SUMMARY
Background: Teratosphaeria nubilosa is a serious leaf pathogen of several Eucalyptus spp. This review considers the taxonomic history, epidemiology, host associations and molecular biology of T. nubilosa.
Taxonomy: Kingdom Fungi; Phylum Ascomycota; Class Dothideomycetes; Order Capnodiales; Family Teratosphaeriaceae; genus Teratosphaeria; species nubilosa.
Identification: Pseudothecia hypophyllous, less so amphigenous, ascomata black, globose becoming erumpent, asci aparaphysate, fasciculate, bitunicate, obovoid to ellipsoid, straight or incurved, eight‐spored, ascospores hyaline, non‐guttulate, thin walled, straight to slightly curved, obovoid with obtuse ends, medially one‐septate, slightly constricted at the median septum, tapering to both ends, ascospore germination type F, germinating from both ends, germ tubes growing parallel to the long axis of the spore with distortion of the primary ascospore cell.
Host range: Teratosphaeria nubilosa is a primary pathogen of several Eucalyptus spp., including E. botryoides, E. bicostata, E. bridgesiana, E. cypellocarpa, E. dunnii, E. globulus ssp. bicostata, E. globulus ssp. globulus, E. globulus ssp. maidenii, E. globulus ssp. pseudoglobulus, E. grandis, E. gunnii, E. nitens, E. pilularis, E. quadrangulata, E. viminalis, E. grandis × E. resinifera and E. urophylla × E. globulus.
Disease symptoms: Leaf spots predominantly occur on juvenile Eucalyptus foliage; however, T. nubilosa has also recently been found on mature Eucalyptus foliage. Leaf spots are amphigenous, varying in size from small spots that are round to irregular. Lesions enlarge and coalesce to form larger blotches over the leaf surface. Initial lesions appear as pale‐green spots surrounded by purple margins and, once mature, are generally yellow to pale brown with dark‐brown raised borders.
Useful websites: Mycobank, http://www.mycobank.org; Mycosphaerella identification website, http://www.cbs.knaw.nl/mycosphaerella/BioloMICS.aspx
INTRODUCTION
Many species of the ascomycete genera Mycosphaerella and Teratosphaeria infect leaves of Eucalyptus spp., where they cause a disease broadly referred to as Mycosphaerella leaf disease (MLD) (Burgess et al., 2007; Carnegie et al., 2007; Crous, 1998; Crous et al., 2004a, 2007a, 2006b,b). The predominant symptoms of MLD are leaf spots on the abaxial and/or adaxial leaf surfaces that vary in size, shape and colour (Crous, 1998). The leaf spots often enlarge and coalesce to form larger blotches across the leaf surface, reducing the photosynthetic capability of trees, which leads to premature leaf abscission (Park, 1988b; Pinkard and Mohammed, 2006). Premature defoliation reduces the growth of susceptible Eucalyptus species, causing a decrease in the eventual wood volume of infected trees (Carnegie and Ades, 2003; Lundquist and Purnell, 1987; Milgate et al., 2005a). MLD therefore poses a continued threat to the commercial propagation of Eucalyptus species by forestry companies.
One of the most virulent species of Teratosphaeria causing disease on Eucalyptus is Teratosphaeria nubilosa (= Mycosphaerella nubilosa). Since it was first identified in south‐eastern Australia, T. nubilosa has been reported from several countries in Africa and Europe, where it has become a major impediment to the continued propagation of cold‐tolerant Eucalyptus spp. For example, the commercial propagation of E. globulus in South Africa was abandoned in the 1930s because of its susceptibility to T. nubilosa (Lundquist and Purnell, 1987). Eucalyptus globulus was later replaced with E. nitens in South Africa as a favoured cold‐tolerant species grown at higher altitudes. Some provenances of E. nitens, particularly those from Victoria, Australia, are also susceptible to infection by T. nubilosa during the first 2–3 years of growth. Thus, the selection of planting stock resistant to this disease has proven to be of paramount importance.
Heavy outbreaks of MLD caused by T. nubilosa have also occurred in Australia, including more than 95% defoliation of plantations of E. globulus in north‐eastern New South Wales (Carnegie, 2007b). Teratosphaeria nubilosa was identified as the main cause of severe damage and defoliation in an E. globulus plantation in Tasmania (Milgate et al., 2005a). In most instances, however, severe outbreaks of MLD in Australia are associated with T. nubilosa and T. cryptica occurring together (Barber et al., 2008; Carnegie et al., 1994; Carnegie and Ades, 2002; Park, 1988b). Eucalyptus globulus was replaced by E. nitens in north‐western Tasmania as the preferred plantation species because of MLD (Mohammed et al., 2003). Severe defoliation (over 75%) has been observed in young E. globulus plantations in the Otways in Victoria in recent years. Previously only known from juvenile and intermediate foliage (Crous, 1998; Park and Keane, 1982a), T. nubilosa has recently been identified from adult Eucalyptus foliage in eastern Australia (Kularatne et al., 2004; Maxwell et al., 2001), an aspect which could substantially increase the damage caused by this pathogen.
Considerable research has been conducted on T. nubilosa during the course of the past 25 years. Most of the early research was focused on the taxonomy, host associations and the disease caused by the pathogen. More recent studies have included aspects related to the epidemiology, phylogeny, intraspecific variation and population biology of this pathogen. The focus of this paper is to provide an overview of the literature pertaining to T. nubilosa. Furthermore, we present opinions regarding the status of knowledge and priorities for research on this increasingly important pathogen.
TAXONOMIC HISTORY
Sphaerella cryptica and S. nubilosa were originally described from diseased Eucalyptus leaves collected near Melbourne, Australia (Cooke, 1891). These two ascomycetous fungi were to become two of the most well‐recognized and important Eucalyptus leaf pathogens. Their placement in Sphaerella was incorrect and, after examination of the type material, S. cryptica and S. nubilosa were transferred to Mycosphaerella (Hansford, 1956).
South Africa was one of the first countries to establish Eucalyptus spp. in plantations for timber production. Initial surveys of Eucalyptus in this country led Doidge (1950) to identify Mycosphaerella molleriana from several Eucalyptus spp. Although morphologically similar, M. molleriana and M. nubilosa were shown to represent two distinct species (Crous et al., 1991; Crous and Wingfield, 1996), with M. juvenis being a synonym of M. nubilosa (Crous et al., 2004a; Hunter et al., 2004a,b). DNA sequence comparisons for four nuclear gene regions led Hunter et al. (2006b) to suggest that Mycosphaerella was not monophyletic (Crous et al., 2001b; Goodwin et al., 2001). By employing nuclear large subunit sequence data for many Mycosphaerella species from diverse hosts, Crous et al. (2007a) confirmed the polyphyletic nature of Mycosphaerella, and revealed various species occurring on Eucalyptus to be more appropriately accommodated in Teratosphaeria. Thus, M. nubilosa was shown to be most appropriately accommodated in Teratosphaeria as T. nubilosa.
DISTRIBUTION OF T. NUBILOSA
Teratosphaeria nubilosa has been observed causing damage in plantations in most states of Australia, including Victoria (Barber et al., 2008; Carnegie et al., 1994), Tasmania (Dungey et al., 1997; Milgate et al., 2001), Western Australia (Jackson et al., 2008; Maxwell, 2004), New South Wales (Carnegie, 2007b) and South Australia (Carnegie, 2000). Herbarium records confirmed by A. J. Carnegie reveal that T. nubilosa was identified in Queensland on E. globulus in the 1960s, but significant damage has not been reported. Teratosphaeria nubilosa has also been identified from New Zealand (Dick, 1982; Dick and Gadgil, 1983), although T. cryptica is the most damaging species in this country (Dick, 1982; Dick and Gadgil, 1983; Hood et al., 2002), as the main hosts planted are less susceptible to T. nubilosa.
Several countries in Africa have identified T. nubilosa from commercial Eucalyptus plantations. Doidge (1950) first identified T. nubilosa (as M. molleriana) from several Eucalyptus species in South Africa. Currently, T. nubilosa is widespread within South Africa on various Eucalyptus species and can be found in several provinces, namely Gauteng, Kwa‐Zulu Natal, Limpopo, Mpumalanga, Eastern Cape and Western Cape (Crous, 1998; Crous et al., 2004a; Crous and Wingfield, 1996; Hunter et al., 2004a,b; G. Perez, FABI, Pretoria, South Africa, unpublished data).
Recent surveys in other African countries have also resulted in the confirmation of T. nubilosa. Teratosphaeria nubilosa has been identified from south, south‐western and western Ethiopia, causing severe defoliation on E. globulus (Gezahgne et al., 2006). Eucalyptus plantations in Kenya, Tanzania and Zambia have also been affected by T. nubilosa, where it has been identified causing defoliation of E. globulus (Crous et al., 2004a; Hunter et al., 2008).
