Abstract
Background: Limited evidence exists on the impact of adding a taxane, using endocrine therapy and carrying a deleterious germline BRCA mutation on ovarian reserve measured by anti-müllerian hormone (AMH) levels of young breast cancer patients receiving (neo)adjuvant cyclophosphamide- and anthracycline-based chemotherapy.
Methods: This is a biomarker analysis including young (≤ 40 years) early breast cancer patients with known germline BRCA mutational status and available prospectively collected frozen plasma samples before and after chemotherapy. Chemotherapy consisted of either six cycles of FEC (5 fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2) or three cycles of FEC followed by three cycles of docetaxel (D, 100 mg/m2). Endocrine therapy consisted of tamoxifen (±GnRH agonists). AMH levels at baseline, 1 and 3 years after diagnosis were compared according to type of chemotherapy (FEC only vs. FEC-D), use of endocrine therapy (yes vs. no) and deleterious germline BRCA mutations (mutated vs. negative).
Results: Out of 148 included patients, 127 (86%) received D following FEC chemotherapy, 90 (61%) underwent endocrine therapy, and 35 (24%) had deleterious germline BRCA mutations. In the whole cohort, AMH levels drastically dropped 1 year after diagnosis (p < 0.0001) with a slight but significant recovery at 3 years (p < 0.0001). One year after diagnosis, patients treated with FEC only had higher median AMH levels than those who received FEC-D (0.22 vs. 0.04 μg/L, p = 0.0006); no difference was observed at 3 years (0.06 and 0.18 μg/L, p = 0.47). Patients under endocrine therapy had significantly higher AMH levels than those who did not receive this treatment 1 year after diagnosis (0.12 vs. 0.02 μg/L; p = 0.008), with no difference at 3 years (0.11 and 0.20 μg/L, p = 0.22). AMH levels were similar between BRCA-mutated and BRCA-negative patients at baseline (1.94 vs. 1.66 μg/L, p = 0.53), 1 year (0.09 vs. 0.06 μg/L, p = 0.39) and 3 years (0.25 vs. 0.16 μg/L; p = 0.43) after diagnosis.
Conclusions: In breast cancer patients receiving FEC chemotherapy, adding D appeared to negatively impact on their ovarian reserve in the short-term; no further detrimental effect was observed for endocrine therapy use and presence of a deleterious germline BRCA mutation.
Keywords: breast cancer, AMH, taxane, endocrine therapy, BRCA mutations
Introduction
As a consequence of the higher incidence of aggressive tumor subtypes and the negative prognostic value of young age at diagnosis (1, 2), a significant proportion of young women with early breast cancer are candidates to receive adjuvant or neoadjuvant chemotherapy. In young patients, a major potential drawback associated with the use of systemic cytotoxic therapy is represented by the risk of causing gonadal damage with subsequent premature ovarian insufficiency (POI) and infertility (3). Considering the substantial quality of life implications associated with the development of these side effects, appropriate oncofertility counseling is now considered mandatory with all cancer patients diagnosed during their reproductive years (4–6).
Age and use of cyclophosphamide-based chemotherapy are the two major known determinants influencing the risk of gonadal damage following the use of anticancer treatments in young women with breast cancer (3). On the contrary, the impact of other treatment- or patient-related factors remains controversial. Specifically, while the effect of cyclophosphamide- and anthracycline-based chemotherapy regimens is well-established, it remains unclear if the addition of a taxane can further increase the risk of gonadal damage (7). Similarly, in young patients with hormone receptor-positive disease, the gonadotoxic impact of using endocrine therapy following chemotherapy remains debated (7, 8). Finally, recent evidence suggests that carrying a deleterious germline BRCA mutation may have a negative impact on women ovarian reserve raising the important concern of a potential increased gonadotoxicity with the use of anticancer treatments in this patient population (9, 10).
