SUMMARY
Pantoea stewartii subsp. stewartii (Pnss) causes Stewart's bacterial wilt of sweet corn and leaf blight of maize. The pathogenicity of Pnss depends on synthesis of extracellular polysaccharide and an Hrp type III secretion system. WtsE, a type III secreted effector protein, is essential for the virulence of Pnss on corn. It belongs to the AvrE family of effectors, which includes DspA/E from Erwinia amylovora and AvrE1 from Pseudomonas syringae. Previously, WtsE was shown to cause disease‐associated cell death in its host plant, sweet corn. Here, we examine the biological activity of WtsE in several non‐host plants. WtsE induced cell death in Nicotiana benthamiana, tobacco, beet and Arabidopsis thaliana when it was transiently produced in plant cells following agroinfiltration or translocated into plant cells from Pnss, Escherichia coli or Pseudomonas syringae pv. phaseolicola (Pph). WtsE‐induced cell death in N. benthamiana, tobacco and beet resembled a hypersensitive response and in N. benthamiana it was delayed by cycloheximide. Interestingly, WtsE strongly promoted the growth of Pnss in N. benthamiana prior to the onset of cell death. Deletion derivatives of WtsE that failed to induce cell death in N. benthamiana and tobacco also did not complement wtsE mutants of Pnss for virulence in sweet corn, indicating a correlation between the two activities. WtsE also induced cell death in A. thaliana, where it suppressed basal defences induced by Pph. Thus, WtsE has growth‐promoting, defence‐suppressing and cell death‐inducing activities in non‐host plants. Expression of WtsE also prevented the growth of yeast, possibly due to an innate toxicity to eukaryotic cells.
INTRODUCTION
Pantoea stewartii subsp. stewartii (Pnss) causes Stewart's bacterial wilt and leaf blight of sweet corn and maize (Zea mays L.). This disease is a serious problem in the north‐central and eastern United States (Pateky, 2003). Pnss is transmitted by the corn flea beetle (Chaetocnema pulicaria) and it overwinters in the beetle's gut (Claflin, 1999). Bacteria grow in the xylem vessels of the corn plant causing wilt and in the intercellular spaces of the leaves causing ‘water‐soaked’ lesions. An important virulence factor for wilting is extracellular polysaccharide (EPS) slime (Dolph et al., 1988). The earliest symptom of infection is water‐soaked lesions on young leaves. Water‐soaking (Wts) results from the release of water and nutrients following cell membrane damage. Bacterial EPS holds the fluids in the leaf tissue so that the lesions appear water‐soaked for several days before turning necrotic. Using inoculation procedures that distinguish between Wts and wilting, we have shown that the Hrp type III secretion system (TTSS) is needed to produce both symptoms. Other classical virulence factors, such as degradative enzymes, toxins and growth regulators, are not known to be produced by Pnss. At this point, WtsE is the only TTSS effector protein known to be required for virulence of Pnss (D. Majerczak and D. Coplin, unpublished data). WtsE belongs to the widespread and conserved AvrE‐family of type III effectors (Frederick et al., 2001, Ham et al., 2006). In most cases, loss of an AvrE‐family effector by an erwinia or pseudomonad plant pathogen greatly reduces virulence. This is especially true for Pnss WtsE, Pantoea agglomerans DspE (Mor et al., 2001), Erwinia amylovora (Ea) DspA/E (Bogdanove et al., 1998, Gaudriault et al., 1997) and Pseudomonas syringae AvrE (Lorang et al., 1994).
In a previous study (Ham et al., 2006), WtsE was shown to induce cell death in susceptible corn seedlings when it was delivered by Escherichia coli MC4100 carrying the heterologous TTSS from Dickeya dadantii (Dda, formerly Erwinia chrysanthemi) (Ham et al., 1998). The WtsF chaperone protein was needed for this delivery system, but was not required for secretion by Pnss. Similarly, other AvrE family proteins have been shown to cause symptoms in both host and non‐host plants due to either virulence or avirulence activities. Boureau et al. (2006) have shown that transiently expressed Ea DspA/E induces necrosis in both apple and tobacco leaves. Oh et al. (2007) have reported that DspA/E causes programmed cell death in Nicotiana benthamiana when it is transiently expressed by agroinfiltration and that this response is dependent on the defence signalling protein SGT1. Surprisingly, despite inducing what appears to be a hypersensitive response (HR), DspA/E enhances E. amylovora growth in the first day following infection of N. benthamiana. Badel et al. (2006) reported that Agrobacterium‐mediated transient expression of P. syringae pv. tomato AvrE1 elicits cell death in tobacco leaves as well as in the susceptible host, tomato. At this point, it is unclear if the response elicited in non‐hosts by AvrE family effectors is always an HR and if susceptible and non‐host responses involve the same mechanisms. For example, Ea DspE/A may act as a virulence factor to enhance bacterial grown in N. benthamiana prior to inducing a non‐host HR (Oh et al., 2007). (Here, the term ‘virulence’ will be used to designate the properties of effectors that promote bacterial growth, elicit disease‐associated cell death or suppress host defences.)
In the present study, we characterized the biological activities of WtsE in various non‐host plants using different delivery systems. We found that, in addition to the host plant corn, WtsE‐induced cell death (WICD) also occurs in non‐host plants including tobacco, N. benthamiana, beet and A. thaliana. Furthermore, we demonstrate virulence activities for WtsE in both N. benthamiana and A. thaliana. Our results indicate that WtsE and other AvrE family members may have a similar target(s) that accounts for their virulence activity in susceptible hosts and their avirulence activity in resistant plants and that N. benthamiana and A. thaliana are relevant model systems to study the activities of WtsE.
RESULTS
Pnss causes cell death in non‐host plants in a wtsE‐dependent manner
In sweet corn, Pnss causes Wts within 12 h when introduced into corn leaves by vacuum infiltration at 2 × 108 CFU/mL. This response was dependent on WtsE (Frederick et al., 2001, Ham et al., 2006). However, in tobacco leaves, Pnss can only elicit cell death when the hrp genes are pre‐induced, either by growth in an inducing medium that mimics the environmental conditions in plant apoplastic spaces or when hrpS, a transcriptional activator of the hrp genes, is constitutively expressed under a strong promoter in plasmid pRF205 (Frederick et al., 1993). In this study, we characterized the ability of Pnss wild‐type and mutant strains, all carrying pRF205 (Plac‐hrpS +), to induce cell death in a variety of non‐host plants. When infiltrated into leaves at 2 × 108 CFU/mL, wild‐type Pnss caused rapid, HR‐like cell death in N. benthamiana, tobacco and beet as soon as 12 h later and Wts in A. thaliana after 24 h (Fig. 1A–D). To determine the WtsE‐dependence of Pnss‐induced cell death in these plants, we compared wild‐type Pnss (DC283) with a WtsE− strain (AA005) (Ham et al., 2006). AA005 failed to cause cell death in N. benthamiana, beet and A. thaliana, but still caused cell death in tobacco (Fig. 1B). As AA005 also produces harpin (HrpN) that alone can elicit an HR in tobacco (Ahmad et al., 2001), we tested an hrpN mutant (DM760) and a wtsE hrpN double mutant (DM2860). DM760 also caused cell death in tobacco (data not shown), but DM2860 did not (Fig. 1B). These results indicate that Pnss‐induced cell death in N. benthamiana, beet and A. thaliana is dependent on WtsE, whereas cell death in tobacco can be elicited by either WtsE or harpin.
