Summary
Magnaporthe oryzae causes blast disease, which is one of the most devastating infections in rice and several important cereal crops. Magnaporthe oryzae needs to coordinate gene regulation, morphological changes, nutrient acquisition and host evasion in order to invade and proliferate within the plant tissues. Thus far, the molecular mechanisms underlying the regulation of invasive growth in planta have remained largely unknown. We identified a precise filamentous‐punctate‐filamentous cycle in mitochondrial morphology during Magnaporthe–rice interaction. Interestingly, disruption of such mitochondrial dynamics by deletion of genes regulating either the mitochondrial fusion (MoFzo1) or fission (MoDnm1) machinery, or inhibition of mitochondrial fission using Mdivi‐1 caused significant reduction in M. oryzae pathogenicity. Furthermore, exogenous carbon source(s) but not antioxidant treatment delayed such mitochondrial dynamics/transition during invasive growth. In contrast, carbon starvation induced the breakdown of the mitochondrial network and led to more punctate mitochondria in vitro. Such nutrient‐based regulation of organellar dynamics preceded MoAtg24‐mediated mitophagy, which was found to be essential for proper biotrophic development and invasive growth in planta. We propose that precise mitochondrial dynamics and mitophagy occur during the transition from biotrophy to necrotrophy and are required for proper induction and establishment of the blast disease in rice.
Keywords: Atg24, Dnm1, Fzo1, Magnaporthe oryzae–rice interaction, mitochondrial fusion and fission, mitophagy, rice blast
Introduction
Mitochondria, the semi‐autonomous double‐membrane bound organelles, generate most of the adenosine triphosphate (ATP) for diverse cellular functions and are involved in various physiological processes, including lipid metabolism, redox signalling, calcium and iron homeostasis, and programmed cell death (Nunnari and Suomalainen, 2012; Zemirli and Morel, 2018). Depending on the cellular physiology and environment, mitochondria exhibit a variety of morphologies, ranging from elongated and interconnected networks to small spherical organelles. The mitochondrial shape is dynamic and depends on the balance between two opposing processes, fusion and fission, which occur continuously during the growth cycle (Westermann, 2010). Maintaining the mitochondrial morphology in steady state by the balance of fusion and fission activities is critical for living cells. When this equilibrium is broken, mitochondrial shape and dynamics are disturbed, leading to important physiological consequences, including increased cellular stress and various diseases (Chang and Doering, 2018; Delettre et al., 2000; Guan et al., 1993; Kijima et al., 2005; Ma et al., 2009; Mozdy et al., 2000; Rapaport et al., 1998; Sesaki and Jensen, 2001; Zemirli and Morel, 2018).
The fusion and fission machineries of mitochondria are well conserved from yeast to mammals. In yeast, mitochondrial fusion mainly depends on the transmembrane GTPase Fzo1, membrane‐anchored dynamin GTPase Mgm1, and Ugo1, which links the outer and inner membrane fusion machineries (Guan et al., 1993; Rapaport et al., 1998; Sesaki and Jensen, 2001). In yeast, the loss of Fzo1, Mgm1 or Ugo1 leads to numerous small fragmented mitochondria due to a block in fusion and amidst ongoing fission of mitochondria (Guan et al., 1993; Rapaport et al., 1998; Sesaki and Jensen, 2001). The Fis1‐Mdv1/Caf4‐Dnm1 complex constitutes the major mitochondrial fission pathway in yeast (Griffin et al., 2005; Mozdy et al., 2000). Fis1, a tail‐anchored outer membrane protein, functions as a membrane receptor, and Mdv1/Caf4 serves as an adaptor to recruit dynamin‐related protein Dnm1 to the fission sites in mitochondria (Griffin et al., 2005; Mozdy et al., 2000). Dnm1 is the key mediator of membrane scission during mitochondrial division (Mozdy et al., 2000). Loss of either Fis1 or Dnm1 blocks fission, resulting in highly interconnected fishnet‐like mitochondria (Mozdy et al., 2000).
In addition to fusion and fission machineries, mitochondrial homeostasis requires proper mitophagy, which is the selective sequestration of mitochondria by autophagosomes followed by their degradation in vacuoles/lysosomes (Liu et al., 2014). Mitophagy is a key mechanism in organellar quality control and is responsible for the removal of damaged or unwanted mitochondria (Liu et al., 2014). In yeast, the mitochondrial outer membrane receptor Atg32 is essential for mitophagy (Kanki et al., 2009; Okamoto et al., 2009). In response to nitrogen starvation or inhibition of mTOR following growth in a non‐fermentable carbon source, Atg32 directs mitochondria to the autophagosome through its interaction with the core autophagic machinery, including Atg8 and Atg11, to induce mitophagy (Kanki et al., 2009; Okamoto et al., 2009). Such receptors sense stimuli that induce mitophagy and couple mitochondrial dynamics to the quality control machinery (Mao and Klionsky, 2013).
Although the fusion and fission machineries are highly conserved, diverse mechanisms ensure proper organellar dynamics and distribution to optimize mitochondrial function in response to changing environments and cellular needs. The entire mitochondrial network is fused during G1‐S phase transition and fragmented/punctate in the late S and M phase depending on the cellular environment in rat kidney cells (Mitra et al., 2009). In addition, the mitochondrial dynamics are modulated in response to certain types and/or severity of stresses and adapt their form by promoting fusion or fission (Shutt and McBride, 2013). When cells are subjected to mild stresses, such as moderate nutrient starvation, protein synthesis inhibition or mTOR inhibition induced autophagy, mitochondria tend to become more fused to increase ATP production and escape from mitophagy (Gomes et al., 2011; Li et al., 2015; Tondera et al., 2009). Conversely, the mitochondrial fission machinery is activated upon prolonged nutrient stress, leading to degradation via mitophagy or apoptosis (Frank et al., 2001; Toyama et al., 2016). It is clear that mitochondrial dynamics and mitophagy are directly associated with metabolic status and stress conditions (Mao and Klionsky, 2013; Toyama et al., 2016; Twig et al., 2008).
Magnaporthe oryzae is a hemibiotroph that initially establishes a close biotrophic association to acquire nutrients from the live host cells, but later switches to the necrotrophic killing phase to obtain nutrients from dead plant tissues (Fernandez and Orth, 2018). During the infection cycle in M. oryzae, the three‐celled conidia are deposited by rain splashes and stick to the rice leaf surface. Under proper conditions, such conidia germinate and form appressoria to assist in the breach of the rigid rice cuticle. Once inside the host cell, M. oryzae differentiates into invasive hyphae and spreads to neighbouring cells, resulting in typical lesion formation. During invasive growth, M. oryzae needs to coordinate the nutrient sensing, gene expression regulation and morphological changes, acquiring nutrients from rice cells and eluding the plant immunity to adapt to the host milieu (Marroquin‐Guzman et al., 2017). The molecular mechanisms involved in regulating mitochondrial homeostasis during invasive growth have not been explored in depth. Recent studies have shown that the complex composed of MoDnm1, MoFis1 and MoMdv1 regulates the mitochondrial fission in M. oryzae (Zhong et al., 2016). Disruption of MoDNM1 or MoFIS1 results in defects in mitochondria fission and pathogenicity (Khan et al., 2015; Zhong et al., 2016). However, the regulation and function of mitochondrial dynamics during in planta development in M. oryzae remains to be investigated further. Our recent analyses showed that the sorting nexin MoAtg24 regulates mitophagy in the foot cells and is necessary for proper asexual differentiation (He et al., 2013). Whether mitophagy occurs during invasive growth and whether MoAtg24 functions during in planta development in M. oryzae needs to be explored further.
In this study, a unique filamentous‐punctate‐filamentous cycle in mitochondrial morphology and dynamics was observed during the early infectious growth of M. oryzae. To uncover the role of this specific cycle and mitophagy, mutants defective in mitochondrial fusion, fission and mitophagy were generated via deletion of MoFZO1, MoDNM1 or MoATG24, respectively. Characterization of Modnm1∆, Mofzo1∆ and Moatg24Δ strains, and Mdivi‐1‐based chemical inhibition of mitochondrial division revealed that mitochondrial fusion and fission machineries and mitophagy are required for maintaining mitochondrial dynamics and are necessary for proper infection and pathogenesis in M. oryzae. We provide evidence that carbon starvation triggers such specific mitochondrial dynamics during the early stages of rice blast. Overall, our study demonstrates that tightly controlled mitochondrial dynamics and mitophagy are required for proper invasive growth during establishment of the blast disease in rice.
