SUMMARY
Xanthomonas axonopodis pv. citri utilizes the type III effector protein PthA to modulate host transcription to promote citrus canker. PthA proteins belong to the AvrBs3/PthA family and carry a domain comprising tandem repeats of 34 amino acids that mediates protein–protein and protein–DNA interactions. We show here that variants of PthAs from a single bacterial strain localize to the nucleus of plant cells and form homo‐ and heterodimers through the association of their repeat regions. We hypothesize that the PthA variants might also interact with distinct host targets. Here, in addition to the interaction with α‐importin, known to mediate the nuclear import of AvrBs3, we describe new interactions of PthAs with citrus proteins involved in protein folding and K63‐linked ubiquitination. PthAs 2 and 3 preferentially interact with a citrus cyclophilin (Cyp) and with TDX, a tetratricopeptide domain‐containing thioredoxin. In addition, PthAs 2 and 3, but not 1 and 4, interact with the ubiquitin‐conjugating enzyme complex formed by Ubc13 and ubiquitin‐conjugating enzyme variant (Uev), required for K63‐linked ubiquitination and DNA repair. We show that Cyp, TDX and Uev interact with each other, and that Cyp and Uev localize to the nucleus of plant cells. Furthermore, the citrus Ubc13 and Uev proteins complement the DNA repair phenotype of the yeast Δubc13 and Δmms2/uev1a mutants, strongly indicating that they are also involved in K63‐linked ubiquitination and DNA repair. Notably, PthA 2 affects the growth of yeast cells in the presence of a DNA damage agent, suggesting that it inhibits K63‐linked ubiquitination required for DNA repair.
INTRODUCTION
Citrus canker, caused by the bacterial pathogen Xanthomonas axonopodis pv. citri, is characterized by water‐soaked eruptions and pustule formation on the surface of the host plant (Brunings and Gabriel, 2003). Although it is known that canker lesions result from the intense division and expansion of the host cells at the site of infection, the molecular events leading to cell hypertrophy and hyperplasia are not fully comprehended. Previous studies have shown that X. axonopodis pv. citri infection changes the transcription of a large set of host genes associated with cell division and expansion, including genes involved in ribosome biogenesis, cell wall remodelling, vesicle trafficking, and the synthesis and mobilization of auxin and gibberellin (Cernadas et al., 2008). The expression of the cell wall remodelling genes, as well as those involved in the synthesis and mobilization of auxin and gibberellin, was shown to be similarly regulated by auxin and gibberellin, indicating that X. axonopodis pv. citri changes the active contents of both hormones to promote cell division and enlargement (Cernadas and Benedetti, 2009). Indeed, it was found that both auxin and gibberellin are required for canker development and that polar transport of auxin and vesicle trafficking mediate this process (Cernadas and Benedetti, 2009; Cernadas et al., 2008).
The transcriptional changes associated with the growth and expansion of citrus cells in response to X. axonopodis pv. citri infection are thought to be triggered by the Type III effector protein PthA. PthA is recognized as the major determinant of X. axonopodis pv. citri pathogenicity (Al‐Saadi et al., 2007; Brunings and Gabriel, 2003). This protein is required for pathogen growth and canker elicitation, and is sufficient to promote hypertrophy and hyperplasia in citrus (Duan et al., 1999; 1991, 1992). PthA belongs to the AvrBs3/PthA protein family. Members of this family, also known as transcription activator‐like (TAL) effectors, are translocated by the Type III secretion system and function as transcriptional activators in the host (Kay et al., 2007; Römer et al., 2007; Sugio et al., 2007; Yang et al., 2006).
One of the most surprising features of AvrBs3/PthA proteins is that they have an internal region comprising variable, nearly identical tandem repeats of 34 amino acids that determine both pathogenicity and avirulence (Kay and Bonas, 2009; Swarup et al., 1992; Yang and Gabriel, 1995). For instance, AvrBs3, the most well‐characterized TAL effector, dimerizes through its repeat region and interacts with an α‐importin (α‐Imp) that mediates its transfer to the nucleus of the host cell (Gürlebeck et al., 2005; Szurek et al., 2001). In the nucleus, AvrBs3 recognizes plant promoters and activates the transcription of a helix–loop–helix factor required for cell enlargement in susceptible plants, whereas, in resistant plants, it transactivates its cognate Bs3 resistance gene, triggering a hypersensitive reaction (Kay et al., 2007; Römer et al., 2007). Most remarkably, it is the repeat region that mediates DNA recognition, and the amino acid polymorphism found within the repeat units determines the DNA sequence specificity of TAL effectors (Boch et al., 2009; Moscou and Bogdanove, 2009).
Many Xanthomonas pathogens contain multiple variants of AvrBs3/PthA proteins. The existence of multiple copies of avrBs3/pthA genes in a single strain is thought to facilitate recombination and rapid adaptation to new hosts (Yang and Gabriel, 1995). However, in the light of the findings of Boch et al. (2009) and Moscou and Bogdanove (2009), it is expected that multiple variants of TAL effectors would also directly affect the transcription of target genes in the host and, consequently, the outcome of the disease process. Accordingly, it has been shown that multiple TAL effectors contribute additively to symptom development (Yang and White, 2004; Yang et al., 1996). The X. axonopodis pv. citri strain 306 (da Silva et al., 2002) contains four related PthA proteins (PthA 1–4) that differ from each other essentially by the number of their 34‐amino‐acid repeats and by the polymorphic residues found within the repeat units (Fig. S1A,B, see Supporting information). These PthA variants are the equivalents of the four functionally characterized PthAs of strain 3213 (Al‐Saadi et al., 2007). Interestingly, it was found that all X. axonopodis pv. citri strains carry one PthA variant with 17.5 repeats that determines the pathogenicity on citrus, and that the other variants of different repeat sizes do not complement a knockout mutation of the 17.5‐repeat PthA (Al‐Saadi et al., 2007). Thus, how PthA variants with 15.5 (PthAs 2 and 3) and 16.5 (PthA 1) repeats contribute to symptom development is not yet clear. Microarray analyses of sweet orange epicotyls transformed with individual PthAs have shown that PthA 4 (17.5 repeats) up‐regulates a larger set of citrus genes associated with cell division and expansion relative to PthA 2. Nevertheless, a number of genes implicated in canker development are up‐regulated by both PthAs, and some are specifically induced by PthA 2 (A. Pereira and C. Benedetti, unpublished data), indicating that they contribute to symptom development in an additive or synergistic manner.
Although the ultimate target of Type III TAL effectors appears to be the plant nuclear DNA (Kay et al., 2007; Römer et al., 2007; Sugio et al., 2007; Yang et al., 2006), it is reasonable to expect that these proteins might interact with other host factors to direct the transcriptional activation of their target genes. Thus far, α‐Imp is the only TAL effector interactor known (Szurek et al., 2001). Therefore, considering the sequence variability among the four PthA internal repeats (Fig. S1, see Supporting Information), and given the extraordinary phenotypes elicited by TAL effectors in the host, we hypothesized that the PthA variants might differentially interact with distinct host proteins in addition to α‐Imp.
To gain insights into the functional dynamics of TAL effectors, we combined two‐hybrid assays with in vitro binding and complementation studies to identify and characterize the interactions of each of the X. axonopodis pv. citri PthAs with sweet orange proteins. In addition to the identification of a citrus α‐Imp similar to that responsible for the nuclear import of AvrBs3 (Szurek et al., 2001), we describe new interactions of PthA proteins with components of a citrus protein complex implicated in protein folding and DNA repair.
RESULTS
PthA proteins localize to the nucleus and form homo‐ and heterodimers
Figure 1A shows that all PthA variants localize to the nucleus of Nicotiana benthamiana cells and, in some nuclei, the proteins appear to concentrate into multiple nuclear dots, indicating that they might be associated with sites of increased transcriptional activity.
Figure 1.