Figure 1.
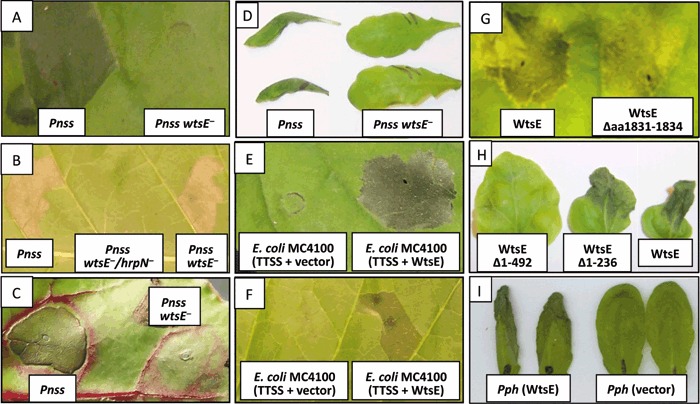
WtsE‐induced cell death in a variety of non‐host plants. (A–D) Wild‐type or mutant P. stewartii strains carrying pRF205 were infiltrated at 2 × 108 CFU/mL into leaves of (A) N. benthamiana, (B) tobacco, (C) beet and (D) A. thaliana. (E,F) E. coli MC4100 (pCPP2156/pJH021) expressing the D. dadantii TTSS and WtsE or E. coli MC4100 (pCPP2156) without WtsE were infiltrated at 1 × 109 CFU/mL into leaves of (E) N. benthamiana and (F) tobacco. (G,H) Wild‐type wtsE or derivatives were transiently expressed by infiltrating 109 CFU/mL of Ag. tumefaciens C58C1 into leaves of N. benthamiana. (I) P. syringae pv. phaseolicola NPS3121 expressing WtsE or carrying a control vector were infiltrated at 1 × 108 CFU/mL into leaves of 4‐week‐old A. thaliana. Pictures were taken at 24 h (A–F) or 48 h (H–I) after infiltration or 24 h after spraying with dexamethsone at 48 h after infiltration (G) and are representative of three or more separate experiments.
WtsE alone is sufficient for causing cell death in non‐host plants and yeast
We previously showed that WtsE alone was capable of causing Wts in corn leaves in the absence of other possible TTSS effectors produced by Pnss. In these experiments, we used a heterologous delivery system to introduce WtsE into plant cells. This system consisted of E. coli MC4100 carrying cosmid pCPP2156 that contains the entire hrp‐gene cluster from Dda. This Hrp TTSS is known to promiscuously secrete type III effectors from a number of plant and animal pathogens into culture supernatants and deliver them into plant cells (Anderson et al., 1999, Ham et al., 1998). We found that the associated WtsF chaperone protein was also needed for translocation of WtsE by this system. Here, we used this delivery system to determine the effect of WtsE on non‐host plant cells. As shown in Fig.1 E,F, E. coli MC4100 (pCPP2156/pJH021 wtsEF +) caused rapid WICD in N. benthamiana and tobacco leaves, while E. coli MC4100 (pCPP2156) alone did not cause any symptoms in these plants. As described below, MC4100 (pCPP2156/pJH021) also caused electrolyte leakage in tobacco leaves. In contrast, MC4100 (pCPP2156/pJH021) did not cause WICD in beet leaves. Unfortunately, we were unable to assess the activity of WtsE in A. thaliana using this delivery system because MC4100 (pCPP2156) caused tissue collapse in A. thaliana, possibly due to the activity of harpin produced by the Dda hrp gene cluster (data not shown).
A second heterologous delivery system for introducing WtsE into non‐host plants was transient expression mediated by Agrobacterium tumefaciens C58C1. We used the pBDex (Allen et al., 2004) and pGD (Goodin et al., 2002) binary vector systems to express WtsE and its derivatives. Infiltration of Ag. tumefaciens carrying the wild‐type wtsE clone (pJH101) or a derivative lacking the first 236 amino acids (pJH028 or pJH102) induced WICD in tobacco (data not shown) and N. benthamiana leaves (Fig. 1G–H). Experiments with Agrobacterium‐mediated transient expression of WtsE did not result in cell death in A. thaliana and beet. However, agroinfiltration seems inefficient at least in wild‐type A. thaliana due to its resistance to Ag. tumefaciens (Zipfel et al., 2006).
In addition, WtsE activity in yeast cells was examined by cloning wtsE in a conditional expression vector derived from pMyr (Stratagene) and expressing it under control of the GAL1 promoter, which is induced by galactose. As shown in Fig. 2A, the Saccharomyces cerevisiae strain cdc25H expressing full‐length WtsE did not grow in galactose medium, while yeast cells expressing the N‐ or C‐terminal halves of WtsE grew normally. The fact that the clones encoding the two halves of WtsE have been used to identify specific interacting proteins by yeast two‐hybrid assays indicates that these derivatives are expressed and stable in yeast. In addition, we introduced the wtsE gene into the chromosome of strain Y7092 and found that it also inhibited growth when induced by galactose (Fig. 2B). This activity of WtsE in yeast cells indicates that it may be toxic to eukaryotic cells in general.
Figure 2.
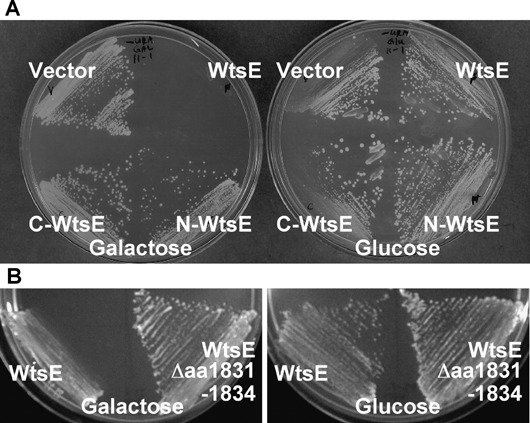
WtsE inhibited the growth of yeast. S. cerevisiae strains cdc25H (A) or Y7092 (B) did not proliferate when conditional expression of wild‐type wtsE was induced by galactose. Expression of the N‐terminal (N‐WtsE, amino acids 1–964) or C‐terminal (C‐WtsE, amino acids 964–1835) halves of WtsE (A) or of WtsE‐Δaa1831‐1834 (B) had no detrimental effect on yeast growth. Growth on suppressive glucose media demonstrated the viability of uninduced yeast.