Results
Mitochondrial dynamics during M. oryzae–rice interaction
We first examined mitochondrial morphology during in planta growth of wild‐type (WT) M. oryzae using the Mito‐GFP [as the mitochondrial marker (He et al., 2013; Patkar et al., 2012)] strain. The conidia of the Mito‐GFP strain were inoculated on sheaths from 21‐day‐old susceptible rice seedlings (Oryza sativa cultivar CO39) and incubated in a humid chamber at room temperature. Mitochondrial morphology was examined at the following three time points post‐inoculation: 30 h post‐inoculation (hpi), when the fungus successfully penetrated the rice epidermis, 48 hpi, when most of the invasive hyphae spread into the neighbouring rice cells and necrotrophy starts to occur, and 72 hpi, when necrotrophy/lesion formation could be observed. At 30 hpi, the majority of mitochondria (81.2 ± 1.9%) were in a tubular or filamentous network (Fig. 1). In contrast, most mitochondria (83.9 ± 9.9%) were fragmented or punctate at 48 hpi (Fig. 1). Interestingly, about half of the mitochondria (50.5 ± 5.9%) appeared to be filamentous or tubular again at 72 hpi (Fig. 1). However, such specific and dynamic changes in the mitochondrial network were not evident during appressorium formation (Fig. S1). Such temporal and dramatic changes in mitochondrial morphology indicated that M. oryzae likely faces dynamic environmental or cellular changes that significantly impact mitochondrial form/function during the first 72 h of in planta growth.
Figure 1.
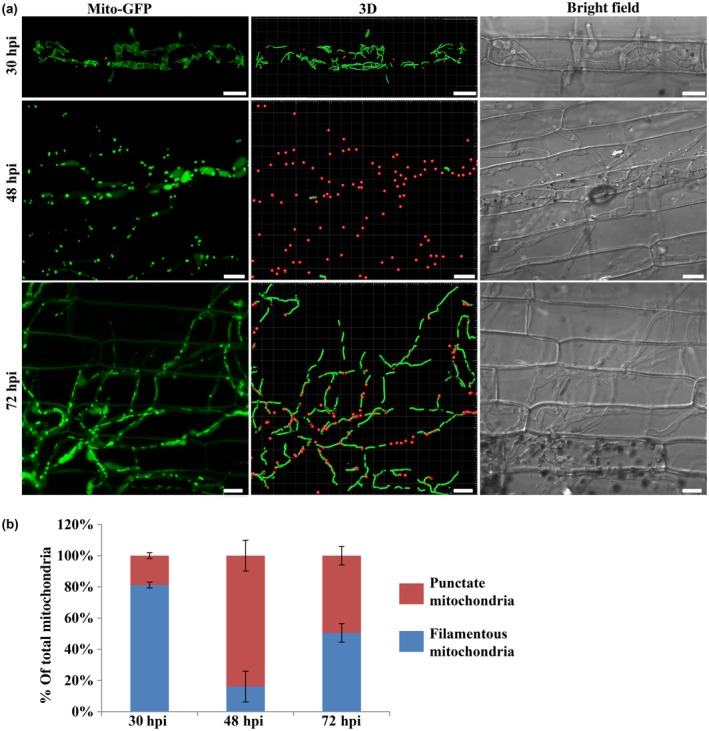
Specific changes in mitochondrial morphology during in planta growth of Magnaporthe oryzae. (a) Mitochondrial morphology in M. oryzae during infection. The conidial suspension of the Mito‐GFP strain was inoculated on rice sheath (Oryza sativa cultivar CO39). Confocal microscopy was carried at 30, 48 and 72 h post‐inoculation (hpi). The 3D reconstruction of the mitochondrial morphology was performed in Bitplan Imaris. Red spots and green filaments represent punctate and filamentous mitochondria, respectively. Scale bar: 8 μm. (b) Quantification of the different morphologies of mitochondria in the wild‐type Mito‐GFP strain during infection. Error bars represent mean ± SD from three independent replicates. Sample size is more than 200 appressoria penetration sites/host tissue per analysis.
The role of mitochondrial dynamics in invasive growth in M. oryzae
Mitochondrial dynamics through fusion and fission during invasive growth occurred prior to lesion development, raising the possibility that such organellar dynamics might play an important role in the establishment and spread of the blast disease. To determine the role of such changes in the morphology and dynamics of mitochondria during M. oryzae infection, we generated mutants defective in mitochondrial fission (Modnm1∆, Fig. S2) or inhibited mitochondrial fission using Mdivi‐1, or disrupted the mitochondrial fusion (Mofzo1∆, Fig. S3) to alter the overall mitochondrial network dynamics, and examined their invasive growth and the pathogenicity.
MoDNM1 is known as an important mitochondria fission gene in M. oryzae (Zhong et al., 2016). In our study, MoDNM1 was simply used as a marker gene for analysing the loss of mitochondrial fission in M. oryzae. A gene‐deletion mutant of MoDNM1 was generated in the Mito‐GFP strain. As previously reported (Zhong et al., 2016), the Modnm1∆ strain exhibited the characteristic tubular or fishnet‐like mitochondrial structures (Fig. 2a), suggesting that the Modnm1∆ is indeed incapable of mitochondrial fission. To verify the role of mitochondrial fission in fungal pathogenicity, the conidial suspension from WT, Modnm1∆ or Modnm1∆ complemented strain was used for blast infection assays on rice seedlings. The Modnm1∆ strain showed highly reduced pathogenicity and formed small and highly restricted lesions at 7 days post‐inoculation (dpi) (Fig. 2b,c). Furthermore, mitochondria in Modnm1∆ were tubular or filamentous at 30, 48 and 72 hpi, while a majority of mitochondria were fragmented/punctate in the WT at 48 hpi (Fig. 3). Since the Modnm1∆ strain has pleiotropic defects in M. oryzae, we also performed the mitochondrial fission inhibitor treatment to confirm the role of mitochondrial fission during blast infection. Treatment with Mdivi‐1, which inhibits mitochondrial fission in M. oryzae (Zhong et al., 2016), resulted in extensive tubular mitochondrial structures in M. oryzae and significantly reduced the invasive growth in rice cells (Fig. 4). Based on these results, we conclude that mitochondrial fission plays an important role in invasive growth and lesion formation by M. oryzae.
Figure 2.
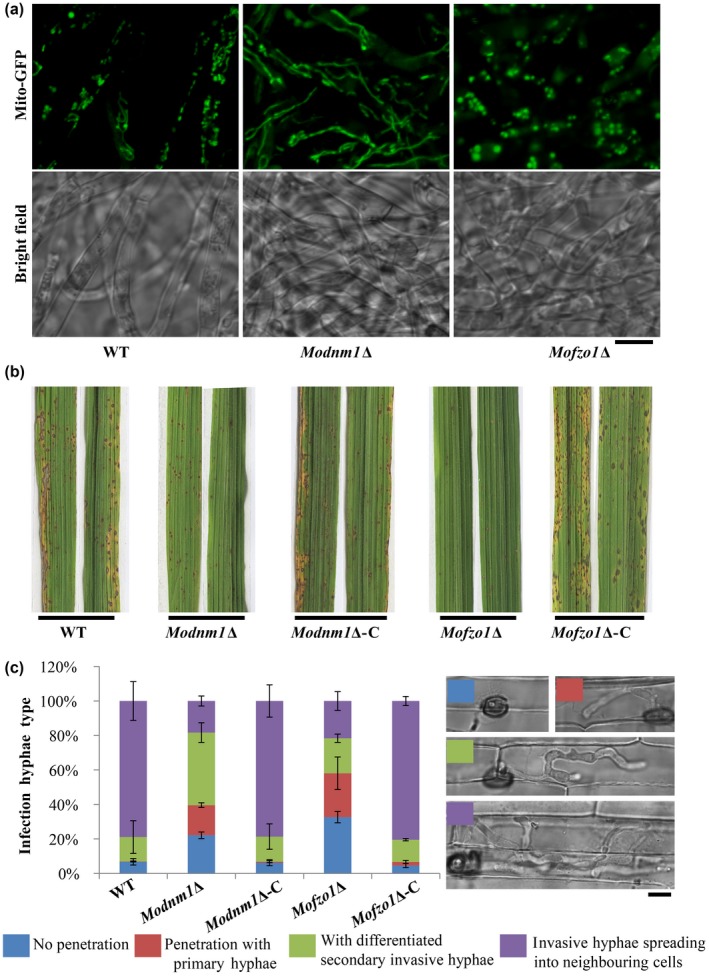
Mitochondrial fusion and fission are required for proper pathogenesis of Magnaporthe oryzae. (a) The function of MoDNM1 and MoFZO1 in mitochondrial fission and fusion. Two‐day‐old liquid CM‐grown mycelia of the indicated strains were used for imaging with confocal microscopy. Most of the mitochondria in Modnm1Δ formed elongated or interconnected fishnet‐like structures, while the mitochondria were punctate or fragmented in Mofzo1Δ in vegetative mycelia. (b) The rice seedling (Oryza sativa cultivar CO39) infection assay of wild‐type (WT), Modnm1Δ, Modnm1Δ complementation strain (Modnm1Δ‐C), Mofzo1Δ and Mofzo1Δ complemented strain (Mofzo1Δ‐C). (c) Detailed observation and statistical analysis of invasive growth in rice sheath cells at 40 h post‐inoculation. Four types (illustrated in the right panel with corresponding colour labels: no penetration, penetration with primary hyphae, with differentiated secondary invasive hyphae, and invasive hyphae spreading into neighbouring cells) were quantified. Data represent mean ± SD of three independent experiments, with n = 200 appressoria per analysis. Scale bar represents 5 μm.
