SUMMARY
A small open reading frame, termed ‘pipo’, is embedded in the P3 cistron of potyviruses. Currently, knowledge on pipo and its role(s) in the life cycle of potyviruses is limited. The P3 and helper‐component proteinase (HC‐Pro) cistrons of Soybean mosaic virus (SMV) harbour determinants affecting virulence on functionally immune Rsv1‐genotype soybeans. Interestingly, a key virulence determinant of SMV on Rsv1‐genotype soybeans (i.e. soybeans containing the Rsv1 resistance gene) that resides at polyprotein codon 947 overlaps both P3 and a pipo‐encoded codon. This raises the question of whether PIPO or P3 is the virulence factor. To answer this question, the corresponding pipo of an avirulent and two virulent strains of SMV were studied by comparative genomics, followed by syntheses and analyses of site‐directed mutants. Our data demonstrate that the virulence of SMV on Rsv1‐genotype soybeans is affected by P3 and not the overlapping pipo‐encoded protein.
INTRODUCTION
The genetic determinants of plant viruses mediating virulence on host genotypes containing resistance genes, dominant or recessive, often reside on viral encoded proteins and involve one or more amino acids (Abdul‐Razzak et al., 2009; Decroocq et al., 2009; Diveki et al., 2004; Eggenberger et al., 2008; Hjulsager et al., 2006; Janzac et al., 2010; Malcuit et al., 1999; Meshi et al., 1988; Padgett et al., 1997; Salvador et al., 2008). In the case of viruses belonging to the family Potyviridae, such determinants have been mapped to various viral encoded proteins (Abdul‐Razzak et al., 2009; Decroocq et al., 2009; Eggenberger et al., 2008; Fellers et al., 2002; Hajimorad et al., 2008; Hjulsager et al., 2006; Janzac et al., 2010; 2000, 2002, 2003; Johansen et al., 2001; Krause‐Sakate et al., 2005; Mestre et al., 2000; Salvador et al., 2008).
The family Potyviridae represents one of the largest groups of plant RNA viruses affecting major crops worldwide (Adams et al., 2005). The genome of the member species of the genus Potyvirus, including that of Soybean mosaic virus (SMV), is approximately 10 kb and contains a single long open reading frame (ORF) and a very small overlapping ORF, termed ‘pipo’ (Chung et al., 2008; Wen and Hajimorad, 2010). On expression, the long polyprotein is cleaved post‐translationally by three viral encoded proteinases to yield a number of multifunctional mature proteins, including helper‐component proteinase (HC‐Pro) and P3 (Urcuqui‐Inchima et al., 2001). The small ORF pipo embedded in the P3 cistron of potyviruses has the potential to encode a protein in the +2 frame relative to the polyprotein ORF. It has been proposed that pipo is expressed in planta as a protein of approximately 25 kDa fused to the N‐terminus P3 (P3N+PIPO) (Chung et al., 2008); however, the presence of P3 amino acid sequences in the detected protein, serologically or by sequencing, remains to be demonstrated. The putative pipo of SMV is 225 nucleotides long, contains a GA6 motif at its 5′ end and has the potential to encode 75 amino acids (Chung et al., 2008; Wen and Hajimorad, 2010). Knowledge on pipo and its role(s) in the life cycle of potyviruses is limited (Choi et al., 2005; Chung et al., 2008; Wei et al., 2010; Wen and Hajimorad, 2010).
The major host of SMV is soybean, and cultivars harbouring the Rsv1 locus are functionally immune to strains G1–G6, but not to G7 or its experimentally evolved variant G7d (Bernard et al., 1991; Hajimorad et al., 2003). SMV‐N, an isolate of strain G2, is avirulent on Rsv1‐genotype soybeans (Hajimorad and Hill, 2001). Studies based on comparative genomics analyses between avirulent SMV‐N (avrN) and virulent SMV‐G7 (virG7), as well as experimental adaptation of avrN‐derived P3 chimeras containing P3 sequences from virG7 or virulent G7d (virG7d), and direct adaptation of avrN to Rsv1‐genotype soybeans, have all identified numerous virulence determinants of SMV residing on HC‐Pro and P3 cistrons (Eggenberger et al., 2008; 2008, 2011). Interestingly, a key virulence determinant within the P3 cistron at polyprotein codon 947 (Eggenberger et al., 2008; Hajimorad et al., 2011) also overlaps one of the pipo‐encoded codons. This raises the possibility that PIPO, but not P3, is the virulence factor of SMV on Rsv1‐genotype soybeans. In this article, virulence is defined as the genetic ability of a strain or a variant of SMV to overcome functional immunity mediated by the Rsv1 locus (Shaner et al., 1992). On the basis of this definition, with respect to Rsv1‐genotype soybeans, SMV‐G7 and SMV‐G7d are both virulent, whereas SMV‐N is avirulent (Hajimorad et al., 2006).
To answer the question of whether pipo plays any role in the virulence of SMV, we have taken advantage of the differential interactions of avrN, virG7 and virG7d with Rsv1‐genotype soybeans (Hajimorad et al., 2003; Hajimorad and Hill, 2001). In this article, using a comparative genomics approach combined with site‐directed mutagenesis, we present evidence that determinants of SMV effective for virulence on Rsv1‐genotype soybeans residing on the P3 cistron are positioned on P3‐ and not the pipo‐encoded protein.
RESULTS AND DISCUSSION
Genetic differences among pipo of avrN, virG7 and virG7d are limited
Alignment of the nucleotide sequence of the putative pipo of avrN with those of virG7 and virG7d showed that they are all 225 nucleotides in length (Fig. 1A). The position of pipo of avrN has been mapped to the genomic nucleotides 2882–3106 (Wen and Hajimorad, 2010). However, in the context of virG7 and virG7d genomes, pipo is located at nucleotides 2885–3109. This is the result of a lack of a single codon in the P1 cistron of avrN relative to those of virG7 and virG7d, which is positioned upstream of pipo (Hajimorad et al., 2006). It has been proposed that AAA encoding lysine (Lys) located within the highly conserved GA6 motif serves as the first codon for the putative SMV pipo‐encoded protein, mainly because of the presence of an adjacent pipo in‐frame stop codon (TGA) (Wen and Hajimorad, 2010) (Fig. 1A). Moreover, another pipo in‐frame stop codon (TGA) located upstream of the GA6 motif is conserved among all three SMV strains (Fig. 1A). Downstream of the GA6 motif, the first pipo in‐frame stop codon (TGA) in the genome of avrN is located at nucleotide positions 3107–3109, whereas those of virG7 and virG7d are at positions 3110–3112 (Fig. 1A). This stop codon probably serves as the terminal signal for the expression of pipo‐encoded protein.
Figure 1.