WtsE‐induced cell death in non‐hosts is delayed by cycloheximide
Our previous work demonstrated that WICD in corn differs in nature from defence‐related cell death. Specifically, cycloheximide (CHX) inhibited a gene‐for‐gene HR elicited by Xanthomonas oryzae pv. oryzae with avrRxo1 in B73 maize, but failed to inhibit Pnss‐induced WICD in sweet corn (Ham et al., 2006). In this study, we tested the sensitivity of WICD in non‐host plants to CHX. In tobacco, P. syringae pv. tomato strain DC3000 induces a non‐host HR that was inhibited by CHX (Bozso et al., 1999). Similarly, macroscopic necrosis induced by MC4100 (pCPP2156/pAA008), expressing the Dda TTSS and WtsE, was delayed and significantly reduced by CHX (data not shown). To quantify the CHX‐induced attenuation of WICD, we measured electrolyte leakage (EL) at various times after infiltration of tobacco with bacteria and CHX. WtsE‐induced EL increased up to 19 h and then levelled off (Fig. 3 and data not shown). EL was reduced two‐fold by CHX between 6 and 19 h, but at 24 h, CHX alone caused considerable EL. Although visible symptoms were not seen in the MC4100 (pCPP2156) controls, this strain did cause significant EL. This may be due to the production and secretion of harpin by the Dda hrp genes, as this EL was also inhibited two‐fold by CHX. Similar results were obtained in N. benthamiana, but CHX was somewhat toxic to this plant and this resulted in high variability (data not shown). WICD caused by Pnss DC283 (pRF205) in beet was likewise inhibited by coinfiltration with CHX. In contrast, we did not see an effect of CHX on WICD in A. thaliana. However, interpretation of this result is difficult as CHX also failed to inhibit the HR induced by known gene‐for‐gene activation of RPM1 and RPS2 by AvrRpm1 and AvrRpt2, respectively (data not shown).
Figure 3.
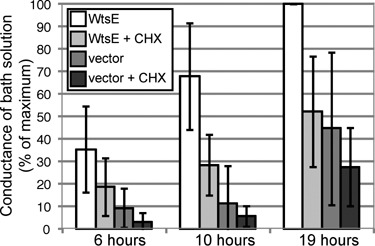
WtsE‐induced cell death in tobacco was inhibited by cycloheximide (CHX). Electrolyte leakage was used quantitatively to measure cell death caused by E. coli MC4100 (pCPP2156/pJH021), expressing the D. dadantii type III secretion system and wtsE, or E. coli MC4100 (pCPP2156) without wtsE in tobacco leaves. Leaves were infiltrated with bacteria (1 × 109 CFU/mL) ± 100 µg/mL CHX. Infiltrated leaf discs were placed in water and conductivity was measured over time. For each treatment, the conductivity value of the corresponding negative control (buffer or buffer with CHX) was subtracted. Then each value was converted to a percentage of the maximum leakage obtained with MC4100 (pCPP2156/pJH021) at 19 h in the same experiment; the later values ranged from 18.5 to 60.3 µS/cm. Values shown are the mean of five experiments ± SD.
Deletion analysis revealed important portions of WtsE for both cell death and viruence activities
WtsE contains several putative sequence motifs, including a leucine zipper, a coiled‐coil domain, a nuclear localization signal (NLS), and two endoplasmic reticulum membrane retention/retrieval signals (ERMRS) (Ham et al., 2006). Among these motifs, only the C‐terminal ERMRS and an NLS are present in most AvrE family proteins. However, the location of the NLS is not conserved. We previously reported that the C‐terminus of WtsE, which contains an ERMRS motif (LKKEGFEMKS) predicted by PSORTII, is important for the virulence function of WtsE. This was based on the non‐pathogenic phenotype of a miniTn5gus insertion in wtsE that removes the last 12 amino acids (Ham et al., 2006). In this study, we have precisely deleted the penultimate four amino acids (1831–1834, FEMK) from the putative ERMRS. When delivered by the E. coli MC4100 (pCPP2156) system this mutant protein (WtsE‐Δaa1831–1834) did not cause WICD in tobacco (Table 1) and its activity was greatly reduced when highly expressed after agroinfiltration in N. benthamiana (Fig. 1G). In yeast, the WtsE‐Δaa1831–1834 protein was not toxic and the yeast strain grew normally (Fig. 2). Finally, the WtsE‐Δaa1831–1834 protein did not restore the virulence of Pnss wtsE mutants in sweet corn, when it was used to complement Pnss DM5101 wtsE::miniTn5gus (data not shown) and AA005 wtsE::aphT (Table 1). These findings confirm the importance of the C‐terminus of WtsE for its ability to induce cell death in plants and yeast.
Table 1.
Summary of results for cell‐death and virulence activities of wild‐type WtsE and its derivatives.
| Derivative | Cell death in N. benthamiana and tobacco using the E. coli delivery system* | Cell death in N. benthamiana by agroinfiltration† | Complementation of Pnss AA005 wtsE for virulence§ |
|---|---|---|---|
| no WtsE | – | – | 0.1 ± 0.1 |
| wild‐type WtsE | + | + | 2.4 ± 0.6 |
| WtsE‐Δaa51‐236‡ | + | + | 2.4 ± 0.8 |
| WtsE‐Δaa51‐492‡ | – | – | 0.5 ± 0.5 |
| WtsE‐Δaa1831‐1834 | – | – | 0.7 ± 0.6 |
| WtsE NLS‐ (KKHK1358‐61AAAA) | + | not done | 2.4 ± 0.6 |
Results for cell death when the indicated WtsE derivatives (or empty vector, no WtsE) were delivered from *E. coli MC4100 (pCPP2156) or†A. tumefaciens, as described in Fig. 1. ‡For agroinfiltration, derivatives of WtsE were Δaa1‐236 and Δaa1‐492. §Derivatives of WtsE (or empty vector, no WtsE) were tested for their ability to restore the virulence of Pnss AA005 wtsE::aphT on sweet corn. Six‐day‐old seedlings were inoculated by placing the inoculum into the whorls and disease symptoms were rated 4 days later. Results of the representative experiment shown are the average and standard deviation from 18–22 plants. The experiment was done four times.
To determine if the NLS is also involved in cell death or virulence, the amino acid residues KKHK, which constitute the NLS of WtsE, were changed to AAAA and the resulting mutant plasmid, pJH049, was tested for WICD activity in the same manner. This mutation affected neither the ability of WtsE to cause WICD using the MC4100 (pCPP2156) delivery system in tobacco and N. benthamiana nor its ability to complement AA005, which has an aphT insertion in the middle of wtsE, for virulence in sweet corn (Table 1). Intriguingly, the NLS mutant did not consistently complement DM5101, which has a mini‐Tn5 insertion at the beginning of wtsE (data not shown).
A region near the N‐terminus of WtsE was also required for WICD in both sweet corn and non‐hosts. Using agroinfiltration, we found that a derivative of WtsE lacking the N‐terminal 236 amino acids (pJH102) still produced WICD in N. benthamiana. However, a deletion removing the first 492 amino acids (pJH103) did not (Fig. 1H). This indicates that the region of WtsE between A237 and F492 is required for WICD. This finding was confirmed using the MC4100 delivery system. As Hrp secretion signals are typically at the N‐terminus (Petnicki‐Ocwieja et al., 2002), we added back the first 50 amino acids to these two N‐terminal deletion mutants. Plasmids pJH041 wtsE‐Δaa51‐236 and pJH043 wtsE‐Δaa51‐492 were constructed in pBluescript SK and introduced into E. coli MC4100 (pCPP2156). WtsEΔ51‐236 caused WICD confirming the agroinfiltration result and indicating that this protein was indeed translocated. In contrast, WtsE‐Δaa51‐492 did not cause WICD, confirming the importance of amino acids 237–492 (Table 1). To determine if this portion of WtsE is also required for the virulence of Pnss, the internal deletion mutations were cloned into a broad host range and low copy number plasmid, pRK415, and introduced into Pnss AA005 wtsE for complementation tests in sweet corn. Similarly, WtsE‐Δaa51‐236, but not WtsE‐Δaa51‐492, restored the virulence of the wtsE mutant (Table 1). Overall, the ability of the above mutants to cause WICD in non‐hosts was positively correlated with their ability to complement a Pnss wtsE mutant for virulence in sweet corn.
