Abstract
Purpose
Endoglin (CD105) is an endothelial cell membrane receptor highly expressed on proliferating tumor vasculature, including that of HCC, and is associated with poor prognosis. Endoglin is essential for angiogenesis and its expression is induced by hypoxia and VEGF pathwayinhibition. TRC105 is a chimeric IgG1 CD105 monoclonal antibody that inhibits angiogenesis and causes antibody-dependent cellular cytotoxicity (ADCC) and apoptosis of proliferating endothelium.
Experimental Design
Patients with HCC (Childs Pugh A/B7), ECOG 0/1, were enrolled in a phase I study of TRC105 at 3, 6, 10, 15mg/kg q 2wks given with sorafenib 400mg bid. Correlative biomarkers included DCE-MRI and plasma levels of angiogenic factors, including soluble endoglin. Pharmacokinetics were assessed in serum.
Results
26 patients were enrolled, of whom 25 received treatment, 15 with cirrhosis. Hep B/C: 3/15; M:F 19:6; Mean age of 60 (range 18–76); 1 DLT (grade 3 AST) occurred at 10mg/kg. The most frequent toxicity was low grade epistaxis, a known toxicity of TRC105. One patient experienced an infusion reaction and was replaced. One patient with coronary stenosis developed a fatal myocardial infarction and one patient developed G3 cerebral tumor hemorrhage. Maximum tolerated dose was not established and DL4 (15mg/kg) was expanded. The overall response rate in 24 evaluable patients at all 4 dose levels was 21% (95% CI: 7.142.2%), and 25% (95% CI: 8.7–49.1%) in patients with measureable disease. Four patients had confirmed stable disease, one of whom was treated for 22 months. Median PFS for 24 patients evaluable for PFS was 3.8 months (95% CI: 3.2–5.6 months); median OS was 15.5 months (95% CI: 8.5–26.3 months).
Conclusions
TRC105 combined with sorafenib was well tolerated at the recommended single agent doses of both drugs. Encouraging evidence of activity to date (PR rate 25%) was observed and the study is now continuing to recruit in the phase 2 stage as a multicenter study to confirm activity of the combination.
Introduction
The publication of the SHARP study in 2008 demonstrated that sorafenib improved overall survival in patients with HCC compared to placebo, and resulted in sorafenib becoming the standard of care for disease that occurs in the setting of preserved liver function which is not amenable to surgery, ablation or chemoembolization1. It also led to an enhanced focus on the development of anti-angiogenic therapies for HCC2. Since then, the results of several phase III studies which attempted to build on the initial promise of sorafenib have been disappointing, although recently regorafenib, another multikinase inhibitor, has shown a survival benefit in the second-line setting3. Single agent sorafenib remains the only approved treatment for the firstline treatment of advanced HCC
Endoglin (CD105), is an endothelial cell membrane receptor that is highly expressed on tumor vasculature, including that of HCC, and associated with poor prognosis4,5. Endoglin is essential for angiogenesis and its expression is upregulated by hypoxia and inhibitors of the VEGF pathway. Preclinical genetic knock-down and knock-out models implicate endoglin as a mechanism of resistance to VEGF pathway inhibition6,7. TRC105 is a chimeric IgG1 monoclonal endoglin antibody that inhibits angiogenesis (through the competitive inhibition of the activating endoglin ligand bone morphogenetic protein (BMP)) in addition to mediating antibodydependent cellular cytotoxicity (ADCC)8,9. We had previously assessed TRC105 in a phase 2 study in HCC, demonstrating lack of significant single-agent activity10. However, based on a strong scientific rationale for combination with another anti-angiogenic strategy and the finding of preclinical efficacy forendoglin antibody when combined with sorafenib in a murine syngeneic model of HCC we conducted an open-label single-arm phase I study and expansion cohort to assess the safety and efficacy of TRC105 combined with sorafenib in a sorafenib-naïve HCC patient population.
Patients and Methods
Preclinical experiments
Female BALB/c mice, 6–8 weeks of age, were obtained from NCI–Frederick (Frederick, MD, USA). BNL, a murine HCC cell line, was kindly provided from University of Navarra, Pamplona Spain11. Mycoplasma testing was performed by SAIC Frederick two months prior to experiments Cells were routinely kept in culture for no more than 8 to 10 passages. Mice were injected s.c. with 1 x106 BNL cells. One week after tumor inoculation when tumors are palpable, mice received a daily oral gavage of sorafenib (Bayer) at a dose of 10 mg/kg. Sorafenib stock solution (4x) was freshly prepared every 4 days using Cremophor EL/ethanol (50:50; Sigma). The final 1x dosing concentration was prepared by diluting with sterile water immediately prior to administration to mice. Control mice received vehicle. Endoglin antibody (clone MJ7/18), which binds murine endoglin, was purchased from the Developmental Studies Hybridoma Bank at the University of Iowa and purified at NCI. 100 mg/mouse was given i.p. every other day. Tumors were measured every other day using digital calipers. All mice were handled, fed, and housed in accordance with the U.S. Department of Health and Human Services institutional guidelines.
