year survival probability= 72% and 15%, for low-grade and high-grade tumors, respectively) [33]. Given that DM patients have a multitude of medical problems that require close medical follow-up early in their disease course, it is possible that longer survival after DM-associated brain cancer diagnosis may be due to lead-time bias. That said, we cannot at present exclude the possibility that the biological aggressiveness of DM-associated brain tumors may differ from their sporadic counterparts.
Of further note, our data did not support a possible connection between the risk of brain tumor and congenital DM. Specifically, none of the brain cancer patients carried a co-diagnosis of severe cognitive impairment, versus 1.2% of the non-cases. Severe cognitive impairment is one of the common features of congenital and childhood DM, the most severe form of this disease [34].
This study’s strengths include its population-based design, nearly complete follow-up, the objective confirmation of cancer diagnoses, and the overall high diagnostic accuracy of the Swedish registries [29, 35]. The main limitations include: small sample sizes that limit study power relative to specific cancer sites, and the lack of genetic information resulting in our inability to distinguish between DM1 and DM2, or to evaluate possible correlations between trinucleotide repeat length and cancer risk. However, we expect that most of the cases in this study were DM1, because it is more prevalent, and it was identified and molecularly characterized before DM2. Longer repeat length does correlate positively with DM severity, so it might be predicted that patients with longer repeats might be at increased risk of developing neoplasms. Findings from our study using data collected by the US Myotonic Dystrophy Registry did not support this hypothesis [36], but an adequately powered analysis of this question has yet to be performed.
In conclusion, based on a larger number of events, we have confirmed that the risk of brain neoplasms is significantly elevated in DM patients, and have demonstrated that the associated lifetime absolute risk is small (approximately 2.0% by age 60). DM-related brain tumor development is almost exclusively an adult-onset event, and was not associated with severe cognitive impairment/congenital DM in our data set. Since brain tumors in DM patients are relatively rare, and there is no proven brain cancer screening/early detection strategy that has been proven to improve patient survival, we do not currently recommend any specific brain tumor related screening program for DM patients. Nonetheless, it is important to carefully evaluate CNS-symptoms suggestive of brain cancers in DM patients, particularly in light of the possibility that DM-associated brain tumors may have a better than expected prognosis.
Acknowledgments:
The authors thank Ms. Shiva Ayoubi, the National Board of Health and Welfare, Stockholm, Sweden, Ms. Emily Carver and Mr. Joseph Barker, Information Management Services, Silver Spring, MD, for important contributions to the development of this database.
Funding: This work was supported by the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the National Cancer Institute, USA (contract N02CP31003–3).
Footnotes
Conflict of Interest: None of the manuscript authors has conflict of interest to disclose
REFRENCES
- [1].Harper PS. Myotonic dystrophy. Philadelphia: WB Saunders, 2001. [Google Scholar]
- [2].Brook JD, Mccurrach ME, Harley HG, et al. Molecular-Basis of Myotonic-Dystrophy - Expansion of a Trinucleotide (Ctg) Repeat at the 3’ End of a Transcript Encoding a Protein-Kinase Family Member. Cell. 1992. 68: 799–808. [DOI] [PubMed] [Google Scholar]
- [3].Fu YH, Pizzuti A, Fenwick RG Jr., et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992. 255: 1256–1258. [DOI] [PubMed] [Google Scholar]
- [4].Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3’ untranslated region of the gene. Science. 1992. 255: 1253–1255. [DOI] [PubMed] [Google Scholar]
- [5].Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001. 293: 864–867. [DOI] [PubMed] [Google Scholar]
- [6].Gadalla SM, Lund M, Pfeiffer RM, et al. Cancer risk among patients with myotonic muscular dystrophy. JAMA. 2011. 306: 2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Win AK, Perattur PG, Pulido JS, Pulido CM, Lindor NM. Increased cancer risks in myotonic dystrophy. Mayo Clin Proc. 2012. 87: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mohamed S, Pruna L, Kaminsky P. [Increasing risk of tumors in myotonic dystrophy type 1]. Presse Med. 2013. 42: e281–284. [DOI] [PubMed] [Google Scholar]
- [9].Gadalla SM, Pfeiffer RM, Kristinsson SY, et al. Quantifying cancer absolute risk and cancer mortality in the presence of competing events after a myotonic dystrophy diagnosis. PLoS One. 2013. 8: e79851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zampetti A, Silvestri G, Manco S, et al. Dysplastic nevi, cutaneous melanoma, and other skin neoplasms in patients with myotonic dystrophy type 1: a cross-sectional study. J Am Acad Dermatol. 2015. 72: 85–91. [DOI] [PubMed] [Google Scholar]