Europe has also seen the introduction of T. nubilosa into its commercial Eucalyptus plantations. Collections of diseased E. globulus leaves from seven locations in Spain identified T. nubilosa causing leaf spots and premature defoliation (Crous et al., 2004a). Similarly, T. nubilosa has also been identified on E. globulus from northern Portugal (Hunter et al., 2008). More recently, T. nubilosa has also been identified from E. globulus and E. dunnii plantations in Uruguay (G. Perez, FABI, Pretoria, South Africa, unpublished data), and on E. globulus in Brazil (P. W. Crous and A. C. Alfenas, unpublished data).
SYMPTOMATOLOGY AND MORPHOLOGY OF T. NUBILOSA
Leaf spots caused by T. nubilosa predominantly occur on juvenile and intermediate Eucalyptus foliage (Carnegie and Ades, 2002; Crous, 1998; Park and Keane, 1982a). However, recent studies have also identified this pathogen from adult Eucalyptus foliage (Kularatne et al., 2004; Maxwell et al., 2001). Teratosphaeria nubilosa leaf spots are amphigenous, varying in size from small to large spots that are round to irregular (Fig. 1C). Lesions often enlarge and coalesce to form large blotches covering the leaf surface and, in severe cases, large lesions can lead to leaf blight. Initial lesions appear as pale‐green spots surrounded by purple margins and, once mature, are generally yellow to pale brown with dark‐brown raised borders (Fig. 1D) (Crous, 1998; Crous et al., 2004a; Park and Keane, 1982b). Defoliation of Eucalyptus trees tends to occur from the lower crown moving upwards (i.e. bottom‐up) (Fig. 1B).
Figure 1.

Typical leaf symptoms caused by Teratosphaeria nubilosa on Eucalyptus. (A, B) Typical premature defoliation of young Eucalyptus trees caused by T. nubilosa, indicating a bottom‐up defoliation pattern. (C, D) Small, round to irregular leaf spots that coalesce to form leaf blotches over the leaf surface.
Ascomata of T. nubilosa are generally hypophyllous, but have also rarely been observed to occur amphigenously (Barber et al., 2008; Carnegie and Ades, 2002). Ascomata are black, globose to punctiform, immersed with a papillate ostiole, becoming erumpent with age, measuring 40–90 µm in diameter. Teratosphaeria nubilosa asci are bitunicate with a multilayered endotunica, ellipsoidal to obclavate, straight or curved, subsessile, eight‐spored, 30–60 × 9–18 µm. Ascospores are two‐ to three‐seriate, oblique, ellipsoidal to obovoid, usually straight in the ascus, colourless, smooth, guttulate, one‐septate, not or slightly constricted at the median septum, widest in the middle of the upper cell, tapering more strongly towards the lower end, (11–)13–16 × 3–3.5(–4.5) µm surrounded by a non‐persistent mucous sheath (Fig. 2) (Crous, 1998; Crous et al., 2004a, 2007a).
Figure 2.
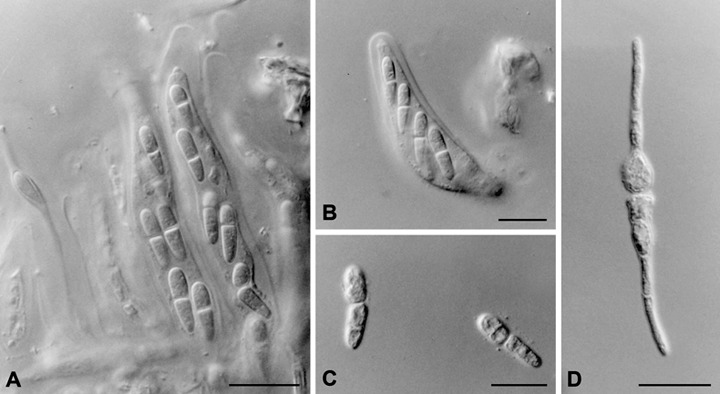
Morphological characteristics of Teratosphaeria nubilosa. (A, B) Eight‐spored bitunicate ascii. (C) Ellipsoidal to obovoid, guttulate, one‐septate ascospores. (D) Typical type F ascospore germination pattern of T. nubilosa. Scale bars, 10 µm.
Ascospore germination patterns have been used as diagnostic features for various Mycosphaerella spp. on Eucalyptus (Crous, 1998; Park and Keane, 1982a). Examination of fresh T. nubilosa ascospores that had been allowed to germinate for 24 h and longer showed that T. nubilosa has a typical type F pattern (Crous et al., 2004a), germinating from both ends with germ tubes growing parallel to the long axis of the spore, with prominent distortion of the original ascospore. However, recent collections of T. nubilosa from northern New South Wales, Australia, have resulted in ascospores that produce multiple germ tubes and, as such, do not exhibit the classical type F form (Carnegie, 2007a).
INFECTION PROCESS AND EPIDEMIOLOGY
Knowledge of the infection process and disease development in species of Mycosphaerella and Teratosphaeria on Eucalyptus is largely based on studies of T. cryptica and T. nubilosa (Park, 1988a,b; Park and Keane, 1982a,b). These studies have investigated the ability of these pathogens to infect Eucalyptus leaves both in situ and in vitro. Apart from the infection processes followed by these pathogens, various environmental conditions have also been investigated in order to understand how they affect the ability of T. nubilosa and T. cryptica to infect Eucalyptus.
Both ascospores and conidia in species of Mycosphaerella and Teratosphaeria can initiate disease on Eucalyptus leaves. Ascospores, however, act as the primary source of inoculum for the majority of these, whereas some infect primarily by means of conidia (Crous et al., 2007a; Park, 1988b; Park and Keane, 1987; Wingfield et al., 1996). The source of ascospores is predominantly from attached infected leaves, or from fallen overwintered leaf litter (Park and Keane, 1987). Within the leaf tissue, ascogonia of Mycosphaerella and Teratosphaeria species have been shown to remain viable for several months, thereby providing sufficient inoculum for successive infection cycles (Cheah and Hartill, 1987; Park, 1988b; Park and Keane, 1982b, 1987).
The infection of Eucalyptus leaves by T. nubilosa predominantly occurs during the vegetative period of the host during the summer and autumn months (Cheah and Hartill, 1987; Ganapathi, 1979). Park (1988a) showed that young expanding leaves of E. globulus (less than 46 days old), were particularly susceptible to T. nubilosa. As Eucalyptus leaves age, they become progressively more resistant to infection as a result of the deposition of resistant compounds (Park, 1988a).
A few hours after ascospores have been deposited onto leaf surfaces, they germinate to form germ tubes. The germ tubes of T. nubilosa will often branch and enter several stomata (Park and Keane, 1982b). The germination of T. nubilosa ascospores on the Eucalyptus leaf surface occurs between 3 and 30 °C, with an optimum temperature of 20 °C (Park, 1988a; Park and Keane, 1982b). Infection of the Eucalyptus leaf surface by the spores of Mycosphaerella and Teratosphaeria spp. may be direct or indirect. Direct penetration involves the penetration of the leaf through the leaf cuticle. This is achieved by the ability of the fungal spore to form an infection peg at the end of a germination tube. Through turgor pressure, the infection peg is able to penetrate the cuticle and gain access to the interior of the leaf. However, in indirect penetration, the fungal spore is unable to produce an infection peg and instead relies on natural openings in the leaf surface, such as stomata, to gain access to the leaf interior. Teratosphaeria nubilosa employs an indirect penetration strategy, where infection occurs through the stomata and germ tubes produce hyphal swellings within the stomatal pores and substomatal cavities (Park and Keane, 1982b).
The levels of moisture in the environment play an important part in the ability of T. nubilosa to infect Eucalyptus leaves. In an inoculation experiment, Park (1988b) found that premature leaf defoliation and large lesions were caused by T. nubilosa on leaves of E. globulus ssp. globulus when plants were exposed to a wetting period of 5–7 days. He also showed that the severity of disease increased with longer periods of leaf wetness.
After penetrating a leaf, fungal hyphae grow along the vascular bundles and colonize the leaf tissue, becoming established throughout the leaf. Following chlorosis, hyphae grow intercellularly throughout the spongy mesophyll and eventually aggregate in the substomatal cavities (Park and Keane, 1982b). These hyphal aggregates then develop into immature ascomata with trichogynes (Park and Keane, 1982b).