Experimental protocol was approved by NCI Animal Care and Use Committee (ACUC).
Clinical Trial
Eligible patients were at least 18 years old with histopathological confirmation of hepatocellular carcinoma (HCC) by the Laboratory of Pathology of the NCI. Other eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; adequate bone marrow, liver, and renal function; disease not amenable to potentially curative liver transplantation, resection or ablative techniques, and progression following or not be amenable to transhepatic arterial chemoembolization (TACE). Prior progressive disease on sorafenib was excluded. If liver cirrhosis was present, patients must have had a Child-Pugh A or B (7 points) classification. In addition, patients with cirrhosis were required to have had esophagogastric endoscopy within 6 months prior to study entry for the assessment of varices. Concomitant treatment of underlying cancer was prohibited. All patients provided written informed consent. This study was approved by the National Cancer Institute Institutional Review Board.
ClinicalTrials.gov identifier: NCT01306058.
Study design
Patients who satisfied the eligibility criteria were enrolled in a 3 + 3 dose escalation phase I study of TRC105 at dose cohorts of 3, 6, 10 and 15mg/kg given every two weeks in combination with sorafenib 400mg bid. A phase 2 cohort of the trial was opened afterwards at the MTD to establish the response rate to therapy. Prior to each TRC105 infusion patients were premedicated with dexamethasone, acetaminophen, H2-blockade and an anti-histamine prior to initial dosing and dexamethasone was then discontinued in the absence of infusion reactions. Staging was performed by either contrast-enhanced CT or, in select cases, MRI scan every 8 weeks. Objective response and progression were evaluated in this study using the international criteria proposed by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. DLT criteria included treatment-related grade 3 non-hematologic toxicities or grade 4 hematological toxicities occurring within the first 28 days of treatment. First dose infusion reactions, a known toxicity of TRC105, were not considered DLT. Patients were considered evaluable for safety if they received any study treatment and were considered evaluable for efficacy if they received at least one week of treatment with sorafenib and TRC105.
Safety
All adverse events and serious adverse events (SAEs) occurring within 30 days of the last dose were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Pharmacodynamic studies
Correlative biomarkers of TRC-105 effect were evaluated with radiologic techniques as well as assays performed on peripheral blood. All tests were performed at multiple time points including baseline and during the first and second 4-week cycles of treatment. Contrast enhanced magnetic resonance imaging (MRI) was employed to detect effects on tumor vasculature. Imaging with MRI was performed at two time points (at baseline and during cycle 2 day 1 +/− 2 days). Normalized signal intensity in unenhanced and enhanced MRIs were compared at each available time point with calculation of measured percentage of signal change to reflect tumor vascularity. MR imaging was performed on a 3T MR system (Philips Achieva, Best, The Netherlands) with a dedicated receive-only phased array coil.
Blood samples were collected in EDTA-containing Vacutainer at pretreatment (baseline), day 15 of the 1st cycle, day 1 of the 2nd cycls, and following treatment discontinuation. After centrifugation, plasma samples were immediately frozen and stored at −80°C. Plasma biomarker tests were performed for vascular endothelial growth factor (VEGF) and placental-derived growth factor (PlGF)using assay plates from Meso-Scale Discovery (MSD, Gaithersburg, MD) according to the product manual. The concentrations of the cytokines were determined with recombinant standards and expressed as pg/ml.
ELISA (R&D Systems) was used to determine the specific concentrations of soluble CD105 in plasma samples. The addition of TRC105 in vitro inhibited the detection of soluble CD105 and only plasma samples without detectable TRC105 serum concentrations were considered for analysis. ELISA was done per the manufacturer’s instructions.
Pharmacokinetics
TRC105 serum concentrations were measured using a validated ELISA with a lower limit of quantification (LLOQ) of 200 ng/mL. Pharmacokinetic samples were drawn just prior to, and ~5 minutes following IV infusion of TRC105 on days 1, 15, 30, 45 and 60.