- [11].Vielhaber S, Jakubiczka S, Gaul C, et al. Brain 1H magnetic resonance spectroscopic differences in myotonic dystrophy type 2 and type 1. Muscle Nerve. 2006. 34: 145–152. [DOI] [PubMed] [Google Scholar]
- [12].Fukuda H, Horiguchi J, Ono C, Ohshita T, Takaba J, Ito K. Diffusion tensor imaging of cerebral white matter in patients with myotonic dystrophy. Acta Radiol. 2005. 46: 104–109. [DOI] [PubMed] [Google Scholar]
- [13].Kuo HC, Hsieh YC, Wang HM, Chuang WL, Huang CC. Correlation among subcortical white matter lesions, intelligence and CTG repeat expansion in classic myotonic dystrophy type 1. Acta Neurol Scand. 2008. 117: 101–107. [DOI] [PubMed] [Google Scholar]
- [14].Kornblum C, Reul J, Kress W, et al. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol. 2004. 251: 710–714. [DOI] [PubMed] [Google Scholar]
- [15].Di Costanzo A, Di Salle F, Santoro L, Tessitore A, Bonavita V, Tedeschi G. Pattern and significance of white matter abnormalities in myotonic dystrophy type 1: an MRI study. J Neurol. 2002. 249: 1175–1182. [DOI] [PubMed] [Google Scholar]
- [16].Antonini G, Mainero C, Romano A, et al. Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004. 75: 1611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meola G, Sansone V, Perani D, et al. Reduced cerebral blood flow and impaired visual-spatial function in proximal myotonic myopathy. Neurology. 1999. 53: 1042–1050. [DOI] [PubMed] [Google Scholar]
- [18].Kuo HC, Hsiao KM, Chen CJ, Hsieh YC, Huang CC. Brain magnetic resonance image changes in a family with congenital and classic myotonic dystrophy. Brain Dev. 2005. 27: 291–296. [DOI] [PubMed] [Google Scholar]
- [19].Romeo V, Pegoraro E, Ferrati C, et al. Brain involvement in myotonic dystrophies: neuroimaging and neuropsychological comparative study in DM1 and DM2. J Neurol. 2010. 257: 1246–1255. [DOI] [PubMed] [Google Scholar]
- [20].Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007. 36: 294–306. [DOI] [PubMed] [Google Scholar]
- [21].Sant M, Minicozzi P, Lagorio S, et al. Survival of European patients with central nervous system tumors. Int J Cancer. 2012. 131: 173–185. [DOI] [PubMed] [Google Scholar]
- [22].Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012. 14 Suppl 5: v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crocetti E, Trama A, Stiller C, et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012. 48: 1532–1542. [DOI] [PubMed] [Google Scholar]
- [24].Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012. 14: 1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malmer B, Henriksson R, Gronberg H. Familial brain tumours-genetics or environment? A nationwide cohort study of cancer risk in spouses and first-degree relatives of brain tumour patients. Int J Cancer. 2003. 106: 260–263. [DOI] [PubMed] [Google Scholar]
- [26].Kyritsis AP, Bondy ML, Rao JS, Sioka C. Inherited predisposition to glioma. Neuro Oncol. 2010. 12: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Du H, Cline MS, Osborne RJ, et al. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nat Struct Mol Biol. 2010. 17: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuyumcu-Martinez NM, Cooper TA. Misregulation of alternative splicing causes pathogenesis in myotonic dystrophy. Prog Mol Subcell Biol. 2006. 44: 133–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011. 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Coviello VB M Cumulative incidence estimation in the presence of competing risks. Stata Journal. 2004. 4: 103–112. [Google Scholar]
- [31].Kaufman DK, Kimmel DW, Parisi JE, Michels VV. A familial syndrome with cutaneous malignant melanoma and cerebral astrocytoma. Neurology. 1993. 43: 1728–1731. [DOI] [PubMed] [Google Scholar]
- [32].Bahuau M, Vidaud D, Kujas M, et al. Familial aggregation of malignant melanoma/dysplastic naevi and tumours of the nervous system: an original syndrome of tumour proneness. Ann Genet. 1997. 40: 78–91. [PubMed] [Google Scholar]
- [33].Mathiesen T, Peredo I, Lonn S. Two-year survival of low-grade and high-grade glioma patients using data from the Swedish Cancer Registry. Acta Neurochir (Wien). 2011. 153: 467–471. [DOI] [PubMed] [Google Scholar]
- [34].Echenne B, Bassez G. Congenital and infantile myotonic dystrophy. Handb Clin Neurol. 2013. 113: 1387–1393. [DOI] [PubMed] [Google Scholar]
- [35].Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta RadiolOncol. 1984. 23: 305–313. [DOI] [PubMed] [Google Scholar]
- [36].Das M, Moxley RT 3rd, Hilbert JE, et al. Correlates of tumor development in patients with myotonic dystrophy. J Neurol. 2012. 259: 2161–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]