Informing young women with newly diagnosed breast cancer about the actual gonadal damage associated with the use of the proposed anticancer treatments is even more complex considering that the majority of the studies that addressed this issue relied only on the presence or absence of menstrual function after the end of chemotherapy (3). However, this cannot be considered a surrogate to determine treatment-induced gonadotoxicity (11). Being the most accurate indicator of remaining ovarian reserve, anti-müllerian hormone (AMH) is considered a promising biomarker of treatment-induced gonadotoxicity (12). Nevertheless, to date, there is limited evidence on the actual gonadal damage associated with the use of the proposed anticancer treatments through AMH assessment in young breast cancer patients (12). This is crucial information to be acquired to better inform these patients about the adverse events associated with the proposed therapies as well as on the need of pursuing fertility preservation strategies before treatment initiation. To acquire more insights on this unmet medical issue, we conducted the present study aiming to evaluate the impact of adding a taxane, using endocrine therapy as well as carrying a deleterious germline BRCA mutation on the ovarian reserve measured by AMH levels at baseline and up to 3 years after diagnosis in young early breast cancer patients treated with (neo)adjuvant anthracycline- and cyclophosphamide-based chemotherapy.
Materials and Methods
Patients and Treatments
This is a biomarker analysis conducted within a cohort of consecutive patients with early breast cancer diagnosed at ≤ 40 years that underwent (neo)adjuvant chemotherapy between January 2008 and December 2016 at the Henri Becquerel Cancer Center (Rouen, France). Patients with known germline BRCA mutational status and prospectively collected and available frozen plasma samples before and after chemotherapy were eligible for inclusion in the present analysis.
Chemotherapy consisted of either six cycles of FEC (five fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2) or three cycles of FEC followed by three cycles of docetaxel (D, 100 mg/m2). Adjuvant endocrine therapy consisted of tamoxifen exclusively or associated with gonadotropin-releasing hormone (GnRH) agonists. None of the patients received GnRH agonists during chemotherapy for ovarian function and/or fertility preservation.
All patients signed a consent form allowing the conservation and study of their biological samples. The present study was approved by the Institutional Scientific and Ethics Committees of Henri Becquerel Centre (registering order N°1807B).
AMH Measurements
As per routine practice at our center, plasma samples are prospectively collected at fixed timepoints during follow-up in all breast cancer patients and are stored in our plasma bank at −20°C.
For the purpose of the present analysis, plasma samples of eligible patients were used to assess AMH levels at baseline (i.e., before starting chemotherapy), ~1 year and over 3 years after diagnosis.
AMH measurements were centrally performed at Henri Becquerel Cancer Center using fully automated ultra-sensitive Elecsys AMH assay on the Cobas e601 instrument (Roche Diagnostics). The detection and quantification limits were 0.01 and 0.03 μg/L, respectively, with an intra-assay imprecision coefficient of variation equal to 1.2% at 1.19 μg/L and 0.9% at 5.89 μg/L.
Study Objectives
The main objective of this study was to assess the impact of adding a taxane (D), using endocrine therapy and carrying a deleterious germline BRCA mutation on the ovarian reserve measured by AMH levels of young breast cancer patients after (neo)adjuvant cyclophosphamide- and anthracycline-based chemotherapy (FEC). AMH levels at baseline, 1 and 3 years after diagnosis were compared according to type of chemotherapy (FEC only vs. FEC-D), use of endocrine therapy (yes vs. no) and deleterious germline BRCA mutations (mutated vs. negative).
Statistical Analysis
Baseline characteristics of patients were compared according to type of chemotherapy or endocrine treatment administered and BRCA mutational status. Quantitative variables were reported as median with interquartile range (IQR, Q1–Q3). Differences were tested using χ2, Fisher's exact test or two-sample t-tests as appropriate.
Comparisons of the evolution of AMH values over time were restricted to the same patients, using paired tests (Wilcoxon).
A p-value of <0.05 was considered statistically significant. Tests were performed using the R® statistical software version 3.4.3. Figures were made using the ggplot2 package (R Core Team 2017, https://www.R-project.org/).
Results
Patient Characteristics
Between January 2008 and December 2016, out of 262 patients diagnosed at ≤ 40 years who underwent (neo)adjuvant chemotherapy for early breast cancer, 148 had plasma samples and BRCA mutational status available to be included in the present analysis (Figure 1).