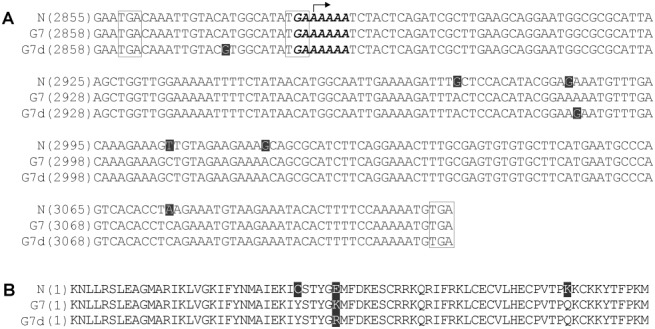
(A) Partial nucleotide sequences of the P3 cistron of the infectious cDNA clones of Soybean mosaic virus (SMV) strains N (N), G7 and G7d containing the full‐length sequences of pipo. The arrow indicates the starting nucleotide of pipo. The nucleotide sequences within the boxes are pipo in‐frame stop codons upstream of the conserved GA6 motif and the termination codon at the 3′‐end of pipo. The sequences of the GA6 motif at the 5′‐terminus of pipo are shown in italic and boldface. The unique nucleotide of each strain not shared with the others is highlighted. (B) Alignment of the deduced primary amino acid sequences of the PIPO protein of N, G7 and G7d. The unique amino acid of each strain not shared with the others is highlighted. It should be noted that the genomic positions of pipo of G7 and G7d differ from that of N because of the presence of one additional codon upstream of the P3 cistron (GenBank accession numbers AY216010, AY216987 and D00507, respectively).
There are a total of five and six nucleotide substitutions between the putative pipo of avrN and those of virG7 and virG7d, respectively (Fig. 1A). There is only one nucleotide substitution in the pipo of virG7 when compared with that of virG7d (Fig. 1A). These nucleotide differences result in a total of three amino acid substitutions in the pipo‐encoded protein of avrN when compared with those of virG7 and virG7d (Fig. 1B). These unique amino acids of avrN not shared with virG7 and virG7d are located at positions 30, 35 and 65 of the PIPO protein. The PIPO protein of virG7 differs from that of virG7d by only a single amino acid substitution (Fig. 1B). This unique amino acid that is not shared with avrN as well as virG7 is located at position 35 of PIPO (Fig. 1B).
PIPO of SMV lacks a determinant of virulence on Rsv1‐genotype soybean
Recently, it has been shown that single mutations in P3 convert avrN to virN on L800 (Rsv1), but not PI96983 (Rsv1) (Hajimorad et al., 2011). For the virulence of avrN on PI96983 (Rsv1), simultaneous mutations in both HC‐Pro and P3 are required (2008, 2011). L800 (Rsv1) is a recombinant soybean line selected from a population resulting from the crossing of SMV‐susceptible Lee68 (rsv1) with PI96983 (Rsv1) (Hayes et al., 2004). The lower strength of resistance of L800 (Rsv1) to avrN, relative to PI96983 (Rsv1), is possibly a result of the possession of only one of the six nucleotide binding/leucine (Leu)‐rich repeat‐type candidate resistance genes at the Rsv1 chromosomal region from PI96983 (Rsv1) (Hayes et al., 2004). However, the resultant phenotypes of L800 (Rsv1) against avrN, virG7 and virG7d resemble the phenotypic responses of PI96983 (Rsv1) (Fig. 2) (2003, 2006). As manipulation of the P3 cistron alone could convert avrN to virN on L800 (Rsv1) (Hajimorad et al., 2011), we utilized this Rsv1‐genotype soybean to examine whether pipo carries virulence determinants of SMV.
Figure 2.

Phenotypic responses of soybean L800 (Rsv1) to mechanical inoculation with progeny derived from the infectious cDNA clones of Soybean mosaic virus (SMV) strains N (SMV‐N), G7 (SMV‐G7) and G7d (SMV‐G7d). Sap containing progeny of the viruses from biolistically inoculated Williams82 served as the inoculum. The inoculated plants were photographed 14 days post‐inoculation. It should be noted that plants inoculated with SMV‐N are functionally immune, do not exhibit any symptoms and the virus cannot be recovered from the inoculated plants. In contrast, L800 (Rsv1) inoculated with SMV‐G7 and SMV‐G7d is symptomatic. SMV‐G7 induces stunting and systemic necrosis visible on the first trifoliate leaf. In contrast, SMV‐G7d causes a systemic mosaic pattern.
Reciprocal exchanges of the unique nucleotide at genomic positions 2970 (G to A) of avrN and 2973 (A to G) of virG7 and virG7d resulted in nonsynonymous substitutions in pipo and the overlapping P3 codons of all the strains (Fig. 3). This was because the unique nucleotide at this position served as the first residue of the overlapping P3 codon, whereas it constituted the second residue of the pipo‐encoded codon (Fig. 3). Thus, NC30Y, G7Y30C and G7dY30C all had an amino acid substitution in their respective P3. These were A947T in P3 of avrN and T948A in those of virG7 and virG7d (3, 4; Table 1). All these mutants easily infected the rsv1‐soybean genotypes (Table 1) (Hajimorad et al., 2011). NC30Y gained virulence on L800 (Rsv1) (Fig. 4; Table 1); however, the virulence of G7Y30C and G7dY30C was severely compromised (Table 1). When Y30 in PIPO of virG7 was substituted with histidine (His) by changing pipo‐encoded codon TAC to CAC (Fig. 3), G7Y30H remained virulent on L800 (Rsv1) (Table 1). This substitution [threonine (Thr) to His], unlike tyrosine (Tyr) to cysteine (Cys) replacement, did not alter the overlapping P3 codon (Table 1). Thus, the loss of virulence on L800 (Rsv1) by G7Y30C and G7dY30C is probably a result of alteration in P3 and not PIPO. We also replaced Cys at position 30 of PIPO of avrN with arginine (Arg) and generated the NC30R mutant. This mutation did not alter the overlapping P3 codon as it was synonymous (Table 1; Fig. 3). However, NC30R was not stable and did not infect the rsv1‐genotype soybeans efficiently, as two of the six biolistically inoculated Williams 82 (rsv1) and none of the Lee68 (rsv1) soybeans became infected (Table 1). Genotyping of the viral progeny from the infected Williams 82 (rsv1) soybean showed that those from one of the plants had a mutation in P3 at genomic position 2970 (G to U), which resulted in A947S and C30L replacements in P3 and PIPO, respectively. Analysis of the progeny from the second infected Williams 82 (rsv1) soybean showed reversion of the introduced substitution to the wild‐type. As the C30R substitution did not alter the overlapping P3 codon, it is possible that this mutation affected a previously unrecognized cis‐acting RNA sequence element. Regardless, NC30R was not virulent on L800 (Rsv1) (Table 1). Hajimorad et al. (2011) have shown recently that NA947V, similar to NA947T, is virulent on L800 (Rsv1) (Fig. 4; Table 1). Unlike A947T substitution in the avrN genome, which changes the overlapping PIPO amino acid at position 30 from Cys to Tyr (Fig. 3), A947V substitution in P3 is synonymous for the pipo‐encoded codon. This is because C to T substitution at genomic position 2971 of avrN alters the overlapping pipo codon TGC to TGT (Fig. 3). Both of these codons encode Cys. This clearly demonstrates that the determinant of virulence at this genomic position, involved in Rsv1‐mediated resistance‐breaking ability, resides on P3 and not PIPO.