Figure 3.
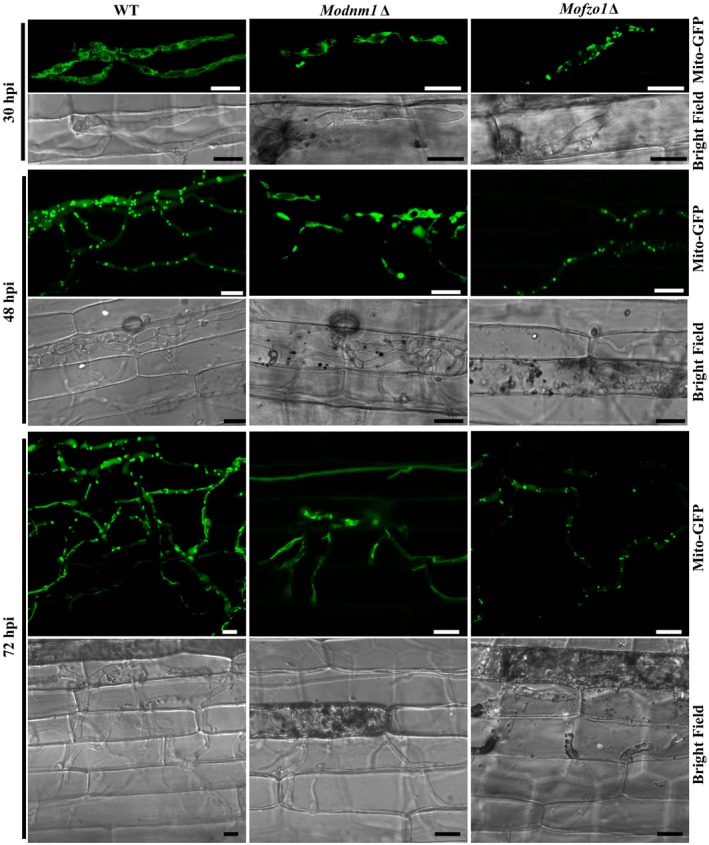
The mitochondrial morphology in Magnaporthe oryzae wild‐type (WT), Modnm1Δ and Mofzo1Δ during the infection process. Invasive hyphal growth of Modnm1Δ or Mofzo1Δ was significantly slower than WT. hpi, h post‐inoculation. Scale bar = 10 μm.
Figure 4.
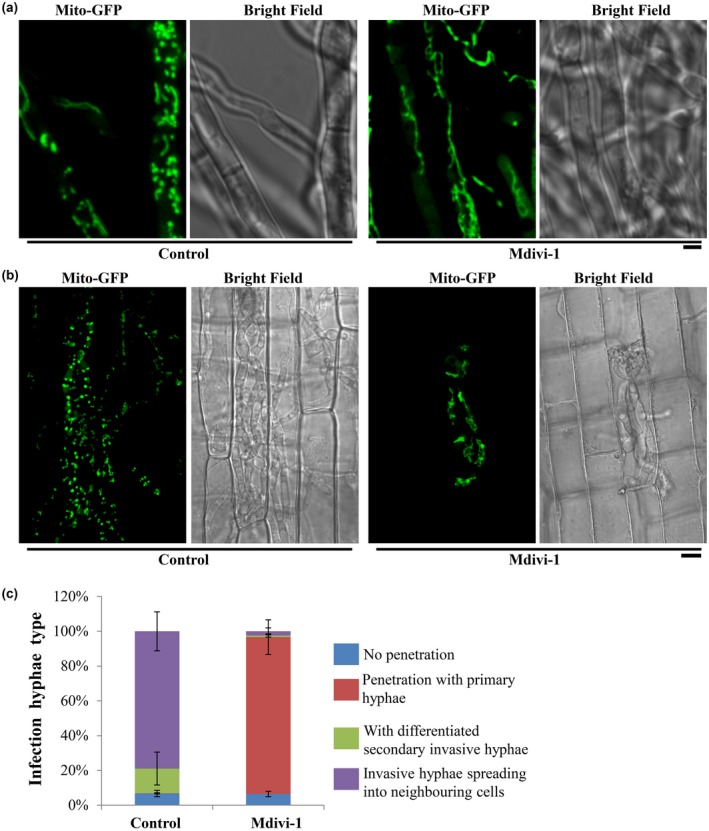
Chemical inhibition of mitochondrial fission reduces invasive hyphal growth of Magnaporthe oryzae. (a) The mitochondria were predominantly tubular or filamentous upon Midvi‐1 treatment. Scale bar represents 2 μm. (b) The mitochondrial morphology of the Mito‐GFP strain with/without Midvi‐1 at 48 h post‐inoculation. Scale bar = 8 μm. (c) Detailed observation and statistical analysis of invasive growth in rice sheath cells at 40 hpi. The different types of invasive hyphae that were analysed/quantified are described in Fig. 2c.
In Saccharomyces cerevisiae, Fzo1 is the first known mediator of mitochondrial fusion (Fritz et al., 2001; Rapaport et al., 1998). Deletion mutant of the orthologous MoFZO1 harboured punctate mitochondria (Fig. 2a), thus indicating a mitochondrial fusion defect in this M. oryzae mutant. Similar to Modnm1∆, the Mofzo1∆ strain formed small and restricted blast lesions on rice plants (Fig. 2b). Further microscopic observations showed that more than 90% of appressoria penetrated successfully and about 80% infectious hyphae extended to neighbouring cells in the WT and the complemented strain at 40 dpi, while only 67.5% of appressoria penetrated successfully and 21.6% of invasive hyphae spread to surrounding cells in the Mofzo1∆ strain (Fig. 2c; P < 0.005). We further analysed the mitochondrial dynamics during invasive growth in M. oryzae. As shown in Fig. 3, the mitochondria in Mofzo1∆ were punctate at all the time points tested. These results indicate that mitochondrial fusion within the blast pathogen is required for proper invasive growth and lesion formation.
Taken together, we conclude that the mitochondrial fission and fusion machineries are involved in invasive growth in M. oryzae, and that mitochondrial dynamics plays a crucial role during in planta growth and development in M. oryzae.
Carbon source depletion triggers mitochondrial fragmentation
Mitochondrial fragmentation can be triggered by multiple environmental factors such as oxidative stress and carbon source depletion (Zemirli and Morel, 2018). During host invasion, the fungal pathogen generally encounters the plant defence response, oxidative stress and metabolic stress. We therefore hypothesized that such host response, oxidative and/or metabolic stress triggers the specific mitochondrial fragmentation during invasive growth in planta.
To test whether the live host factors trigger such changes in mitochondrial fragmentation, we first examined the mitochondrial morphology in the blast fungus in live host tissue and compared it to that in heat‐killed rice sheath. The heat treatment was used to first kill the rice sheath cells before inoculating with the blast fungal strain of interest. Mitochondrial fragmentation was evident in invasive hyphae in heat‐killed rice sheath at 48 hpi. However, the percentage of filamentous mitochondria was significantly higher than the control samples at 48 hpi and did not show any difference at 72 hpi (Fig. 5). These results indicate that the mitochondrial fragmentation observed during M. oryzae invasive growth is dependent in part on the active defence response in addition to other factors in live host plants.