Statistical methods
The primary objective of the phase 1 portion of the study was to determine the maximum tolerated dose (MTD) for TRC105 when given with sorafenib in HCC. Once this was established preliminary evidence of efficacy was assessed in an expansion phase 2 cohort to increase the experience with the combination and to determine if it was associated with a response rate which was likely to exceed that of sorafenib alone. Results from the SHARP study suggested that an overall response rate for sorafenib alone in this patient population was 2%1. The aim in the expansion phase II cohort was to rule out an unacceptably low PR + CR rate of 5% (p0=0.05) in favor of an improved PR + CR rate of 25% (p1=0.25). With alpha=0.10 (probability of accepting a poor treatment=0.10) and beta = 0.20 (probability of rejecting a good treatment=0.20), the trial was designed to enroll 6 evaluable patients in the first stage and if at least one response was noted, to continue enrollment until a total of 23 patients were enrolled, in which case 3 or more responses would be considered adequate demonstration of efficacy. Other secondary objectives of this trial were to evaluate progression-free and overall survival by the Kaplan-Meier method as well as pharmacodynamic markers of drug effect. Paired data from angiogenic biomarkers obtained on study and following study treatment were compared to pretreatment results using a Wilcoxon signed rank test. All p-values are two-tailed and presented without adjustment for multiple comparisons. Plasma biomarker analysis was performed with GraphPad Prism 7.0 as well as SAS version 9.3.
Results
Preclinical experiments
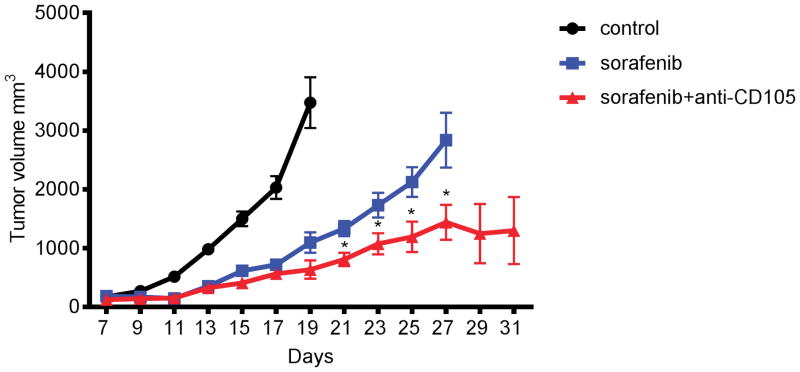
Based on the hypothesis that endoglin expression is upregulated by hypoxia and inhibitors of the VEGF pathway, such as sorafenib, and acts as a mechanism of resistance,6,7 we explored the potentially complementary roles of combined VEGF pathway and endoglin inhibition in a preclinical experiment. Seven days after BNL tumor inoculation, BALB/c mice were given daily oral gavage of sorafenib (10 mg/kg). Endoglin antibody (100 mg/mouse) was injected i.p. every other day. Tumor sizes are presented as mean ± SEM (n=14 for control, n=10 for sorafenib, n=10 for sorafenib with endoglin). As shown in Figure 1 the combination of sorafenib and endoglin antibody resulted in enhanced anti-tumor activity compared to sorafenib alone.
Figure 1.
Tumor volume over time following BNL tumor inoculation in BALB/c mice given daily oral gavage of sorafenib (10 mg/kg) with or without anti-CD105 antibody (100 mg/mouse injected i.p. every other day) or no treatment. Tumor sizes are presented as mean ± SEM (n=14 for control, n=10 for sorafenib, n=10 for sorafenib+anti-CD105).
Patient characteristics (Table 1)
Table 1.
Patient characteristics
| Total | 25 |
| 24/1 | |
|
| |
| Age | |
| HCC/Fibrolamellar | |
| Median (range) | 60 (18–76) |
|
| |
| Sex | |
| Male | 19 |
| Female | 6 |
|
| |
| ECOG | |
| 0 | 8 |
| 1 | 17 |
|
| |
| Liver Cirrhosis | |
| Yes | 15 |
| No | 10 |
|
| |
| Etiology of HCC | |
| HBV | 3 |
| HCV | 15 |
| Cryptogenic | 6 |
| Hemochromatosis | 1 |
|
| |
| Baseline Child Pugh Score | |
| 5 | 10 |
| 6 | 4 |
| 7 | 1 |
| NA | 10 |
|
| |
| Extrahepatic disease | |
| Yes | 17 No8 |
|
| |
| Prior therapies* | |
| No prior intervention | 9 |
| ≥2 locoregional procedures | 7 previous TACE8 |
| Surgery/Transplant | 5/2 |
| Ablation | 2 |
| Radioembolization | 2 |
Of note, some patients received more than one prior therapeutic intervention
Twenty-six patients were enrolled in a clinical trial evaluating escalating doses of TRC105 in combination with sorafenib given at standard doses of 400mg twice daily. One patient signed consent but developed rapid disease progression and did not receive any treatment. The baseline characteristics of the remaining evaluable (N=25) patients are presented in Table 1. The majority were male (M:F 19:6) with a median age of 60 (range 18–76). One patient had fibrolamellar variant HCC. Cirrhosis was present either by clinical or pathologic diagnosis in fifteen patients with a median Child-Pugh score of 5. The most common etiology for HCC was viral hepatitis. Fifteen patients had hepatitis C. Three patients had hepatitis B, all of whom were on anti-viral medication at the time of enrollment. All of the patients were Barcelona Clinic Liver Cancer (BCLC) stage C, for whom sorafenib was indicated. Seventeen of the patients had extrahepatic disease. Two patients had prior liver transplant and four had recurred following partial hepatectomy. Eight patients had prior locoregional therapies, which consisted of TACE, radioembolization or radiofrequency ablation.