WtsE has virulence activity in non‐host plants
Although the response of N. benthamiana to WtsE superficially resembles an HR, our results do not preclude that WtsE also carries out virulence functions in this non‐host. Oh et al. (2007) showed that DspA/E promotes the early growth of Ea in N. benthamiana, despite later inducing a non‐host HR. We have similarly compared the growth of wild‐type and wtsE Pnss strains in N. benthamiana (Fig. 4). The population of the wild‐type strain increased over one log during the first 12 h after infiltration, but declined after the onset of necrosis at 24 h. In contrast, the wtsE mutant declined steadily after only 6 h. Thus, WtsE has a virulence activity that can promote the growth of Pnss in N. benthamiana prior to inducing HR‐like necrosis.
Figure 4.
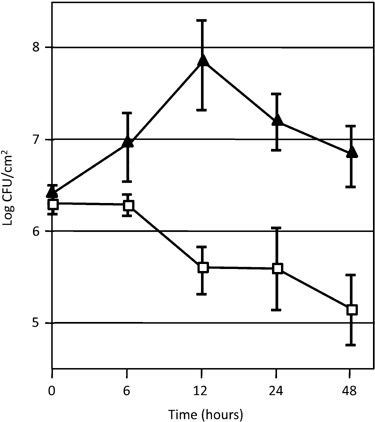
WtsE promotes early growth of P. stewartii in N. benthamiana. Wild‐type (solid triangles) and wtsE (open squares, strain DM5101) Pnss were hand‐infiltrated at 5 × 108 CFU/mL and viable cell counts were monitored over the course of 2 days. Both strains carried pRF205, which induces Hrp gene expression. The experiment has been done three times with similar results. The bacterial populations ± SD shown are the combined data from two of the experiments. Similar results were obtained with strain AA005 wtsE (not shown).
We also sought to determine if WtsE can suppress basal defences in A. thaliana, where it causes a Wts‐like necrosis. WtsE was translocated into A. thaliana leaf cells by P. syringae pv. phaseolicola (Pph) NPS3121. In ecotype Col‐0, this Pph strain causes minimal cell death and induces strong basal defence responses, including induction of the defence protein PR‐1 and deposition of two morphologically distinct types of callose (small and big callose) (Ham et al., 2007). When infiltrated into Col‐0 leaves at 1 × 108 CFU/mL, NPS3121 (pJH082 wtsEF +) caused water‐soaked lesions (Fig. 1I). In addition to causing symptoms, WtsE also suppressed early Pph‐induced defence responses. Induction of PR1 was significantly reduced when Pph expressed WtsE (Fig. 5A). Also, prior to the appearance of symptoms (15 h after infiltration) formation of big callose deposits was almost completely suppressed and formation of small callose deposits was significantly reduced (Fig. 5B).
Figure 5.
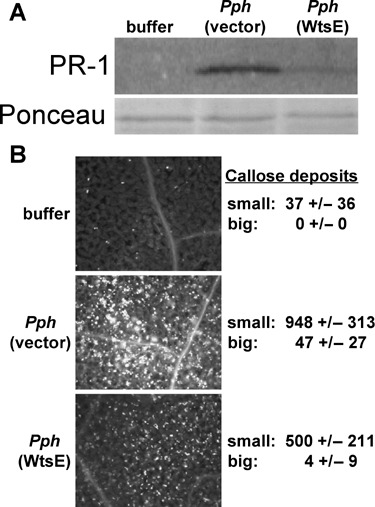
WtsE suppressed basal defence responses in A. thaliana. (A) WtsE suppressed P. syringae pv. phaseolicola (Pph)‐induced accumulation of PR‐1 protein. Buffer or Pph expressing either WtsE (from pJH082) or carrying a control vector were infiltrated into A. thaliana leaves at 1 × 108 CFU/mL. After 48 h, total protein extracts were prepared from infiltrated leaves and subjected to anti‐PR‐1 Western blotting. The lower panel shows Ponceau‐S staining to control for protein loading. Results are representative of three or more experiments. (B) WtsE suppressed Pph‐induced callose deposition. Leaves infiltrated as in (A) were collected 15 h after infiltration and callose was stained with aniline blue. Both small (<20 µm) and big (>20 µm) callose deposits were significantly reduced when Pph expressed wtsE. Both types of callose deposits were counted in representative areas of five leaves for each treatment from three separate experiments and the average and standard deviation from all counts are shown.
DISCUSSION
AvrE family effector proteins are important for the full virulence of most Gram‐negative plant pathogenic bacteria in the Enterobacteriaceae and Pseudomonadaceae (reviewed in Ham et al., 2006), but they were first discovered due to their ability to induce an HR in non‐hosts. The prototype for this group, P. syringae pv. tomato AvrE, was initially identified as an avirulence gene in soybean. Later, this family was shown to cause both cell death in susceptible hosts and the HR in many non‐hosts. For example, Badel et al. (2006) reported that transient expression of AvrE1 elicited cell death in tobacco and N. benthamiana leaves, as well as susceptible tomato leaves. Likewise, Ea DspA/E, an essential pathogenicity factor, was reported to induce cell death in host (apple) and non‐host (tobacco) plants when transiently expressed (Boureau et al., 2006). We likewise reported that WtsE alone can cause Wts in sweet corn (Ham et al., 2006). Plant responses to DspA/E and AvrE effectors have not been well studied in susceptible hosts because they have not been associated with a disease of an ideal model plant and/or the pathogen has other effectors with a redundant function, e.g. HopM1 in P. syringae pv. tomato DC3000 (DebRoy et al., 2004).
Here, we show that WtsE alone has cell death activity in a variety of non‐host plants. During the course of these studies, we developed one or more delivery systems for these non‐hosts so that we will be able to study the mode of action of WtsE in several model plants. First, Pnss strains constitutively expressing hrpS caused cell death in N. benthamiana, A. thaliana and beet in a wtsE‐dependent manner. In tobacco, however, both wtsE and hrpN Pnss mutants induced cell death, but a wtsE hrpN double mutant did not, indicating that either WtsE or harpin can induce cell death in this plant. In contrast, N. benthamiana and A. thaliana were not responsive to the amount of harpin produced by Pnss in planta. Second, Agrobacterium‐mediated transient expression of wtsE elicited WICD in tobacco and N. benthamiana. Third, translocation of WtsE from E. coli MC4100 (pCPP2156 hrpDda +) resulted in WICD in N. benthamiana and tobacco, but not in beet leaves. Unfortunately, the E. coli delivery system could not be used in A. thaliana because MC4100 (pCPP2156) alone induced tissue collapse and agroinfiltration did not work in A. thaliana Col‐0 and some plantings of tobacco did not respond well. However, WtsE did cause Wts symptoms in A. thaliana when it was delivered by a fourth system, Pph NPS3121. From these results, we conclude that expression of WtsE alone is sufficient to cause cell death in a variety of host and non‐host plants. Conditional expression of WtsE also arrested the growth of yeast cells, indicating it is toxic to a broad spectrum of eukaryotic cells.