Ganapathi (1979) described the development of the ascomata of T. cryptica (as M. nubilosa) in detail. His studies showed that the ascocarp initials comprise a group of cells. Developing ascomata have the appearance of stromata with the presence of trichogynes, which grow towards the stomatal apex. During ascogonial development, the stroma matures and breaks through the host surface. The developing trichogynes grow through the top of the stroma and are fertilized by spermatia. Spermatia are formed in a gelatinous matrix that seeps from the spermatogonial ostiole onto the leaf surface (Ganapathi and Corbin, 1979). After fertilization, ascogonia mature through successive developmental steps from asci and ascospores. Mature ascogonia of Teratosphaeria and Mycosphaerella spp. generally have large, thick, elongated cells impregnated with melanin that form the outer layers of the ascocarp wall (Niyo et al., 1986). Although cells making up the inner ascocarp walls generally contain lower melanin levels than those of the outer ascogonial walls, similar cellular organelles are observed in both cell types (Niyo et al., 1986).
The liberation of ascospores of Mycosphaerella and Teratosphaeria spp. is dependent on moisture. For example, ascospores of T. cryptica are discharged when the relative humidity is greater than 95% and not when it is below 90% (Park and Keane, 1982b). Rainfall acts as the main stimulus for the release of ascospores from mature ascomata, and longer periods of rainfall lead to the discharge of greater numbers of spores (Park, 1988b). The discharge of ascospores continues in the presence of sufficient moisture and relative humidity until the asci within the ascomata are exhausted of ascospores (Cheah and Hartill, 1987).
Park and Keane (1982b) found that the optimum temperature for ascospore discharge in T. nubilosa was 25 °C and, in field conditions in south‐eastern Australia, the release of T. nubilosa ascospores occurred between 5 and 15 °C. These spores can be ejected up to a distance of 12–15 mm above the ascomata. This allows the spores to be wind dispersed for considerable distances (Park and Keane, 1982b).
HOST RANGE AND SUSCEPTIBILITY
Increased surveys of Eucalyptus plantations in various parts of the world during the course of the past two decades have led to a substantially increased number of reported Eucalyptus hosts for T. nubilosa. This pathogen now has a relatively wide host range in the Eucalyptus subgenus Symphyomyrtus series Viminalis and Resiniferae (Carnegie, 2007a; Carnegie and Keane, 1994, Crous, 1998, Jackson et al., 2005; Park and Keane, 1982a,b). To date, this pathogen has been isolated from E. botryoides, E. bridgesiana, E. cypellocarpa, E. dunnii, E. globulus ssp. bicostata, ssp. globulus, ssp. maidennii and ssp. pseudoglobulus, E. grandis, E. gunnii, E. nitens, E. quadrangulata, E. viminalis, E. grandis×E. resinifera and E. urophylla×E. globulus (Carnegie and Keane, 1994; Crous, 1998; Crous et al., 2004a; Hunter et al., 2004a,b; Jackson et al., 2005; Park and Keane, 1982a). However, some species, such as E. globulus, are considerably more susceptible than others.
Numerous studies have reported wide variation in susceptibility to MLD amongst Eucalyptus species, provenances or families (Carnegie and Ades, 2005; Carnegie et al., 1994, 1998, 2004; Dungey et al., 1997; Hood et al., 2002; Milgate et al., 2005a; Purnell and Lundquist, 1986; Wilcox, 1982). In an assessment of MLD on the juvenile foliage of 14 species of Eucalyptus, Carnegie et al. (1998) reported that E. globulus, E. nitens and E. cypellocarpa had significantly more disease than all other species. Wide variation has been reported at the provenance level for both E. globulus and E. nitens.
During initial provenance trials of E. nitens in South Africa, Purnell and Lundquist (1986) observed that New South Wales provenances of E. nitens outperformed Victorian provenances. They found that New South Wales provenances exhibited higher average levels of height, diameter at breast height and volume than Victorian provenances. New South Wales provenances also showed higher survival and lower MLD than Victorian provenances (Lundquist and Purnell, 1987). Purnell and Lundquist (1986) therefore suggested that provenances to be planted in South Africa should be selected on the basis of growth rate, stem form, wood quality and disease resistance. Carnegie et al. (1998) also reported significant variation in susceptibility to MLD amongst provenances of E. nitens, planted in Victoria, Australia, although there was little disease on the adult foliage of these trees assessed several years later, and no variation amongst provenances (Carnegie and Ades, 2005).
Carnegie et al. (1994) investigated the variation within several provenances of the four E. globulus subspecies, and determined that E. globulus ssp. globulus provenances (two from Tasmania and one from Victoria), together with one E. globulus spp. bicostata provenance from Victoria, were significantly more susceptible to MLD than an E. globulus ssp. pseudoglobulus provenance from Victoria and an E. globulus ssp. maidenii provenance from New South Wales. Dungey et al. (1997) reported that King Island, Tasmania, provenances of E. globulus ssp. globulus were less susceptible than E. globulus ssp. globulus provenances from Taranna, Tasmania. However, Dungey et al. (1997) reported that a single, unknown provenance of E. globulus ssp. bicostata showed relative resistance to MLD, and Carnegie (2007b) reported that E. globulus ssp. maidenii was highly susceptible to T. nubilosa in northern New South Wales, contrasting with the earlier observations by Carnegie et al. (1994), most probably as a result of the differences in provenances between these studies. Significant variation at the family level has also been observed for E. globulus (Carnegie and Ades, 2005; Dungey et al., 1997; Milgate et al., 2005a).
The use of hybrids in Eucalyptus forestry has gained importance over the past couple of decades (Denison and Kietzka, 1993; Lee, 2007). However, hybrids can often be more susceptible to pests and diseases than their respective parents. First‐generation progeny of E. globulus and E. nitens hybrids exhibit higher levels of susceptibility to MLD than their respective parents (Carnegie and Ades, 2002; Dungey et al., 1997).
Genetic parameters for resistance to MLD have been calculated for several species. Narrow‐sense heritability estimates of severity on juvenile foliage (caused by T. cryptica) for E. nitens range from 0.12 to 0.21 (Dungey et al., 1997). For E. globulus, heritability for MLD on juvenile foliage (caused by both T. nubilosa and T. cryptica) ranges from 0.12 to 0.36, and from 0.17 to 0.36 for T. cryptica on adult foliage (Carnegie and Ades, 2005; Dungey et al., 1997). For defoliation of the juvenile crown, heritability estimates range from 0.28 to 0.48 (Carnegie and Ades, 2005). Milgate et al. (2005a) reported heritability of 0.60 for the severity of T. nubilosa on E. globulus, similar to that reported for disease severity on E. globulus × E. nitens hybrids (0.51; Dungey et al., 1997).
Variation in resistance to infection amongst provenances of Eucalyptus spp. has been shown at the histological level. Smith et al. (2006) showed that the E. nitens provenance (Ebor) from northern New South Wales was more resistant than the E. nitens provenance (Tallaganda) from southern New South Wales. This was a result of the higher degree of packed parenchyma cells within leaves of more resistant provenances that were able to divide more quickly and form an impenetrable necrophylactic periderm (Smith et al., 2006).
T. NUBILOSA INTRASPECIFIC VARIATION
The internal transcribed spacer (ITS) region of the rDNA operon has traditionally been targeted for DNA sequence comparisons in Mycosphaerella species. Studies on T. nubilosa using sequences for the ITS region have identified variation within this taxon, resolving it into two distinct phylogenetic clades (Crous et al., 2004a; Maxwell, 2004). Hunter (2007) termed these two clades ITS lineage 1 and ITS lineage 2, respectively (Fig. 3). ITS lineage 1 accommodates isolates of T. nubilosa from New Zealand, south‐eastern Australia (Tasmania and Victoria) and south‐western Australia (Western Australia), whereas ITS lineage 2 accommodates isolates from a broader geographical area, including south‐eastern Australia (Victoria), eastern Australia (New South Wales), south‐western Australia (Western Australia), Tanzania, South Africa, Ethiopia, Spain, Portugal and Kenya (Hunter, 2007; Maxwell, 2004). ITS lineage 1 of T. nubilosa can be distinguished from ITS lineage 2 by four fixed base‐pair polymorphisms, two transitions and two transversions, occurring at nucleotide positions 13, 37, 255 and 334, respectively, of the ITS gene region (Hunter, 2007).
Figure 3.