Safety
Overall treatment was well tolerated. One patient developed a severe infusion reaction on the first dose of TRC105 and continued on sorafenib alone. The most frequent toxicities were headache and also chronic, intermittent grade 1 oral cavity bleeding or epistaxis, a known toxicity of TRC105, reflecting mucocutaneous telangiectasia. One patient who remained on therapy for over 2 years at the higher dose level required multiple tooth extractions, possibly related to his chronic gingival bleeding. The headache tended to occur in the first few days following TRC105 infusion and was only moderately responsive to analgesics. Two patients required anti-migraine medication. Three patients were treated at dose levels 1 (3mg/kg TRC105) or 2 (6mg/kg TRC105). (See supplemental Table 1 for dose level enrollment.) One patient developed DLT (grade 3 AST elevation) at dose level 3 (10mg/kgTRC105) and this dose level was expanded to six patients. One patient at dose level 2 developed a grade 3 cerebral hemorrhage attributed to a brain metastasis found on MRI scan, although a contributory effect of the investigational therapy could not be excluded. In addition, one patient at dose level 2 experienced fatal myocardial ischemia six weeks after starting on study, an event which was considered at least possibly attributable to therapy, although emergent angiography revealed extensive coronary artery disease. Dose escalation continued to dose level 4 (15 mg/kg TRC105) without further DLT, and this was determined to be the maximum tolerated dose. In total, twelve patients were treated at this dose level. Grade 3 or 4 treatment-related toxicities for all study participants are summarized in Table 2.
Table 2.
Toxicity
| Treatment-emergent adverse events (n =25) | ||||
|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Grade 5 | |
| Headache | 20 (80%) | |||
| Epistaxis | 19 (76%) | |||
| Increased aspartate transaminase | 18 (72%) | 5 (20%) | ||
| Rash, other | 18 (72%) | 3 (12%) | ||
| Hypophosphatemia | 18 (72%) | 7 (28%) | ||
| hypoalbuminemia | 17 (68%) | 2 (8%) | ||
| Anemia | 16 (64%) | 1 (4%) | ||
| Fatigue | 15 (60%) | |||
| Increased alkaline phosphatase | 15 (60%) | 5 (20%) | ||
| Diarrhea | 15 (60%) | 1 (4%) | ||
| Increased blood bilirubin | 14 (56%) | 6 (24%) | 1 (4%) | |
| Nausea | 14 (56%) | |||
| Increased alanine transaminase | 13 (52%) | |||
| Oral mucositis/pain | 12 (48%) | |||
| thrombocytopenia | 10 (40%) | 1 (4%) | ||
| Amylase | 10 (40%) | 2 (8%) | 1 (4%) | |