Figure 3.
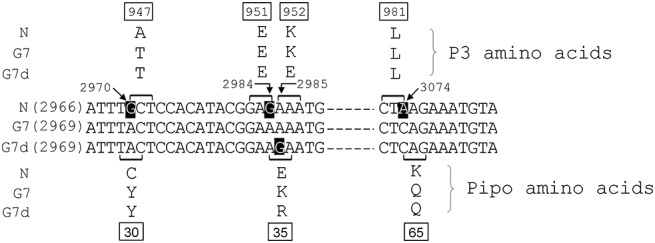
Partial nucleotide sequences of the P3 cistron of the infectious cDNA clones of Soybean mosaic virus strains N, G7 and G7d harbouring codons expressing amino acids of P3 and the overlapping pipo‐encoded protein. The square brackets above and below the nucleotide sequences show the codons encoding P3 and the unique PIPO amino acids, respectively. The unique nucleotide of each strain not shared with the others is highlighted. It should be noted that the genomic positions of pipo of G7 and G7d differ from that of N because of the presence of one additional codon upstream of the P3 cistron (GenBank accession numbers AY216010, AY216987 and D00507, respectively).
Figure 4.
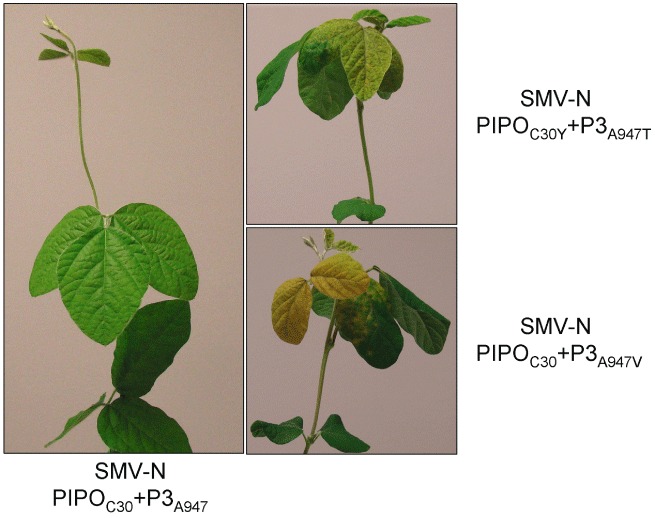
Phenotypic responses of soybean L800 (Rsv1) to biolistic inoculation with the infectious cDNA clones of avirulent Soybean mosaic virus (SMV) strain N (SMV‐N) and two SMV‐N‐derived virulent derivatives harbouring a parallel mutation in polyprotein codon 947 located in the P3 cistron. It should be noted that alanine (Ala) (A) to threonine (Thr) (T) substitution at position 947 leads to alteration in the overlapping PIPO codon (C30Y), whereas replacement with valine (Val) (V), instead of Thr, does not. This demonstrates that the determinant of virulence on L800 (Rsv1) resides on P3 and not the overlapping pipo‐encoded protein. Plants were photographed at 14 days post‐inoculation.
Table 1.
Abilities of Soybean mosaic virus (SMV) strains N, G7 and G7d, as well as their derivative PIPO, P3 and PIPO+P3 mutants ,to infect systemically rsv1‐ and Rsv1‐genotype soybeans following biolistic or mechanical inoculations.*
| Viruses/mutants | Substitutions† | rsv1 | Rsv1 | |||
|---|---|---|---|---|---|---|
| Nucleotide | P3 (aa) | PIPO (aa) | Lee68 | Williams82 | L800 | |
| SMV‐N | (3/3)‡ 3/3§ | (4/4) 4/4 | (0/10) 0/4 | |||
| SMV‐G7 | (3/3) 9/9 | (3/3) 3/3 | (3/3) 4/4 | |||
| SMV‐G7d | ND¶ 6/6 | (3/3) 6/6 | (3/3) 4/4 | |||
| SMV‐NC30Y | G2970A | A947T** | C30Y | ND 5/5 | (3/4) 5/5 | (2/3) 6/6 |
| SMV‐NC30R | T2969C | NA †† | C30R | (0/2) ND | (2a ‡‡/6) ND | (0/7) 0/3 |
| SMV‐NE35K | G2984A | NA | E35K | (2/2) 3/3 | (6/6) 4/4 | (1a/5) 1a/10 |
| SMV‐NK65Q | A3074C | NA | K65Q | (3/3) 3/3 | (4/4) 2a/2 | (0/4) 0/4 |
| SMV‐NE35K+K65Q | G2984A+A3074C | NA | E35K | (3/3) 6/6 | (4/4) 3/3 | (0/4) 1a/10 |
| K65Q | ||||||
| SMV‐NE35R | G2984A+A2985G | K952E | E35R | ND 3/3 | (6/6) 3a/3 | (0/4) 0/6 |
| SMV‐NE35G | A2985G | K952E | E35G | ND 7/7 | (4/4) 7/7 | (0/3) 1a/7 |
| SMV‐NK816E+E35K | A2577G+G2984A | K816E | E35K | ND 3/3 | (4/4) 4/4 | (3/4a) 6/6 |
| SMV‐G7Y30C | A2973G | T948A | Y30C | ND 3/3 | (3/3) 4a/4 | (0/4) 1a/12 |
| SMV‐G7Y30H | T2972C | NA | Y30H | (1/3) 5/5 | (5/6) 4/5 | (2a/7) 6/6 |
| SMV‐G7K35E | A2987G | NA | K35E | (3/3) 3/3 | (4/4) 3/3 | (4a/4) 7/8 |
| SMV‐G7Q65K | C3077A | NA | Q65K | (3/3) 3/3 | (3/3) 3/3 | (4a/4) 6/8 |
| SMV‐G7K35R | A2988G | K953E | K35R | ND 3/3 | (4/4) 3/3 | (3/4) 5/5 |
| SMV‐G7K35E+Q65K | A2987G+C3077A | NA | K35E | (3/3) 6/6 | (4/4) 3/3 | (4a/4) 9/9 |
| Q65K | ||||||
| SMV‐G7dY30C | A2973G | T948A | Y30C | ND 4/5 | (6/6) 2a/4 | (0/4) 1a/4 |
| SMV‐G7dR35E | A2987G+G2988A | E953K | R35E | ND 5/5 | (6/6) 3a/4 | (3/4) 5/5 |
| SMV‐G7dR35K | G2988A | E953K | R35K | ND 5/5 | (7/7) 2a/2 | (3/4) 4/4 |
| SMV‐G7dR35G | A2987G | NA | R35G | ND 6/6 | (3/3) 7a/7 | (4/4) 4/4 |
| SMV‐G7dQ65K | C3077A | NA | Q65K | ND 3/3 | (4/4) 3/3 | (4/4a) 4/4 |
| SMV‐NK816E | A2577G | K816E | NA | ND 3/3 | (4/4) 4/4 | (2a/4) 6/6 |
| SMV‐NV822M | G2595A | V822M | NA | ND 5/5 | (3/3) 4/4 | (2/3) 4a/4 |
| SMV‐NR945G ** | A2964G | R945G | K28R | ND 5/5 | (3/3) 2/3 | (2/3) 4/5 |
| SMV‐NA947V ** | C2971T | A947V | NA | ND 6/6 | (3/3) 7/7 | (3/3) 8/9 |
| SMV‐NP948L ** | C2974T | P948L | NA | ND 7/7 | (3/3) 7/7 | (1/4) 4/5 |
| SMV‐NV1045A ** | T3265C | V1045A | NA | ND 6/6 | (3/3) 3/3 | (1/3) 4/5 |
For biolistic inoculation, plasmid containing full‐length infectious cDNA clones was delivered into fully expanded primary leaves of 2‐week‐old soybean seedlings. For mechanical inoculation, infectious sap from biolistically inoculated Williams82 (rsv1) was used as inoculum and rub‐inoculated onto fully expanded primary leaves of 2‐week‐old soybean seedlings. The inoculated plants were maintained in a growth chamber (22 °C) until evaluation for infection based on symptom expression. Absence of virus in asymptomatic plants was confirmed by antigen‐coated indirect enzyme‐linked immunosorbent assay (ELISA).