In non‐hosts, WICD could either be an HR due to the avirulence activity of WtsE or be similar to the disease‐associated Wts response in corn. These responses are difficult to distinguish based solely on symptoms and timing. Moreover, the mechanisms needed to elicit either one could be similar if R‐proteins are guarding one or more of the intended cellular targets of WtsE. In our previous study, we demonstrated that WtsE‐induced Wts in corn seedlings was not inhibited by CHX, which blocks de novo protein synthesis in eukaryotic cells (Ham et al., 2006). In the same study, a control HR in corn seedlings, derived from a known gene‐for‐gene interaction, was inhibited by CHX, indicating the susceptible Wts response differs from a defence‐associated HR. In an attempt to determine whether or not WICD in non‐host plants involves an HR, we again tested for inhibition by CHX. Interestingly, WICD was delayed but not fully inhibited in the leaves of N. benthamiana, tobacco and beet treated with CHX. Thus, we hypothesize that WtsE is capable of inducing two types of cell death in non‐host plants. First, some hosts, such as tobacco and beet, may respond with an HR typified by CHX‐sensitive, dry, collapsed necrosis. Second, they may exhibit disease‐associated WICD, if an HR is inhibited by CHX (or corresponding R‐genes are absent). Thus, the delayed appearance of mild necrosis in CHX‐treated tobacco, N. benthamiana and beet may have been due to the unmasking of disease‐associated WICD, once an HR was blocked. Further work is needed to characterize these responses. However, the ability of wild‐type, but not wtsE, strains of Pnss to grow initially in N. benthamiana suggests that the initial response of this plant may be disease‐associated cell death that later triggers an HR. In A. thaliana, however, our efforts to use CHX to determine the nature of WICD were uninformative because it failed to inhibit both WICD and known HRs. At this point, we favour the hypothesis that the response in A. thaliana represents disease‐associated cell death because, when delivered by Pph, it induces tissue collapse that is phenotypically distinct from known HRs and resembles the disease‐associated cell death induced by AvrE1 and HopM1 when they are delivered by Pph (data not shown).
The delivery systems we developed were used to determine the effects of various mutations in wtsE on virulence in Pnss and on non‐host cell death. Deletion analysis revealed that the first 236 amino acids of WtsE were dispensable for the cell death activity when WtsE was transiently expressed in N. benthamiana following agroinfiltration (Fig. 1H). This was confirmed by the ability of WtsE lacking amino acids 51–236 to induce cell death comparable with wild‐type WtsE in N. benthamiana when delivered by E. coli MC4100 (pCPP2156) and to fully complement a wtsE strain of Pnss (Table 1). In addition, these results indicate that the entire secretion signal is probably contained within the first 50 amino acids of WtsE. Extending this deletion to amino acids 492 disrupted the ability of WtsE to cause cell death and to complement a wtsE strain of Pnss, indicating that amino acids 237–492 are important for both the avirulence and the virulence activities of WtsE. In contrast, mutation of the putative NLS did not significantly affect the cell death or virulence activities of WtsE (Table 1). Our preliminary finding that the putative ERMRS is needed for virulence (Ham et al., 2006) was confirmed by a more precise deletion of the penultimate four amino acids at the C‐terminus of WtsE, which removes the right half of this motif. This change resulted in a large reduction of both cell death and virulence activities. It is unusual that WtsE would have this retention signal, as it lacks an apparent ER targeting motif. An interesting possibility is that WtsE provides the retention signal in trans to an ER‐resident protein with which it interacts. Meng et al. (2006) have shown that Ea DspA/E interacts with several leucine‐rich‐repeat receptor‐like kinases (LRR RLKs) from apple in the yeast two‐hybrid system and we have similar preliminary data for WtsE (J. Ham et al., unpublished). As these potential defence signalling molecules are probably trafficked from the ER to their active site at the plasma membrane, it may be that WtsE blocks their function by retaining them in the ER. This may be an important defence‐suppressing activity, as the expression of numerous LRR RLKs is up‐regulated during plant basal defences (Navarro et al., 2004, Thilmony et al., 2006).
It is significant that mutations in WtsE have so far failed to separate the induction of HR‐like cell death in non‐hosts from its virulence activities in sweet corn. This suggests that elicitation of both an HR and disease‐associated WICD involve the same mechanism. As the AvrE family effectors are conserved in many plant pathogenic bacteria and essential for their virulence, it is reasonable that plants would have evolved a corresponding family of R‐proteins. Our finding is consistent with the guard hypothesis, which proposes that plant defence systems indirectly detect effector proteins by recognizing perturbation of a host target(s) associated with their virulence activity. In response, the very large AvrE family effectors appear to have also incorporated the ability to suppress some host defences. AvrE1 has been shown to suppress defences in A. thaliana (DebRoy et al., 2004) and we have shown the same for WtsE here. This may simply be a second function of WtsE that disarms basal resistance, but it also raises the possibility that the Wts caused by WtsE in corn and A. thaliana may be a form of controlled, partially suppressed programmed cell death. In conclusion, we have demonstrated that WtsE elicits cell death in N. benthamiana, tobacco, A. thaliana and beet, that it carries out both growth‐promoting and defence‐suppressing virulence functions in N. benthamiana and A. thaliana, and that it is toxic to yeast. Thus, the WtsE delivery systems that we have developed for the model plants, A. thaliana and N. benthamiana, may be valuable for studying the functions of an important family of bacterial effectors proteins that is conserved in many bacterial pathogens of plants.
EXPERIMENTAL PROCEDURES
Bacterial and yeast strains and their growth conditions
Bacterial and yeast strains and plasmids used in this study are described in Table 2. E. coli, Pnss and Ag. tumefaciens strains were routinely grown and maintained in Luria–Bertani (LB) broth or agar (Sambrook et al., 1989) at 28 °C for Pnss and Ag. tumefaciens strains and at 37 °C for E. coli strains. P. syringae pv. phaseolicola strains were grown in King's B agar (Schaad et al., 2001) at 28 °C. Appropriate antibiotics were supplied as needed at the following concentrations (µg/mL): ampicillin (Ap), 200; chloramphenicol (Cm), 34; kanamycin (Km), 100 for P. s. pv. phaseolicola and 50 for Pnss and E. coli; nalidixic acid (Nal), 20; spectinomycin (Sp), 50; rifampicin (Rif), 100; and tetracycline (Tc), 25. S. cerevisiae strains were grown and maintained in yeast extract‐peptone‐dextrose (YPD) agar or appropriate complete minimal (CM) dropout media (Ausubel et al., 1995).
Table 2.