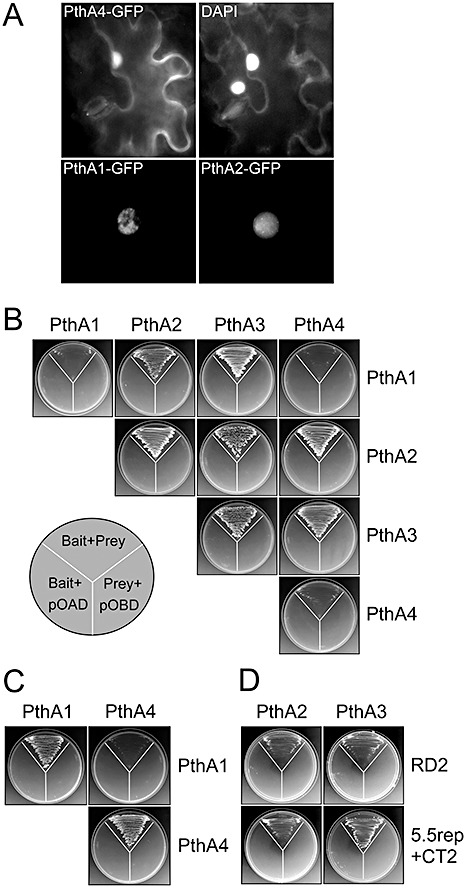
PthA proteins localize to the nucleus and form homo‐ and heterodimers. (A) Fluorescence of PthA–green fluorescent protein (GFP) fusions in the nucleus of transiently transfected Nicotiana benthamiana cells 72 h after Agrobacterium tumefaciens infiltration. The top panels show the PthA4–GFP and 4′,6‐diamidino‐2‐phenylindole (DAPI) fluorescence of the same cell at ×400 magnification. The bottom panels show the localization of PthA1– and PthA2–GFP fusions in nuclear dots at ×1000 magnification. (B) Yeast two‐hybrid assays showing that PthAs 2 and 3 form homodimers but also heterodimerize with each other and with PthAs 1 and 4. Yeast cells co‐transformed with the bait and prey constructs are shown at the top of the plate, whereas cells carrying the bait construct plus the empty pOAD or the prey construct plus the empty pOBD are shown at the sides, as indicated. (C) Under less stringent conditions of interaction (synthetic complete medium lacking histidine but containing adenine, SC − His + Ade), PthAs 1 and 4 homodimerize but do not self‐interact. (D) The repeat domain (RD2) as well as the 5.5rep + CT2 of PthA2 were sufficient for homo‐ and heterodimerization with PthAs 2 and 3, respectively. In (B–D), bait and prey are indicated horizontally and vertically, respectively.
As AvrBs3 dimerizes in the cytosol of the host cell prior to its nuclear import (Gürlebeck et al., 2005), we hypothesized that the four PthA variants would possibly form homo‐ and/or heterodimers in the host cell. To test this assumption, we used two‐hybrid assays to verify all possible interactions among the PthA proteins. We observed that all PthAs are capable of forming homodimers, but only PthAs 2 and 3 heterodimerize with each other and with PthAs 1 and 4 (Fig. 1B). Surprisingly, PthAs 1 and 4 do not form heterodimers even under less stringent conditions of interaction, i.e. in the presence of adenine (Fig. 1C). As shown for AvrBs3, the repeat domain of PthA was sufficient for homo‐ and heterodimerization (Fig. 1D). In addition, a truncated version of PthA2 (5.5rep + CT2), carrying the last 5.5 repeat units plus the C‐terminus (Fig. S1C, see Supporting Information), also formed homo‐ and heterodimers with PthAs 2 and 3, respectively (Fig. 1D).
Identification of sweet orange interactors of PthA
To identify interactors of PthAs, we performed two‐hybrid screening of a citrus cDNA library using PthAs 2, 3 and 4 as bait (Fig. S1C, see Supporting Information). The majority of the identified proteins can be classified into four major functional categories, including protein folding, nuclear transport, transcriptional control and DNA repair. The screenings made with PthA 4 retrieved primarily proteins associated with transcriptional control, RNA stabilization and DNA repair, carrying either DNA‐ or RNA‐binding motifs. These interactions will, however, be reported elsewhere. Below, we describe the interactions of the four PthAs with the citrus proteins implicated in protein folding, K63‐linked ubiquitination and nuclear transport, identified with PthAs 2 and 3.
Of particular relevance was the identification of interactions with α‐Imp, which is 87% identical to the Capsicum annuumα‐Imp shown to interact with AvrBs3 and to mediate its nuclear import (Szurek et al., 2001), and with citrus cyclophilin (Cyp), thioredoxin (TDX) and ubiquitin‐conjugating enzyme variant (Uev). The citrus Cyp has a single peptidyl‐prolyl cis–trans isomerase (PPiase) domain and is 84% identical to ROC1, an Arabidopsis thaliana Cyp required for the activation of AvrRpt2 inside the host cell (Coaker et al., 2005). TDX (tetratricopeptide domain‐containing thioredoxin) is a two‐domain protein with disulphide reductase activity found only in plants (Vignols et al., 2003). Its N‐terminus contains a tetratricopeptide repeat (TPR) found in the co‐chaperone Hsp70‐interacting protein HIP, whereas its C‐terminus carries a thioredoxin (TRX) domain (Fig. 2B). The citrus Uev is closely related to the yeast methylmethanesulphonate 2 (MMS2) and its human orthologue Uev1a, which act in conjunction with Ubc13 to promote K63‐linked ubiquitination of components of the DNA repair machinery (Hofmann and Pickart, 1999; Petroski et al., 2007; Windheim et al., 2008).
Figure 2.
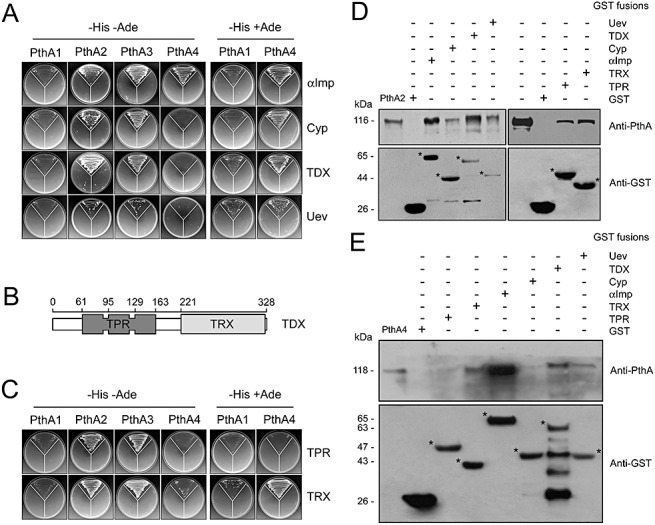
Differential interactions between PthA proteins with the citrus α‐importin (α‐Imp), cyclophilin (Cyp), thioredoxin (TDX) and ubiquitin‐conjugating enzyme variant (Uev). (A) Yeast two‐hybrid assays showing that PthAs 2 and 3 interact most strongly with the citrus proteins, whereas PthAs 1 and 4 interact with the prey under less stringent conditions (synthetic complete medium lacking histidine but containing adenine, SC − His + Ade). The disposition of the yeast co‐transformants in the plates is the same as shown in Fig. 1. (B) Schematic representation of the tetratricopeptide repeat (TPR) and thioredoxin (TRX) domains of TDX with the numbers representing the positions of the amino acid residues. The TPR (residues 1–178) and TRX (residues 179–328) domains were subcloned into the pOAD vector for two‐hybrid assays. (C) Differential interactions between the four PthA proteins with the TDX domains, showing the preferential interaction of PthAs 2 and 3 with both TPR and TRX domains, relative to PthAs 1 and 4. PthA 4 and the TRX domain of TDX self‐interacted in the presence of adenine. (D, E) Glutathione transferase (GST) pulldown assays with PthAs and the citrus proteins. 6 × His–PthAs 2 and 4 were expressed in Escherichia coli and purified by affinity chromatography. The purified proteins were subjected to GST pulldown assays, as described in Experimental procedures, and the resulting samples were separated by electrophoresis and probed with the anti‐PthA and anti‐GST sera. (D) PthA2 binds to all citrus proteins (GST fusions), but more strongly to α‐Imp and TDX. (E) Pronounced interaction of PthA4 with α‐Imp. No interactions were detected between PthA 4 and Cyp or the TPR domain of TDX. Purified PthA proteins used as controls are shown on the left. The GST fusions are indicated by asterisks and the molecular sizes of the proteins are shown on the left. The corresponding sodium dodecylsulphate (SDS) gels are shown in Fig. S2 (see Supporting Information).
To confirm the interactions, the full‐length Cyp, TDX and Uev proteins were used as prey in two‐hybrid assays against all four PthA baits. We also used an α‐Imp construct in which the first 80 residues carrying the N‐terminal auto‐inhibitory region (Harreman et al., 2003) were deleted. Figure 2A shows that both PthAs 2 and 3 interact more strongly than PthAs 1 and 4 with the citrus proteins. Indeed, with the exception of α‐Imp, PthA 4 interacts weakly with Cyp, TDX and Uev, and the interactions of PthA 1 with the citrus prey are even weaker, as observed in synthetic complete medium lacking tryptophan, leucine and histidine but containing adenine (SC − Leu − Trp − His + Ade) (Fig. 2A).
As TDX was the only prey protein displaying two functional domains (Fig. 2B), we tested the requirement of each of its domains for interaction with PthAs. Figure 2C shows that both the TPR and TRX domains of TDX strongly and preferentially interact with PthAs 2 and 3, whereas only the TRX domain interacts with PthAs 1 and 4, and only under less stringent conditions (Fig. 2C).
To confirm the interactions observed in yeast, glutathione transferase (GST) pulldown assays were carried out using PthAs 2 and 4 as representative bait. This choice was based on the differential interaction shown by the PthA variants with the prey proteins, i.e. PthA 1 interacts weakly with the prey, PthAs 2 and 3 show similar interaction patterns with all prey, and both have the same number of repeat units, and PthA4, carrying 17.5 repeat units, is the form required to elicit canker on citrus (Al‐Saadi et al., 2007; Duan et al., 1999). Consistent with the two‐hybrid data, pulldown assays revealed that PthA 2 binds to all prey proteins, including the TPR and TRX domains of TDX. Stronger interactions were observed with α‐Imp and TDX, as observed in yeast (Fig. 2D). Similarly, we observed a good correspondence between the two‐hybrid and in vitro protein interaction assays performed with PthA 4 (Fig. 2E). PthA 4 binds more tightly to α‐Imp than to Cyp and Uev. In addition, only the TDX domain of TDX interacts with PthA 4 in vitro (Fig. 2E).