| Abdominal pain | 9 (36%) | |||
| Hand-foot skin reaction | 8 (32%) | 2 (8%) | ||
| Infusion reaction | 8 (32%) | 1 (4%) | ||
| Neutropenia | 8 (32%) | |||
| Weight loss | 8 (32%) | |||
| Hypertension | 6 (24%) | 1 (4%) | ||
| Vomiting | 6 (24%) | |||
| Hypomagnesemia | 5 (20%) | |||
| Alopecia | 5 (20%) | |||
| Insomnia | 4 (16%) | |||
| Constipation | 3 (12%) | |||
| Intracranial hemorrhage | 1 (4%) | |||
| Myocardial ischemia | 1 (4%) | |||
| Lipase | 1 (4%) | |||
| Hyperglycemia | 1 (4%) | 2 (8%) | ||
| Hyperuricemia | 2 (8%) | |||
Efficacy
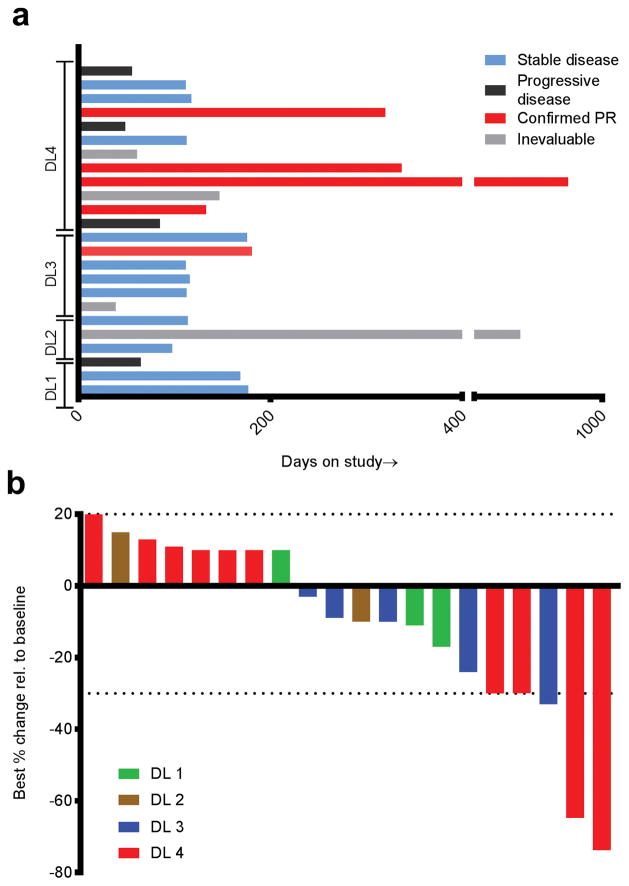
The overall response rate in 24 evaluable patients at all 4 dose levels was 5/24 = 21% (95% CI: 7.1–42.2%), and was 5/20 = 25% (95% CI: 8.7–49.1%) in patients with measureable disease. Four of the five responses occurred at the highest TRC105 dose level (dose level 4 at 15 mg/kg) and one response occurred at dose level 3 (Figure 2a). To obtain a better estimate of efficacy once the MTD was established, we expanded dose level 4 so that a total of N=12 patients were treated at this dose level, of whom four of 10 patients with measureable disease had response by RECIST (Figure 2b). Duration of response ranged from 4.4 months to 27.6 months. Examples of clinical responses are shown in Figure 3. One of the responses (Figure 3e–f) manifested primarily as extensive necrosis. There were no objective responders to study treatment at the lower dose levels. With a median potential follow-up of 36.5 months, the median time to tumor progression in this study was 3.8 months (95% CI: 3.2–5.6 months) with a median overall survival of 15.5 months (95% CI: 8.5–26.3 months); (Supplemental Figure 1), including one patient who died at 33.5 months after initiating treatment.
Figure 2.
Efficacy data for study population; (A) Swimmer’s plot showing time on study and nature of response; Red line = partial response; Blue = SD; Black = PD; Grey = non-evaluable; (B) Waterfall plot showing magnitude of best response as reflected by change in target lesion sum over time as per RECIST.
Figure 3.
(A, B and D): CT scans in a 61 year old gentleman with HCV-related HCC reduction in target lesion (white arrow) over time (A- baseline; B- 8 weeks; D – 12 months). 3C shows peripheral blood aFP measurement over time for the same gentleman; (E–F) MRI scan at baseline and after 4 months of treatment for another responding patient with HCV-related HCC showing marked necrotic response in tumor (denoted T).