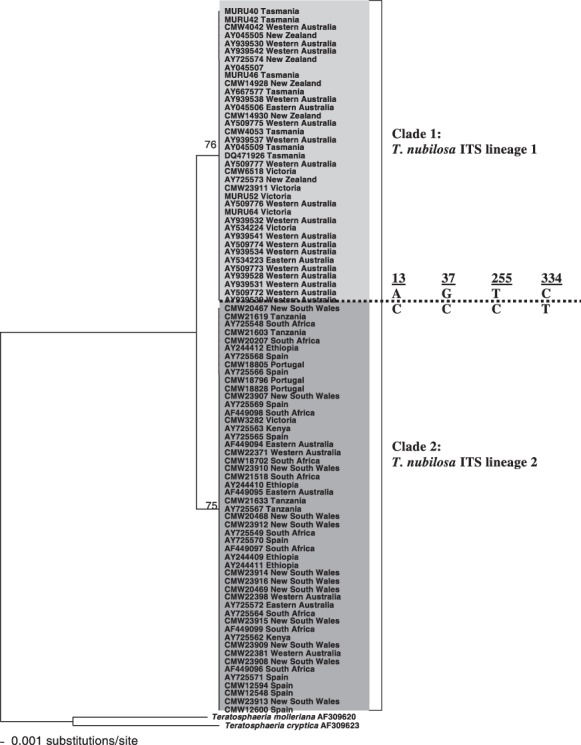
Neighbour‐joining phylogram obtained from a distance analysis using the Hasegawa–Kishino–Yano (HKY) substitution model on internal transcribed spacer (ITS) sequence data of Teratosphaeria nubilosa isolates. Bootstrap values after 1000 replicates are shown above the branches. Teratosphaeria molleriana and T. cryptica were used as outgroups. Fixed sequence polymorphisms, separating clade 1 from clade 2, and their base‐pair positions are indicated at the broken line.
Gene regions other than the ITS rDNA operon have been considered for the possible variation that their sequences might reflect for isolates of T. nubilosa. However, only a limited number of alternative loci have been studied, and these have shown little phylogenetic signal for variation within this species. Some variation within the β‐tubulin (Bt) 2 gene region has been observed, where isolates of T. nubilosa can be distinguished from each other on the basis of the presence of three base‐pair insertions at nucleotide position 191 (ACA/XXX) and a transition mutation at nucleotide position 215 (A/G) (Hunter, 2007). Although the translation elongation factor‐1α (EF‐1α) gene region has also been used to compare isolates of T. nubilosa, it appears that this gene region is conserved within the taxon, showing no polymorphisms (Hunter, 2007).
Variation within T. nubilosa has also been observed at the phenotypic level. This has notably been seen in patterns of germination in the fungus. Although T. nubilosa most typically has an F‐type ascospore germination, a recent study has shown that specimens from northern New South Wales in Australia produce multiple germ tubes on germination (Carnegie, 2007a), as well as typical type F germination.
Culture morphology is another plastic character in T. nubilosa. Early studies of this species in Australia by Park and Keane (1982b) showed two distinct culture morphologies. In one of these, the mycelium is black and submerged, producing dark‐green aerial hyphae. In the other culture form, the mycelium is submerged, with white to olive green aerial mycelium (Park and Keane, 1982b). Similar findings were noted by Hunter (2007), who found that the ex‐epitype isolate of T. nubilosa (isolated in Victoria, south‐eastern Australia) produced a colony morphology identical to the second type described by Park and Keane (1982b). However, two other colony morphologies were noted by Hunter (2007) for isolates from Victoria. In one of these colonies, uneven and irregular margins showing folding and sectoring with predominant white mycelial tufts occurred as extensive aerial mycelium. These colonies were pale greenish grey on the surface and pale olivaceous grey on the reverse. In the second type, colonies grew extremely slowly and produced irregular margins, extensive folding and convolutions with sparse aerial mycelium and submerged mycelium. These colonies were olivaceous grey on the surface and iron‐grey on the reverse (Hunter, 2007). In addition, cultures of T. nubilosa from northern New South Wales grew similar in colour to the second type described by Park and Keane (1982b), but much more slowly, with a somewhat raised appearance (A. J. Carnegie, unpublished data).
POPULATION BIOLOGY
Several studies on the population biology of species of Mycosphaerella occurring on cereal hosts have been published over the past several years. However, very little research into the population biology of Mycosphaerella and Teratosphaeria species occurring on Eucalyptus has been undertaken, apart from two studies into the population biology of T. cryptica and T. nubilosa (Hunter, 2007; Hunter et al., 2008; Maxwell, 2004; Milgate et al., 2005b).
Hunter et al. (2006a) developed 10 polymorphic microsatellite markers for T. nubilosa sensu stricto. These markers were used to study the movement and population biology of several T. nubilosa populations at different hierarchical levels from five different countries, including Australia, Portugal, Spain, South Africa and Tanzania. The population parameters that were investigated included gene diversity, genotypic diversity, population differentiation and gene flow.
The centre of origin of T. nubilosa has not been confirmed. This pathogen was originally identified from south‐eastern Australia near Melbourne in the state of Victoria (Cooke, 1891). By examining the gene diversity of a T. nubilosa population collected in New South Wales, eastern Australia, Hunter et al. (2008) were able to show that this population had a significantly higher gene diversity (0.506) when compared with the gene diversity of T. nubilosa populations collected in South Africa, which exhibited moderate to high diversity ranging from 0.149 to 0.250. These data substantiated the hypothesis that eastern Australia is indeed the centre of origin for T. nubilosa.
A large number of multilocus haplotypes (MLHs) were observed from the various T. nubilosa populations collected (Hunter et al., 2008). In total, 68 MLHs were observed from all populations from the five countries. A total of 42 MLHs were detected in the South African populations from the various hierarchical levels and, interestingly, one of these was the only one found from the Portuguese and Spanish populations, whereas another South African MLH was the only one detected in a T. nubilosa population from Tanzania (Hunter, 2007; Hunter et al., 2008). A further finding from this study was that the 10 MLHs of T. nubilosa identified from Western Australian (TN59–TN68) and the 16 MLHs identified from New South Wales (TN43–TN58) were unique to these two populations, and did not occur in any of the other countries in which populations were collected (4, 5).
Figure 4.
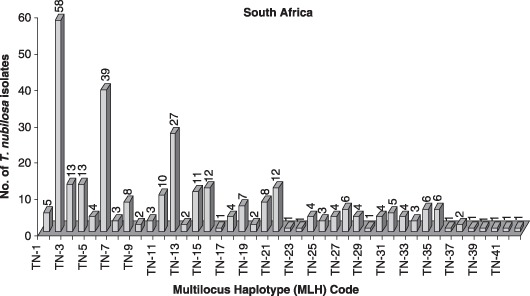
Total number of multilocus haplotypes (MLHs) of Teratosphaeria nubilosa isolated from Eucalyptus nitens in three provinces of South Africa (TN1–TN42) (Hunter et al., 2008).
Figure 5.
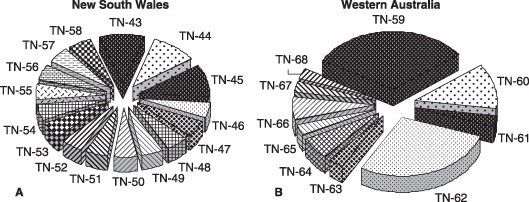
Multilocus haplotypes (MLHs) of Teratosphaeria nubilosa isolated from Australia: (A) 16 MLHs of T. nubilosa (TN43–TN58) isolated from New South Wales, eastern Australia; (B) 10 MLHs of T. nubilosa (TN59–TN68) isolated from Western Australia (Hunter et al., 2008).
Gene flow between T. nubilosa populations has also been examined. Hunter et al. (2008) observed a high rate of gene flow between T. nubilosa populations collected at three plantations in South Africa. However, very little gene flow was observed between the New South Wales and Western Australian T. nubilosa populations examined, and a low level of gene flow occurred between the Western Australian and South African T. nubilosa populations. From all of these data, Hunter et al. (2008) hypothesized that T. nubilosa is native to eastern Australia and that it was introduced into South Africa from Australia. Once in South Africa, T. nubilosa then spread into other countries of Africa, such as Tanzania, and finally into Europe (Fig. 6).
Figure 6.
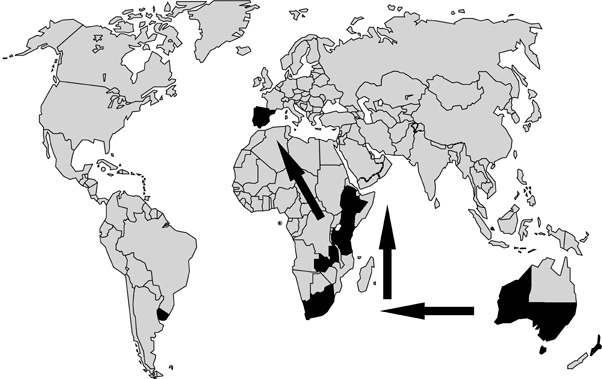
World map indicating the present distribution of Teratosphaeria nubilosa (black shaded areas). Arrows indicate the proposed pathway of gene flow and movement of T. nubilosa from Australia into South Africa and from South Africa into other countries of Africa and, finally, to Europe.