The positions of nucleotides or amino acid (aa) substitutions are based on genomic sequences of SMV strains N, G7 and G7d (GenBank accession nos. D00507, AY216010 and AY216987, respectively). For the position of PIPO amino acids, see 1, 3.
Number of plants systemically infected/number of plants inoculated biolistically.
Number of plants systemically infected/number of plants sap inoculated mechanically.
Not done.
Published data (Hajimorad et al., 2011).
Not affected (substitution is either synonymous or located outside pipo).
The superscript letter ‘a’ indicates that, for each mutant, total RNA was extracted from one infected plant of the indicated genotype and subjected to reverse transcriptase‐polymerase chain reaction (RT‐PCR). The stability of the introduced mutation(s) and the lack of any newly emerged mutations in progeny viruses were confirmed by sequencing the entire P3 cistron of progeny viruses. Infection of one Williams82 (Rsv1) with SMV‐NC30R was associated with an additional mutation in P3 at position C2970T, which resulted in A947S and C30L substitutions in P3 and PIPO, respectively. Progeny viruses from the second infected Williams82 with SMV‐NC30R lacked the introduced mutation and the mutation had reverted to the wild‐type. No mutation in P3 of the progenies derived from the single infected L800 (Rsv1) inoculated biolistically with SMV‐NE35K was detected; however, these progenies failed to infect additional L800 (Rsv1) when inoculated mechanically. Progeny from L800 (Rsv1) mechanically inoculated with sap derived from biolistically inoculated Williams82 (rsv1) with SMV‐NE35K contained a sequence polymorphism at position 2577. Thus, both codons AAG and GAG encoding lysine (Lys) and glutamic acid (Glu), respectively, were present at codon position 816 located in P3 (Fig. S1). Infection of L800 (Rsv1) with SMV‐NE35K+K65Q was associated with C2671T substitution, which resulted in A847V mutation in P3. Infection of a single mechanically inoculated L800 (Rsv1) with SMV‐G7Y30C was associated with only limited vein necrosis on the third trifoliate leaf at 21 days post‐inoculation without any sign of necrosis on the leaf blade or any other visible symptoms; however, no additional mutation in P3 was detected. Infection of L800 (Rsv1) by SMV‐NE35G was associated with a sequence polymorphism in P3 at position 2709, where both TCA and ACA codons encoding serine (Ser) and threonine (Thr), respectively, were present at polyprotein position 860. No newly emerged mutations in P3 cistrons of other progenies derived from any of the other molecularly cloned mutant viruses were detected.
Reciprocal substitutions between avrN and virG7 at position 35 of PIPO were synonymous for the overlapping P3 codons of these two viruses. This was because the unique nucleotide of pipo, corresponding to genomic position 2984 of avrN, constituted the third and first residues of the overlapping P3 and PIPO codons, respectively (Fig. 3). Thus, NE35K and G7K35E were generated without alteration in P3 amino acids. Inoculation of NE35K to L800 (Rsv1) resulted in one of five biolistically and one of 10 mechanically inoculated plants (Table 1). However, sequences of progeny viruses from the mechanically inoculated L800 (Rsv1) showed polymorphism in the P3 amino acid at position 816 (Fig. S1, see Supporting Information). One additional passage of these progeny viruses in L800 (Rsv1) resulted in the fixation of Lys to glutamic acid (Glu) at the polyprotein position 816 (Fig. S1). This genomic position is located upstream of pipo of avrN (data not shown). When the K816E mutation was introduced into P3 of avrN and the mutant was inoculated to L800 (Rsv1), NK816E was virulent (Table 1). We further reconstructed K816E+E35K mutations in avrN. Biolistic inoculation of NK816E+E35K, as well as mechanical inoculation of its derivative progeny to L800 (Rsv1), showed that NK816E+E35K, similar to NK816E, was virulent (Table 1). Thus, E35K substitution in PIPO of avrN alone does not confer virulence on L800 (Rsv1). The observation that the virulence of G7K35E on L800 (Rsv1) and PI96983 (Rsv1) was not compromised (Table 1; data not shown) further allowed us to conclude that the PIPO residue at position 35 is not involved in the virulence of virG7 on Rsv1‐genotype soybeans.
Reciprocal exchanges at position 35 of PIPO between virG7d and avrN, as well as virG7 and virG7d, were problematic. This was because the position of the unique nucleotide of pipo of virG7d constituted the first residue of the overlapping P3 codon (Fig. 3). The overlapping P3 codon in avrN and virG7 at this position, AAA, encodes Lys, whereas that of virG7d, GAA, codes for Glu (Fig. 3). Thus, the synthesis of NE35R also resulted in K952E substitution in P3 (Fig. 3; Table 1). However, NE35R+K952E, similar to avrN, remained avirulent on L800 (Rsv1) (Table 1). We also generated NE35G that contained the K952E substitution in P3 as well; however, NE35G+K952E, similar to NE35R+K952E, remained avirulent on L800 (Rsv1) (Table 1). Synthesis of G7K35R also resulted in a substitution in P3 at position 953 (Lys to Glu) (Fig. 3). However, similar to virG7, G7K35R, which contained the K953E substitution in P3, remained virulent on L800 (Rsv1) (Table 1). We also exchanged the amino acid at position 35 in PIPO of virG7d and generated G7dR35E, G7dR35K and G7dR35G. Both G7dR35E and G7dR35K contained a mutation in P3 at position 953 (Glu to Lys), whereas G7dR35G lacked this mutation (Table 1). However, inoculation of these three mutants on L800 (Rsv1) showed that all remained virulent on L800 (Rsv1) (Table 1). G7dR35G also remained virulent on PI96983 (Rsv1) (data not shown).