Cyp, TDX and Uev interact with each other
As described in the Discussion, Cyp, TDX and Uev have been associated with protein folding, activation and quality control. We thus tested whether they could interact with each other. Figure 3A shows that TDX interacts with Cyp and Uev, and that both TPR and TRX domains contribute to these interactions. Although the binding of TDX to Uev is relatively weak in the in vitro pulldown assays relative to the binding with Cyp, it is notable that significantly larger amounts of Uev were pulled down with TDX in the presence of Cyp (Fig. 3B). Similarly, larger amounts of Cyp were detected when Uev was added in the pulldown with TDX (Fig. 3B). Considering that the interaction between Cyp and Uev is relatively weak (Fig. 3A,B), this synergism of interaction is best interpreted as evidence of the formation of a protein complex involving TDX, Cyp and Uev.
Figure 3.
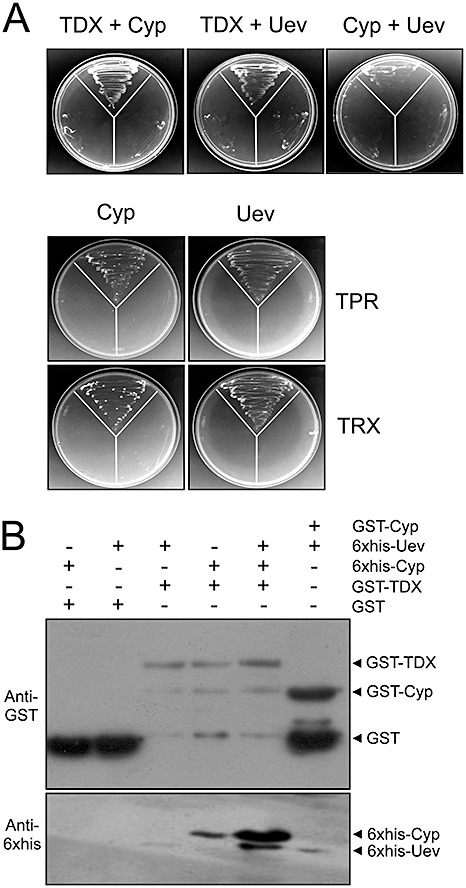
The citrus proteins cyclophilin (Cyp), thioredoxin (TDX) and ubiquitin‐conjugating enzyme variant (Uev) interact with each other. (A) Yeast two‐hybrid assays showing interactions between TDX, Cyp and Uev, as well as between Cyp and Uev. The tetratricopeptide repeat (TPR) and thioredoxin (TRX) domains of TDX also interact with Cyp and Uev. The disposition of the yeast co‐transformants in the plates is the same as shown in Fig. 1. (B) Western blot of glutathione transferase (GST) pulldown assays using GST–TDX or GST–Cyp as bait and 6 × His–Cyp and 6 × His–Uev as prey. Consistent with the in vivo assay shown in (A), stronger interactions between TDX and Cyp are observed in the presence of Uev, suggesting the existence of a protein complex formed by TDX, Cyp and Uev. The corresponding sodium dodecylsulphate (SDS) gel is shown in Fig. S2 (see Supporting Information).
The citrus Ubc13 interacts with PthA2 and forms a heterodimer with Uev
The role of the Uev–Ubc13 heterodimer in the K‐63 ubiquitination of components of the DNA damage repair machinery has been well documented in yeast, animals and plants (Broomfield et al., 1998; Hofmann and Pickart, 1999; Motegi et al., 2008; Panier and Durocher, 2009; 2006, 2008). Given the high degree of identity shared by Ubc13 proteins, we were able to identify the citrus Ubc13 orthologue. We therefore investigated whether the citrus Ubc13 would interact with Uev, as has been observed in yeast and vertebrates. Figure 4A shows that Ubc13 strongly interacts with Uev, but not with TDX or Cyp. In addition, consistent with the results shown in Fig. 2, Ubc13 interacts with PthAs 2 and 3 only (Fig. 4A). These interactions were also confirmed by in vitro pulldown assays, where we detected strong binding of Ubc13 to Uev and PthA2, but no binding to PthA4 (Fig. 4B).
Figure 4.
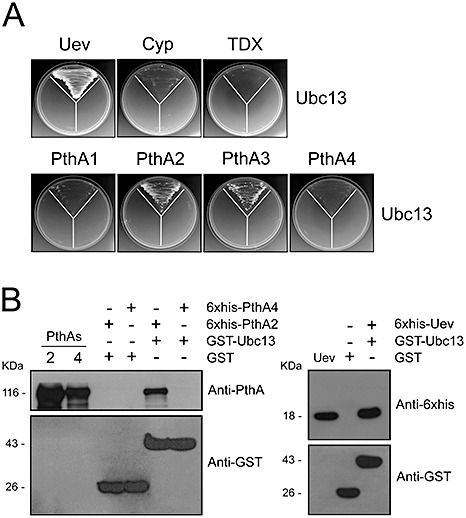
The citrus Ubc13 interacts with ubiquitin‐conjugating enzyme variant (Uev) and PthA proteins. (A) Sweet orange Ubc13 interacts with Uev and with PthAs 2 and 3. The disposition of the yeast co‐transformants in the plates is the same as shown in Fig. 1. (B) Western blots of glutathione transferase (GST) pulldown assays using GST–Ubc13 as bait and 6 × His–Uev and 6 × His–PthAs 2 and 4 as prey. According to the assay shown in (A), Ubc13 binds to Uev and PthA2, but not to PthA 4. Purified PthA proteins used as controls and the molecular sizes of the proteins are shown on the left. The corresponding sodium dodecylsulphate (SDS) gels are shown in Fig. S2 (see Supporting Information).
The PthA repeat domain and leucine‐rich repeat (LRR) are important for protein–protein interactions
Yeast two‐hybrid assays showed that the repeat domain of PthA2 (RD2), as well as the 5.5rep + CT2 fragment (Fig. S1C, see Supporting Information), interacts with Cyp, TDX, Uev, Ubc13 and α‐Imp, except that the interaction of RD2 with α‐Imp is less pronounced (Fig. 5A). Interestingly, LRR, which is located adjacent to the repeat region and is invariable among PthAs (Fig. S1, see Supporting Information), interacts only with α‐Imp (Fig. 5A). These results were also confirmed by GST pulldown assays, which showed that both RD2 and 5.5rep + CT2 bind to the citrus proteins (Fig. 5B). The C‐terminus of PthAs transactivated the yeast reporter genes and so could not be used in the in vivo assays. In this case, we demonstrated by GST pulldown that the C‐terminus of PthA2 (CT2), carrying LRR and the nuclear localization signal (NLS) (Fig. S1, see Supporting Information), binds to α‐Imp only (Fig. 5B). Taken together, the results indicate that the repeat region, which is sufficient for homo‐ and heterodimerization of PthAs (Fig. 1C), also contributes to the interactions with Cyp, TDX, Uev and Ubc13, whereas LRR and NLS appear to be required for interaction with α‐Imp only.
Figure 5.
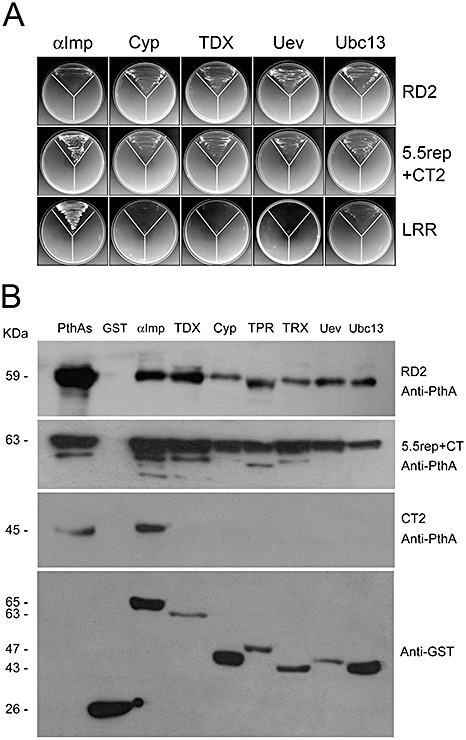
The repeat domain and leucine‐rich repeat (LRR) are important for protein–protein interactions. (A) Yeast two‐hybrid assays showing the interactions of the internal repeat domain of PthA2 (RD2) and the 5.5rep + CT2 construct with all citrus proteins. LRR interacted with α‐importin (α‐Imp) only. (B) Western blot of glutathione transferase (GST) pulldown assays showing that both RD2 and 5.5rep + CT2 constructs bind to citrus proteins (GST fusions). The C‐terminus of PthA2 (CT2), carrying LRR and the nuclear localization signal (NLS), binds to α‐Imp only. Purified PthA proteins used as controls and the molecular sizes of the proteins are shown on the left. The corresponding sodium dodecylsulphate (SDS) gels are shown in Fig. S2 (see Supporting Information).