Figure 1.
The flow diagram of participants. GnRHa, gonadotropin-releasing hormone agonists.
Median age was 35 years (IQR 31.5–38; Table 1). A total of 127 (86%) patients received D in addition to FEC chemotherapy, 90 (61%) underwent endocrine therapy, and 35 (24%) had deleterious germline BRCA mutations.
Table 1.
Baseline patients' and tumor characteristics.
| Patient characteristics | All patients (n = 148) |
|---|---|
| Age at diagnosis, median [IQR] | 35.5 [31.5–38] |
| AMH, median [IQR] | 1.68 [1.00–3.30] |
| AMH, mean [SD] | 2.55 [2.81] |
| Body mass index (kg/m2), median [IQR] | 22.7 [21.0–26.2] |
| Smoker, n (%) | 52 (35%) |
| Genetic mutation, n (%) | 35 (24%) |
| BRCA1 | 22 (63%) |
| BRCA2 | 13 (37%) |
| Tumor characteristics, n (%) | |
| Histological grade | |
| Grade I | 6 (4%) |
| Grade II | 54 (36%) |
| Grade III | 87 (59%) |
| Not available | 1 (<1%) |
| Hormone receptor positivity | 92 (62%) |
| Oestrogen receptor | 90 (61%) |
| Progesteron receptor | 65 (44%) |
| HER2-positive | 27 (18%) |
| Triple-negative | 52 (35%) |
| Pathological nodal status positivity | 77 (52%) |
| Tumor size (T) | |
| T1 | 49 (33%) |
| T2 | 72 (49%) |
| T3 | 23 (15%) |
| T4 | 4 (3%) |
| Surgical treatment, n (%) | |
| Conservative | 82 (55%) |
| Mastectomy | 66 (45%) |
| Adjuvant treatment, n (%) | |
| Radiation therapy | 144 (97%) |
| Endocrine therapya | 90 (61%) |
| Chemotherapy | 148 (100%) |
| Chemotherapy regimen, n (%) | |
| 3 FEC−3 D | 127 (86%) |
| 6 FEC | 21 (14%) |
| Endocrine therapy, n (%) | |
| Tamoxifen | 82 (91%) |
| Tamoxifen + GnRH agonists | 8 (9%) |
| Fertility history, n (%) | |
| Pregnancy before treatment | 129 (84%) |
| Childbirth before treatment | 128 (84%) |
2 patients with hormone receptor-positive tumors refused endocrine therapy.
AMH, anti-mullerian hormone; IQR, interquartile range; SD, standard deviation; FEC, fluorouracil, epirubicin, cyclophosphamide; D, docetaxel; GnRH, gonadotropin-releasing hormone.
AMH Evolution Under Chemotherapy in the Whole Population
At baseline, median AMH level was 1.68 μg/L (IQR 1.00–3.30). Higher age was associated with significant lower AMH levels (p = 0.047; Figure S1).
One year after diagnosis (median 387 days, IQR 363–426), AMH levels drastically dropped to a median value of 0.06 μg/L (IQR 0–0.25; p < 0.0001). Three years after diagnosis (median 1,132 days, IQR 1,099–1,186), a slight but significant recovery of AMH value was observed with a median level of 0.17 μg/L (IQR 0.04–0.41; p < 0.0001; Figure S2).
Impact of Taxanes
Baseline characteristics according to type of chemotherapy (FEC only vs. FEC-D) are reported in Table S1.