Pharmacokinetics
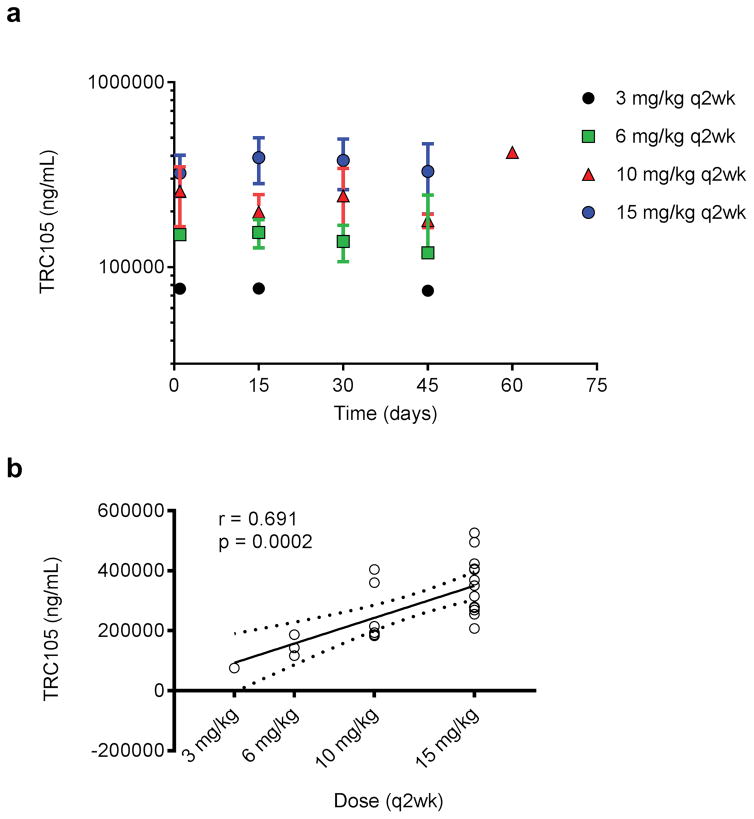
Mean peak TRC105 serum concentrations were plotted over time by dose level to assess accumulation. Using data from all 24 evaluable patients, it appeared that there was a slight accumulation from day 1 (first dose) to day 15 (second dose), but none thereafter (Figure 4a). Peak TRC105 serum concentrations were moderately-well correlated (Spearman r =0. 7) with dose, increasing in an apparent linear (dose-proportional) manner (Figure 4b). All TRC105 trough concentrations on dose level 1 (3 mg/kg) were below the lower limit of quantification (LLOQ) of 200 ng/mL. Only 1 of 3 patients at dose level 2 (6mg/kg), 3 of 6 patients at dose level 3 (10 mg/kg), and 8 of 12 patients at dose level 4 (15 mg/kg) had measurable trough levels of TRC105.
Figure 4.
Pharmacokinetics. (A) Mean peak TRC105 serum concentrations; (B) Dose proportional increases in mean per patient TRC105 peak serum concentrations
Pharmacodynamics
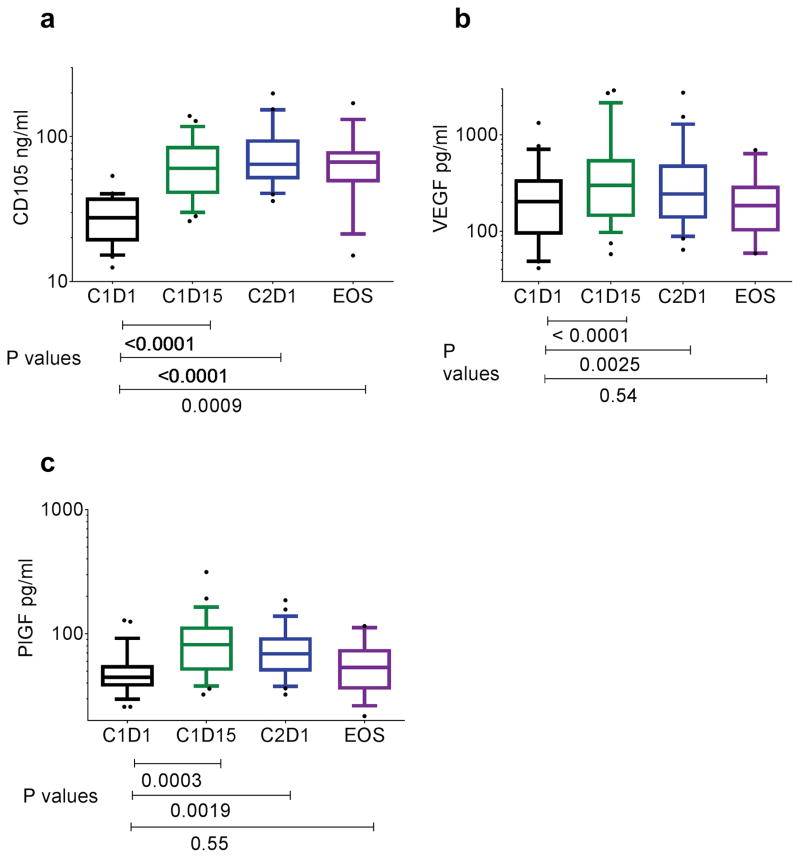
Because TRC105 interfered with the R&D Systems ELISA, soluble endoglin was only assessed in patient samples without detectable TRC105 concentrations. Median soluble endoglin levels increased prior to dosing in cycle 2 compared to baseline (64.5 versus 27.5 ng/ml p<0.0001) (Figure 5a). Median plasma levels of VEGF and PlGF increased after four weeks of therapy [243.4 versus 202.5 (p=0.0025) and 68.8 versus 44.6 (p=0.0019), respectively] (Figure 5b and c).