Strains and plasmids used in this study.
| Description | Source/Reference | |
|---|---|---|
| Agrobacterium tumefaciens | ||
| C58C1 | Vir‐, prototrophic strain, Rifr | (Van Larebeke et al., 1974) |
| Escherichia coli | ||
| MC4100 | araD139 Δ(argF‐lac)205 λ−flbB5301 ptsF25 relA1 rpsL150 deoC1 | CGSC* (Casadaban, 1976) |
| DB3.1™ | F‐ gyrA462 endA1 Δ (sr1‐recA) mcrB mrr hsdS20(rB‐, mB‐) supE44 ara‐14 galK2 lacY1 proA2 rpsL20(SmR) xyl‐5 λ‐ leu mtl1 | Invitrogen |
| Pantoea stewartii subsp. stewartii | ||
| DC283 | A Nalr derivative of wild‐type strain SS104 | (Coplin et al., 1986) |
| DM5101 | DC283 wtsE::miniTn5gus, Kmr ; miniTn5gus insertion at amino acid 43 | (Ham et al., 2006) |
| AA005 | A wtsE::aphT derivative of DC283, Kmr; aphT insertion at amino acid 962 | (Ham et al., 2006) |
| Pseudomonas syringae pv. phaseolicola | ||
| NPS3121 | Wild‐type strain, Rifr | (Lindgren et al., 1986) |
| Saccharomyces cerevisiae | ||
| cdc25H | MATαura3‐52 his3‐200 ade2‐101 lys2‐801 trp1‐901 leu2‐3, 112 cdc25‐2 GAL + | (Petitjean et al., 1990) |
| Y7092 | MATα can1Δ::STE2pr‐SpΔhis5 his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 lyp1Δtrp1Δ::GAL‐IpgB1‐URA3 | (Alto et al., 2006) |
| Plasmids | ||
| pAA008 | Clone of the 8.9‐kb EcoRI/BamHI fragment carrying wtsE and wtsF in pBluescript SK, Apr | (Ham et al., 2006) |
| pAS1 | Bait vector for yeast two‐hybrid system, PADH1, ColE1 origin and Apr marker for use in E. coli and 2 µ origin and LEU2 selectable marker for use in S. cerevisiae | ABRC†, (Fan et al., 1997) |
| pBDex | A derivative of pTA7002 (Aoyama and Chua, 1997) with the Basta plant selection marker replaced by the hygromycin selection marker, Kmr | (Allen et al., 2004) |
| pBluescript KS and SK (+) | ColE1 α‐lacZ, Apr | Stratagene |
| pCPP2156 | pCPP19 cosmid carrying the D. dadantii hrp cluster, Spr | (Ham et al., 1998) |
| pCR®4‐TOPO® | TOPO® vector for cloning PCR products, Apr, Kmr | Invitrogen Corp |
| pDSK519 | Broad‐host‐range cloning vector; Kmr | (Keen et al., 1988) |
| pENTR/D‐TOPO® | An entry vector for the Gateway® cloning system, Kmr | Invitrogen |
| pENTR::N‐WtsE | Clone of the 0.5‐kb 5’‐end portion of wtsE in pENTR/D‐TOPO®, Kmr | This study |
| pENTR::WtsE | Wild type wtsE clone in pENTR/D‐TOPO®, Kmr | This study |
| pENTR::WtsE‐ΔERMRS | ΔERMRS derivative of wtsE clone in pENTR/D‐TOPO®, Kmr | This study |
| pGD | Binary vector for agroinfiltration, Kmr | (Goodin et al., 2002) |
| pJH001 | pCR®4‐TOPO® clone with a 6.4‐kb EcoR1/BamHI fragment containing the wtsEF operon and its native promoter, Apr, Kmr | This study |
| pJH003 | Clone of the 6.4‐kb EcoRI/BamH1 fragment from pJH001 in pRK415 carrying the wtsEF operon and its native promoter, Tcr | This study |
| pJH021 | pBluescript SK(+) clone of the 6.4‐kb EcoRI/BamHI fragment from pJH001 carrying the wtsEF operon and its native promoter, Apr | This study |
| pJH023 | pJH021 with wtsE‐Δaa1831‐1834, Apr | This study |
| pJH024 | pJH003 with wtsE‐Δaa1831‐1834, Tcr | This study |
| pJH028 | pBDex::wtsE‐Δaa1‐236, Kmr | This study |
| pJH040 | pJH021 with wtsE‐Δaa51‐236, Apr | This study |
| pJH041 | pJH003 with wtsE‐Δaa51‐236, Tcr | This study |
| pJH042 | pJH021 with wtsE‐Δaa51‐492, Apr | This study |
| pJH043 | pJH003 with wtsE‐Δaa51‐492, Tcr | This study |
| pJH048 | pJH021 with wtsE NLS‐ (KKHK1358‐61AAAA), Apr | This study |
| pJH049 | pJH003 with wtsE NLS‐ (KKHK1358‐61AAAA), Tcr | This study |
| pJH082 | Clone of the 6.4‐kb EcoRI/BamH1 fragment from pJH001 in pDSK519 carrying the wtsEF operon and its native promoter, Kmr | This study |
| pJH087 | Derivative of pJH028 with Δaa1831‐1834 in WtsE, Kmr | This study |
| pJH101 | pGD::wtsE, Kmr | This study |
| pJH102 | pGD::wtsE‐Δaa1‐236, Kmr | This study |
| pJH103 | pGD::wtsE‐Δaa1‐492, Kmr | This study |
| pMyr | Prey vector for CytoTrapTM yeast two‐hybrid system, PGAL1, ColE1 origin and Cmr marker for use in E. coli and 2 µ origin and URA3 selectable marker for use in S. cerevisiae | Stratagene |
| pMyr‐BD | A modified pMyr vector (Stratagene); the GAL4‐BD and multicloning site of pAS1 was substituted for the Myr fusion protein and multicloning site of pMyr. Cmr, URA3. | This study |
| pMyr‐BD::C‐WtsE | pMyr‐BD expressing the C‐terminal half of WtsE (amino acids 964–1835) in S. cerevisiae, Cmr, URA3. | This study |
| pMyr‐BD::N‐WtsE | pMyr‐BD expressing N‐terminal half of WtsE (amino acids 1–964) in S. cerevisiae. Cmr, URA3. | This study |
| pMyr‐BD::WtsE | pMyr‐BD expressing the full‐length WtsE in S. cerevisiae. Cmr, URA3. | This study |
| pPGA | Modified destination vector for the Gateway® cloning of wtsE, Cmr | Dr. C. Boone, Univ. of Toronto |
| pPGA::WtsE | Wild type wtsE construct in pPGA for the integration of GAL1‐wtsE‐URA3 into the Y7092 genome | This study |
| pPGA::WtsE‐ΔERMRS | ΔERMRS derivative wtsE construct in pPGA for the integration of GAL1‐wtsE‐URA3 into the Y7092 genome | This study |
| pRF205 | pVK100 with a 1.7‐kb HindIII fragment containing Plac‐hrpS + | (Frederick et al., 1993) |
| pRK415 | IncP α‐lacZ, Tcr | (Keen et al., 1988) |
E. coli Genetic Stock Center.
†Arabidopsis Biological Resource Center at The Ohio State University.