Citrus Uev and Ubc13 complement the Δubc13 and Δmms2/uev1a yeast mutants
The yeast mutants Δubc13 and Δmms2/uev1a are defective in K63‐linked ubiquitination and, as a result, are unable to grow in the presence of MMS as they are defective in DNA damage repair (Broomfield et al., 1998; Hofmann and Pickart, 1999). To test whether the corresponding citrus proteins are also involved in DNA repair, we expressed the citrus Ubc13 and Uev genes in the corresponding yeast mutants (Fig. 6A). We observed that the growth of both mutants in MMS gradient plates can be rescued by the citrus proteins, strongly indicating that the citrus Ubc13 and Uev are functional homologues of the yeast proteins (Fig. 6B).
Figure 6.
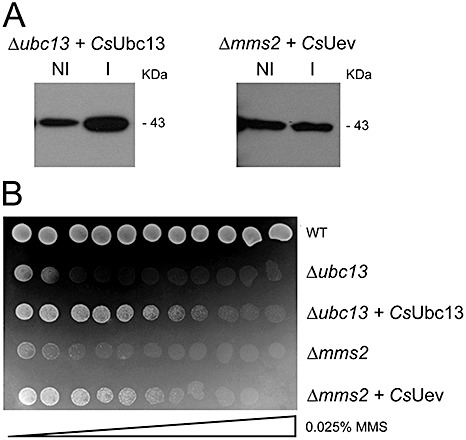
The citrus Ubc13 and ubiquitin‐conjugating enzyme variant (Uev) proteins complement yeast Δubc13 and Δmms2/uev1a mutants. (A) The Citrus sinensis Ubc13 and Uev genes under the control of a copper‐induced promoter were expressed in the yeast mutants Δubc13 and Δmms2/uev1a as glutathione transferase (GST) fusions. The proteins (43 kDa) were induced with 0.5 mm copper sulphate for 2 h, purified by affinity chromatography and probed with anti‐GST serum. Induced (I) and noninduced (NI) samples are shown. The amounts of protein loaded on the gels are shown in Fig. S2 (see Supporting Information). (B) Growth of the yeast strain SUB62 and its derivative mutants Δubc13 and Δmms2/uev1a in synthetic complete (SC) medium in the presence of increasing amounts of methylmethanesulphonate (MMS). The growth of both mutants in MMS gradient plates can be rescued by the corresponding citrus Ubc13 and Uev genes.
PthA proteins are not K63‐linked ubiquitinated in vivo
The fact that the citrus Uev and Ubc13 complemented the yeast Δubc13 and Δmms2/uev1a mutants suggested that PthA could be ubiquitinated in vivo by the yeast Ubc13–Uev complex. To test this hypothesis, we expressed PthAs 2 and 4 as GST fusions in the wild‐type yeast and mutant derivatives. The proteins were purified by affinity chromatography and detected by Western blot. Blots developed with the anti‐PthA serum revealed a major band with the expected size for the GST–PthA fusions (Fig. 7A). Both PthAs were expressed in the wild‐type and mutant cells at relatively equal amounts, and the presence of lower molecular weight bands detected by the anti‐PthA and anti‐ubiquitin sera indicates that the PthAs were degraded in the yeast cells (Fig. 7A). Although a band corresponding to the size of the GST–PthA2 fusion was predominantly labelled by the anti‐ubiquitin serum in the wild‐type yeast, relative to the mutants and relative to the GST–PthA4 fusions, no bands were detected by an anti‐K63‐linked ubiquitin monoclonal antibody (not shown), indicating that the PthAs were not ubiquitinated through K63‐linked ubiquitin chains.
Figure 7.
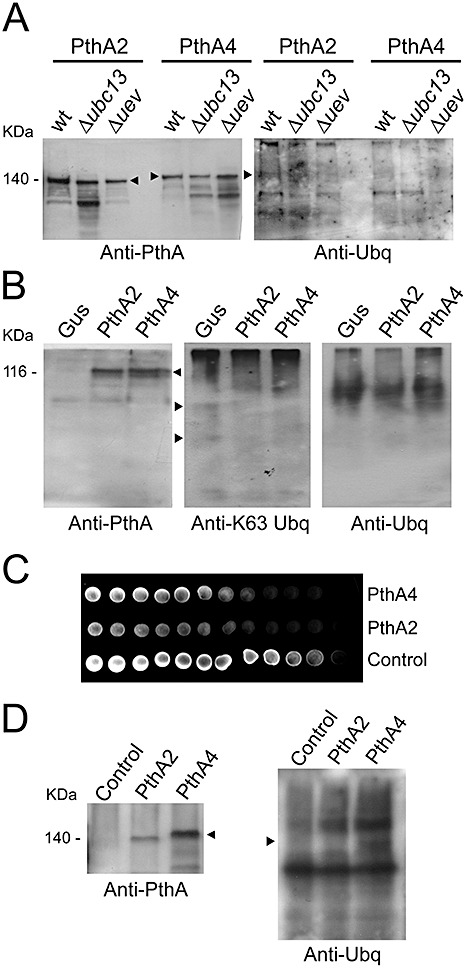
PthA proteins are not K63‐linked ubiquitinated in vivo. (A) PthAs 2 and 4 were expressed in the wild‐type yeast strain SUB62 and its derivative mutants Δubc13 and Δmms2/uev1a as glutathione transferase (GST) fusions (∼140 and 147 kDa, respectively). The proteins were purified by affinity tag and subjected to sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) followed by Western blot detection with anti‐PthA or anti‐ubiquitin sera. Major PthA bands of the expected size for the GST fusions are indicated by the arrows. (B) PthAs 2 and 4 were expressed in sweet orange epicotyl sections using Agrobacterium tumefaciens transformation. A β‐glucuronidase (GUS) construct was used as control. Both PthA proteins (∼116 kDa) were detected by anti‐PthA serum (arrow), but not by anti‐ubiquitin serum. (C) Growth of yeast strain SUB62 (control expressing GST only) and SUB62 separately transformed with PthA 2 and 4 GST fusions in yeast peptone dextrose (YPD) medium in the presence of a methylmethanesulphonate (MMS) gradient. (D) The anti‐PthA serum confirms the presence of PthA proteins in the yeast extracts (arrow), whereas the anti‐ubiquitin serum reveals a missing band in the yeast extract expressing PthA2 (arrow). The amounts of proteins loaded on the sodium dodecylsulphate (SDS) gels are shown in Fig. S2 (see Supporting Information).
To test whether PthA is ubiquitinated in planta, sweet orange hypocotyls were transformed with PthA 2, PthA 4 or β‐glucuronidase (GUS) as control. Both PthAs 2 and 4 were detected by the anti‐PthA serum, but not by the anti‐K63‐linked ubiquitin or by the general anti‐ubiquitin antibodies (Fig. 7B). Taken together, these results indicate that PthA proteins are not K63‐linked ubiquitinated in vivo.
PthAs inhibit K63‐linked ubiquitination and affect DNA repair
The fact that the PthA proteins were not ubiquitinated in vivo suggests that they might, on interaction with the Ubc13–Uev complex, inhibit or promote the K63‐linked ubiquitination of target proteins. Indeed, the presence of extra bands detected by the anti‐K63‐linked ubiquitin antibody in citrus cells transformed with GUS relative to PthAs (Fig. 7B) suggests that PthAs might inhibit K63‐linked ubiquitination. Consistent with this idea, the expression of PthAs 2 and 4 significantly inhibited the growth of normal yeast cells in the presence of MMS (Fig. 7C), indicating that DNA repair mechanisms were affected by the PthA proteins. Notably, the negative effect of PthA2 expression on DNA repair was more pronounced than that of PthA4 expression, although the intracellular levels of PthA4 were higher in comparison with the levels of PthA2 (Fig. 7D). This is in agreement with the observation that PthA2 preferentially interacts with the Ubc13–Uev proteins. In addition, although the anti‐K63‐linked ubiquitin antibody detected no bands in yeast extracts, blots developed with the general anti‐ubiquitin serum revealed a missing band in the yeast extract expressing PthA2 relative to control and PthA4 (Fig. 7D). Taken together, these results indicate that PthA2 preferentially inhibits K63‐linked ubiquitination required for DNA repair.
DISCUSSION
The presence of four PthA variants in a single bacterial strain suggests that they might interact with distinct host factors. Here, in addition to the interaction with α‐Imp, the only protein interactor of this class of effectors known to date (Szurek et al., 2001), we describe new and differential interactions of PthA variants with the citrus proteins Cyp, TDX and the Uev–Ubc13 pair. In agreement with previous reports (Szurek et al., 2001), all PthAs interacted with α‐Imp and were localized to the nucleus. This interaction appears to be mediated primarily by NLS found at the C‐terminus of the protein. However, we noted that LRR located adjacent to NLS, which has been shown to influence AvrBs3 dimerization (Gürlebeck et al., 2005), was sufficient to promote an interaction with α‐Imp. In addition, as dimerization of TAL effectors is required for their nuclear import (Gürlebeck et al., 2005), the existence of related PthA proteins in a single pathogen also suggested that they might interact with each other. Indeed, all PthAs formed homodimers, but only PthAs 2 and 3 heterodimerized with each other and with PthAs 1 and 4. As the repeat region was sufficient to promote homo‐ and heterodimerization, these interactions may reflect variations in the number of repeat units.