IDENTIFICATION TECHNIQUES
Classical taxonomic techniques are able to distinguish many of the species of Mycosphaerella and Teratosphaeria from Eucalyptus. The main basis is ascospore morphology, germination pattern, cultural characteristics and anamorph associations (Crous, 1998; Park and Keane, 1982a), which have been used to describe many species (Carnegie and Keane, 1994, 1998; Crous, 1998; Crous et al., 1993; Park and Keane, 1982a, 1984). These techniques are still able to distinguish species, but molecular analysis is now routinely used to confirm morphological identifications and to identify cryptic species.
Randomly amplified polymorphic DNA (RAPD) is effective in distinguishing between morphologically closely related Mycosphaerella and Teratosphaeria spp. Carnegie et al. (2001) employed RAPDs (Table 1) to effectively distinguish between T. cryptica, M. gregaria, T. nubilosa and M. marksii. DNA‐based methods, such as RAPD, which can distinguish between species based on differences in DNA sequence, are particularly advantageous. The reliability and standardization of RAPD across laboratories, however, are problematic and represent the major disadvantages of this technique.
Table 1.
Sequences of primers that have been used in molecular biology techniques in the identification and study of Teratosphaeria nubilosa.
| Primer | Usage | Sequence (5′−3′) | Annealing temperature | Product size (bp) | Polymorphisms | Reference |
|---|---|---|---|---|---|---|
| OPW‐06 | RAPD | AGGCCCGATG | 40 | 250–2000 | 8–11* | Carnegie et al. (2001) |
| OPV‐08 | RAPD | GGACGGCGTT | 40 | 250–2000 | 8–11* | |
| OPV‐18 | RAPD | TGGTGGCGTT | 40 | 250–2000 | 8–11* | |
| OPV‐19 | RAPD | GGGTGTGCAG | 40 | 250–2000 | 8–11* | |
| OPX‐01 | RAPD | CTGGGCACGA | 40 | 250–2000 | 8–11* | |
| OPX‐07 | RAPD | GAGCCAGGCT | 40 | 250–2000 | 8–11* | |
| MNF | SSP | CGTCGGAGTAATACAACC | 50 | 199 | N/A | Kularatne et al. (2004) |
| MNR | AGGCTGGAGTGGTGAAATG | |||||
| MnubF | SSP | CAACCCCATGTTTTCCCACCACG | 62 | 395 | N/A | Glen et al. (2007) |
| MnubR | CGCCAGACCGGTCCCCGTC | |||||
| MN1F | SSP | GCGCCAGCCCGACCTCC | 57 | 404 | N/A | Maxwell et al. (2005) |
| MN1R | GGTCCCCGTCAGCGAAACAGT | 56 | ||||
| MN‐1 | SSR | TCCTGAAATGAGTGCAGACG | 60 | 257–271 | (AG)10(TG)10 † | Hunter et al. (2006a) |
| TCCTCATCCTCTGTGGAACC | ||||||
| MN‐2 | SSR | CATTGCTTCGGCGGTTATAG | 60 | 182–266 | (ACT)859bp(AC)11 † | |
| ATGCACGAAGTCGTTGTTTG | ||||||
| MN‐3 | SSR | GACTCAACCGTCGTCGAAAC | 60 | 306–320 | (AC)13 † | |
| CGAACTGAATCCGCTGTGTA | ||||||
| MN‐4 | SSR | TGTCACAAGACTTTGGATTGC | 60 | 137–165 | (ATTGTGG)10 † | |
| CCACCACAATCTCCTCACAA | ||||||
| MN‐7 | SSR | CGCCTCACAGTTACACATGG | 60 | 377–395 | (TGTA)6 † | |
| CGAAAGGCTGAGGCTGAA | ||||||
| MN‐8 | SSR | TTCTATATACTATATTCTATTTAGG | 53 | 202–322 | (CTCTCTATA)20 † | |
| ATATACTATATCTAAAAGAGGTAG | ||||||
| MN‐9 | SSR | CGAATGGGCTATCAGAAACG | 60 | 211–221 | (CT)20 † | |
| ACAGGGCAAGGACCTCGTAT | ||||||
| MN‐10 | SSR | ACACCTCGAAATCGCTCATC | 60 | 136–144 | (TC)11 † | |
| TAGCTCTGTGCTGCCTTTGA | ||||||
| MN‐11 | SSR | CTCACCAGTGCCGTCTAGGT | 60 | 193–223 | (TTGGTG)5 † | |
| GGAAATCCTGCCCTAACCTC | ||||||
| MN‐14 | SSR | TCGACTACCGTAGGGGACTACT | 60 | 100–112 | (AC)13 † | |
| ATGCACGAAGTCGTTGTTTG |
RAPD, randomly amplified polymorphic DNA; SSP, species‐specific primer; SSR, simple sequence repeat/microsatellite marker.
Number of scoreable polymorphic amplicons used to identify Teratosphaeria nubilosa with RAPD.
Core microstallite region amplified with microsatellite primers and exhibiting size polymorphisms in repeat number.
Species‐specific DNA primers are effective for distinguishing between morphologically similar species of Mycosphaerella and Teratosphaeria occurring on Eucalyptus. Maxwell et al. (2005) developed species‐specific primers for T. cryptica, M. lateralis, M. marksii, T. nubilosa (Table 1) and T. parva. Similarly, Glen et al. (2007) were also able to develop species‐specific primers for T. cryptica, T. nubilosa and M. tasmaniensis (Table 1). All of these species are known to occur in Australia and several other countries in which they cause MLD (Crous, 1998). The developed primers were used to amplify DNA from the selected Mycosphaerella spp. and from diseased Eucalyptus leaves infected with these specific Mycosphaerella and Teratosphaeria spp. In the case of T. nubilosa, it is interesting that the species‐specific primers developed by Maxwell et al. (2005) and Glen et al. (2007) were able to detect the presence of T. nubilosa in Eucalyptus leaves that showed hardly any symptoms of T. nubilosa infection. This technique provides a useful tool in quarantine facilities and in nurseries, where it is necessary to detect Mycosphaerella spp. in planta. Kularatne et al. (2004) developed a polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) technique for the effective identification of T. nubilosa (Table 1) and T. cryptica. This technique also allowed for these species to be detected directly from infected Eucalyptus leaf tissue.
DNA sequencing comparison for various DNA gene regions and phylogenetic inference has become the most commonly used approach to confirm the morphological diagnosis of Mycosphaerella and Teratosphaeria spp. occurring on Eucalyptus spp. The ITS rDNA operon was the first and is the most commonly used gene region to identify these fungi (e.g. Arzanlou et al., 2008; Crous et al., 2000, 2001a, 2001b, 2006a; Hunter et al., 2004a,b). However, the ITS gene region does not always provide sufficient resolution to distinguish between Mycosphaerella spp. and their anamorphs (Verkley and Starink‐Willemse, 2004). Therefore, several other nuclear gene regions, such as Actin (ACT), EF‐1α and Bt, have also recently been used to consider species’ boundaries and cryptic taxa within Mycosphaerella (Crous et al., 2006b, 2007a; Hunter et al., 2006b). It is certain that the generation of greater numbers of DNA sequence data sets of various nuclear and mitochondrial gene regions, and the combination of these data sets, will contribute substantially to our understanding of species’ concepts within Mycosphaerella and Teratosphaeria in the future. They should also provide a more refined understanding of teleomorph and anamorph morphologies that are phylogenetically informative.
DISEASE MANAGEMENT
The management of MLD is difficult because commercial Eucalyptus plantations cover thousands of hectares (over 451 000 ha of E. globulus in Australia alone; Parsons et al., 2006). This, combined with the fact that T. nubilosa produces wind‐dispersed ascospores (Park and Keane, 1982b), complicates efforts to control the disease. Nonetheless, various management strategies have been used for the control of MLD and, in combination, they can lead to a decrease in losses caused by T. nubilosa.
The most effective means to deal with diseases of plantation‐grown trees is by deploying resistant planting stock. The selection of less susceptible Eucalyptus species has been used to combat MLD in several countries following severe epidemics during early plantings. For example, E. nitens has replaced the more susceptible E. globulus in South Africa (Lundquist and Purnell, 1987) and north‐western Tasmania, Australia (Mohammed et al., 2003). Early plantations in New Zealand, using E. delegatensis and E. regnans, were severely damaged by MLD (T. cryptica), which led to the use of more resistant species, such as E. nitens.
Provenance variation has been utilized to further reduce the impact of MLD. New South Wales provenances of E. nitens have been selected for planting over Victorian provenances in South Africa (Purnell and Lundquist, 1986). Several authors have recommended the use of the inherent resistance in E. globulus for planting in high‐risk areas for MLD in south‐eastern Australia (Carnegie et al., 1994; Milgate et al., 2005a).