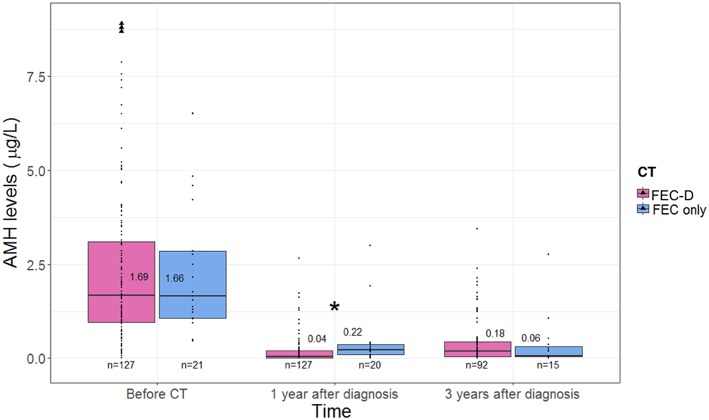
At baseline, no difference in AMH levels was observed between patients treated with FEC only (1.66 μg/L, IQR 1.06–2.85) or FEC-D (1.69 μg/L, IQR 0.98–3.33; p = 0.83) (Figure 2).
Figure 2.
The evolution of anti-mullerian hormone levels according to type of chemotherapy. *Statistical significant difference. AMH, anti-mullerian hormone; CT, chemotherapy; FEC, fluorouracil, epirubicin, cyclophosphamide; D, docetaxel.
One year after diagnosis, patients treated with FEC only had higher median AMH levels (0.22 μg/L, IQR 0.10–0.36) as compared to those who received FEC-D (0.04 μg/L, IQR 0.00–0.21; p = 0.0006), respectively.
Three years after diagnosis, no difference in median AMH levels was observed between patients treated with FEC only (0.06 μg/L, IQR 0.04–0.32) or FEC-D (0.18 μg/L, IQR 0.04–0.43; p = 0.47). Patients treated with FEC only had no recovery of AMH values between 1 and 3 years (0.22 vs. 0.06 μg/L, respectively, p = 0.81) while patients treated by FEC-D had a slight but significant recovery (0.04 vs. 0.18 μg/L, p < 0.0001).
Impact of Endocrine Therapy
Baseline characteristics according to use of endocrine therapy (yes vs. no) are reported in Table S2. Among the 90 (61%) patients who received endocrine therapy, 82 (91%) underwent tamoxifen alone and 8 (9%) tamoxifen combined with GnRH agonists (Table 1). At the 3-year timepoint, all but three patients who started endocrine therapy were still under treatment.
At baseline, no difference in AMH values was observed between patients who received endocrine therapy (1.94 μg/L, IQR 1.01–3.76) and those who did not (1.50 μg/L, IQR 0.96–2.77; p = 0.17) (Figure 3).
Figure 3.
The evolution of anti-mullerian hormone levels according to use of endocrine therapy. *Statistical significant difference. AMH, anti-mullerian hormone; ET, endocrine therapy.
One year after chemotherapy, patients under endocrine therapy had significantly higher AMH levels (0.12 μg/L, IQR 0.02–0.29) than those who did not receive this treatment (0.02 μg/L, IQR 0.00–0.12; p = 0.008).
No difference was observed 3 years after diagnosis, with comparable values of AMH for patients undergoing endocrine therapy (0.11 μg/L, IQR 0.03–0.43) or not (0.20 μg/L, 0.07–0.40; p = 0.22).
Impact of Carrying a Deleterious Germline BRCA Mutation
Baseline characteristics according to BRCA mutational status (mutated vs. negative) are reported in Table S3. Among the 35 (24%) BRCA-mutated breast cancer patients, 22 and 13 harbored deleterious BRCA1 or BRCA2 mutations, respectively. Patients in the BRCA-mutated cohort were younger than those without mutation (p = 0.027).
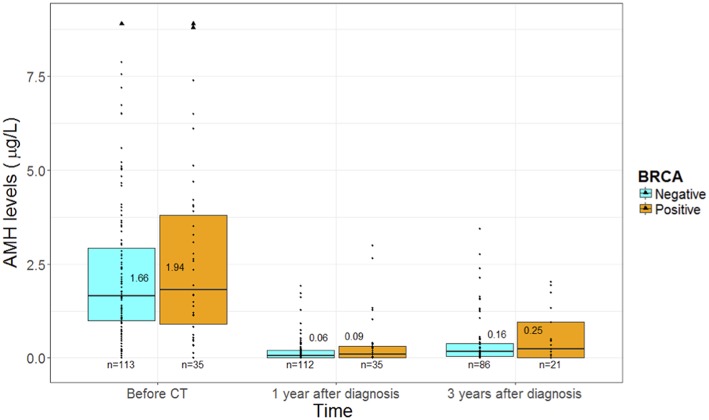
At baseline, no difference in AMH values was observed between BRCA-mutated (1.94 μg/L, IQR 0.98–3.96) and BRCA-negative (1.66 μg/L, IQR 1.00–3.02) patients (p = 0.53) (Figure 4).