The pattern of homo‐ and heterodimerization of PthAs may be correlated with the preferential interaction of PthAs 2 and 3, relative to PthAs 1 and 4, with Cyp, TDX and Uev. Again, this seems to reflect variations in the repeat region as this region also contributed to the interactions with the citrus proteins. Therefore, the results indicate that the repeat region may not only be critical to match citrus target promoters, as expected considering the role played by AvrBs3 (Boch et al., 2009; Kay et al., 2009; Römer et al., 2009), but may also play a role in the interaction with host proteins.
The PthA‐interacting proteins Cyp, TDX and the Uev–Ubc13 pair have all been associated with protein folding, activation and quality control. In this study, we have presented evidence indicating that they interact to form a protein complex. This is consistent with the fact that Cyps are targets of TRX (Laxa et al., 2007; Motohashi et al., 2003) and interact with the TPR domain of Hsp90 and Hsc70 (Carrello et al., 2004; Ratajczak and Carrello, 1996). In addition, Uev1a, which lacks ubiquitin‐conjugating activity, associates with Ubc13 to form a functional heterodimer that promotes K63‐linked ubiquitination of target proteins implicated in DNA repair, signal transduction and homologous recombination (Hofmann and Pickart, 1999; Wang et al., 2001; Zhao et al., 2007). The Uev1a–Ubc13 pair has also been described as a component of the quality control C‐terminus of Hsp70‐interacting protein (CHIP) U‐box ubiquitin ligase complex, which comprises a TPR domain that mediates interactions with Hsp90 and Hsp70 (Zhang et al., 2005). As some effectors are transferred by secretion systems in an unfolded or inactive state and are activated by plant Cyps related to the citrus Cyp (Coaker et al., 2005; Deng et al., 1998), we initially hypothesized that the citrus proteins might be part of a chaperone or quality control protein complex required for the folding and/or activation of PthAs. However, recombinant PthAs produced in Escherichia coli are structured and have DNA‐binding activity (M. Domingues and C. Benedetti, unpublished results), suggesting that proline isomerization by the plant Cyp is not critical for PthA folding/action. Accordingly, PthAs are not ubiquitinated in vivo, but apparently inhibit K63‐linked ubiquitination associated with DNA repair. Thus, our data strongly point to a model in which PthAs would inhibit the activity of a citrus protein complex associated with DNA repair. Interestingly, other citrus proteins identified as PthA4 interactors, including a high mobility group (HMG) protein, a homologue of the mammalian translin‐associated factor (TRAX) and an SMC (structural maintenance of chromosomes), are associated with DNA repair and chromatin stability (Erdemir et al., 2002; Hirano, 2002; Prasad et al., 2007).
Although the role of Uev–Ubc13 in response to DNA damage has been well established in yeast, animals and plants (Broomfield et al., 1998; Hofmann and Pickart, 1999; Motegi et al., 2008; Panier and Durocher, 2009; 2006, 2008), recent studies have shown that K63‐linked ubiquitination also serves as a targeting signal for the 26S proteasome (Saeki et al., 2009). This is in line with recent findings showing that CHIP targets the degradation of mammalian SRC‐3, a transcriptional co‐activator associated with tumour progression (Kajiro et al., 2008). It is thus attractive to speculate that, if a CHIP‐related complex operates in citrus, its inhibition by PthAs might favour canker development.
Alternatively, it is notable that citrus Cyp is 64% identical to yeast Cyp A, a protein that decreases gene silencing (Arévalo‐Rodríguez and Heitman, 2005; Arévalo‐Rodríguez et al., 2000) and interacts with Ess1, a prolyl‐isomerase that affects chromatin remodelling and the process of transcription through isomerization of prolines of the C‐terminal domain of the RNA polymerase II (Hani et al., 1999; Krishnamurthy et al., 2009; Wu et al., 2000). Interestingly, Ess1 activity is affected by a ubiquitin ligase that promotes K63 ubiquitination and the degradation of RNA polymerase II (Somesh et al., 2007; Wu et al., 2001). As the ubiquitination and degradation of RNA polymerase II occur in response to DNA damage and during transcriptional elongation arrest, as a mechanism to avoid the transcription of damaged chromatin and prolonged transcriptional arrest of active genes (Daulny and Tansey, 2009; 2005, 2007), we propose that PthAs might increase the rates of transcription through the modulation of the activity of a protein complex associated with chromatin remodelling and transcriptional control. This idea is in agreement with the expected role of PthAs as transcriptional activators, and is supported by the fact that both Cyp and Uev identified here localize to the nucleus (Fig. S3, see Supporting Information). A possible role of the citrus Cyp in PthA‐dependent transcription is under investigation.
EXPERIMENTAL PROCEDURES
Plant material and bacterial infiltration
Six‐month‐old plants of sweet orange (Citrus sinensis) were obtained from certified nurseries and kept in a growth room at 25–28 °C under 14 h/day fluorescent light. Leaves were infiltrated with suspensions of X. axonopodis pv. citri, as described previously (Cernadas et al., 2008). Leaf sectors were infiltrated with approximately 0.3 mL of an aqueous suspension of bacterial cells [optical density at 600 nm (OD600 nm) = 0.5].
Leaves of N. benthamiana were infiltrated with Agrobacterium tumefaciens suspensions (OD600 nm= 0.5) carrying the constructs pBI‐35S::PthA(1‐4)‐GFP, pBI‐35S::GFP, pBI‐35S::GUS, pBI‐35S::DsRed, pBI‐35S::Cyp‐DsRed or pBI‐35S::Uev‐DsRed. The subcellular localization of the fusion proteins was visualized by fluorescence microscopy equipped with filters for green fluorescent protein (GFP), red fluorescent protein (DsRed) and 4′,6‐diamidino‐2‐phenylindole (DAPI) fluorescence.
Citrus cDNA library construction and yeast two‐hybrid assays
Messenger RNA was extracted from sweet orange leaves 48 h after X. axonopodis pv. citri infiltration using Trizol and FastTrack (Invitrogen). RNA samples from noninfiltrated and infiltrated leaf sectors were reverse‐transcribed using an oligo‐dT with a NotI restriction site. cDNAs were ligated to SalI adaptors, digested with SalI/NotI and ligated into a modified pOAD vector (Uetz et al., 2000) carrying a NotI site between SalI and PstI. The generated cDNA library containing approximately 0.8 × 106 independent clones was moved into the Saccharomyces cerevisiae strain PJ694a (MAT a trp1‐901 leu2‐3 112 ura3‐52 his3‐200 gal4Δ gal80Δ LYS2::GAL1‐HIS3 GAL2‐ADE2 met2::GAL7‐lacZ) (James et al., 1996) by high‐efficiency lithium acetate transformation (Gietz and Woods, 2002).
The genes corresponding to PthAs 1–4 were amplified from X. axonopodis pv. citri plasmids and cloned into the NdeI/EcoRI sites of pET28 (Novagen). The genes were subsequently digested with NotI and subcloned into pOBD/pOAD vectors (Uetz et al., 2000). This created an N‐terminal truncation of the proteins lacking the first 128 amino acid residues (Fig. S1C, see Supporting Information). This construct was chosen to avoid the transactivation of the yeast HIS3 reporter gene by the N‐terminus (not shown). The constructs were verified by DNA sequencing and used as bait/prey in two‐hybrid assays.
The initial two‐hybrid screening was performed on synthetic complete medium lacking tryptophan, leucine, histidine and adenine (SC − Trp − Leu − His − Ade), supplemented with 5 mm 3‐aminotriazole (3AT), using PthAs 2 and 3 as bait. pOAD plasmids recovered from positive clones were sequenced and, when required, the full‐length citrus cDNAs, including Ubc13, were obtained by reverse transcription‐polymerase chain reaction (RT‐PCR) and subcloned downstream of and in frame with the fusion proteins Gal4 (pOAD), GST (pGEX‐4T1) and 6 × His (pET28). Bait (pOBD) and prey (pOAD) constructs, including controls (empty pOBD + pOAD prey and pOBD bait + empty pOAD), were used to co‐transform PJ694a cells. The cells were grown on SC − Trp − Leu − His (±Ade) containing 0, 3 or 5 mm 3AT for 5 days at 30 °C. The cDNA sequences of the citrus Cyp, TDX, Uev, Ubc13 and α‐Imp were deposited in the GenBank database as GQ853548, GQ853549, GQ455410, GQ862814 and GQ475487, respectively.