Hybrids between susceptible and resistant Eucalyptus spp. have been very useful in avoiding damage caused by various diseases. Hybrids between Eucalyptus spp., such as E. globulus (susceptible to T. nubilosa), and distantly related species, such as E. grandis (tolerant to T. nubilosa), are only now emerging from breeding programmes. These hybrids between E. globulus and E. grandis are displaying very high levels of resistance to MLD in Uruguay (M. J. Wingfield, unpublished data). This is in contrast with the parent E. globulus which is often severely affected. However, although hybrids provide an exciting opportunity to avoid MLD caused by T. nubilosa, there is also cause to be cautious. For example, Dungey et al. (1997) and Carnegie and Ades (2002) found that F1 E. globulus × E. nitens hybrids were more susceptible to MLD than either parents of the cross. Carnegie and Ades (2002) further suggested that such Eucalyptus hybrids should not be used in environments where MLD is severe. This enhanced susceptibility may be related to the fact that the parent trees both carried genes for susceptibility to infection. Similarly, in New Zealand, hybrids of E. regnans × E. delegatensis were more severely damaged by MLD (T. cryptica) (Wilcox, 1982). The caveat is that hybridization between species can result in a complex suite of traits, and the testing of progeny carefully prior to commercial deployment is an essential prerequisite.
Available moisture levels play an important part in the development of ascomata and ascospores. Knowing the optimal amount of leaf moisture necessary for the development and maturation of ascomata and ascospores of Mycosphaerella and Teratosphaeria spp., the frequency and length of watering in nursery systems can be adjusted to decrease the levels of inoculum (Mondal and Timmer, 2002). Furthermore, any dead or decomposing leaf material present in a nursery or plantation acts as an inoculum source, as ascomata are capable of developing in such material (Park and Keane, 1987). It is therefore beneficial to remove any such material from a nursery system. Overhead irrigation mechanisms should also be avoided in nurseries, as water accumulates on leaf surfaces and stimulates the production of ascospores. Alternative drip irrigation or hydroponic systems for irrigation of nursery stock should be used, thereby avoiding the high humidity levels and the periods of water accumulation on leaf surfaces.
Fungicide applications can provide a means to control the development of T. nubilosa. In a nursery environment, this method is most feasible because of the smaller size of the Eucalyptus seedlings and the growth tunnels in which they are housed. Furthermore, it has been suggested that the cost of fungicide applications can be reduced by spraying during the vegetative period of the host (Park, 1988b). Carnegie and Ades (2003) showed that spraying both a protectant and systemic fungicide significantly reduced the development of MLD on juvenile and adult foliage of E. globulus in the field. Disease forecasting systems can also be used to determine the most appropriate time for fungicide application (Jacome et al., 1991). However, once deployed into the field and at a plantation level, applications are unlikely to be economically viable for forestry companies (Carnegie and Ades, 2003). They are also detrimental to the environment and are typically prohibited by groups that certify forest operations, such as the Forestry Stewardship Council (FSC, http://www.fsc.org/).
Carnegie (2007b) proposed three strategies for the management of foliar fungi in Eucalyptus plantations: (i) risk‐site mapping, i.e. identifying high‐risk sites for disease and deploying more tolerant genotypes into these areas; (ii) tree resistance, as discussed above; and (iii) increasing tree tolerance and recovery, i.e. reducing the impact of defoliation events by ensuring that trees are growing optimally, either through correct site selection or remedial fertilization following a defoliation event.
CONCLUSIONS AND FUTURE PERSPECTIVES
Mycosphaerella and Teratosphaeria spp. were traditionally regarded as host‐specific fungi. However, recent studies have identified several species of Mycosphaerella and Teratosphaeria that have undergone host jumps and are now able to infect new susceptible hosts. For example, M. citri, a recognized pathogen of Citrus, has been identified from leaves of Acacia mangium in Thailand, and several other plant hosts, such as Musa sp., Aeglopsis spp., Fortunella and Poncirus (Crous et al., 2004b). Mycosphaerella communis is a Eucalyptus pathogen that has also been found on Protea spp. (Crous et al., 2004a). With increased surveys, there is no doubt that more Mycosphaerella and Teratosphaeria spp. will be found on a larger number of hosts. Similarly, T. nubilosa, which has hitherto only been known from Eucalyptus spp., has been isolated from Acacia in Thailand (Crous and Groenewald, 2005). The full implications of this finding is not yet known and, until it has been established whether T. nubilosa can also cause disease on hosts other than Eucalyptus, it must be accepted that it can also act as a facultative saprobe on leaf spots caused by other species of Mycosphaerella and Teratosphaeria.
Over the past few years many fungal genomes have been sequenced. Genomic sequencing is becoming more affordable, and several important plant pathogenic fungi are candidates for future genomic sequencing. The genomes of various ascomycete fungi have also been sequenced, including species of Mycosphaerella, such as M. graminicola (http://genome.jgi‐psf.org/Mycgr1/Mycgr1.home.html) and M. fijiensis (http://genome.jgi‐psf.org/Mycfi1/Mycfi1.home.html), two important plant pathogens. Considering the importance of T. nubilosa as a severe pathogen of Eucalyptus spp., it would represent a good candidate for complete genomic sequencing. A genome sequence of T. nubilosa would offer several advantages. Virulence genes could be relatively easily mapped, and such information would aid in the selection and breeding of resistant or tolerant Eucalyptus species and clones. Furthermore, many more microsatellite regions could be easily located and targeted, thereby increasing the number of markers to be used in population studies of this important pathogen.
Leaf lesions caused by species of Mycosphaerella and Teratosphaeria generally show the presence of more than one species within a lesion. It is possible, therefore, that mycelium of different Mycosphaerella and Teratosphaeria species may come into contact within a lesion, leading to hyphal anastomosis and the exchange of genetic material. Through this process, hybrids between different species may be formed. No research has thus far been undertaken to identify hybrids within Mycosphaerella or Teratosphaeria species occurring on Eucalyptus, although multigene DNA sequence data suggest that there may be evidence of hybridization events on this host.
The movement of infected plant material between countries and continents is increasing rapidly (Wingfield et al., 2001). Therefore, many fungal pathogens will most probably be introduced into new environments (Wingfield, 1999). Quarantine measures should consequently be strictly implemented and updated to reduce the risk of fungal pathogens, such as T. nubilosa, being introduced into new environments. For example, T. nubilosa has recently been identified in South America (G. Perez, FABI, Pretoria, South Africa, unpublished data), an area from which it was previously unknown. Therefore, it is important that Mycosphaerella and Teratosphaeria spp. be incorporated into quarantine regulations and actionable lists.
ACKNOWLEDGEMENTS
We thank the members of the Tree Protection Co‐operative Programme (TPCP), Centre of Excellence in Tree Health Biotechnology (CTHB), National Research Foundation (NRF) and the THRIP initiative of the Department of Trade and Industry, South Africa for financial support. We also thank Mr. Ian Smith for figures 1A and 1B and Mr. David Smith for figure 1C.