Figure 5.
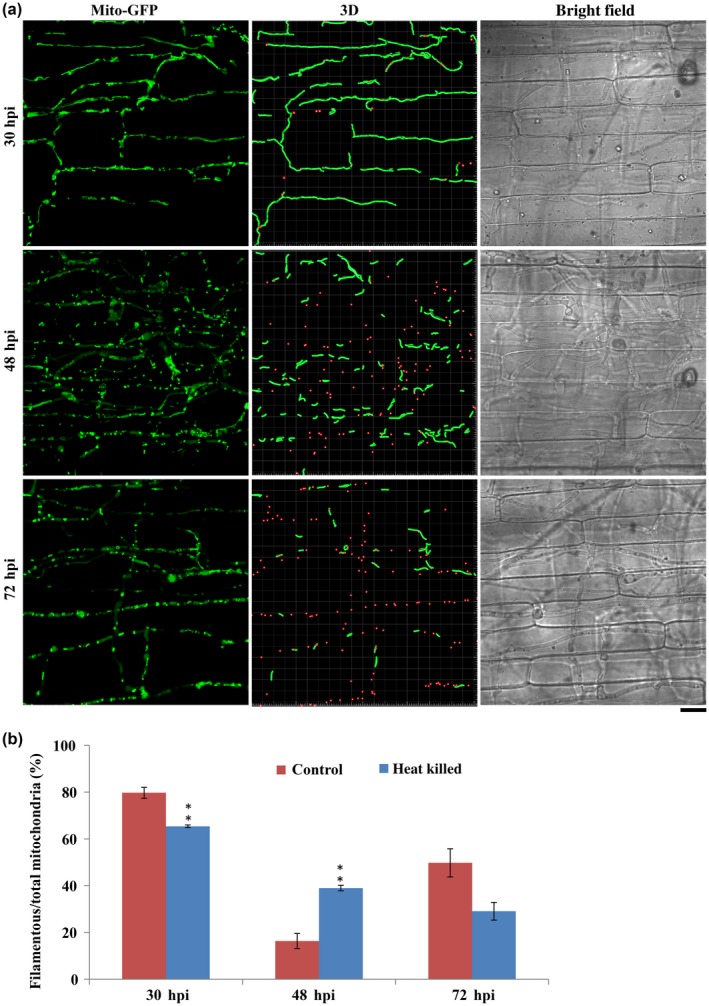
The mitochondrial morphology and dynamics in the Magnaporthe oryzae Mito‐GFP strain in heat‐killed rice sheath. (a) Confocal microscopy images of the Mito‐GFP strain in dead rice sheath cells at 30, 48 and 72 h post‐inoculation (hpi). Scale bar = 12 μm. In the 3D image, red spots and green filaments highlight punctate and filamentous mitochondria, respectively. (b) Quantitative analysis of mitochondria of different morphologies in the Mito‐GFP strain in heat‐killed rice cells. Values represent the mean ± SD from three independent experiments. Sample size is more than 200 appressoria penetration sites per analysis. **P < 0.005 compared with wild‐type at indicated time point.
Oxidative stress could trigger the mitochondrial fragmentation in M. oryzae (Fig. S4). We further tested whether oxidative stress triggers the mitochondrial fragmentation during invasive growth by imaging the mitochondrial morphology at 30, 48 and 72 hpi in the presence of the exogenous antioxidant. The antioxidant treatment was initiated at 24 hpi. In the presence of 2.5 mM glutathione (GSH) as an exogenous antioxidant, around 81% mitochondria still became punctate or fragmented at 48 hpi (Fig. S5). At 72 hpi, filamentous or tubular mitochondria were apparent in GSH‐treated invasive hyphae. Likewise, N‐acetyl cysteine (NAC) treatment did not alter the mitochondrial fragmentation regime at 48 hpi (Fig. S5). Therefore, we inferred that reactive oxygen species/oxidative stress is unlikely to be the trigger for mitochondrial fragmentation during M. oryzae invasive growth.
Next, we examined the role of carbon source depletion on mitochondrial fragmentation as exogenous carbon sources have been reported to alter metabolic stresses (Toyama et al., 2016). The carbon source, glucose or sucrose, was individually added into inoculated conidia droplets on the rice sheath surface at 24 hpi. Mitochondrial morphology was assessed using a confocal microscope at 30, 48 and 72 hpi. We found that excess glucose or sucrose significantly delayed the mitochondrial fragmentation (Figs 6 and 7), which indicates that carbon source depletion might be the major factor triggering mitochondrial fragmentation during in planta growth in M. oryzae. In the control experiments (no additional carbon source), around 84% mitochondria appeared fragmented at 48 hpi (Figs 6 and 7), whereas mitochondrial fragmentation occurred at 72 hpi in the presence of the indicated exogenous carbon source. At 72 hpi, 69% and 67% of mitochondria were punctate upon additional supply of glucose or sucrose, respectively (Figs 6 and 7), while more than 50% of mitochondria in the WT appeared filamentous again. Lastly, carbon starvation could also induce fragmentation of the mitochondrial network and led to an increase in punctate mitochondria in WT M. oryzae grown in liquid culture (Fig. S6). Based on these data, we conclude that the depletion of carbon source as well as the presence of excess sugar impacts mitochondrial dynamics (and/or function) in the invasive hyphae of M. oryzae. Since the aforementioned changes in carbon homeostasis delayed mitochondrial fragmentation to some extent, it is possible that downstream molecules in the carbon metabolic pathway(s) regulate mitochondrial dynamics during blast infection. Accordingly, the important carbon metabolic intermediate glucose‐6‐phosphate (G6P) was added to the inoculated conidial suspension at 24 hpi, and the mitochondrial morphology was assessed at 48 and 72 hpi. Nearly 85% and 64% of total mitochondria remained tubular or filamentous in the presence of G6P at 48 hpi (P < 0.001) and 72 hpi (Figs 6 and 7; P < 0.01). Taken together, these results indicate that carbon source depletion and the live host factors, but not oxidative stress per se, trigger mitochondrial fragmentation during the invasive growth phase in M. oryzae.
Figure 6.
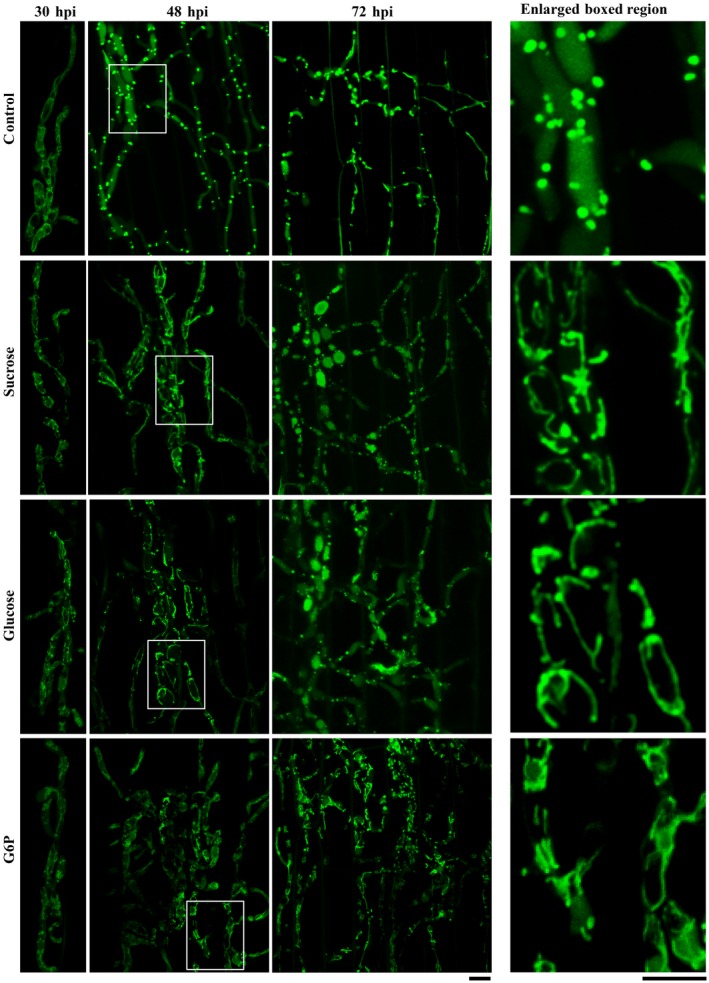
Carbon‐replete condition delays mitochondrial fragmentation in planta. Conidia of the Magnaporthe oryzae Mito‐GFP strain were inoculated onto the rice sheaths. At 24 h post‐inoculation (hpi) the fluid in the conidial suspension was replaced with sterile H2O (control), 8 mg/mL sucrose, 50 mg/mL glucose or 1.5 mg/mL glucose‐6‐phosphate (G6P). Confocal microscopy was carried out at 30, 48 and 72 hpi. The right‐hand panels show an enlarged view of the boxed region in the left‐hand panel. Scale bar equals 5 μm.