Reciprocal substitution at position 65 of PIPO was synonymous in relation to P3; hence, it did not alter the overlapping encoded P3 amino acid in any of the strains. This was because the unique nucleotide of avrN, corresponding to genomic position 3074, constituted the third residue with regard to the P3 codon (Fig. 3). However, the virulence of the resultant mutants did not vary from that of the parental viruses, as NK65Q remained avirulent, whereas G7Q65K and G7dQ65K were both virulent on L800 (Rsv1) (Table 1). G7Q65K also remained virulent on PI96983 (Rsv1) (data not shown). We also introduced double mutations in PIPO of avrN and virG7 without any alterations in the amino acid of their respective P3, and generated NE35K+K65Q and G7K35E+Q65K. Inoculation of these two mutants, each with double substitutions in PIPO, to L800 (Rsv1) showed that NE35K+K65Q remained avirulent, whereas G7K35E+Q65K was virulent (Table 1). Furthermore, G7K35E+Q65K also remained virulent on PI96983 (Rsv1) (data not shown).
CONCLUSIONS
The main purposes of this study were to exploit the differential interactions of SMV strains avrN, virG7 and virG7d with Rsv1‐genotype soybeans and to answer the question of whether the virulence determinants of SMV on functionally immune Rsv1‐genotype soybeans reside on P3 or the overlapping pipo‐encoded protein. Thus, this study did not aim to provide an understanding of the functional role(s) of mutations in virulence or to reveal the underlying mechanism(s) of functional immunity mediated by the Rsv1 locus against SMV‐N.
The reciprocal exchanges of the unique amino acid of PIPO, without alteration in P3 residues, among the three strains did not alter their phenotypes on L800 (Rsv1). Previously, via the experimental adaptation of avrN to Rsv1‐genotype soybeans, we have identified several mutations in the P3 cistron that convert avrN to virN on L800 (Rsv1) (Hajimorad et al., 2011). These include substitutions at polyprotein positions 945 [Arg to glycine (Gly)], 948 [proline (Pro) to Leu], 947 [alanine (Ala) to Thr], 947 [Ala to valine (Val)] and 1045 (Val to Ala) (Hajimorad et al., 2011) (Table 1). In the present study, we also identified two additional mutations in the P3 cistron, V822M and K816E, which converted avrN to virN on L800 (Rsv1) (Table 1). Analyses of all of these mutations in relation to P3 and PIPO showed that substitution at polyprotein position 1045 is located downstream of pipo; hence, it affects only P3. However, substitutions at polyprotein positions 816 and 822 are upstream of pipo; thus none has a direct impact on pipo‐encoded protein. However, R945G, A947T, A947V and P948L substitutions all overlap pipo‐encoded codons. Among these mutations, R945G and A947T substitutions altered the overlapping PIPO codons as well. R945G substitution resulted in K28R substitution in PIPO of avrN. As avrN, virG7 and virG7d all encode a Lys at position 28 of PIPO, gain of virulence by NR945G on L800 (Rsv1) is probably a result of substitution in P3 and not PIPO. However, A947T substitution resulted in C30Y replacement in PIPO. Interestingly, a parallel mutation at this site, A947V, which converted avrN to virN on L800 (Rsv1), altered only P3 but not the overlapping pipo‐encoded amino acid (Fig. 3; Table 1). However, both NC30Y+A947T and NA947V were virulent on L800 (Rsv1) and induced similar phenotypes (Hajimorad et al., 2011) (Fig. 4). Hence, these data collectively demonstrate that virulence determinants of SMV on Rsv1‐genotype soybean reside on P3 and not the overlapping pipo‐encoded protein.
In a number of other potyviral pathosystems, the P3 cistron is involved in adaptations to new hosts or in conferring ability to overcome resistance or tolerance. We analysed the reported virulence determinants of these viruses to determine whether pipo is involved in any of these interactions. A mutation (A1047V) in the P3 cistron of Tobacco etch virus (TEV), which was associated with the adaptation of TEV to Arabidopsis thaliana (Agudelo‐Romero et al., 2008), is located downstream of pipo. The determinants of virulence of Turnip mosaic virus (TuMV) to overcome two resistance genes in Brassica napus are mapped to the P3 cistron (2002, 2003), but are positioned outside pipo. An important determinant of TuMV to infect Brassica spp. and/or Raphanus sativus, which is positioned in the P3 cistron (Suehiro et al., 2004), does not overlap pipo. The N‐terminal half of the P3 cistron of TuMV, which induces hypersensitive response‐like cell death in A. thaliana (Kim et al., 2010), is positioned upstream of pipo. Most of the mutations in the P3 cistron of TuMV essential for adaptation to R. sativus (Tan et al., 2005) do not alter PIPO, except the C3172T substitution that changes the overlapping pipo‐encoded amino acid. Multiple determinants in P3 of Pea seed‐borne mosaic virus, involved in virulence against sbm‐2 resistance in Pisum sativum (Hjulsager et al., 2006), are positioned upstream of pipo. Tolerance breaking of Zucchini yellow mosaic virus in tolerant zucchini cultivars is related to a point mutation in the P3 cistron (Desbiez et al., 2003; Glasa et al., 2007); however, the substitution at position 917 is located adjacent to the conserved pipo motif that affects only P3. Thus, in all of these pathosystems, except TuMV–R. sativus, the determinants are mapped to the P3 protein and pipo is not involved. However, the role of pipo of TuMV in the adaptation of the virus to R. sativus remains to be determined.
EXPERIMENTAL PROCEDURES
Viruses, soybean genotypes, inoculation and SMV detection
The plasmids pSMV‐N (GenBank Accession No. D00507), pSMV‐G7 (GenBank Accession No. AY216010), pSMV‐G7d (GenBank Accession No. AY216987), pSMV‐NV822M, pSMV‐NA947T and pSMV‐NA947V have been described previously (2003, 2006, 2011; Wang et al., 2006). All plasmids were propagated in ElectroMax DH5α‐E (Invitrogen, Carlsbad, CA, USA) and were purified using a QiaPrep Spin MiniPrep Kit (Qiagen, Valencia, CA, USA). To establish infection with plasmid DNA, fully expanded primary leaves of soybean seedlings were biolistically inoculated as described previously (2003, 2008). Sap containing viral progenies in 50 mm phosphate buffer, pH 7.0, derived from the infected tissues of biolistically inoculated soybeans, was used as inoculum to mechanically inoculate carborundum (600‐mesh)‐dusted primary leaves of soybeans (Hajimorad and Hill, 2001). Soybean (Glycine max) genotypes Williams82 (rsv1) and Lee68 (rsv1), both universally susceptible to all SMV strains, and Rsv1‐soybeans PI96983 and L800, both functionally immune to avrN, were used in this study (Bernard et al., 1991; Hayes et al., 2004). PI96983 (Rsv1) is a soybean plant introduction (PI) containing the Rsv1 resistance locus, and was originally collected in Hwanghae Puk, South Korea, and introduced to the USA in 1932. Soybean L800 (Rsv1) is a recombinant line containing only one of the six nucleotide binding/Leu‐rich repeat‐type candidate resistance genes at the Rsv1 chromosomal region from PI96983 (Rsv1) (Hayes et al., 2004). L800 (Rsv1) was selected from a population resulting from the crossing of Lee68 (rsv1) with PI96983 (Rsv1) (Hayes et al., 2004). All seeds were obtained from glasshouse‐ or field‐grown plants shown to be free of SMV by indexing. The inoculated plants were maintained in a growth chamber operating at 22 °C with a photoperiod of 16 h. The detection of SMV in the inoculated plants was performed by symptomatology, enzyme‐linked immunosorbent assay (ELISA) (Malapi‐Nelson et al., 2009) or reverse transcriptase‐polymerase chain reaction (RT‐PCR) (Hajimorad et al., 2008).