Protein purification and GST pulldown
The 6 × His‐tagged proteins PthA2, PthA4, 5.5rep + CT2, CT2 and Uev were expressed in E. coli BL21(DE3) cells and purified by metal affinity chromatography. Briefly, cultures were grown at 25 °C in Luria–Bertani (LB) medium containing kanamycin (50 µg/mL) to OD600 nm= 0.6, followed by induction with 0.4 mm isopropyl‐thio‐β‐d‐galactopyranoside (IPTG) for 3 h. Cells harvested by centrifugation were suspended in binding buffer [20 mm Tris‐HCl, pH 8.0, 300 mm NaCl, 5 mm imidazole, 20% glycerol, 1 mm phenylmethylsulphonylfluoride (PMSF), 0.5 mm dithiothreitol (DTT)] and incubated on ice with lysozyme (1 mg/mL) for 30 min. Bacterial cells were sonicated and the clarified supernatants were incubated with nickel or cobalt resins for 4 h at 4 °C under slow agitation. The beads were washed three times with two column volumes of washing buffer (20 mm Tris‐HCl, pH 8.0, 300 mm NaCl, 15 mm imidazole, 20% glycerol, 1 mm PMSF, 0.5 mm DTT) and the retained proteins were eluted with washing buffer containing up to 200 mm imidazole.
Prey proteins, subcloned into the SalI/NotI sites of pGEX‐4T, were expressed in BL21(DE3) cells on induction with IPTG for 2 h at 30–37 °C. Cell pellets were suspended in phosphate‐buffered saline (PBS) containing 1 mm DTT and lysozyme. After sonication and centrifugation, soluble fractions of GST fusions were immobilized on glutathione resin and nonbound proteins were removed with three PBS washes. Thirty micrograms of the purified 6 × His fusion proteins were incubated with the resins containing GST or the GST fusion proteins for 2 h at 4 °C. The beads were washed four times with PBS and the resin‐bound proteins were resolved on 10–13% sodium dodecylsulphate‐polyacrylamide electrophoresis (SDS‐PAGE) gels. Proteins were transferred onto nylon membranes, probed with anti‐PthA (1 : 5000), anti‐6 × His (1 : 3000) or anti‐GST (1 : 1000) sera and developed with the ECL kit (GE Healthcare).
Functional complementation of yeast mutants
The yeast strain SUB62 (MATa lys2‐801leu2‐3, 112 ura3‐52 his3‐D200 trp1‐1[am]) and its single mutant derivatives Δubc13 and Δmms2 (uev1a) (Hofmann and Pickart, 1999) were transformed with the citrus Ubc13 or Uev genes cloned into pYEX‐4T (Clontech). Transformants were grown on SC − Leu − Ura plates at 30 °C for 4 days. For qualitative analysis of MMS sensitivity, liquid cultures were grown overnight to exponential phase in yeast peptone dextrose (YPD) medium and plated in YPD supplemented with 0.025% MMS gradient (Pastushok et al., 2005). The plates were incubated at 30 °C for 24 h.
In vivo ubiquitination
PthA ubiquitination in yeast was evaluated using the wild‐type yeast strain SUB62 and the corresponding mutant derivatives ubc13Δ and UevΔ. The strains were transformed with PthA 2 and 4 GST fusions cloned into pYEX4T‐1 and grown in SC − Leu − Ura for 4 days at 30 °C. Liquid cultures were grown to OD600nm= 0.8 and protein expression was induced with 0.5 mm CuSO4 for 2 h. Cells were collected by centrifugation (6000 g for 15 min at 4 °C), resuspended in PBS containing 1 mm PMSF and lysed with glass beads and freeze–thaw cycles. Recombinant proteins were purified using glutathione sepharose beads. Purified proteins were analysed by 7% SDS‐PAGE and probed with the anti‐PthA and anti‐ubiquitin sera.
To evaluate possible PthA ubiquitination in planta, PthAs 2 and 4 were expressed in etiolated epicotyls of sweet orange ‘Hamlin’ according to de Oliveira et al. (2009). Surface‐sterilized seeds were sown in half‐strength Murashige and Skoog (MS) basal medium supplemented with 50 mg/L myo‐inositol and 25 g/L sucrose. Seeds were incubated at 27 ± 2 °C under dark conditions for 6 weeks. Epicotyl sections of etiolated seedlings were cut transversally into 1‐cm segments and incubated for 15 min with Agrobacterium tumefaciens EHA105 (OD600nm= 1.0) carrying the pBI121‐PthA2, pBI121‐PthA4 or pBI121‐GUS construct in MS basal medium containing 100 µm acetosyringone. Excess of Agrobacterium suspension was removed by blotting, and co‐cultivation was carried out in the dark at 26 ± 2 °C for 2 days. Epicotyls were macerated in liquid nitrogen and resuspended in SDS‐PAGE sample buffer. Soluble proteins were recovered by centrifugation (16 000 g for 30 min at 4 °C), resolved by 10% SDS‐PAGE and detected by the anti‐PthA, anti‐ubiquitin (Sigma) and anti‐polyubiquitin K63‐linkage‐specific clone HWA4C4 (Biomol International) antibodies.
Inhibition of K63‐linked ubiquitination‐mediated DNA repair in yeast
PthAs 2 and 4 were expressed in the SUB62 wild‐type strain. Transformants were selected on SC − Leu − Ura plates at 30 °C for 4 days. Liquid cultures were grown overnight to exponential phase in YPD medium and plated in YPD supplemented with a 0.025% MMS gradient. The plates were incubated at 30 °C for 24 h and the expression of PthAs 2 and 4 was detected by Western blot with anti‐PthA serum.
Supporting information
Fig. S1 PthA proteins and constructs used in two‐hybrid and pulldown assays. (A) Schematic representation of the four PthAs from Xanthomonas axonopodis pv. citri strain 306. Dark‐grey bars indicate the 34‐amino‐acid repeats depicted in (B). Light‐grey boxes indicate the C‐terminal leucine‐rich repeat (LRR) and the acidic domain (AD) flanking three nuclear localization signals (NLSs). The numbers represent the positions of the amino acid residues and the letters indicate residue variation outside the repetitive domain. The proteins were expressed in Escherichia coli as 6 × His fusions and used in glutathione transferase (GST) pulldown assays. (B) Sequence alignment of the 34‐amino‐acid repeats of the four PthA proteins, depicting the regions of increased amino acid variation (*). Comma indicates ‘and’, whereas hyphen indicates ‘to’. (C) Schematic representation of the PthA constructs in pOBD/pOAD used as bait/prey in the two‐hybrid assays. The internal repeat domain of PthA2 (RD2), the invariable LRR and the C‐terminal (CT2) and C‐terminal with the last 5.5 repeats of PthA2 (5.5rep + CT2) were also expressed as 6 × His fusions for pulldown assays.
Fig. S2 Coomassie blue‐stained sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels corresponding to the Western blots shown in Figs 2D, E, 3B, 4B, 5B, 6A, 7A, B and D, showing the relative amounts of proteins loaded in each lane. The molecular size markers are indicated on the left. The glutathione transferase (GST) fusion proteins used in the pulldown assays of Figs 2D, E, 3B, 4B and 5B are indicated by the asterisks. The gels corresponding to Fig. 6A show the copper‐induced expression (I) of citrus ubiquitin‐conjugating enzyme variant (Uev) and Ubc13 in the yeast mutants, relative to noninduced samples (NI). Arrows indicate the induced bands. The gels corresponding to Fig. 7A show the GST–PthA samples purified from wild‐type and mutant yeast cells. NI (noninduced), I (induced), S (soluble), FT (flow‐through), W (wash) and E (elution). The ‘E’ samples were loaded side by side in the blot shown in Fig. 7A. The gels corresponding to Fig. 7B and D show the amounts of proteins loaded from plant and yeast cells expressing PthAs 2 and 4. Arrows indicate the positions of PthA bands.