Figure 7.
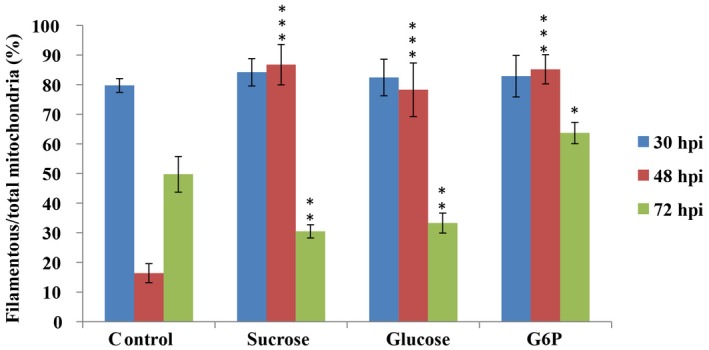
Mitochondrial morphology with or without exogenous sucrose, glucose or glucose‐6‐phosphate (G6P). Values represent the mean ± SD from three independent experiments. ***P < 0.001; **P < 0.005; *P < 0.01 in comparison to control (H2O) at the same time points. Sample size is more than 200 appressoria penetration sites per analysis.
Mitophagy is necessary for blast infection
As shown in Figs 1, 3, 6 and 8d, the vacuolar localization of Mito‐GFP (mitochondrial marker) was observed at 48 and 72 hpi (Fig. S7), indicating that mitophagy is likely induced to degrade mitochondria during the initial stages of establishment of the blast disease. Our previous study showed that MoAtg24 is specifically required for mitophagy and is necessary for proper asexual differentiation (He et al., 2013). To determine whether mitophagy plays any role during infection, the pathogenicity of Moatg24Δ was tested using rice seedling infection assays. Compared to the WT, which caused the characteristic spindle‐shaped blast lesions with grey centres, the Moatg24Δ showed highly reduced pathogenicity in rice (Fig. 8a). Typical blast disease lesions were not elaborated in the susceptible rice cultivar inoculated with Moatg24Δ conidia, while only small lesions were occasionally evident (Fig. 8a).
Figure 8.
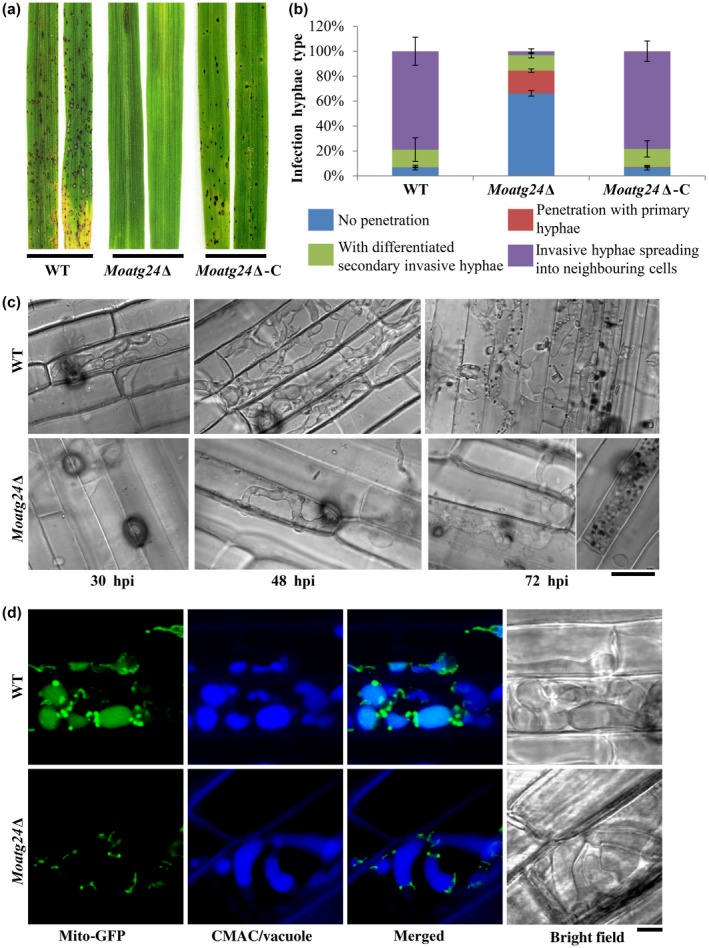
MoAtg24‐mediated mitophagy is necessary for Magnaporthe oryzae infection. (a) Loss of MoATG24 gene leads to reduction in pathogenicity. Blast infection assays of wild‐type (WT), Moatg24Δ or Moatg24Δ complementation strain (Moatg24Δ‐C) were performed using rice seedlings (Oryza sativa cultivar CO39). Images were taken at 7 days post‐inoculation. (b) Developmental defects in the invasive hyphae of Moatg24Δ strain. The invasive hyphae in rice sheath cells were quantified as described in Fig. 2c. Data represents the mean ± SD from three independent experiments. More than 200 appressoria from each indicated strain were assessed each time. (c) Invasive hyphal growth in WT and Moatg24Δ strains at 30, 48 and 72 h post‐inoculation (hpi). Scale bar = 12 μm. (d) MoAtg24 is required for mitophagy during M. oryzae infection. WT or Moatg24Δ strain expressing Mito‐GFP was inoculated into rice sheaths for 60 h. The vacuoles in invasive hyphae were visualized by staining with CMAC. Scale bar = 2.5 μm.
To understand the differences between infection by Moatg24Δ and WT conidia, invasive hyphae were observed under the microscope at 30, 48 and 72 hpi. At 40 hpi, nearly 90% of WT appressoria successfully penetrated the rice sheath. By contrast, less than 20% of Moatg24Δ appressoria were capable of invading the rice sheath (Fig. 8b; P < 0.001). At 48 hpi, although the penetration rates of appressoria in WT and Moatg24Δ were comparable, the secondary invasive hyphae were highly reduced in the Moatg24Δ (<2%) compared to WT (around 80%) (Fig. 8b; P < 0.001). At 72 hpi, the difference in invasive hyphae in Moatg24Δ and WT was more pronounced. The invasive hyphae of WT had successfully spread into five to seven rice cells, whereas the invasive hyphae of Moatg24Δ were mainly restricted to the first invaded cells in the rice epidermis (Fig. 8c). In rare cases, the invasive hyphae of Moatg24Δ could be found within the neighbouring cells surrounding the primary infected rice epidermal cell. Based on these results, we inferred that the highly reduced pathogenicity of Moatg24Δ is a result of lack of spread of invasive hyphae from the site of host entry/invasion.
Since MoAtg24 is essential for mitophagy, and the Moatg24∆ showed highly reduced invasive growth in this study, it became important to assess and confirm whether MoAtg24‐based mitophagy occurs naturally during blast infection. Therefore, mitophagy was assessed using confocal microscopy in the secondary invasive hyphae, which formed in rare instances in the Moatg24Δ mutant. As shown in Fig. 8d, Mito‐GFP signal could be detected in vacuoles (CMAC, 7‐amino‐4‐chloromethylcoumarin, staining) at 60 hpi in invasive hyphae (Fig. 8d, upper panel). In contrast, such a Mito‐GFP signal did not colocalize with the vacuoles in the rare invasive hyphae in Moatg24Δ (Fig. 8d, lower panel), indicating that mitophagy is blocked during the infection‐related growth in the Moatg24Δ mutant. Considering that the Moatg24Δ mutant is defective in invasive growth and failed to form blast lesions, these results show that MoATG24‐mediated mitophagy plays a critical role in the infection process of M. oryzae.