Construction of point mutants by site‐directed mutagenesis
The megaprimer PCR‐based mutagenesis method (Sambrook and Russell, 2001) was used for the synthesis of all site‐directed mutants employing PrimeStar HS DNA polymerase (Takara Bio, Madison, WI, USA). All the oligonucleotide primers used are listed in Table S1 (see Supporting Information).
To generate SMV‐NC30R, SMV‐NE35K, SMV‐NE35R, SMV‐NE35G, SMV‐NK65Q and SMV‐NK816E, the mutagenic primers N‐2969s, N‐2984s, N‐ERs, N‐2985s, N‐3074s and N‐2577a (Table S1), respectively, were used in PCRs in the presence of N‐3264a and pSMV‐N, which served as the template. The resultant PCR products with each primer set served as megaprimers in the presence of SMV‐2289s and pSMV‐N to generate genomic fragments spanning nucleotides 2289–3264 and containing the desired mutations. The resultant amplified fragments were digested with KpnI and SpeI and ligated into pSMV‐N. To generate SMV‐NK816E+E35K, the mutagenic primer N‐2577a (Table S1) was used in the presence of N‐2289s and pSMV‐NE35K as the template in the first PCR. The resultant amplified fragment served as a megaprimer in the presence of SMV‐3264a and pSMV‐N in the second PCR. The mutagenized fragment was digested with KpnI and SpeI and ligated into pSMV‐N. To generate SMV‐NE35K+K65Q, the mutagenic primer N‐3074s was used in the presence of N‐3264a primer and pSMV‐NE35K as the template in the first PCR. The resultant amplified fragment served as a megaprimer in the presence of SMV‐2289s and pSMV‐N in the second PCR. The mutagenized fragment was digested with KpnI and SpeI and ligated into pSMV‐N.
To generate SMV‐G7Y30H, SMV‐G7Y30C, SMV‐G7K35E, SMV‐G7K35R and SMV‐G7Q65K, the mutagenic primers G7‐2972s, G7‐2973s, G7‐2987s, G7‐KRs and G7‐3077s (Table S1), respectively, were initially used in PCRs in the presence of primer G‐3263a and pSMV‐G7 as the template. The resultant PCR products with each of the primer sets served as megaprimers in the presence of SMV‐2289s and pSMV‐G7 to generate genomic fragments spanning nucleotides 2289–3263 containing the desired mutations. Subsequently, the fragments were digested with KpnI and SpeI and ligated into pSMV‐G7. To generate SMV‐G7K35E+Q65K, the mutagenic primer G7‐3077s was used in the presence of primer G7‐3263a and pSMV‐G7K35E, which served as the template. The resultant PCR products served as megaprimers in the presence of SMV‐2289s and pSMV‐G7 to generate the mutagenized fragment containing two mutations; this was subsequently digested with KpnI and SpeI and ligated into pSMV‐G7.
To generate SMV‐G7dY30C, SMV‐G7dR35G, SMV‐G7dR35E, SMV‐G7dR35K and SMV‐G7dQ65K, the mutagenic primers d‐2973s, d‐2987s, d‐2988s, d‐RKs and d‐3077s (Table S1), respectively, were used in PCRs in the presence of primer G‐3263a and pSMV‐G7d as the template. The resultant PCR product with each of the primer sets served as a megaprimer in the presence of SMV‐2289s and pSMV‐G7d to generate mutagenized genomic fragments spanning nucleotides 2289–3264 containing the desired mutations. The resultant amplified fragments were digested with KpnI and SpeI and ligated into pSMV‐G7d.
To ensure the absence of any undesired PCR‐generated mutation, the entire amplified regions of each of the mutant viruses were sequenced and analysed. The infectivity of all the mutants was tested on Williams82 by biolistic inoculation.
RT‐PCR and sequencing
To verify the stability of the introduced mutations in planta, the genomic region of all the progeny viruses containing the entire P3 cistron was reverse transcribed in the presence of primer SMV‐3910a (Table S1) with SuperScript reverse transcriptase (Invitrogen). PCR amplification of the entire P3 citrons was performed using primers SMV‐2289s and SMV‐3840a (Table S1) in the presence of Ex Taq polymerase (Takara Bio). The PCR products were purified with a QIAquick‐PCR Purification Kit (Qiagen) and sequenced using the same primers. Sequencing was performed at the University of Tennessee DNA Sequencing Facility, Knoxville, TN, USA. The sequences were edited and analysed using Vector NTI (Invitrogen).
Supporting information
Fig. S1 Electropherograms showing the sequences of reverse transcriptase‐polymerase chain reaction (RT‐PCR) products from progenies derived from SMV‐NE35K. Primary leaves of Williams 82 (rsv1) were biolistically inoculated with pSMV‐NE35K, and systemically infected trifoliate leaves harvested 21 days post‐inoculation were either subjected to RT‐PCR analysis or used as the source of inoculum to inoculate L800 (Rsv1). (A) Partial P3 sequences generated by a forward primer are shown. SMV‐N nucleotides, the encoded amino acids and their positions within the genome and the viral polyprotein, located in the P3 cistron, are shown above the sequences, and soybean genotypes are given on the left side of the electropherograms. The polymorphism at nucleotide position 2577 (indicated by an arrow) in the sequences derived from the first passage of progenies in L800 (Rsv1), but not in those from Williams82 (rsv1), and fixation of the mutation in those derived from the second and fifth passages of progenies should be noted. (B) Electropherogram showing the sequences of an RT‐PCR product from the first passage in L800 (Rsv1) generated with a reverse primer to demonstrate the polymorphism shown in (A), and generated with a forward primer; this is not a sequencing artefact.