General DNA manipulation techniques
DNA isolation, digestion with restriction enzymes and ligation were done following standard protocols (Sambrook et al., 1989). Purification of DNA fragments from agarose gels or PCR was performed using appropriate Qiagen kits (Qiagen, Valencia, CA). Cloning of PCR products was done with Platinum® Taq DNA polymerase High Fidelity and TOPO® vectors (Invitrogen, Carlsbad, CA) and all clones of PCR products were confirmed by DNA sequencing with fluorescent dye terminator chemistry and the ABI 3700 capillary electrophoresis sequencer (PE‐Applied Biosystems, Wellesley, MA) at The Ohio State University Plant‐Microbe Genomics Facility. Plasmids were introduced into bacterial cells by either electroporation using a GenePulser™ (Bio‐Rad Laboratories, Hercules, CA) apparatus set to 200 Ω and 1.5 kV or triparental mating as previously described (Merighi et al., 2003). Yeast transformation was conducted following a standard protocol using lithium acetate (Ausubel et al., 1995).
WtsE constructs
Genomic DNA of Pnss DC283 was used as the template for the PCRs used to generate WtsE constructs, if not otherwise stated. wtsE clones and mutants are described in Table 2. These were prepared by standard PCR, restriction digestion and ligation procedures cited above and each construction usually involved multiple cloning steps. Details are given in the supporting information. For expression of wtsE in yeast, we modified Stratagene's pMyr vector, by replacement of the multicloning site and coding region for the Myr fusion protein with the multicloning site and GAL4 DNA binding protein from pAS1. Alternatively, wtsE was introduced into the chromosome of yeast strain Y7092 (Alto et al., 2006). For delivery by E. coli MC4100 (pCPP2156) constructs were made in pBluescript SK(+), for delivery by Pph NS3121 they were made in pDSK519, and for agroinfiltration they were made in the pGD and pBDex binary vectors (Allen et al., 2004, Goodin et al., 2002).
Plants
Sweet corn seedlings (Zea mays cv. Seneca Horizon), tobacco (N. tabaccum cv. Bottom Special), N. benthamiana and beet (Beta vulgaris cv. Red Ace Hybrid) were grown and maintained in growth chambers set to 30 °C and an 18/6‐h day/night cycle. A. thaliana (ecotype Col‐0) plants were grown in a growth room at 23 °C for 8 h in the light and at 16 °C for 16 h in the dark.
Virulence and cell death assays
Virulence assays for Pnss mutants in sweet corn seedlings were performed as previously described (Frederick et al., 2001). Briefly, bacterial suspensions containing 0.2% Tween 40 were added to the whorls of 8‐day‐old sweet corn seedlings without wounding and disease severity was rated on a 0 to 3 scale (0 = no symptoms, 1 = a few lesions but no ooze, 2 = many lesions and some ooze, and 3 = confluent lesions and ooze). Agrobacterium‐mediated transient expression experiments were conducted following Goodin et al. (2002). Procedures for preparation and infiltration of bacterial suspensions were as described previously, except needleless syringes were used to infiltrate bacterial suspensions into tobacco, N. benthamiana, beet and A. thaliana (Ham et al., 2006).
Electrolyte leakage assays
Bacteria were grown overnight on plates, resuspended in fresh LB broth, and were further cultured at 28 °C with vigorous shaking (150 r.p.m.) until OD600 values reached c. 0.6 for E. coli strains. They were then washed and resuspended in 0.01 m potassium phosphate buffer (pH 7.0) at c. 1 × 109 CFU/mL. CHX was added to some treatments at 100 µg/mL. N. benthamiana or tobacco leaves were sampled directly after infiltration, using an 0.8‐cm‐diameter cork borer. Two leaf discs were removed from the infiltrated area from each of four leaves from two different plants, briefly washed in deionized water, and placed into a plastic tube containing 15 mL of deionized water, which was then held in the light at room temperature. At intervals the conductivity of the fluid in each tube was measured using a WTW model Cond 330i conductivity meter with a TetraCon 325 probe (WTW, Weilheim, Germany).
Supporting information
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
We thank S. V. Beer for the anti‐DspEEa antibodies, D. Kliebenstein for the anti‐PR‐1 antibodies, C. Boone for pPGA, J. Dangl and H. Kaminaka for pBDex, and S. Y. He for valuable discussions. This project was supported by the National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education, and Extension Service, grant number 2005‐35319‐15328 and the Ohio Agricultural Research and Development Center.
REFERENCES
- Ahmad, M. , Majerczak, D.R. , Pike, S. , Hoyos, M. , Novacky, A. and Coplin, D.L. (2001) Biological activity of harpin produced by Pantoea stewartii subsp. stewartii . Mol. Plant–Microbe Interact. 14, 1223–1234. [DOI] [PubMed] [Google Scholar]
- Allen, R.L. , Bittner‐Eddy, P.D. , Grenville‐Briggs, L.J. , Meitz, J.C. , Rehmany, A.P. , Rose, L.E. and Beynon, J.L. (2004) Host‐parasite coevolutionary conflict between Arabidopsis and downy mildew. Science, 306, 1957–1960. [DOI] [PubMed] [Google Scholar]
- Alto, N.M. , Shao, F. , Lazar, C.S. , Brost, R.L. , Chua, G. , Mattoo, S. , McMahon, S.A. , Ghosh, P. , Hughes, T.R. , Boone, C. and Dixon, J.E . (2006) Identification of a bacterial type III effector family with G protein mimicry functions. Cell, 124, 133–145. [DOI] [PubMed] [Google Scholar]
- Anderson, D.M. , Fouts, D.E. , Collmer, A. and Schneewind, O. (1999) Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc. Natl Acad. Sci. USA, 96, 12839–12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama, T. and Chua, N.‐H. (1997) A glucocorticoid‐mediated transcriptional induction system in transgenic plants. Plant J. 11, 605–612. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M. , Brent, R. , Kingston, R.E. , Moore, D.D. , Seidman, J.G. , Smith, J.A. and Struhl, K . (1995) Current Protocols in Molecular Biology. New York: John Wiley & Sons. [Google Scholar]
- Badel, J.L. , Shimizu, R. , Oh, H.S. and Collmer, A. (2006) A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant–Microbe Interact. 19, 99–111. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A. , Kim, J.F. , Wei, Z. , Kolchinsky, P. , Charkowski, A.O. , Conklin, A.K. , Collmer, A. and Beer, S.V. (1998) Homology and functional similarity of an hrp‐linked pathogenicity locus, dspEF, of Erwinia amylovora and the avirulence locus avrE of Pseudomonas syringae pathovar tomato. Proc. Natl Acad. Sci. USA, 95, 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boureau, T. , ElMaarouf‐Bouteau, H. , Garnier, A. , Brisset, M.N. , Perino, C. , Pucheu, I. and Barney, M.A. (2006) DspA/E, a type III effector essential for Erwinia amylovora pathogenicity and growth in planta, induces cell death in host apple and nonhost tobacco plants. Mol. Plant–Microbe Interact. 19, 16–24. [DOI] [PubMed] [Google Scholar]