Taken together, our data support that mitochondrial dynamics and mitophagy are important intermediate events between nutrient sensing and homeostasis in M. oryzae leading to the establishment and extent of the devastating blast disease in rice.
Discussion
The adaptation of mitochondrial fusion and fission to cellular demands is critical for a number of important physiological processes (Zemirli and Morel, 2018). In M. oryzae, the complex composed of MoDnm1, MoFis1 and MoMdv1 regulates mitochondrial fission and plays important roles in pathogenesis (Khan et al., 2015; Zhong et al., 2016). In this study, the regulation and function of mitochondrial dynamics during M. oryzae growth and development in planta was determined. Mitochondrial dynamics were first observed during the early stages of the infection cycle of M. oryzae. Interestingly, we uncovered a unique filamentous‐punctate‐filamentous transition cycle in mitochondrial morphology during in planta growth. We demonstrated that the key regulators of mitochondrial fusion and fission are essential for proper mitochondrial dynamics and invasive growth in M. oryzae. These results suggest that mitochondrial fusion and fission are tightly controlled during blast infection and that such sequential change in organellar morphology is important for pathogenesis in M. oryzae.
Mitochondrial fragmentation could be triggered by multiple environmental factors or stressors. The blast pathogen generally encounters host defence, oxidative stress and metabolic stress that may trigger such mitochondrial fragmentation. We propose that carbon source depletion is one of the important factors triggering mitochondrial fragmentation during in planta growth of M. oryzae. First, mitochondrial fragmentation was observed in the invasive hyphae in heat‐killed rice sheath, which is incapable of mounting the defence response. Second, exogenous antioxidants did not inhibit or delay such fragmentation processes during infection. Carbon sources (such as glucose or sucrose) or the important metabolic intermediate G6P delayed mitochondrial fragmentation, whereas prolonged nutrient starvation induced the breakdown of mitochondrial network in M. oryzae (Fig. S8). In addition, we found that NH4NO3 treatment did not change the mitochondrial morphology (Fig. S9), indicating that nitrogen starvation is likely not an important factor that leads to mitochondrial fragmentation during blast infection. Strigolactone is a plant hormone which is associated with mitochondrial biogenesis, fission, fusion, spore germination and hyphal branching in some fungal genera (Besserer et al., 2006). Strigolactone (GR24) treatment did not inhibit the mitochondrial fragmentation processes during Magnaporthe infection (Fig. S9).
Magnaporthe oryzae initially acquires nutrients from living host cells, but switches to the necrotrophic killing phase to acquire nutrients from dead tissues between 48 and 72 hpi (Fig. S9c). During the transition to necrotrophy, the filamentous invasive hyphae of M. oryzae maintain viability as the fungal lifestyle changes and lesion development when host cell death is occurring (Fernandez and Orth, 2018; Jones et al., 2016; Kankanala et al., 2007). Magnaporthe oryzae thus needs to adapt to and overcome nutrient stress prior to switching to the necrotrophic phase. Our results show that glucose or sucrose supplementation promotes proliferation of invasive hyphae and decreases the cell death in rice during early infection of M. oryzae (Fig. S10). These results suggest that carbon source depletion occurs during infection and is likely a major factor which triggers the biotrophy–necrotrophy transition. However, carbon starvation and then carbon source acquisition from dead plant tissues may not be the only factors that trigger mitochondria fragmentation and rebuilding of the network, since addition of glucose every 6 h after 24 hpi simply delayed the fragmentation of mitochondria (Figs 6 and 7). It is possible that other signals cooperate with carbon homeostasis machinery to regulate and control the mitochondrial morphology/function during the blast infection in rice. In conclusion, our study suggests that carbon source depletion with other factor(s) trigger(s) mitochondrial fragmentation and biotrophic–necrotrophic phase switch during infection of M. oryzae.
Our previous study showed that M. oryzae mitophagy requires MoAtg24 and is important for proper asexual differentiation (He et al., 2013). In this study, we found that mitophagy is induced along with precise mitochondrial fragmentation during invasive growth in M. oryzae. Furthermore, mitophagy plays a critical role in the invasive growth of M. oryzae in response to energy demands and nutrient homeostasis. It has been suggested that mitophagy requires efficient fission to separate out damaged or unwanted mitochondria to fit into the autophagosomes (Mao and Klionsky, 2013). Thus, it is possible that mitochondrial fragmentation together with ensuing mitophagy is the strategy employed by M. oryzae to separate and degrade the damaged/excess mitochondria in order to protect itself in the hostile environment in planta.
In conclusion, our study revealed that a unique filamentous‐punctate‐filamentous cycle in mitochondrial morphology controlled by fission and fusion machinery is important for pathogenesis of M. oryzae. Such morphological transitions are likely coupled with nutrient homeostasis (particularly carbon source) and biotrophy‐to‐necrotrophy switch during M. oryzae infection. Lastly, mitophagy regulates the precise turnover of mitochondria and plays a critical role during the initiation of the devastating blast disease in rice.
Experimental Procedures
Fungal strains and culture media
The M. oryzae WT strain B157 (field isolate, mat1‐2) was a kind gift from the Indian Institute of Rice Research (Hyderabad, India). The M. oryzae strains Mito‐GFP, Moatg24Δ and Moatg24Δ‐C have been described in our previous reports (He et al., 2013; Patkar et al., 2012; Ramos‐Pamplona and Naqvi, 2006).
Magnaporthe oryzae strains were grown on prune agar (yeast extract 1 g/L, lactose 2.5 g/L, sucrose 2.5 g/L, prune juice 40 mL/L, agar 20 g/L, pH 6.5) medium at 28 °C in the dark for 2 days, followed by growth under continuous light for 5 days to collect conidia for infection assay. Mutants generated by Agrobacterium tumefaciens‐mediated transformation (ATMT) were selected on either complete medium (CM: casein hydrolysate 6 g/L, sucrose 10 g/L, yeast extract 6 g/L, agar 20 g/L) containing hygromycin (250 μg/mL) or basal medium (BM: asparagine 2.0 g/L, yeast nitrogen base 1.6 g/L, NH4NO3 1.0 g/L, glucose 10 g/L, agar 20 g/L, pH 6.0) with chlorimuron‐ethyl (50 μg/mL) or with ammonium glufosinate (50 μg/mL).
Construction of Modnm1Δ and Mofzo1Δ strains, and complementation analyses
The MoDNM1 gene (MGG_06361) deletion mutant was generated using the standard one‐step gene replacement strategy. Briefly, about 1 kilobase (kb) of 5' UTR (untranslated region) and 3' UTR regions were PCR amplified and ligated sequentially to flank the ILV2SUR sulfonylurea‐resistance cassette in pFGL820 (Addgene, 58221) (Fig. S2a). The following primers were used to amplify the 5' and 3' UTR of the MoDNM1 gene: Dnm1‐5F (5ʹ‐GAGAGTGTT GAATTC CTCACGGGATGGGCTTCTG‐3ʹ) Dnm1‐5R (5ʹ‐GAGAGTGTT GGTACC GGCGAAAATCGGTTCCGTGGTC‐3ʹ), Dnm13‐F (5ʹ‐GAGAGTGTT GTCGAC TGAAGCTGTTTGCGCCATG‐3ʹ), and Dnm13‐R (5ʹ‐GAGAGTGTT GCATGC TACCTATGATCAGCCCGC‐3ʹ). Underlined sequences are restriction sites introduced for cloning purpose. The final plasmid construct was confirmed by sequencing and subsequently introduced into the Mito‐GFP strain by ATMT to replace the MoDNM1 gene (Yang and Naqvi, 2014). All the correct transformants in this study were ascertained by locus‐specific PCR and/or Southern blot analysis (Figs S2b, S3b). For complementation analysis, the full‐length genomic copy with promoter of MoDNM1 was amplified with MoDnm1‐F (5ʹ‐AATT GAATTC GTTGAGCAGGCCGAGCGAC‐3ʹ) and MoDnm1‐R (5ʹ‐AATT GAATTC CACTGGCATTTGATTACGCAAGG‐3ʹ) inserted into pFGL822 (Addgene, 558226) and introduced into the Modnm1Δ strain.