Fig. S3 Nuclear localization of cyclophilin (Cyp) and ubiquitin‐conjugating enzyme variant (Uev) in Nicotiana benthamiana cells. Fluorescence of Uev–red fluorescent protein (DsRed) and Cyp–DsRed fusions in the nucleus of transiently transfected N. benthamiana cells 72 h after Agrobacterium tumefaciens infiltration. 4′,6‐Diamidino‐2‐phenylindole (DAPI) fluorescence of the same cells is shown alongside.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We thank Dr Robert Cohen and Dr Candice Carlile for providing the yeast strain SUB62 and the Δubc13 and Δmms2 mutants. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grants 98/14138‐2; 00/10266‐8; 03/08316‐5; 07/06686‐0). MND, TAS, RAC, MLPO, CD and CEB received fellowships from FAPESP and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
- Al‐Saadi, A. , Reddy, J.D. , Duan, Y.P. , Brunings, A.M. , Yuan, Q. and Gabriel, D.W. (2007) All five host‐range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats that determines pathogenicity on citrus, but none determine host‐range variation. Mol. Plant–Microbe Interact. 20, 934–943. [DOI] [PubMed] [Google Scholar]
- Arévalo‐Rodríguez, M. and Heitman, J. (2005) Cyclophilin A is localized to the nucleus and controls meiosis in Saccharomyces cerevisiae . Eukaryot. Cell, 4, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo‐Rodríguez, M. , Cardenas, M.E. , Wu, X. , Hanes, S.D. and Heitman, J. (2000) Cyclophilin A and Ess1 interact with and regulate silencing by the Sin3‐Rpd3 histone deacetylase. EMBO J. 19, 3739–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch, J. , Scholze, H. , Schornack, S. , Landgraf, A. , Hahn, S. , Kay, S. , Lahaye, T. , Nickstadt, A. and Bonas, U. (2009) Breaking the code of DNA‐binding specificity of TAL‐Type III effectors. Science, 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Broomfield, S. , Chow, B.L. and Xiao, W. (1998) MMS2, encoding a ubiquitin‐conjugating‐enzyme‐like protein, is a member of the yeast error‐free postreplication repair pathway. Proc. Natl. Acad. Sci. USA, 95, 5678–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Carrello, A. , Allan, R.K. , Morgan, S.L. , Owen, B.A. , Mok, D. , Ward, B.K. , Minchin, R.F. , Toft, D.O. and Ratajczak, T. (2004) Interaction of the Hsp90 cochaperone cyclophilin 40 with Hsc70. Cell Stress Chaperones, 9, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernadas, R.A. and Benedetti, C.E. (2009) Role of auxin and gibberellin in citrus canker development and in the transcriptional control of cell‐wall remodeling genes modulated by Xanthomonas axonopodis pv citri . Plant Sci. 177, 190–195. [Google Scholar]
- Cernadas, R.A. , Camillo, L.R. and Benedetti, C.E. (2008) Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii . Mol. Plant Pathol. 9, 609–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coaker, G. , Falick, A. and Staskawicz, B. (2005) Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science, 308, 548–550. [DOI] [PubMed] [Google Scholar]
- Daulny, A. and Tansey, W.P. (2009) Damage control: DNA repair, transcription, and the ubiquitin‐proteasome system. DNA Repair, 8, 444–448. [DOI] [PubMed] [Google Scholar]
- Deng, W. , Chen, L. , Wood, D.W. , Metcalfe, T. , Liang, X. , Gordon, M.P. , Comai, L. and Nester, E.W. (1998) Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proc. Natl. Acad. Sci. USA, 95, 7040–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y.P. , Castañeda, G.Z. , Erdos, G. and Gabriel, D.W. (1999) Expression of a single, host‐specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant–Microbe Interact. 12, 556–560. [Google Scholar]
- Erdemir, T. , Bilican, B. , Oncel, D. , Goding, C.R. and Yavuzer, U. (2002) DNA damage‐dependent interaction of the nuclear matrix protein C1D with translin‐associated factor X (TRAX). J. Cell Sci. 115, 207–216. [DOI] [PubMed] [Google Scholar]
- Gietz, R.D. and Woods, R.A. (2002) Transformation of yeast by lithium acetate/single‐stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96. [DOI] [PubMed] [Google Scholar]
- Gürlebeck, D. , Szurek, B. and Bonas, U. (2005) Dimerization of the bacterial effector protein AvrBs3 in the plant cell cytoplasm prior to nuclear import. Plant J. 42, 175–187. [DOI] [PubMed] [Google Scholar]
- Hani, J. , Schelbert, B. , Bernhardt, A. , Domdey, H. , Fischer, G. , Wiebauer, K. and Rahfeld, J.U. (1999) Mutations in a peptidylprolyl‐cis/trans‐isomerase gene lead to a defect in 3′‐end formation of a pre‐mRNA in Saccharomyces cerevisiae . J. Biol. Chem. 274, 108–116. [DOI] [PubMed] [Google Scholar]
- Harreman, M.T. , Hodel, M.R. , Fanara, P. , Hodel, A.E. and Corbett, A.H. (2003) The auto‐inhibitory function of importin alpha is essential in vivo . J. Biol. Chem. 278, 5854–5863. [DOI] [PubMed] [Google Scholar]
- Hirano, T. (2002) The ABCs of SMC proteins: two‐armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 16, 399–414. [DOI] [PubMed] [Google Scholar]
- Hofmann, R.M. and Pickart, C.M. (1999) Noncanonical MMS2‐encoded ubiquitin‐conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell, 96, 645–653. [DOI] [PubMed] [Google Scholar]
- James, P. , Halladay, J. and Craig, E.A. (1996) Genomic libraries and a host strain designed for highly efficient two‐hybrid selection in yeast. Genetics, 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiro, M. , Hirota, R. , Nakajima, Y. , Kawanowa, K. , So‐ma, K. , Ito, I. , Yamaguchi, Y. , Ohie, S.H. , Kobayashi, Y. , Seino, Y. , Kawano, M. , Kawabe, Y. , Takei, H. , Hayashi, S. , Kurosumi, M. , Murayama, A. , Kimura, K. and Yanagisawa, J. (2008) The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat. Cell Biol. 11, 312–319. [DOI] [PubMed] [Google Scholar]
- Kay, S. and Bonas, U. (2009) How Xanthomonas type III effectors manipulate the host plant. Curr. Opin. Microbiol. 12, 37–43. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Wieduwild, R. and Bonas, U. (2009) Detailed analysis of the DNA recognition motifs of the Xanthomonas type III effectors AvrBs3 and AvrBs3Deltarep16. Plant J. 59, 859–871. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, S. , Ghazy, M.A. , Moore, C. and Hampsey, M. (2009) Functional interaction of the Ess1 prolyl isomerase with components of the RNA polymerase II initiation and termination machineries. Mol. Cell. Biol. 29, 2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxa, M. , König, J. , Dietz, K.J. and Kandlbinder, A. (2007) Role of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20‐3 in peptidyl‐prolyl cis–trans isomerase and redox‐related functions. Biochem. J. 401, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M.J. and Bogdanove, A.J. (2009) A simple cipher governs DNA recognition by TAL effectors. Science, 326, 1501. [DOI] [PubMed] [Google Scholar]
- Motegi, A. , Liaw, H.J. , Lee, K.Y. , Roest, H.P. , Maas, A. , Wu, X. , Moinova, H. , Markowitz, S.D. , Ding, H. , Hoeijmakers, J.H. and Myung, K. (2008) Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl. Acad. Sci. USA, 105, 12 411–12 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi, K. , Koyama, F. , Nakanishi, Y. , Ueoka‐Nakanishi, H. and Hisabori, T. (2003) Chloroplast cyclophilin is a target protein of thioredoxin. Thiol modulation of the peptidyl‐prolyl cis–trans isomerase activity. J. Biol. Chem. 278, 31 848–31 852. [DOI] [PubMed] [Google Scholar]
- De Oliveira, M.L.P. , Febres, V.J. , Costa, M.G.C. , Moore, G.A. and Otoni, W.C. (2009) High‐efficiency Agrobacterium‐mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep. 28, 387–395. [DOI] [PubMed] [Google Scholar]
- Panier, S. and Durocher, D. (2009) Regulatory ubiquitylation in response to DNA‐double strand breaks. DNA Repair, 8, 436–443. [DOI] [PubMed] [Google Scholar]
- Pastushok, L. , Moraes, T.F. , Ellison, M.J. and Xiao, W. (2005) A single Mms2 ‘key’ residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. J. Biol. Chem. 280, 17 891–17 900. [DOI] [PubMed] [Google Scholar]
- Petroski, M.D. , Zhou, X. , Dong, G. , Daniel‐Issakani, S. , Payan, D.G. and Huang, J. (2007) Substrate modification with lysine 63‐linked ubiquitin chains through the UBC13‐UEV1A ubiquitin‐conjugating enzyme. J. Biol. Chem. 282, 29936–29945. [DOI] [PubMed] [Google Scholar]