Figure 4.
The evolution of anti-mullerian hormone levels according to BRCA mutational status. AMH, anti-mullerian hormone.
One year after chemotherapy, AMH values drastically dropped in both groups to 0.09 μg/L (IQR 0.00–0.30) and 0.06 μg/L (IQR 0.00–0.21) in the BRCA-mutated and negative cohorts, respectively (p = 0.39).
Recovery at 3 years from diagnosis was observed in a similar extent in both groups with levels of 0.25 μg/L (IQR 0.02–0.95) and 0.16 μg/L (IQR 0.04–0.39) in the BRCA-mutated and negative cohorts, respectively (p = 0.43).
When the analyses were repeated to take into account the different age at diagnosis, similar results were shown with no difference in AMH levels between the BRCA-mutated and the BRCA-negative cohorts both at diagnosis and after treatment (Supplementary Data). Similarly, within the BRCA-mutated cohort, no impact of the type of BRCA mutation (BRCA1 vs. BRCA2) was observed (Figure S3).
Discussion
In this study, we assessed the ovarian reserve measured by AMH levels at baseline and up to 3 years after diagnosis in young early breast cancer patients treated with (neo)adjuvant anthracycline- and cyclophosphamide-based chemotherapy. While prior evidence exists on AMH levels following the use of endocrine therapy, this is the first study to our knowledge addressing the impact on patients' ovarian reserve of adding a taxane to anthracycline- and cyclophosphamide-based chemotherapy and the potential influence on treatment gonadotoxictiy of carrying a deleterious germline BRCA mutation. In early breast cancer patients receiving FEC chemotherapy, adding D appeared to negatively impact on their ovarian reserve in the short-term; no further detrimental effect was observed for endocrine therapy use and presence of a deleterious germline BRCA mutation.
Prior studies investigating treatment impact on patients' ovarian reserve showed that AMH decreases rapidly during the first cycles of chemotherapy reaching the lowest values at the end of systemic cytotoxic therapy (12–16). However, limited data on AMH values beyond 1 year following chemotherapy have been reported so far. Median AMH value at baseline in our study (1.68 μg/L) was in line with published data among non-cancer patients of similar age (median 2.3 μg/L) (17). With an estimated AMH value decline of 5.6%/year among non-cancer patients ≤ 40 years (17), the physiological decline in our population would have led to a median AMH value 3 years after diagnosis of 1.4 μg/L, which is far more elevated than the value observed (0.17 μg/L). Thus, our findings confirmed a deep and persistent impact of anthracycline- and cyclophosphamide-based chemotherapy on AMH levels up to 3 years after diagnosis. In addition, the specific features of our homogenous patient cohort provided a unique opportunity to investigate the influence on AMH levels of adding a taxane, using endocrine therapy as well as carrying a deleterious germline BRCA mutation.
A sequential treatment with anthracycline- and cyclophosphamide-based chemotherapy followed by a taxane is the current standard (neo)adjuvant chemotherapy regimen in young breast cancer patients (5). Despite being a widely used regimen since many years, the gonadotoxicity of such treatment remains controversial. While a prior meta-analysis showed no statistically significant increased risk of amenorrhea with the addition of a taxane (7), larger studies have recently shown a potential negative effect (18, 19). However, limited evidence exists on the actual and potential damage induced by taxanes on patients' ovarian reserve measured by AMH levels (20). Our findings, for the first time with a clear comparison between FEC only and FEC-D, provide some evidence on the potential increased gonadotoxic burden of administering a taxane following anthracycline- and cyclophosphamide-based chemotherapy. However, partial AMH recovery between 1 and 3 years after diagnosis was observed for FEC-D while a further decrease was shown for FEC only: hence, there was no difference between the two treatment options at 3 years. Notably, the dose of cyclophosphamide for patients treated with FEC only was approximately twice as compared to the one received by women who underwent FEC-D (6 vs. 3 cycles, respectively). As shown in animal models, taxanes appear to damage specifically the growing follicles with no apparent direct effect on primordial follicles (21). This specific gonadotoxic mechanism may explain our observation on the early (within 1 year) negative impact of taxanes on AMH levels. This is in contrast with cyclophosphamide that causes massive atresia of both primordial and growing follicles, with subsequent longer impact on women ovarian reserve reflected by poorer long-term AMH recovery (22).