For generating the plasmid vector for MoFZO1 (MGG_05209) deletion, about 1 kb of 5' UTR and 3' UTR regions were PCR amplified and ligated sequentially to flank the phosphinothricin acetyl transferase gene cassette in pFGL822 (Fig. S3). The following primers were used to amplify the 5' and 3' UTR of the MoFzo1 gene: Fzo1‐5F (5ʹ‐GAGAGTGTT GAATTC ACTCGGCCGCGATACGCTGC‐3ʹ), Fzo1‐5r (5ʹ‐GAGAGTGTT GGATCC GTGATCGATTTCGTCCAGTC‐3ʹ), Fzo1‐3F (5ʹ‐GAGAGTGTT CTGCAG GCAGAACCATCCTCGTCGTC‐3ʹ), and Fzo1‐3r (5ʹ‐GAGAGTGTT AAGCTT CCTGGCGGCGGCGACATCAAC‐3ʹ). The final plasmid was introduced into the Mito‐GFP strain by ATMT to replace the MoFZO1 gene. The complementation fragment, which contains the full‐length genomic copy with promoter of MoFZO1 gene, was amplified with MoFzo1‐F (5ʹ‐AATT GGATCC GGCTGTCTGCGTGATCCCTG‐3ʹ) and MoFzo1‐R (5ʹ‐AATT TCTAGA GCTGTGGAGCGAGGAGCAGG‐3ʹ) and inserted into pFGL899 to complement the Mofzo1Δ strain (Yang and Naqvi, 2014).
Infection assays
For blast infection assay, conidial suspension (106/mL) with 0.01% gelatine was sprayed on 21‐day‐old rice seedlings (Oryza sativa cultivar CO39) and incubated in a growth chamber (16 h light/d, 22 °C and 90% humidity). Blast disease in infection assays was assessed and recorded by scanning the leaves at 7 dpi. The blast infection assays were repeated at least three times.
For the host penetration and in planta invasive hyphal development assay, healthy rice seedlings (CO39) at the age of 4 weeks were selected for sheath preparation. Conidial suspension (5 × 104/mL) were inoculated onto rice sheath and incubated on sterile wet tissue paper in 90 mm Petri dishes. The Petri dishes with inoculated rice sheaths were transferred into the growth chamber with a photoperiod of 16 h:8 h light:dark cycle at 25 °C. The inoculated sheath was trimmed manually and observed by using an Olympus BX51 wide field microscope or with a laser scanning confocal microscope at selected time points.
To prepare heat‐killed rice sheaths, the fresh rice sheaths were immersed into sterile water at 70 °C for 25 min (Shipman et al., 2017). The heat‐killed rice sheath has the physical structures of cells, while the abilities of host response to fungal infection are lost.
Carbon sources, antioxidant and Mdivi‐1 treatments
For treatments with excess carbon sources, the conidia from the tested strains were inoculated on to rice sheath and incubated in growth chamber. At 24 hpi, the water on the rice sheath was removed and the following solutions were applied to the sheath: 8 mg/mL sucrose, 50 mg/mL glucose, 1.5 mg/mL G6P (Sigma‐Aldrich, Switzerland), 2.5 mM GSH (L‐glutathione reduced, Sigma‐Aldrich, USA), 40 mM NAC (Sigma‐Aldrich, USA), or 10 µM Mdivi‐1 (Selleck, USA). The rice sheaths were incubated in the growth chamber until observation. These experiments were repeated thrice.
Vacuolar staining
The infected rice sheaths were incubated with CellTracker™ Blue CMAC Dye (7‐amino‐4‐chloromethylcoumarin, Molecular Probes, C2110) at a final working concentration of 10 μM for 2 h at 37 °C. The sample was washed with water prior to microscopic observation.
Live cell imaging and image processing
Live cell epifluorescence microscopy was performed with a Zeiss LSM 700 inverted confocal microscope (Carl Zeiss, Inc.) using a Plan‐Apochromat 63 (Numerical Aperture = 1.40) oil immersion lens. Enhanced GFP (EGFP) and CMAC excitation were performed at 488 nm (Em. 505–530 nm) and 405 nm (Em. 430–470 nm) respectively. For in planta invasive hyphal development observation, a z‐stack that consisted of 0.5 μm‐space sections was captured for each appressorium penetration site. Image processing was processed in Image J, which was downloaded from National Institutes of Health (http://rsb.info.nih.gov/). The maximum projection of z‐stack was obtained by Z projection with maximum intensity in Image J. Three‐dimensional reconstruction, visualization and analysis were performed in Bitplan Imaris with filament and spots program (Zurich, Switzerland). For figure preparation, the images were arranged in Illustrator CS6 (Adobe Systems Incorporated, USA).
Accession numbers
MoDnm1: XP_003717217.1; MoFzo1: XP_003712754.1; MoAtg24: XP_003716251.1
Supporting information
Fig. S1 Mitochondrial morphology during appressorium formation in M. oryzae.
Fig. S2 Generation and verification of Modnm1Δ mutant.
Fig. S3 Generation and verification of Mofzo1Δ mutant.
Fig. S4 Oxidant treatment induces mitochondrial fragmentation.
Fig. S5 Antioxidant treatment does not delay mitochondrial dynamics during invasive growth.
Fig. S6 Carbon source depletion induces mitochondrial fragmentation in M. oryzae.
Fig. S7 The vacuolar localization of Mito‐GFP (mitochondrial marker) during invasive growth.
Fig. S8 Prolonged nutrient starvation induces mitochondrial fragmentation and mitophagy.
Fig. S9 NH4NO3 or strigolactone treatment did not change the mitochondrial fragmentation during infection by M. oryzae.
Fig. S10 Addition of glucose or sucrose promotes spread of invasive hyphae and decreases the cell death in rice during early infection by M. oryzae.
Acknowledgements
We thank the Fungal Pathobiology group at Temasek Life Sciences Laboratory for useful discussion and suggestions. We thank Gu Keyu and Amit Anand for image analyses. This study was supported by the Chinese Academy of Agricultural Sciences under the Elite Youth program and the Agricultural Sciences and Technologies Innovation Program, and the Zhejiang Provincial Natural Science Foundation of China (LQ19C140004 and LQ19C130007), the National Research Foundation, Singapore (Prime Minister's Office, NRF‐CRP7‐2010‐02), and intramural grants from the Temasek Life Sciences Laboratory, Singapore.
Naweed I. Naqvi, Yanjun Kou and Yunlong He planned and designed the research. Yanjun Kou, Yunlong He, Yazhou Shu, Jiehua Qiu, Fan Yang and Yizhen Deng performed experiments, conducted fieldwork, analysed data etc. Yanjun Kou, Yunlong He and Naweed I. Naqvi wrote the manuscript. Yanjun Kou and He Yunlong contributed equally.
Contributor Information
Yanjun Kou, Email: kouyanjun@caas.cn.