- Prasad, R. , Liu, Y. , Deterding, L.J. , Poltoratsky, V.P. , Kedar, P.S. , Horton, J.K. , Kanno, S. , Asagoshi, K. , Hou, E.W. , Khodyreva, S.N. , Lavrik, O.I. , Tomer, K.B. , Yasui, A. and Wilson, S.H. (2007) HMGB1 is a cofactor in mammalian base excision repair. Mol. Cell, 27, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak, T. and Carrello, A. (1996) Cyclophilin 40 (CyP‐40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP‐40 for hsp90 binding. J. Biol. Chem. 271, 2961–2965. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Hahn, S. , Jordan, T. , Strauss, T. , Bonas, U. and Lahaye, T. (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science, 318, 645–648. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Strauss, T. , Hahn, S. , Scholze, H. , Morbitzer, R. , Grau, J. , Bonas, U. and Lahaye, T. (2009) Recognition of AvrBs3‐like proteins is mediated by specific binding to promoters of matching pepper Bs3 alleles. Plant Physiol. 150, 1697–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki, Y. , Kudo, T. , Sone, T. , Kikuchi, Y. , Yokosawa, H. , Toh‐e, A. and Tanaka, K. (2009) Lysine 63‐linked polyubiquitin chain may serve as a targeting signal for the 26S proteasome. EMBO J. 28, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva, A.C. , Ferro, J.A. , Reinach, F.C. , Farah, C.S. , Furlan, L.R. , Quaggio, R.B. , Monteiro‐Vitorello, C.B. , Van Sluys, M.A. , Almeida, N.F. , Alves, L.M. , Do Amaral, A.M. , Bertolini, M.C. , Camargo, L.E. , Camarotte, G. , Cannavan, F. , Cardozo, J. , Chambergo, F. , Ciapina, L.P. , Cicarelli, R.M. , Coutinho, L.L. , Cursino‐Santos, J.R. , El‐Dorry, H. , Faria, J.B. , Ferreira, A.J. , Ferreira, R.C. , Ferro, M.I. , Formighieri, E.F. , Franco, M.C. , Greggio, C.C. , Gruber, A. , Katsuyama, A.M. , Kishi, L.T. , Leite, R.P. , Lemos, E.G. , Lemos, M.V. , Locali, E.C. , Machado, M.A. , Madeira, A.M. , Martinez‐Rossi, N.M. , Martins, E.C. , Meidanis, J. , Menck, C.F. , Miyaki, C.Y. , Moon, D.H. , Moreira, L.M. , Novo, M.T. , Okura, V.K. , Oliveira, M.C. , Oliveira, V.R. , Pereira, H.A. , Rossi, A. , Sena, J.A. , Silva, C. , De Souza, R.F. , Spinola, L.A. , Takita, M.A. , Tamura, R.E. , Teixeira, E.C. , Tezza, R.I. , Trindade dos Santos, M. , Truffi, D. , Tsai, S.M. , White, F.F. , Setubal, J.C. and Kitajima, J.P. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Somesh, B.P. , Reid, J. , Liu, W.F. , Søgaard, T.M. , Erdjument‐Bromage, H. , Tempst, P. and Svejstrup, J.Q. (2005) Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell, 121, 913–923. [DOI] [PubMed] [Google Scholar]
- Somesh, B.P. , Sigurdsson, S. , Saeki, H. , Erdjument‐Bromage, H. , Tempst, P. and Svejstrup, J.Q. (2007) Communication between distant sites in RNA polymerase II through ubiquitylation factors and the polymerase CTD. Cell, 129, 57–68. [DOI] [PubMed] [Google Scholar]
- Sugio, A. , Yang, B. , Zhu, T. and White, F.F. (2007) Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAγ1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 104, 10 720–10 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, S. , De Feyter, R. , Brlansky, R.H. and Gabriel, D.W. (1991) A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of X. campestris to elicit canker like lesions on citrus. Phytopathology, 81, 803–809. [Google Scholar]
- Swarup, S. , Yang, Y. , Kingsley, M.T. and Gabriel, D.W. (1992) An Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol. Plant–Microbe Interact. 5, 204–213. [DOI] [PubMed] [Google Scholar]
- Szurek, B. , Marois, E. , Bonas, U. and Ackerveken, G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Uetz, P. , Giot, L. , Cagney, G. , Mansfield, T.A. , Judson, R.S. , Knight, J.R. , Lockshon, D. , Narayan, V. , Srinivasan, M. , Pochart, P. , Qureshi‐Emili, A. , Li, Y. , Godwin, B. , Conover, D. , Kalbfleisch, T. , Vijayadamodar, G. , Yang, M. , Johnston, M. , Fields, S. and Rothberg, J.M. (2000) A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae . Nature, 403, 623–627. [DOI] [PubMed] [Google Scholar]
- Vignols, F. , Mouaheb, N. , Thomas, D. and Meyer, Y. (2003) Redox control of Hsp70‐Co‐chaperone interaction revealed by expression of a thioredoxin‐like Arabidopsis protein. J. Biol. Chem. 278, 4516–4523. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Deng, L. , Hong, M. , Akkaraju, G.R. , Inoue, J. and Chen, Z.J. (2001) TAK1 is a ubiquitin‐dependent kinase of MKK and IKK. Nature, 412, 346–351. [DOI] [PubMed] [Google Scholar]
- Wen, R. , Newton, L. , Li, G. , Wang, H. and Xiao, W. (2006) Arabidopsis thaliana UBC13: implication of error‐free DNA damage tolerance and Lys63‐linked polyubiquitylation in plants. Plant Mol. Biol. 61, 241–253. [DOI] [PubMed] [Google Scholar]
- Wen, R. , Torres‐Acosta, J.A. , Pastushok, L. , Lai, X. , Pelzer, L. , Wang, H. and Xiao, W. (2008) Arabidopsis UEV1D promotes Lysine‐63‐linked polyubiquitination and is involved in DNA damage response. Plant Cell, 20, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windheim, M. , Peggie, M. and Cohen, P. (2008) Two different classes of E2 ubiquitin‐conjugating enzymes are required for the mono‐ubiquitination of proteins and elongation by polyubiquitin chains with a specific topology. Biochem. J. 409, 723–729. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Wilcox, C.B. , Devasahayam, G. , Hackett, R.L. , Arévalo‐Rodríguez, M. , Cardenas, M.E. , Heitman, J. and Hanes, S.D. (2000) The Ess1 prolyl isomerase is linked to chromatin remodeling complexes and the general transcription machinery. EMBO J. 19, 3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Chang, A. , Sudol, M. and Hanes, S.D. (2001) Genetic interactions between the ESS1 prolyl‐isomerase and the RSP5 ubiquitin ligase reveal opposing effects on RNA polymerase II function. Curr. Genet. 40, 234–242. [DOI] [PubMed] [Google Scholar]
- Yang, Y. and Gabriel, D.W. (1995) Intragenic recombination of a single plant pathogen gene provides a mechanism for the evolution of new host specificities. J. Bacteriol. 177, 4963–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. and White, F.F. (2004) Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant–Microbe Interact. 17, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Yuan, Q. and Gabriel, D.W. (1996) Watersoaking function(s) of XcmH1005 are redundantly encoded by members of the Xanthomonas avr/pth gene family. Mol. Plant–Microbe Interact. 9, 105–113. [Google Scholar]
- Yang, B. , Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Windheim, M. , Roe, S.M. , Peggie, M. , Cohen, P. , Prodromou, C. and Pearl, L.H. (2005) Chaperoned ubiquitylation‐crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP‐Ubc13–Uev1a complex. Mol. Cell, 20, 525–538. [DOI] [PubMed] [Google Scholar]
- Zhao, G.Y. , Sonoda, E. , Barber, L.J. , Oka, H. , Murakawa, Y. , Yamada, K. , Ikura, T. , Wang, X. , Kobayashi, M. , Yamamoto, K. , Boulton, S.J. and Takeda, S. (2007) A critical role for the ubiquitin‐conjugating enzyme Ubc13 in initiating homologous recombination. Mol. Cell, 25, 663–675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 PthA proteins and constructs used in two‐hybrid and pulldown assays. (A) Schematic representation of the four PthAs from Xanthomonas axonopodis pv. citri strain 306. Dark‐grey bars indicate the 34‐amino‐acid repeats depicted in (B). Light‐grey boxes indicate the C‐terminal leucine‐rich repeat (LRR) and the acidic domain (AD) flanking three nuclear localization signals (NLSs). The numbers represent the positions of the amino acid residues and the letters indicate residue variation outside the repetitive domain. The proteins were expressed in Escherichia coli as 6 × His fusions and used in glutathione transferase (GST) pulldown assays. (B) Sequence alignment of the 34‐amino‐acid repeats of the four PthA proteins, depicting the regions of increased amino acid variation (*). Comma indicates ‘and’, whereas hyphen indicates ‘to’. (C) Schematic representation of the PthA constructs in pOBD/pOAD used as bait/prey in the two‐hybrid assays. The internal repeat domain of PthA2 (RD2), the invariable LRR and the C‐terminal (CT2) and C‐terminal with the last 5.5 repeats of PthA2 (5.5rep + CT2) were also expressed as 6 × His fusions for pulldown assays.
Fig. S2 Coomassie blue‐stained sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gels corresponding to the Western blots shown in Figs 2D, E, 3B, 4B, 5B, 6A, 7A, B and D, showing the relative amounts of proteins loaded in each lane. The molecular size markers are indicated on the left. The glutathione transferase (GST) fusion proteins used in the pulldown assays of Figs 2D, E, 3B, 4B and 5B are indicated by the asterisks. The gels corresponding to Fig. 6A show the copper‐induced expression (I) of citrus ubiquitin‐conjugating enzyme variant (Uev) and Ubc13 in the yeast mutants, relative to noninduced samples (NI). Arrows indicate the induced bands. The gels corresponding to Fig. 7A show the GST–PthA samples purified from wild‐type and mutant yeast cells. NI (noninduced), I (induced), S (soluble), FT (flow‐through), W (wash) and E (elution). The ‘E’ samples were loaded side by side in the blot shown in Fig. 7A. The gels corresponding to Fig. 7B and D show the amounts of proteins loaded from plant and yeast cells expressing PthAs 2 and 4. Arrows indicate the positions of PthA bands.
Fig. S3 Nuclear localization of cyclophilin (Cyp) and ubiquitin‐conjugating enzyme variant (Uev) in Nicotiana benthamiana cells. Fluorescence of Uev–red fluorescent protein (DsRed) and Cyp–DsRed fusions in the nucleus of transiently transfected N. benthamiana cells 72 h after Agrobacterium tumefaciens infiltration. 4′,6‐Diamidino‐2‐phenylindole (DAPI) fluorescence of the same cells is shown alongside.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