Figure 5.
Levels of a) CD105 b) VEGF and c) PlGF in plasma of study patients taken at Cycle 1 days 1 and 15, cycle 2 D1 and at end of study (EOS)
We evaluated the perfusion of the tumors with the analysis of normalized signal intensity in unenhanced and enhanced MRIs at each available time point with calculation of measured percentage of signal change to reflect tumor vascularity. Supplementary Figure 2 depicts a waterfall plot showing percentage of signal change in each evaluable patient compared to baseline. While the majority of patients exhibited decrease in signal intensity following treatment, there was no correlation with dose level and in only one case did a significant signal decrease correlate with objective response by conventional imaging.
Discussion
HCC has long been considered unique in terms of its reliance upon hepatic arterial blood supply and the presence of relative hypervascularity12. Indeed, these very features are taken advantage of to aid both diagnosis and treatment. The sole proven drug treatments for advanced disease – sorafenib, and more recently regorafenib – have anti-angiogenesis as their putative main mode of action2. VEGF pathway blockade – either by specific inhibition with antibody or as a result of multikinase inhibition – causes intratumoral hypoxia, which in turn leads to upregulation of hypoxia inducible factor (HIF)-1a and the compensatory, even counteractive, transcription of many pro-angiogenic genes13. There appears to be a close interplay between endoglin and VEGF levels8. Endoglin expression is one of the responses to hypoxia induced by anti-angiogenic, or – more specifically – anti-VEGF pathway agents8. It represents an attractive target in solid tumor oncology given this fact, and also because, by itself, endoglin is essential for endothelial cell proliferation and angiogenesis7. Mice lacking endoglin die in utero from the absence of angiogenesis5. Endoglin is densely expressed on the proliferating endothelial cells of many tumor types and has been correlated with a poor prognosis7. In HCC, endoglin was found to be expressed in 100% of surgically resected specimens (N=113) and highly specific for tumor areas in that neither the normal nor adjacent para-carcinomatous tissue stained positively for endoglin by immunohistochemistry4.
We found that endoglin -directed therapy, in combination with sorafenib, had enhanced antitumor efficacy in a preclinical mouse model. In a phase I clinical trial we found that when the humanized anti-endoglin monoclonal antibody, TRC105 was similarly combined with sorafenib in patients with advanced HCC there was evidence of efficacy, with objective, relatively durable, responses in a proportion of patients. The finding of objective responses was encouraging. In patients with advanced HCC the objective response rate to sorafenib monotherapy, as reported by the SHARP study and the Asian-Pacific study – the two large phase III trials resulting in its early approval – was 2% and 3% respectively1,14. In our study five patients demonstrated confirmed partial responses by standard RECIST criteria with an overall intention-to-treat objective response rate of 5/24 (21%) and a response rate of 5/20= 25% based on the 20 patients evaluable for response. The responses seemed to be dose-dependent in that four of ten evaluable (40%) patients treated at the highest dose level (TRC105 15mg/kg) achieved partial response. The other response occurred at dose level 3 (TRC105 10mg/kg), with no responses observed at the lower dose levels. The combination regimen was relatively well tolerated. One concern with combining anti-angiogenic agents is the bleeding risk, especially in a prone, generally thrombocytopenic HCC population who may have esophageal or gastric varices15. In our study, we mandated an upper endoscopy to exclude those at risk, and perhaps as a result did not observe any high grade bleeding events. One exception was a patient with a cerebral bleed in the setting of a brain metastasis, an event which may have been exacerbated by treatment. The main bleeding issue we experienced – as with sorafenib monotherapy – was chronic low grade, particularly gum bleeding, but also epistaxis, which did seem to impact on patients’ quality of life, particularly at the higher dose levels. In the future development of this combination any randomized design should include quality of life analysis to better assess this.