REFERENCES
- Arzanlou, M. , Groenewald, J.Z. , Fullerton, R.A. , Abeln, E.C.A. , Carlier, J. , Zapater, M.‐F. , Buddenhagen, I.W. , Viljoen, A. and Crous, P.W. (2008) Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia, 20, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, P.A. , Carnegie, A.J. , Burgess, T.I. and Keane, P.J. (2008) Leaf diseases caused by Mycosphaerella species in Eucalyptus globulus plantations and nearby native forest in the Green Triangle Region of southern Australia. Australas Plant Path. 37, 472–481. [Google Scholar]
- Burgess, T.I. , Barber, P.A. , Sufaati, S. , Xu, D. , Hardy, G.E.St.J. and Dell, B. (2007) Mycosphaerella spp. on Eucalyptus in Asia; new species; new hosts and new records. Fungal Divers. 24, 135–157. [Google Scholar]
- Carnegie, A.J. (2000) A study of the species of Mycosphaerella on eucalypts and the impact of Mycosphaerella leaf diseases on Eucalyptus globulus Labill. PhD Thesis. Melbourne: University of Melbourne. [Google Scholar]
- Carnegie, A.J. (2007a) Forest health condition in New South Wales, Australia, 1996–2005. I. Fungi recorded from eucalypt plantations during forest health surveys. Australas Plant Path. 36, 213–224. [Google Scholar]
- Carnegie, A.J. (2007b) Forest health in New South Wales, Australia, 1996–2005. II. Fungal damage recorded in eucalypt plantations during forest health surveys and their management. Australas Plant Path. 36, 225–239. [Google Scholar]
- Carnegie, A.J. and Ades, P.K. (2002) The proportion of leaf spots caused by Mycosphaerella cryptica and M. nubilosa on Eucalyptus globulus, E. nitens and their F1 hybrids in a family trial in Tasmania, Australia. Aust. Mycol. 21, 53–63. [Google Scholar]
- Carnegie, A.J. and Ades, P.K. (2003) Mycosphaerella leaf disease reduces growth of plantation‐grown Eucalyptus globulus . Aust. Forest. 66, 113–119. [Google Scholar]
- Carnegie, A.J. and Ades, P.K. (2005) Variation in Eucalyptus globulus Labill. and E. nitens Dean and Maiden in susceptibility of adult foliage to disease caused by Mycosphaerella cryptica (Cooke) Hansf. Silvae Genet. 54, 174–184. [Google Scholar]
- Carnegie, A.J. and Keane, P.J. (1994) Further Mycosphaerella species associated with leaf diseases of Eucalyptus . Mycol. Res. 98, 413–418. [Google Scholar]
- Carnegie, A.J. and Keane, P.J. (1998) Mycosphaerella vespa sp. nov. from diseased Eucalyptus leaves in Australia. Mycol. Res. 102, 1274–1276. [Google Scholar]
- Carnegie, A.J. , Ades, P.K. and Ford, R. (2001) The use of RAPD‐PCR analysis for the differentiation of Mycosphaerella species from Eucalyptus in Australia. Mycol. Res. 105, 1313–1320. [Google Scholar]
- Carnegie, A.J. , Ades, P.K. , Keane, P.J. and Smith, I.W. (1998) Mycosphaerella diseases of juvenile foliage in a eucalypt species and provenance trial in Victoria, Australia. Aust. Forest. 61, 190–194. [Google Scholar]
- Carnegie, A.J. , Burgess, T.I. , Beilharz, V. and Wingfield, M.J. (2007) New species of Mycosphaerella from Myrtaceae in plantations and native forests in eastern Australia. Mycologia, 99, 461–474. [DOI] [PubMed] [Google Scholar]
- Carnegie, A.J. , Johnson, I.G. and Henson, M. (2004) Variation among provenances and families of blackbutt (Eucalyptus pilularis) in early growth and susceptibility to damage from leaf spot fungi. Can. J. Forest. Sci. 34, 2314–2326. [Google Scholar]
- Carnegie, A.J. , Keane, P.J. , Ades, P.K. and Smith, I.W. (1994) Variation in susceptibility of Eucalyptus globulus provenances to Mycosphaerella leaf disease. Can. J. Forest Res. 24, 1751–1757. [Google Scholar]
- Cheah, L.H. and Hartill, W.F.T. (1987) Ascospore release in Mycosphaerella cryptica (Cooke) Hansford. Eur. J. Forest Pathol. 17, 129–141. [Google Scholar]
- Cooke, M.C. (1891) Australian fungi. Grevillea, 19, 60–62. [Google Scholar]
- Crous, P.W. (1998) Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus . Mycol. Memoirs, 21, 1–170. [Google Scholar]
- Crous, P.W. and Groenewald, J.Z. (2005) Hosts, species and genotypes: opinion versus data. Australas Plant Path. 34, 463–470. [Google Scholar]
- Crous, P.W. and Wingfield, M.J. (1996) Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia, 88, 441–458. [Google Scholar]
- Crous, P.W. , Aptroot, A. , Kang, J.C. , Braun, U. and Wingfield, M.J. (2000) The genus Mycosphaerella and its anamorphs. Stud. Mycol. 45, 107–121. [Google Scholar]
- Crous, P.W. , Braun, U. and Groenewald, J.Z. (2007a) Mycosphaerella is polyphyletic. Stud. Mycol. 58, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Ferreira, F.A. , Alfenas, A. and Wingfield, M.J. (1993) Mycosphaerella suberosa associated with corky leaf spots on Eucalyptus in Brazil. Mycologia, 85, 705–710. [Google Scholar]
- Crous, P.W. , Groenewald, J.Z. , Groenewald, M. , Caldwell, P. , Braun, U. and Harrington, T.C. (2006a) Species of Cercospora associated with grey leaf spot of maize. Stud. Mycol. 55, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Groenewald, J.Z. , Mansilla, J.P. , Hunter, G.C. and Wingfield, M.J. (2004a) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus . Stud. Mycol. 50, 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Groenewald, J.Z. , Pongpanich, K. , Himaman, W. , Arzanlou, M. and Wingfield, M.J. (2004b) Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Stud. Mycol. 50, 457–469. [Google Scholar]
- Crous, P.W. , Hong, L. , Wingfield, B.D. and Wingfield, M.J. (2001a) ITS rDNA phylogeny of selected Mycosphaerella species and their anamorphs occurring on Myrtaceae. Mycol. Res. 105, 425–431. [Google Scholar]
- Crous, P.W. , Kang, J.C. and Braun, U. (2001b) A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia, 93, 1081–1101. [Google Scholar]
- Crous, P.W. , Summerell, B.A. , Carnegie, A.J. , Mohammed, C. , Himaman, N. and Groenewald, J.Z. (2007b) Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus . Fungal Divers. 26, 143–185. [Google Scholar]
- Crous, P.W. , Wingfield, M.J. and Park, R.F. (1991) Mycosphaerella nubilosa, a synonym of M. molleriana . Mycol. Res. 95, 628–632. [Google Scholar]
- Crous, P.W. , Wingfield, M.J. , Mansilla, J.P. , Alfenas, A.C. and Groenewald J.Z. (2006b) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Stud. Mycol. 55, 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison, N.P. and Kietzka, J.A. (1993) The use and importance of hybrid intensive forestry in South Africa. S. Afr. Forest. J. 165, 55–60. [Google Scholar]
- Dick, M. (1982) Leaf inhabiting fungi of eucalypts in New Zealand. New Zeal. J. For. Sci. 12, 525–537. [Google Scholar]
- Dick, M. and Gadgil, P.D. (1983) Eucalyptus leaf spots Pathology in New Zealand No. 1. Rotorua: New Zealand Forest Service, Forest Research Institute. [Google Scholar]
- Doidge, M.E. (1950) The South African fungi and lichens to the end of 1945. Bothalia, 5, 1–1094. [Google Scholar]
- Dungey, H.S. , Potts, B.M. , Carnegie, A.J. and Ades, P.K. (1997) Mycosphaerella leaf disease: genetic variation in damage to Eucalyptus nitens, Eucalyptus globulus, and their F1 hybrid. Can. J. Forest Res. 27, 750–759. [Google Scholar]
- Ganapathi, A. (1979) Studies on the etiology of the leaf blotch disease of Eucalyptus spp. caused by Mycosphaerella nubilosa (Cke.) Hansf. PhD Dissertation. Auckland: Department of Botany, University of Auckland. [Google Scholar]
- Ganapathi, A. and Corbin, J.B. (1979) Colletogloeum nubilosum sp. nov., the imperfect state of Mycosphaerella nubilosa on Eucalyptus in New Zealand. T. Brit. Mycol. Soc. 72, 237–244. [Google Scholar]
- Gezahgne, A. , Roux, J. , Hunter, G.C. and Wingfield, M.J. (2006) Mycosphaerella species associated with leaf disease of Eucalyptus globulus in Ethiopia. Forest Pathol. 36, 253–263. [Google Scholar]
- Glen, M. , Smith, A.H. , Langrell, S.R.H. and Mohammed, C.L. (2007) Development of nested polymerase chain reaction detection of Mycosphaerella spp. and its application to the study of leaf disease in Eucalyptus plantations. Phytopathology, 97, 132–144. [DOI] [PubMed] [Google Scholar]