Table S1 Sequences of oligonucleotide primers used for site‐directed mutagenesis, polymerase chain reaction (PCR), reverse transcriptase‐polymerase chain reaction (RT‐PCR) and sequencing.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
The authors are grateful to Drs A. L. Eggenberger and J. H. Hill (Iowa State University, Ames, IA, USA) for plasmids pSMV‐N and pSMV‐G7. This project was funded by the University of Tennessee College of Agricultural Sciences and Natural Resources and the Tennessee Agricultural Experimental Station, Knoxville, TN, USA.
REFERENCES
- Abdul‐Razzak, A. , Guiraud, T. , Peypelut, M. , Walter, J. , Houvenaghel, M.‐C. , Candresse, T. , Gall, O.L. and German‐Retana, S. (2009) Involvement of the cylindrical inclusion (CI) protein in the overcoming of an elf4E‐mediated resistance against Lettuce mosaic potyvirus . Mol. Plant Pathol. 10, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, M.J. , Antoniw, J.F. and Fauquet, C.M. (2005) Molecular criteria for genus and species discrimination within the family Potyviridae . Arch. Virol. 150, 459–479. [DOI] [PubMed] [Google Scholar]
- Agudelo‐Romero, P. , Carbonell, P. , Perez‐Amador, M.A. and Elena, S.F. (2008) Virus adaptation by manipulation of host's gene expression. PLoS ONE, 3, e2397. doi: 10.137/journal.pone.0002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, R.L. , Nelson, R.L. and Creemens, C.R. (1991) USDA soybean genetic collection: isoline collection. Soybean Genet. Newsl. 18, 27–57. [Google Scholar]
- Choi, I.I.‐R. , Horken, K.M. , Stenger, D.C. and French, R. (2005) An internal RNA element in the P3 cistron of Wheat streak mosaic virus revealed by synonymous mutations that affect both movement and replication. J. Gen. Virol. 86, 2605–2614. [DOI] [PubMed] [Google Scholar]
- Chung, B.Y.‐W. , Miller, W.A. , Atkins, J.F. and Firth, A.E. (2008) An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA, 105, 5897–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroocq, V. , Salvador, B. , Sicard, O. , Glasa, M. , Cosson, P. , Svanella‐Dumas, L. , Revers, F. , García, J.A. and Candresse, T. (2009) The determinant of potyvirus ability to overcome the RTM resistance of Arabidopsis thaliana maps to the N‐terminal region of the coat protein. Mol. Plant–Microbe Interact. 22, 1302–1311. [DOI] [PubMed] [Google Scholar]
- Desbiez, C. , Gal‐On, A. , Girard, M. , Wipf‐Scheibe, C. and Lecoq, H. (2003) Increase in Zucchini yellow mosaic virus symptom severity in tolerant zucchini cultivars is related to a point mutation in P3 protein and is associated with a loss of relative fitness on susceptible plants. Phytopathology, 93, 1478–1484. [DOI] [PubMed] [Google Scholar]
- Diveki, Z. , Salanki, K. and Balazs, E. (2004) The necrotic phenotype of the Cucumber mosaic virus (CMV) Ns strain is solely determined by amino acid 461 of the 1a protein. Mol. Plant–Microbe Interact. 17, 837–845. [DOI] [PubMed] [Google Scholar]
- Eggenberger, A.L. , Hajimorad, M.R. and Hill, J.H. (2008) Gain of virulence on Rsv1‐genotype soybean by an avirulent Soybean mosaic virus requires concurrent mutations in both P3 and HC‐Pro. Mol. Plant–Microbe Interact. 21, 931–936. [DOI] [PubMed] [Google Scholar]
- Fellers, J.P. , Tremblay, D. , Handest, M.F. and Lommel, S.A. (2002) The Potato virus Y MSNR NIb‐replicase is the elicitor of a veinal necrosis hypersensitive response in root knot nematode resistant tobacco. Mol. Plant Pathol. 3, 145–152. [DOI] [PubMed] [Google Scholar]
- Glasa, M. , Svoboda, J. and Nováková, S. (2007) Analysis of the molecular and biological variability of Zucchini yellow mosaic virus isolates from Slovakia and Czech Republic. Virus Genes, 35, 415–421. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. and Hill, J.H. (2001) Rsv1‐mediated resistance against Soybean mosaic virus‐N is hypersensitive response‐independent at the inoculation site, but has the potential to initiate a hypersensitive response‐like mechanism. Mol. Plant–Microbe Interact. 14, 587–598. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Eggenberger, A.L. and Hill, J.H. (2003) Evolution of Soybean mosaic virus‐G7 molecularly cloned genome in Rsv1‐genotype soybean results in emergence of a mutant capable of evading Rsv1‐mediated recognition. Virology, 314, 497–509. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Eggenberger, A.L. and Hill, J.H. (2006) Strain‐specific P3 of Soybean mosaic virus elicits Rsv1‐mediated extreme resistance, but absence of P3 elicitor function alone is insufficient for virulence on Rsv1‐genotype soybean. Virology, 345, 156–166. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Eggenberger, A.L. and Hill, J.H. (2008) Adaptation of Soybean mosaic virus avirulent chimeras containing P3 sequences from virulent strains to Rsv1‐genotype soybeans is mediated by mutations in HC‐Pro. Mol. Plant–Microbe Interact. 21, 937–946. [DOI] [PubMed] [Google Scholar]
- Hajimorad, M.R. , Wen, R.‐H. , Eggenberger, A.L. , Hill, J.H. and Saghai Maroof, M.A. (2011) Experimental adaptation of an RNA virus mimics natural evolution. J. Virol. 85, 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A.J. , Jeong, S.C. , Gore, M.A. , Yu, Y.G. , Buss, G.R. , Tolin, S.A. and Saghai Maroof, M.A. (2004) Recombination within a nucleotide‐binding‐site/leucine‐rich‐repeat gene cluster produces new variants conditioning resistance to soybean mosaic virus in soybeans. Genetics, 166, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjulsager, C.K. , Olsen, B.S. , Jensen, D.M.K. , Cordea, M.I. , Krath, B.N. , Johansen, I.E. and Lund, O.S. (2006) Multiple determinants in the coding region of Pea seed‐borne mosaic virus P3 are involved in virulence against sbm‐2 resistance. Virology, 355, 52–61. [DOI] [PubMed] [Google Scholar]
- Janzac, B. , Montarry, J. , Palloix, A. , Navaud, O. and Moury, B. (2010) A point mutation in the polymerase of Potato virus Y confers virulence toward the Pvr4 resistance of pepper and a high competitiveness cost in susceptible cultivar. Mol. Plant–Microbe Interact. 23, 823–830. [DOI] [PubMed] [Google Scholar]
- Jenner, C.E. , Sanchez, F. , Nettleship, S.B. , Foster, G.D. , Ponz, F. and Walsh, J.A. (2000) The cylindrical inclusion gene of Turnip mosaic virus encodes a pathogenic determinant to the Brassica resistance gene TuRB01 . Mol. Plant–Microbe Interact. 13, 1102–1108. [DOI] [PubMed] [Google Scholar]