Naweed I. Naqvi, Email: naweed@tll.org.sg
References
- Besserer, A. , Puech‐Pages, V. , Kiefer, P. , Gomez‐Roldan, V. , Jauneau, A. , Roy, S. , Portais, J.‐C. , Roux, C. , Bécard, G. and Séjalon‐Delmas, N . (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 4, e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A.L. and Doering, T.L. (2018) Maintenance of mitochondrial morphology in Cryptococcus neoformans is critical for stress resistance and virulence. MBio, 9, e01375–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre, C. , Lenaers, G. , Griffoin, J.M. , Gigarel, N. , Lorenzo, C. , Belenguer, P. , Pelloquin, L , Grosgeorge, J , Turc‐Carel, C , Perret, E. , Astarie‐Dequeker, C. , Lasquellec, L. , Arnaud, B. , Ducommun, B. , Kaplan, J. and Hamel, C.P . (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin‐related protein, is mutated in dominant optic atrophy. Nat. Genet. 26, 207–210. [DOI] [PubMed] [Google Scholar]
- Fernandez, J. and Orth, K. (2018) Rise of a cereal killer: the biology of Magnaporthe oryzae biotrophic growth. Trends Microbiol. 26, 582–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. , Gaume, B. , Bergmann‐Leitner, E.S. , Leitner, W.W. , Robert, E.G. , Catez, F. , Smith, C.L. and Youle, R.J . (2001) The role of dynamin‐related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell, 1, 515–525. [DOI] [PubMed] [Google Scholar]
- Fritz, S. , Rapaport, D. , Klanner, E. , Neupert, W. and Westermann, B. (2001) Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J. Cell. Biol. 152, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, L.C. , Di Benedetto, G. and Scorrano, L. (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, E.E. , Graumann, J. and Chan, D.C. (2005) The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J. Cell. Biol. 170, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, K. , Farh, L. , Marshall, T.K. and Deschenes, R.J. (1993) Normal mitochondrial structure and genome maintenance in yeast requires the dynamin‐like product of the MGM1 gene. Curr. Genet. 24, 141–148. [DOI] [PubMed] [Google Scholar]
- He, Y. , Deng, Y.Z. and Naqvi, N.I. (2013) Atg24‐assisted mitophagy in the foot cells is necessary for proper asexual differentiation in Magnaporthe oryzae . Autophagy, 9, 1818–1827. [DOI] [PubMed] [Google Scholar]
- Jones, K. , Kim, D.W. , Park, J.S. and Khang, C.H. (2016) Live‐cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae . BMC Plant Biol. 16, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankanala, P. , Czymmek, K. and Valent, B. (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell, 19, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki, T. , Wang, K. , Cao, Y. , Baba, M. and Klionsky, D.J. (2009) Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell, 17, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, I.A. , Ning, G. , Liu, X. , Feng, X. , Lin, F. and Lu, J. (2015) Mitochondrial fission protein MoFis1 mediates conidiation and is required for full virulence of the rice blast fungus Magnaporthe oryzae . Microbiol. Res. 178, 51–58. [DOI] [PubMed] [Google Scholar]
- Kijima, K. , Numakura, C. , Izumino, H. , Umetsu, K. , Nezu, A. , Shiiki, T. , Ogawa, M. , Ishizaki, Y. , Kitamura, T. , Shozawa, Y. and Hayasaka, K . (2005) Mitochondrial GTPase mitofusin 2 mutation in Charcot‐Marie‐Tooth neuropathy type 2A. Hum. Genet. 116, 23–27. [DOI] [PubMed] [Google Scholar]
- Li, J. , Wang, Y. , Wang, Y. , Wen, X. , Ma, X.N. , Chen, W. , Huang, F. , Kou, J. , Qi, L.‐W. , Liu, B. and Liu, K . (2015) Pharmacological activation of AMPK prevents Drp1‐mediated mitochondrial fission and alleviates endoplasmic reticulum stress‐associated endothelial dysfunction. J. Mol. Cell. Cardiol. 86, 62–74. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Sakakibara, K. , Chen, Q. and Okamoto, K. (2014) Receptor‐mediated mitophagy in yeast and mammalian systems. Cell Res. 24, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. , Hagen, F. , Stekel, D.J. , Johnston, S.A. , Sionov, E. , Falk, R. , Polacheck, I. , Boekhout, T. and May, R.C . (2009) The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl. Acad. Sci. USA, 106, 12980–12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, K. and Klionsky, D.J. (2013) Mitochondrial fission facilitates mitophagy in Saccharomyces cerevisiae . Autophagy, 9, 1900–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin‐Guzman, M. , Hartline, D. , Wright, J.D. , Elowsky, C. and Bourret, T.J. (2017) The Magnaporthe oryzae nitrooxidative stress response suppresses rice innate immunity during blast disease. Nat. Microbiol. 2, 17054. [DOI] [PubMed] [Google Scholar]
- Mitra, K. , Wunder, C. , Roysam, B. , Lin, G. and Lippincott‐Schwartz, J. (2009) A hyperfused mitochondrial state achieved at G1‐S regulates cyclin E buildup and entry into S phase. Proc. Natl. Acad. Sci. USA, 106, 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy, A.D. , McCaffery, J.M. and Shaw, J.M. (2000) Dnm1p GTPase‐mediated mitochondrial fission is a multi‐step process requiring the novel integral membrane component Fis1p. J. Cell. Biol. 151, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari, J. and Suomalainen, A. (2012) Mitochondria: in sickness and in health. Cell, 148, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K. , Kondo‐Okamoto, N. and Ohsumi, Y. (2009) Mitochondria‐anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell, 17, 87–97. [DOI] [PubMed] [Google Scholar]
- Patkar, R.N. , Ramos‐Pamplona, M. , Gupta, A.P. , Fan, Y. and Naqvi, N.I. (2012) Mitochondrial beta‐oxidation regulates organellar integrity and is necessary for conidial germination and invasive growth in Magnaporthe oryzae . Mol. Microbiol. 86, 1345–1363. [DOI] [PubMed] [Google Scholar]
- Ramos‐Pamplona, M. and Naqvi, N.I. (2006) Host invasion during rice‐blast disease requires carnitine‐dependent transport of peroxisomal acetyl‐CoA. Mol. Microbiol. 61, 61–75. [DOI] [PubMed] [Google Scholar]
- Rapaport, D. , Brunner, M. , Neupert, W. and Westermann, B. (1998) Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae . J. Biol. Chem. 273, 20150–20155. [DOI] [PubMed] [Google Scholar]
- Sesaki, H. and Jensen, R.E. (2001) UGO1 encodes an outer membrane protein required for mitochondrial fusion. J. Cell. Biol. 152, 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman, E.N. , Jones, K. , Jenkinson, C.B. , Kim, D.W. , Zhu, J. and Khang, C.H. (2017) Nuclear and structural dynamics during the establishment of a specialized effector‐secreting cell by Magnaporthe oryzae in living rice cells. BMC Cell Biol. 18, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt, T.E. and McBride, H.M. (2013) Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochem. Biophys. Acta, 1833, 417–424. [DOI] [PubMed] [Google Scholar]
- Tondera, D. , Grandemange, S. , Jourdain, A. , Karbowski, M. , Mattenberger, Y. , Herzig, S. , Da Cruz, S. , Clerc, P. , Raschke, I. , Merkwirth, C. , Ehses, S. , Krause, F. , Chan, D.C. , Alexander, C. , Bauer, C. , Youle, R. , Langer, T. and Martinou, J.‐C . (2009) SLP‐2 is required for stress‐induced mitochondrial hyperfusion. EMBO J. 28, 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama, E.Q. , Herzig, S. , Courchet, J. , Lewis, T.L. Jr , Loson, O.C. , Hellberg, K. , Young, N.P. , Chen, H. , Polleux, F. , Chan, D.C. and Shaw, R.J. (2016) Metabolism. AMP‐activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig, G. , Elorza, A. , Molina, A.J. , Mohamed, H. , Wikstrom, J.D. , Walzer, G. , Stiles, L. , Haigh, S.E. , Katz, S. , Las, G. , Alroy, J. , Wu, M. , Py, B.F. , Yuan, J. , Deeney, J.T. , Corkey, B.E. and Shirihai, O.S . (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann, B. (2010) Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884. [DOI] [PubMed] [Google Scholar]
- Yang, F. and Naqvi, N.I. (2014) Sulfonylurea resistance reconstitution as a novel strategy for ILV2‐specific integration in Magnaporthe oryzae . Fungal Genet. Biol. 68, 71–76. [DOI] [PubMed] [Google Scholar]
- Zemirli, N. and Morel, E. (2018) Mitochondrial dynamics in basal and stressful conditions. Int. J. Mol. Sci. 19, pii: E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, K. , Li, X. , Le, X. , Kong, X. , Zhang, H. , Zheng, X. , Wang, P. and Zhang, Z . (2016) MoDnm1 dynamin mediating peroxisomal and mitochondrial fission in complex with MoFis1 and MoMdv1 Is important for development of functional Appressorium in Magnaporthe oryzae . PLoS Pathog. 12, e1005823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Mitochondrial morphology during appressorium formation in M. oryzae.
Fig. S2 Generation and verification of Modnm1Δ mutant.
Fig. S3 Generation and verification of Mofzo1Δ mutant.
Fig. S4 Oxidant treatment induces mitochondrial fragmentation.
Fig. S5 Antioxidant treatment does not delay mitochondrial dynamics during invasive growth.
Fig. S6 Carbon source depletion induces mitochondrial fragmentation in M. oryzae.
Fig. S7 The vacuolar localization of Mito‐GFP (mitochondrial marker) during invasive growth.
Fig. S8 Prolonged nutrient starvation induces mitochondrial fragmentation and mitophagy.
Fig. S9 NH4NO3 or strigolactone treatment did not change the mitochondrial fragmentation during infection by M. oryzae.
Fig. S10 Addition of glucose or sucrose promotes spread of invasive hyphae and decreases the cell death in rice during early infection by M. oryzae.