- Bozso, Z. , Ott, P.G. , Kecskes, M.L. and Klement, Z. (1999) Effect of heat and cycloheximide treatment of tobacco on the ability of Pseudomonas syringae pv.syringae 61 hrp/hrmA mutants to cause HR. Physiol. Mol. Plant Pathol. 55, 215–223. [Google Scholar]
- Casadaban, M.J. (1976) Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104, 541–555. [DOI] [PubMed] [Google Scholar]
- Claflin, L.E. (1999) Stewart's Bacterial Wilt. St. Paul, MN: American Phytopathological Society. [Google Scholar]
- Coplin, D.L. , Frederick, R.D. , Majerczak, D.R. and Haas, E.S. (1986) Molecular cloning of virulence genes from Erwinia stewartii . J. Bacteriol. 168, 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebRoy, S. , Thilmony, R. , Kwack, Y.B. , Nomura, K. and He, S.Y. (2004) A family of conserved bacterial effectors inhibits salicylic acid‐mediated basal immunity and promotes disease necrosis in plants. Proc. Natl Acad. Sci. USA, 101, 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph, P.J. , Majerczak, D.R. and Coplin, D.L. (1988) Characterization of a gene cluster for exopolysaccharide biosynthesis and virulence in Erwinia stewartii . J. Bacteriol. 170, 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, H.Y. , Hu, Y. , Tudor, M. and Ma, H. (1997) Specific interactions between the K domains of AG and AGLs, members of the MADS domain family of DNA binding proteins. Plant J. 12, 999–1010. [DOI] [PubMed] [Google Scholar]
- Frederick, R.D. , Ahmad, M. , Majerczak, D.R. , Arroyo‐Rodriguez, A.S. , Manulis, S. and Coplin, D.L. (2001) Genetic organization of the Pantoea stewartii subsp. stewartii hrp gene cluster and sequence analysis of the hrpA, hrpC, hrpN and wtsE operons. Mol. Plant–Microbe Interact. 14, 1213–1222. [DOI] [PubMed] [Google Scholar]
- Frederick, R.D. , Majerczak, D.R. and Coplin, D.L. (1993) Erwinia stewartii WtsA, a positive regulator of pathogenicity gene expression, is similar to Pseudomonas syringae pv. phaseolicola HrpS. Mol. Microbiol. 9, 477–485. [DOI] [PubMed] [Google Scholar]
- Gaudriault, S. , Malandrin, L. , Paulin, J.P. and Barny, M.A. (1997) DspA, an essential pathogenicity factor of Erwinia amylovora showing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB‐dependent way. Mol. Microbiol. 26, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Goodin, M.M. , Dietzgen, R.G. , Schichnes, D. , Ruzin, S. and Jackson, A.O. (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–383. [DOI] [PubMed] [Google Scholar]
- Ham, J.H. , Bauer, D.W. , Fouts, D.E. and Collmer, A. (1998) A cloned Erwinia chrysanthemi Hrp (type III protein secretion) system functions in Escherichia coli to deliver Pseudomonas syringae Avr signals to plant cells and to secrete Avr proteins in culture. Proc. Natl Acad. Sci. USA, 95, 10206–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham, J.H. , Kim, M.G. , Lee, S.Y. and Mackey, D. (2007) Layered basal defenses underlie non‐host resistance of Arabidopsis to Pseudomonas syringae pv. phaseolicola . Plant J. 51, 604–616. [DOI] [PubMed] [Google Scholar]
- Ham, J.H. , Majerczak, D.R. , Arroyo‐Rodriguez, A.S. , Mackey, D. and Coplin, D.L. (2006) WtsE, an AvrE‐family effector protein from Pantoea stewartii subsp. stewartii, causes disease‐associated cell death in corn and requires a chaperone protein for stability. Mol. Plant–Microbe Interact. 19, 1092–1102. [DOI] [PubMed] [Google Scholar]
- Keen, N.T. , Tamaki, S. , Kobayashi, D. and Trollinger, D. (1988) Improved broad‐host‐range plasmids for DNA cloning in gram‐negative bacteria. Gene, 70, 191–197. [DOI] [PubMed] [Google Scholar]
- Lindgren, P.B. , Peet, R.C. and Panapoulos, N.J. (1986) Gene cluster of Pseudomonas syringae pv. phaseolicola controls pathogenicity on bean plants and hypersensitivity on nonhost plants. J. Bacteriol. 168, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorang, J.M. , Shen, H. , Kobayashi, D. , Cooksey, D. and Keen, N.T. (1994) AvrA and AvrE in Pseudomonas syringae pv. tomato Pt23 play a role in virulence on tomato plants. Mol. Plant–Microbe Interact. 7, 508–515. [Google Scholar]
- Meng, X. , Bonasera, J.M. , Kim, J.F. , Nissinen, R.M. and Beer, S.V. (2006) Apple proteins that interact with DspA/E, a pathogenicity effector of Erwinia amylovora, the fire blight pathogen. Mol. Plant–Microbe Interact. 19, 53–61. [DOI] [PubMed] [Google Scholar]
- Merighi, M. , Majerczak, D.R. , Stover, E.H. and Coplin, D.L. (2003) The HrpX/HrpY two‐component system activates hrpS expression, the first step in the regulatory cascade controlling the Hrp regulon in Pantoea stewartii subsp. stewartii . Mol. Plant–Microbe Interact. 16, 238–248. [DOI] [PubMed] [Google Scholar]
- Mor, H. , Manulis, S. , Zuck, M. , Nizan, R. , Coplin, D.L. and Barash, I. (2001) Genetic organization of the hrp gene cluster and dspAE/BF operon in Erwinia herbicola pv. gypsophilae . Mol. Plant–Microbe Interact. 14, 431–436. [DOI] [PubMed] [Google Scholar]
- Navarro, L. , Zipfel, C. , Rowland, O. , Keller, I. , Robatzek, S. , Boller, T. and Jones, J.D . (2004) The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene‐dependent defense responses and bacterial pathogenesis. Plant Physiol. 135, 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, C.S. , Martin, G.B. and Beer, S.V. (2007) DspA/E, a type III effector of Erwinia amylovora, is required for early rapid growth in Nicotiana benthamiana and causes NbSGT1‐dependent cell death. Mol. Plant Pathol. 8, 255–265. [DOI] [PubMed] [Google Scholar]
- Pateky, J.K. (2003) Stewart's Wilt of Corn . APSnet. http://www.apsnet.org/online/feature/stewarts/.
- Petitjean, A. , Hilger, F. and Tatchell, K. (1990) Comparison of thermosensitive alleles of the CDC25 gene involved in the cAMP metabolism of Saccharomyces cerevisiae . Genetics, 124, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petnicki‐Ocwieja, T. , Schneider, D.J. , Tam, V.C. , Chancey, S.T. , Shan, L. , Jamir, Y. , Schecter, L.M. , Janes, M.D. , Buell, C.R. , Tang, X. , Collmer, A. and Alfano, J.R. (2002) Genomewide identification of proteins secreted by the Hrp type III protein secretion system of Pseudomonas syringae pv. tomato DC3000. Proc. Natl Acad. Sci. USA, 99, 7652–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, S. and Maniatis, T. (1989) Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press. [Google Scholar]
- Schaad, N.W. , Jones, J.B. and Chun, W. (2001) Laboratory Guide for Identification of Plant Pathogenic Bacteria. St. Paul, MN: APS Press. [Google Scholar]
- Thilmony, R. , Underwood, W. and He, S.Y. (2006) Genome‐wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 46, 34–53. [DOI] [PubMed] [Google Scholar]
- Van Larebeke, N. , Engler, G. , Holsters, M. , Van den Elsacker, S. , Zaenen, I. , Schilperoort, R.A. and Schell, J. (1974) Large plasmid in Agrobacterium tumefaciens essential for crown gall‐inducing ability. Nature, 252, 169–170. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D. , Boller, T. and Felix, G. (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