All young women with hormone receptor-positive breast cancer are candidates to receive adjuvant endocrine therapy (5). For those who are previously exposed to chemotherapy, it is crucial to counsel them on the potential impact of this additional treatment on their ovarian reserve. Several studies have shown an increased risk of post-treatment amenorrhea when tamoxifen was administered after chemotherapy (7, 18, 19). On the contrary, the limited data on endocrine therapy gonadotoxicity measured by AMH levels did not show any difference between patients who received or not tamoxifen following chemotherapy (8, 13, 16, 23). Our findings confirm the lack of detrimental effect of endocrine therapy on patients' ovarian reserve. Interestingly, patients treated with tamoxifen had significantly higher AMH levels at 1 year as compared to those who received chemotherapy alone. Similarly, higher AMH values in patients treated with tamoxifen following chemotherapy has been also observed in two recent studies (8, 23), with one of them showing a faster AMH recovery between 3 and 6 months after the end of systemic cytotoxic therapy for women treated with endocrine therapy (23). Notably, when given concurrently with chemotherapy, preclinical studies have suggested a potential protective effect of tamoxifen against anticancer treatment gonadotoxicity, including of cyclophosphamide-based therapy (24). Taken together, although tamoxifen may cause perturbation in menstrual function after chemotherapy, the available evidence including our findings suggests the lack of detrimental effect on patients' ovarian reserve. Nevertheless, a proper oncofertility counseling in these women is particularly important and should take into account also the need to prolong such treatment up to 10 years after diagnosis (5), with their subsequent ovarian aging. Indeed, patients with hormone receptor-positive breast cancer have lower chances of post-treatment pregnancies as compared to women who are not candidates to endocrine therapy (8, 25). An international study is currently ongoing to investigate the safety of a temporary interruption of endocrine therapy after 18–30 months of treatment to allow a pregnancy (26).
More than 10% of breast cancer cases arising in young women are hereditary tumors related to the presence of a deleterious germline BRCA mutation (27, 28). Considering the high mutation rate in this patient population, current guidelines strongly support a genetic testing in all women diagnosed at a young age irrespectively of their family history (5). Preclinical and clinical evidence has suggested a potential negative impact of these mutations on female reproductive potential (29, 30). In breast cancer patients, three small studies investigated AMH levels in BRCA-mutated breast cancer patients but only at the time of diagnosis without data after chemotherapy (9, 10, 31). Therefore, a major unanswered concern in this setting is the potential increased gonadotoxicity risk of BRCA-mutated patients (29, 30). The only study that assessed chemotherapy-induced amenorrhea in this setting did not show any difference between BRCA-mutated and BRCA-negative patients (32). Our study is on the same line suggesting for the first time the lack of detrimental effect for carrying a deleterious germline BRCA mutation on chemotherapy-induced gonadal damage. Further studies in breast cancer patients with germline mutations in BRCA or other susceptibility genes are warranted to improve their oncofertility counseling in terms of both estimating the risk of gonadotoxicity with the proposed anticancer treatments as well as the efficacy and safety of fertility preservation procedures in this setting (10, 33).