With regard to the correlative, pharmacodynamics studies, as expected we did observe that median soluble endoglin levels increased compared to baseline. This was consistent with our prior study evaluating TRC105 monotherapy and was also reported by Liu et al. who evaluated different doses of TRC105 ranging from 0.3 to 15 mg/kg every 2 weeks as well as some patients receiving 10 and 15 mg/kg weekly8,10. The increase in soluble endoglin levels following TRC105 treatment may be due to several factors, including prolonged stabilization of soluble endoglin due to TRC105 binding or increased shedding of soluble endoglin induced by TRC105 binding at the cell membrane. Hawinkels et al. have shown that endoglin shedding was mediated by matrix metalloproteinase (MMP)-14 and resulted in vitro in reduced spontaneous and vascular endothelial growth factor-induced endothelial sprouting16. Similarly, it would be expected that as a result of increased intratumoral hypoxia caused by anti-angiogenic therapy that levels of angiogenic biomarkers would increase during therapy, and we did observe this for VEGF and PlGF. This finding has not been universal. For example, Liu et al. in a phase I trial of TRC105 noted an decrease in VEGF-A amongst other biomarkers at 4 weeks8 . Of note, in our study there did not appear to be any difference in biomarker levels between responders and non-responders and it is therefore not clear these biomarkers will have predictive benefit. In our pharmacokinetic studies we observed increases in peak TRC105 serum concentrations were moderately well correlated with dose, increasing in an apparent linear (dose-proportional) manner. There were no differences in TRC105 trough concentrations between doses however, suggesting lack of antibody accumulation.
Regarding the ongoing development of TRC105, this agent has been studied in combination with other VEGF pathway inhibitors in early phase clinical trials. TRC105 combined with bevacizumab demonstrated activity in a bevacizumab refractory population in a Phase 1/2 trial17. The combination is being studied in ongoing Phase 2 trials in patients with glioblastoma and choriocarcinoma. The combination of TRC105 and axitinib demonstrated preliminary evidence of activity, including a 29% partial response rate per RECIST in antiangiogenicrefractory patients with renal cell carcinoma in a Phase 1b study, and is being studied in the randomized Phase 2 TRAXAR trial (NCT01806064). The finding of durable compete responses in patients with angiosarcoma, when TRC105 was administered with pazopanib, has led to this combination being studied in the randomized global Phase 3 TAPPAS trial (NCT02979899).
In summary, we found that the combination of TRC105, a human chimeric monoclonal endoglin antibody, and sorafenib were well tolerated and induced objective, durable, responses in a proportion of patients with HCC. This combination is currently under evaluation in a multicenter phase II study to confirm these encouraging early indications of efficacy.
Supplementary Material
Statement of translational relevance.
Endoglin (CD105) is an endothelial cell membrane receptor that is highly expressed on HCC vasculature, and is upregulated by inhibitors of the VEGF pathway. This phase I study assessed the safety and efficacy of an endoglin antibody (TRC105) when combined with sorafenib in a sorafenib-naïve HCC patient population. Encouraging clinical activity was seen, suggesting a potential combinatorial approach to build on the initial promise of sorafenib in patients with HCC.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Duffy A, Greten T. Developing better treatments in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 4:551–60. doi: 10.1586/egh.10.58. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebocontrolled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 4.Yang LY, Lu WQ, Huang GW, Wang W. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer. 2006;6:110. doi: 10.1186/1471-2407-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallas NA, Samuel S, Xia L, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1931–7. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 6.Li DY, Sorensen LK, Brooke BS, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–7. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 7.Rosen LS, Gordon MS, Robert F, Matei DE. Endoglin for targeted cancer treatment. Current oncology reports. 2014;16:365. doi: 10.1007/s11912-013-0365-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Starr MD, Brady JC, et al. Modulation of circulating protein biomarkers following TRC105 (anti-endoglin antibody) treatment in patients with advanced cancer. Cancer Med. 2014;3:580–91. doi: 10.1002/cam4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan-Stevaux O, Zhong W, Culp S, et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PloS one. 2012;7:e50920. doi: 10.1371/journal.pone.0050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy AG, Ulahannan SV, Cao L, et al. A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib. United European Gastroenterol J. 2015;3:453–61. doi: 10.1177/2050640615583587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapanadze T, Gamrekelashvili J, Ma C, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–13. doi: 10.1016/j.jhep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR, Ma ZC. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–26. doi: 10.1007/s004320050296. [DOI] [PubMed] [Google Scholar]
- 13.Rapisarda A, Hollingshead M, Uranchimeg B, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867–77. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, doubleblind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 15.Duffy A, Wilkerson J, Greten TF. Hemorrhagic events in hepatocellular carcinoma patients treated with antiangiogenic therapies. Hepatology. 2013;57:1068–77. doi: 10.1002/hep.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawinkels LJ, Kuiper P, Wiercinska E, et al. Matrix metalloproteinase-14 (MT1-MMP)mediated endoglin shedding inhibits tumor angiogenesis. Cancer research. 2010;70:4141–50. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- 17.Gordon MS, Robert F, Matei D, et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2014;20:5918–26. doi: 10.1158/1078-0432.CCR-14-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.