- Jenner, C.E. , Tomimura, K. , Ohshima, K. , Hughes, S.L. and Walsh, J.A. (2002) Mutations in Turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology, 300, 50–59. [DOI] [PubMed] [Google Scholar]
- Jenner, C.E. , Wang, X. , Tomimura, K. , Ohshima, K. , Ponz, F. and Walsh, J.A. (2003) The dual role of the potyvirus P3 protein of Turnip mosaic virus as a symptom and avirulence determinant in Brassicas. Mol. Plant–Microbe Interact. 16, 777–784. [DOI] [PubMed] [Google Scholar]
- Johansen, I.E. , Lund, O.S. , Hjulsager, C.K. and Laursen, J. (2001) Recessive resistance in Pisum sativum and potyvirus pathotype resolved in a gene‐for‐cistron correspondence between host and virus. J. Virol. 75, 6609–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B.M. , Suehiro, N. , Natsuaki, T. , Inukai, T. and Masuta, C. (2010) The P3 protein of Turnip mosaic virus can alone induce hypersensitive response‐like cell death in Arabidopsis thaliana carrying TuNI . Mol. Plant–Microbe Interact. 23, 144–152. [DOI] [PubMed] [Google Scholar]
- Krause‐Sakate, R. , Redondo, E. , Richard‐Forget, F. , Jadao, A.S. , Houvenaghel, M.‐C. , German‐Retana, S. , Pavan, M.A. , Candresse, T. , Zerbini, F.M. and Gall, O.L. (2005) Molecular mapping of the viral determinants of systemic wilting induced by a Lettuce mosaic virus (LMV) isolate in some lettuce cultivars. Virus Res. 109, 175–180. [DOI] [PubMed] [Google Scholar]
- Malapi‐Nelson, M. , Wen, R.‐H. , Ownley, B.H. and Hajimorad, M.R. (2009) Co‐infection of soybean with Soybean mosaic virus and Alfalfa mosaic virus results in disease synergism and alteration in accumulation level of both viruses. Plant Dis. 93, 1259–1264. [DOI] [PubMed] [Google Scholar]
- Malcuit, I. , Marano, M.R. , Kavanagh, T.A. , De Jong, W. , Forsyth, A. and Baulcombe, D.C. (1999) The 25‐kDa movement protein of PVX elicits Nb‐mediated hypersensitive cell death in potato. Mol. Plant–Microbe Interact. 12, 536–543. [Google Scholar]
- Meshi, T. , Motoyoshi, F. , Adachi, A. , Watanabe, Y. , Takamatsu, N. and Okada, Y. (1988) Two concomitant base substitutions in the putative replicase genes of tobacco mosaic virus confer the ability to overcome the effects of a tomato resistance gene, Tm‐1 . EMBO J. 7, 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre, P. , Brigneti, G. and Baulcombe, D.C. (2000) An Ry‐mediated resistance response in potato requires the intact active site of the NIa proteinase from potato virus Y. Plant J. 23, 653–661. [DOI] [PubMed] [Google Scholar]
- Padgett, H.S. , Watanabe, Y. and Beachy, R.N. (1997) Identification of the TMV replicase sequence that activates the N gene‐mediated hypersensitive response. Mol. Plant–Microbe Interact. 10, 709–715. [Google Scholar]
- Salvador, B. , Delgadillo, M.O. , Saenz, P. , Garcia, J.A. and Simon‐Mateo, C. (2008) Identification of Plum pox virus pathogenicity determinants in herbaceous and woody hosts. Mol. Plant–Microbe Interact. 21, 20–29. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D.W. (2001) Molecular Cloning, a Laboratory Manual, 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Shaner, G. , Stromberg, E.L. , Lacy, G.H. , Barker, K.R. and Pirone, T.P. (1992) Nomenclature and concepts of pathogenicity and virulence. Annu. Rev. Phytopathol. 30, 47–66. [DOI] [PubMed] [Google Scholar]
- Suehiro, N. , Natsuaki, T. , Watanabe, T. and Okuda, S. (2004) An important determinant of the ability of Turnip mosaic virus to infect Brassica spp. and/or Raphanus sativus is in its P3 protein. J. Gen. Virol. 85, 2087–2098. [DOI] [PubMed] [Google Scholar]
- Tan, Z. , Gibbs, A.J. , Tomitaka, Y. , Sánchez, F. , Ponz, F. and Ohshima, K. (2005) Mutations in Turnip mosaic virus genomes that have adapted to Raphanus sativus . J. Gen. Virol. 86, 501–510. [DOI] [PubMed] [Google Scholar]
- Urcuqui‐Inchima, S. , Haenni, A.‐L. and Bernardi, F. (2001) Potyvirus proteins: a wealth of functions. Virus Res. 74, 157–175. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Eggenberger, A. , Hill, J. and Bogdanove, A.J. (2006) Pseudomonas syringae effector avrB confers soybean cultivar‐specific avirulence on Soybean mosaic virus adapted for transgene expression but effector avrPto does not. Mol. Plant–Microbe Interact. 19, 304–312. [DOI] [PubMed] [Google Scholar]
- Wei, T. , Zhang, C. , Hong, J. , Xiong, R. , Kasschau, K.D. , Zhou, X. , Carrington, J.C. and Wang, A. (2010) Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N‐PIPO. PLoS Pathog. 6, e1000962. doi: 10.1371/journal.ppat.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, R.‐H. and Hajimorad, M.R. (2010) Mutational analysis of the putative pipo of soybean mosaic virus suggests disruption of PIPO protein impedes movement. Virology, 400, 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Electropherograms showing the sequences of reverse transcriptase‐polymerase chain reaction (RT‐PCR) products from progenies derived from SMV‐NE35K. Primary leaves of Williams 82 (rsv1) were biolistically inoculated with pSMV‐NE35K, and systemically infected trifoliate leaves harvested 21 days post‐inoculation were either subjected to RT‐PCR analysis or used as the source of inoculum to inoculate L800 (Rsv1). (A) Partial P3 sequences generated by a forward primer are shown. SMV‐N nucleotides, the encoded amino acids and their positions within the genome and the viral polyprotein, located in the P3 cistron, are shown above the sequences, and soybean genotypes are given on the left side of the electropherograms. The polymorphism at nucleotide position 2577 (indicated by an arrow) in the sequences derived from the first passage of progenies in L800 (Rsv1), but not in those from Williams82 (rsv1), and fixation of the mutation in those derived from the second and fifth passages of progenies should be noted. (B) Electropherogram showing the sequences of an RT‐PCR product from the first passage in L800 (Rsv1) generated with a reverse primer to demonstrate the polymorphism shown in (A), and generated with a forward primer; this is not a sequencing artefact.
Table S1 Sequences of oligonucleotide primers used for site‐directed mutagenesis, polymerase chain reaction (PCR), reverse transcriptase‐polymerase chain reaction (RT‐PCR) and sequencing.
Supporting info item
Supporting info item