Some limitations should be considered in the interpretation of our results. This analysis was conducted in a single center and included a relatively small cohort of patients who received FEC only chemotherapy or carried a deleterious germline BRCA mutation. A few post-chemotherapy plasma samples were missing and could not be assessed in the whole study cohort. Data on post-treatment pregnancies and menstrual function were not collected so that no correlation with AMH values could be performed. Nevertheless, despite these limitations, our study represents one of the largest AMH analyses and with the longest follow-up conducted so far among breast cancer patients; plasma samples were prospectively collected at predefined timepoints during treatment and oncologic follow-up up to 3 years after diagnosis in a homogenous cohort of women. In addition, our study provides answers on three relevant questions that are of crucial importance in everyday clinical practice to properly counsel these women on the gonadotoxicity of the proposed anticancer treatments. Future larger prospective studies are needed to address the clinical utility of this biomarker before its incorporation in routine clinical practice including its potential role as predictor of treatment-induced POI and infertility in young cancer patients.
Conclusions
Our study confirmed the deep and prolonged adverse effect of chemotherapy on the ovarian reserve of young breast cancer patients with only partial recovery 3 years after treatment. We showed that adding D following anthracycline- and cyclophosphamide-based chemotherapy (FEC regimen) appeared to cause an early negative impact on their ovarian reserve. Endocrine therapy with tamoxifen and the presence of a deleterious germline BRCA mutation did not appear to further worsen chemotherapy gonadotoxicity. Altogether, although future larger collaborative efforts are needed to validate our results, we provide important data for improving the oncofertility counseling of young breast cancer patients.
Data Availability
The datasets generated for this study are available on reasonable request to the corresponding author.
Ethics Statement
The present study was approved by the local Institutional Review Board (registering order N°1807B). All patients signed a consent form allowing the conservation and study of their biological samples.
Author Contributions
MLa, AP, and FC contributed to the conception and design of the study. CC and AP performed the analysis of all plasma samples. JL performed the statistical analysis. The results were interpreted by MLa, NO, JL, AP, and FC who also drafted the initial manuscript that was then revised critically for important intellectual content and approved by all the authors. All the authors contributed to collection and assembly of data.
Conflict of Interest Statement
MLa served as a consultant for Teva and received honoraria from Theramex and Takeda outside the submitted work. FC received institutional research funds from Astra Zeneca and served as consultant for Astra Zeneca, Merck and BMS, outside of the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
During the conduction of this study, MLa acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium).
Footnotes
Funding. This work was exclusively founded by the Henri Becquerel Cancer Center (Appel d'offre interne 2018).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00575/full#supplementary-material
References
- 1.Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. (2014) 16:427. 10.1186/s13058-014-0427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong Y-N, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. (2016) 34:3308–14. 10.1200/JCO.2015.65.8013 [DOI] [PubMed] [Google Scholar]
- 3.Lambertini M, Goldrat O, Clatot F, Demeestere I, Awada A. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol. (2017) 29:243–52. 10.1097/CCO.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 4.Peccatori FA, Azim HA, Jr., Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24 Suppl 6:vi160–70. 10.1093/annonc/mdt199 [DOI] [PubMed] [Google Scholar]
- 5.Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso M-J, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. (2017) 35:203–17. 10.1016/j.breast.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 6.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. (2018) 36:1994–2001. 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 7.Zavos A, Valachis A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. (2016) 55:664–70. 10.3109/0284186X.2016.1155738 [DOI] [PubMed] [Google Scholar]
- 8.Shandley LM, Spencer JB, Fothergill A, Mertens AC, Manatunga A, Paplomata E, et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril. (2017) 107:243–52.e5. 10.1016/j.fertnstert.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. (2013) 5:172ra21. 10.1126/scitranslmed.3004925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. (2018) 29:237–43. 10.1093/annonc/mdx639 [DOI] [PubMed] [Google Scholar]