- Goodwin, S.B. , Dunkle, L.D. and Zismann, V.L. (2001) Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology, 91, 648–658. [DOI] [PubMed] [Google Scholar]
- Hansford, C.G. (1956) Australian fungi. III. New species and revisions (continued). P. Linn. Soc. N. S. W. 81, 23–51. [Google Scholar]
- Hood, I.A. , Gardner, J.F. , Kimberley, M.O. and Molony, K. (2002) Variation among eucalypt species in early susceptibility to the leaf spot fungi Phaeophleospora eucalypti and Mycosphaerella spp. New Zeal. J. For. Sci. 32, 235–255. [Google Scholar]
- Hunter, G.C. (2007) Taxonomy, phylogeny and population biology of Mycosphaerella species occurring on Eucalyptus , pp. 164–196. PhD Thesis. Pretoria: Department of Microbiology and Plant Pathology, University of Pretoria. [Google Scholar]
- Hunter, G.C. , Cortinas, M.N. , Wingfield, B.D. , Crous, P.W. and Wingfield, M.J. (2006a) Development of polymorphic microsatellite markers for the Eucalyptus leaf pathogen Mycosphaerella nubilosa . Mol. Ecol. Notes, 6, 900–903. [Google Scholar]
- Hunter, G.C. , Crous, P.W. , Roux, J. , Wingfield, B.D. and Wingfield, M.J. (2004a) Identification of Mycosphaerella species associated with Eucalyptus nitens leaf defoliation in South Africa. Australas Plant Path. 33, 349–355. [Google Scholar]
- Hunter, G.C. , Roux, J. , Wingfield, B.D. , Crous, P.W. and Wingfield, M.J. (2004b) Mycosphaerella species causing leaf disease in South African Eucalyptus plantations. Mycol. Res. 108, 672–681. [DOI] [PubMed] [Google Scholar]
- Hunter, G.C. , Van Der Merwe, N.A. , Burgess, T.I. , Carnegie, A.J. , Wingfield, B.D. , Crous, P.W. and Wingfield, M.J. (2008) Global movement and population biology of Mycosphaerella nubilosa infecting leaves of cold‐tolerant Eucalyptus globulus and E. nitens . Plant Pathol. 57, 235–242. [Google Scholar]
- Hunter, G.C. , Wingfield, B.D. , Crous, P.W. and Wingfield, M.J. (2006b) A multi‐gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Stud. Mycol. 55, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, S.L. , Maxwell, A. , Burgess, T.I. , Hardy, G.E.St.J. and Dell, B. (2008) Incidence and new records of Mycosphaerella species within a Eucalyptus globulus plantation in Western Australia. Forest Ecol. Manag. 255, 3931–3937. [Google Scholar]
- Jackson, S.L. , Maxwell, A. , Dell, B. and Hardy, G.E.St.J. (2005) New records of Mycosphaerella leaf disease from Eucalyptus in Western Australia. Australas Plant Path. 34, 423–424. [Google Scholar]
- Jacome, L.H. , Schuh, W. and Stevenson, R.E. (1991) Effect of temperature and relative humidity on germination and germ development of Mycosphaerella fijiensis var. difformis . Phytopathology, 81, 1480–1485. [Google Scholar]
- Kularatne, H.A.G.C. , Lawrie, A.C. , Barber, P.A. and Keane, P.J. (2004) A specific primer PCR and RFLP assay for the rapid identification and differentiation in planta of some Mycosphaerella species associated with foliar diseases of Eucalyptus globulus. Mycol. Res. 108, 1476–1493. [DOI] [PubMed] [Google Scholar]
- Lee, D. (2007) Achievements in forest tree genetic improvement in Australia and New Zealand 2: Development of Corymbia species and hybrids for plantations in eastern Australia. Aust. Forest. 70, 11–16. [Google Scholar]
- Lundquist, J.E. and Purnell, R.C. (1987) Effects of Mycosphaerella leaf spot on growth of Eucalyptus nitens . Plant Dis. 71, 1025–1029. [Google Scholar]
- Maxwell, A. (2004) The taxonomy, phylogeny and impact of Mycosphaerella species on eucalypts in south‐western Australia. PhD Thesis, pp. 93–128. Perth, WA: School of Biological Sciences and Biotechnology, Murdoch University. [Google Scholar]
- Maxwell, A. , Hardy, G.E.St.J. and Dell, B. (2001) First record of Mycosphaerella nubilosa in Western Australia. Aust. Plant Pathol. 30, 65. [Google Scholar]
- Maxwell, A. , Jackson, S.L. , Dell, B. and Hardy, G.E.St.J. (2005) PCR‐identification of Mycosphaerella species associated with leaf diseases of Eucalyptus . Mycol. Res. 109, 992–1004. [DOI] [PubMed] [Google Scholar]
- Milgate, A.W. , Potts, B.M. , Joyce, K. , Mohammed, C. and Vaillancourt, R.E. (2005a) Genetic variation in Eucalyptus globulus for susceptibility to Mycosphaerella nubilosa and its association with tree growth. Australas Plant Path. 34, 11–18. [Google Scholar]
- Milgate, A.W. , Vaillancourt, R.E. , Mohammed, C. , Powell, M. and Potts, B.M. (2005b) Genetic structure of a Mycosphaerella cryptica population. Australas Plant Path. 34, 345–354. [Google Scholar]
- Milgate, A.W. , Yuan, Z.Q. , Vaillancourt, R.E. and Mohammed, C. (2001) Mycosphaerella species occurring on Eucalyptus globulus and Eucalyptus nitens plantations of Tasmania, Australia. Forest Pathol. 31, 53–63. [Google Scholar]
- Mohammed, C. , Wardlaw, T. , Smith, A. , Pinkard, E. , Battaglia, M. , Glen, M. , Tommerup, I. , Potts, B. and Vaillancourt, R. (2003) Mycosphaerella leaf diseases of temperate eucalypts around the southern pacific rim. New Zeal. J. For. Sci. 33, 362–372. [Google Scholar]
- Mondal, S.N. and Timmer, L.W. (2002) Environmental factors affecting pseudothecial development and ascospore production of Mycosphaerella citri, the cause of citrus greasy spot. Phytopathology, 92, 1267–1275. [DOI] [PubMed] [Google Scholar]
- Niyo, K.A. , McNabb, H.S. Jr. and Tiffany, L.H. (1986) Ultrastructure of the ascocarps, asci and ascospores of Mycosphaerella populorum . Mycologia, 78, 202–212. [Google Scholar]
- Park, R.F. (1988a) Effect of certain host, inoculum, and environmental factors on infection of Eucalyptus species by two Mycosphaerella species. T. Brit. Mycol. Soc. 90, 221–228. [Google Scholar]
- Park, R.F. (1988b) Epidemiology of Mycosphaerella nubilosa and M. cryptica on Eucalyptus spp. in south‐western Australia. T. Brit. Mycol. Soc. 91, 261–266. [Google Scholar]
- Park, R.F. and Keane, P.J. (1982a) Three Mycosphaerella species from leaf diseases of Eucalyptus . T. Brit. Mycol. Soc. 79, 95–100. [Google Scholar]
- Park, R.F. and Keane, P.J. (1982b) Leaf diseases of Eucalyptus associated with Mycosphaerella species. T. Brit. Mycol. Soc. 79, 101–115. [Google Scholar]
- Park, R.F. and Keane, P.J. (1984) Further Mycosphaerella species causing leaf diseases of Eucalyptus . T. Brit. Mycol. Soc. 83, 93–105. [Google Scholar]
- Park, R.F. and Keane, P.J. (1987) Spore production by Mycosphaerella species causing leaf diseases of Eucalyptus . T. Brit. Mycol. Soc. 89, 461–470. [Google Scholar]
- Parsons, M. , Gavran, M. and Davidson, J. (2006) Australias Plantations 2006 National Plantation Inventory. Canberra: Bureau of Rural Sciences. [Google Scholar]
- Pinkard, E.A. and Mohammed, C.L. (2006) Photosynthesis of Eucalyptus globulus with Mycosphaerella leaf disease. New Phytol. 170, 119–127. [DOI] [PubMed] [Google Scholar]
- Purnell, R.C. and Lundquist, J.E. (1986) Provenance variation of Eucalyptus nitens on the Eastern Transvaal Highveld in South Africa. S. Afr. Forest. J. 138, 23–31. [Google Scholar]
- Smith, A.H. , Pinkard, E.A. , Hunter, G.C. , Wingfield, M.J. and Mohammed, C.L. (2006) Anatomical variation and defence responses of juvenile Eucalyptus nitens leaves to Mycosphaerella leaf disease. Australas Plant Path. 35, 725–731. [Google Scholar]
- Verkley, G.J.M. and Starink‐Willemse, M. (2004) A phylogenetic study of some Septoria species pathogenic to Asteraceae based on ITS ribosomal DNA sequences. Mycol. Prog. 3, 315–323. [PubMed] [Google Scholar]
- Wilcox, M.D. (1982) Preliminary selection of suitable provenances of Eucalyptus regnans for New Zealand. New Zeal. J. For. Sci. 12, 468–479. [Google Scholar]
- Wingfield, M.J. (1999) Pathogens in exotic plantation forestry. Int. For. Rev. 1, 163–168. [Google Scholar]
- Wingfield, M.J. , Crous, P.W. and Boden, D. (1996) Kirramyces destructans sp. nov., a serious leaf pathogen of Eucalyptus in Indonesia. S. Afr. J. Bot. 62, 325–327. [Google Scholar]
- Wingfield, M.J. , Roux, J. , Coutinho, T. , Govender, P. and Wingfield, B.D. (2001) Plantation disease and pest management in the next century. S. Afr. Forest. J. 190, 67–71. [Google Scholar]