- 11.Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. (2010) 94:638–44. 10.1016/j.fertnstert.2009.03.045 [DOI] [PubMed] [Google Scholar]
- 12.Fréour T, Barrière P, Masson D. Anti-müllerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. Eur J Cancer. (2017) 74:1–8. 10.1016/j.ejca.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, et al. Prospective evaluation of serum anti-Müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer. (2017) 79:72–80. 10.1016/j.ejca.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 14.Trapp E, Steidl J, Rack B, Kupka MS, Andergassen U, Jückstock J, et al. Anti-Müllerian hormone (AMH) levels in premenopausal breast cancer patients treated with taxane-based adjuvant chemotherapy–a translational research project of the SUCCESS A study. Breast. (2017) 35:130–5. 10.1016/j.breast.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 15.Perdrix A, Saint-Ghislain M, Degremont M, David M, Khaznadar Z, Loeb A, et al. Influence of adjuvant chemotherapy on anti-Müllerian hormone in women below 35 years treated for early breast cancer. Reprod Biomed Online. (2017) 35:468–74. 10.1016/j.rbmo.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Anderson RA, Mansi J, Coleman RE, Adamson DJA, Leonard RCF. The utility of anti-Müllerian hormone in the diagnosis and prediction of loss of ovarian function following chemotherapy for early breast cancer. Eur J Cancer. (2017) 87:58–64. 10.1016/j.ejca.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. (2013) 98:1602–11. 10.1210/jc.2012-1829 [DOI] [PubMed] [Google Scholar]
- 18.Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod. (2016) 31:2737–49. 10.1093/humrep/dew224 [DOI] [PubMed] [Google Scholar]
- 19.Lambertini M, Campbell C, Bines J, Korde LA, Izquierdo M, Fumagalli D, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. (2019) 111:86–94. 10.1093/jnci/djy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson RA, Themmen APN, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. (2006) 21:2583–92. 10.1093/humrep/del201 [DOI] [PubMed] [Google Scholar]
- 21.Lopes F, Smith R, Anderson RA, Spears N. Docetaxel induces moderate ovarian toxicity in mice, primarily affecting granulosa cells of early growing follicles. Mol Hum Reprod. (2014) 20:948–59. 10.1093/molehr/gau057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. (2015) 30:2926–35. 10.1093/humrep/dev256 [DOI] [PubMed] [Google Scholar]
- 23.Decanter C, Cloquet M, Dassonneville A, D'Orazio E, Mailliez A, Pigny P. Different patterns of ovarian recovery after cancer treatment suggest various individual ovarian susceptibilities to chemotherapy. Reprod Biomed Online. (2018) 36:711–8. 10.1016/j.rbmo.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 24.Piasecka-Srader J, Blanco FF, Delman DH, Dixon DA, Geiser JL, Ciereszko RE, et al. Tamoxifen prevents apoptosis and follicle loss from cyclophosphamide in cultured rat ovaries. Biol Reprod. (2015) 92:132. 10.1095/biolreprod.114.126136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. (2018) 36:1981–90. 10.1200/JCO.2018.78.0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagani O, Ruggeri M, Manunta S, Saunders C, Peccatori F, Cardoso F, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. (2015) 24:201–7. 10.1016/j.breast.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SM, Ruddy KJ, Tamimi RM, Gelber S, Schapira L, Come S, et al. BRCA1 and BRCA2 mutation testing in young women with breast cancer. JAMA Oncol. (2016) 2:730–6. 10.1001/jamaoncol.2015.5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. (2018) 19:169–80. 10.1016/S1470-2045(17)30891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambertini M, Goldrat O, Toss A, Azim HA, Peccatori FA, Ignatiadis M, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev. (2017) 59:61–70. 10.1016/j.ctrv.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 30.Peccatori FA, Mangili G, Bergamini A, Filippi F, Martinelli F, Ferrari F, et al. Fertility preservation in women harboring deleterious BRCA mutations: ready for prime time? Hum Reprod. (2018) 33:181–7. 10.1093/humrep/dex356 [DOI] [PubMed] [Google Scholar]
- 31.Gunnala V, Fields J, Irani M, D'Angelo D, Xu K, Schattman G, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. (2019) 111:363–71. 10.1016/j.fertnstert.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Valentini A, Finch A, Lubinski J, Byrski T, Ghadirian P, Kim-Sing C, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. (2013) 31:3914–9. 10.1200/JCO.2012.47.7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci. (2018) 25:26–32. 10.1177/1933719117728800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on reasonable request to the corresponding author.